Evaporation, Autoignition and Micro-Explosion Characteristics of RP-3 Kerosene Droplets under Sub-Atmospheric Pressure and Elevated Temperature
Abstract
:1. Introduction
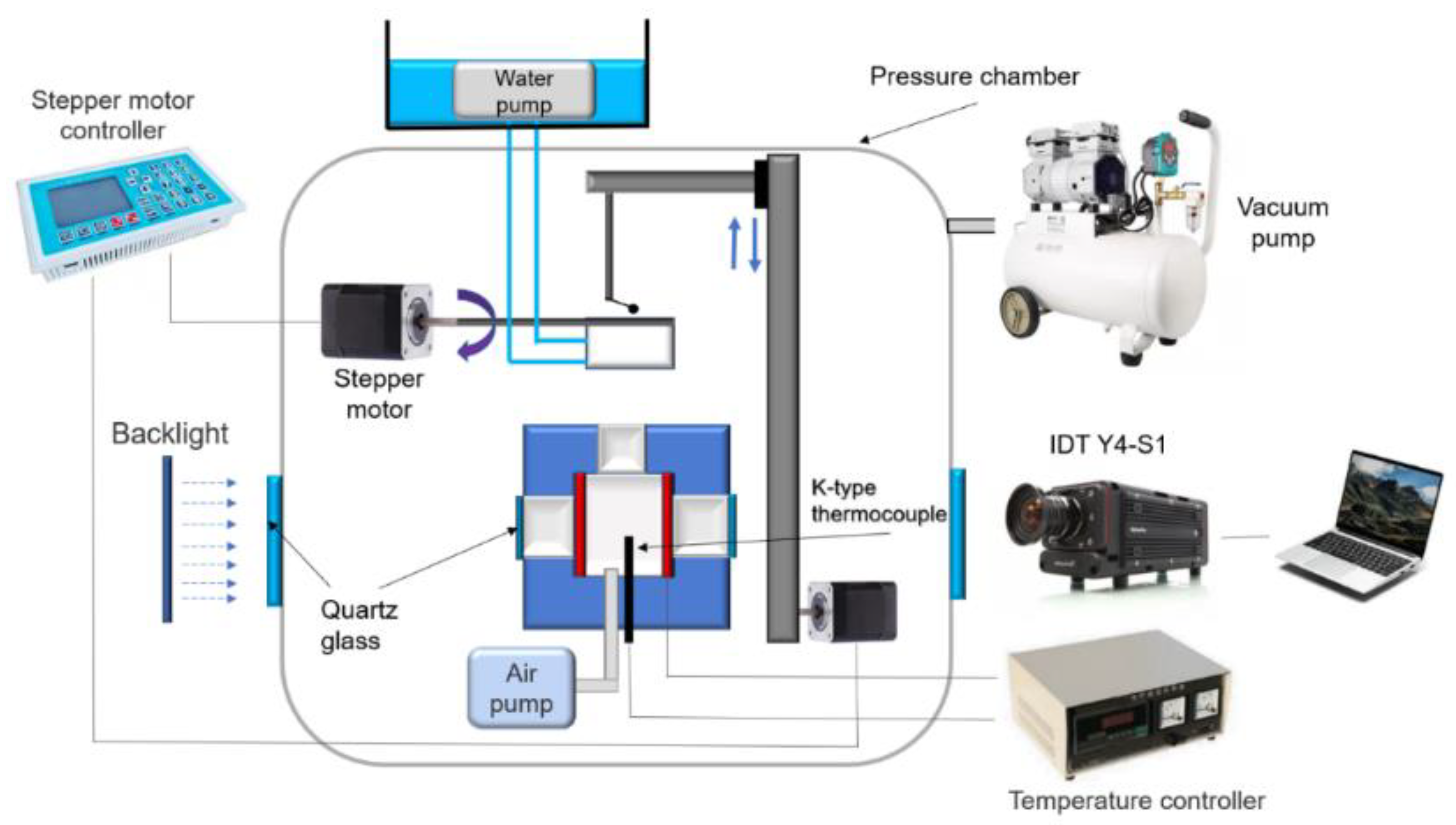
2. Materials and Methods
3. Results and Discussion
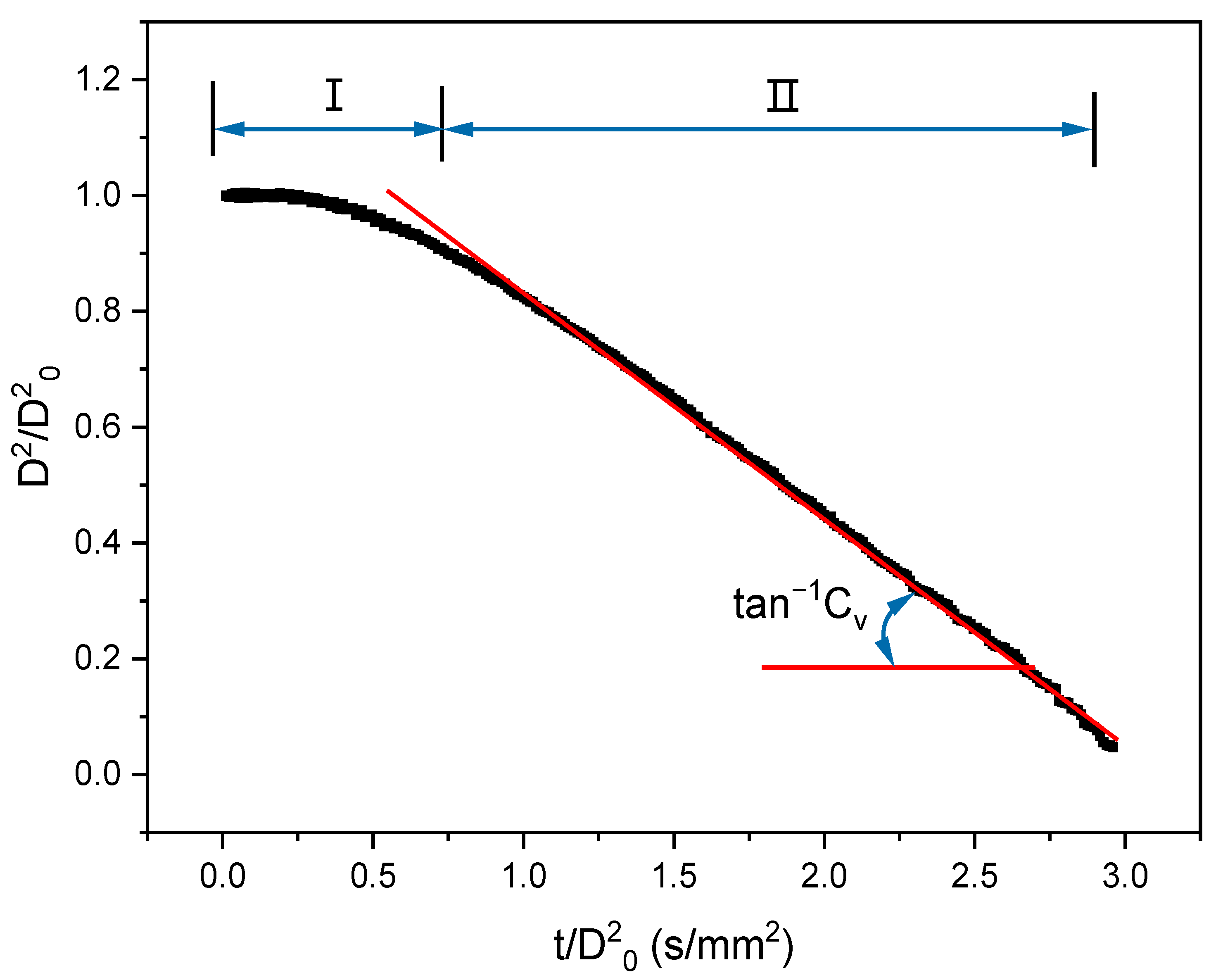
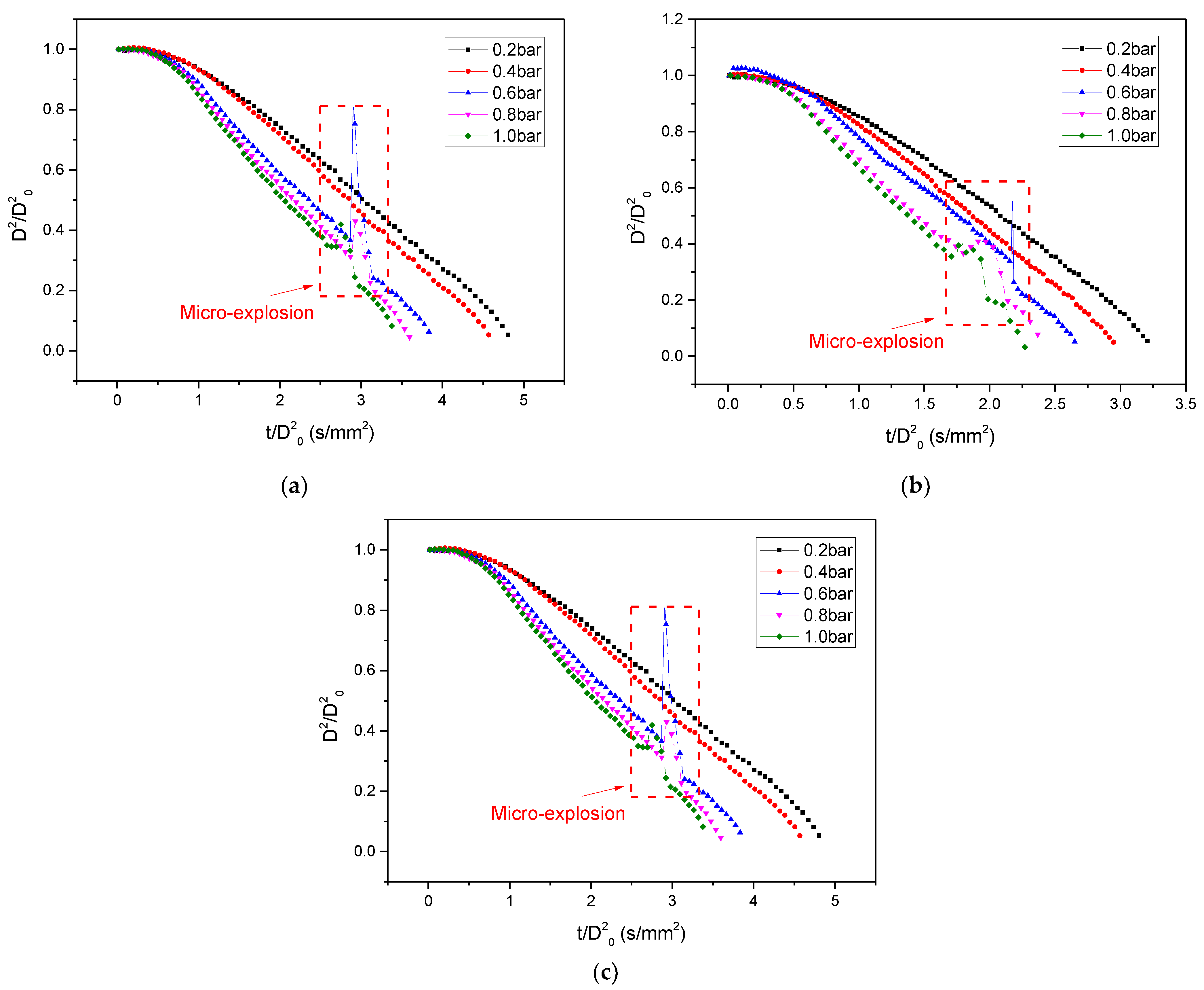
3.1. D2-Law
3.1.1. General Behavior
3.1.2. Temperature Influence
3.1.3. Pressure Influence
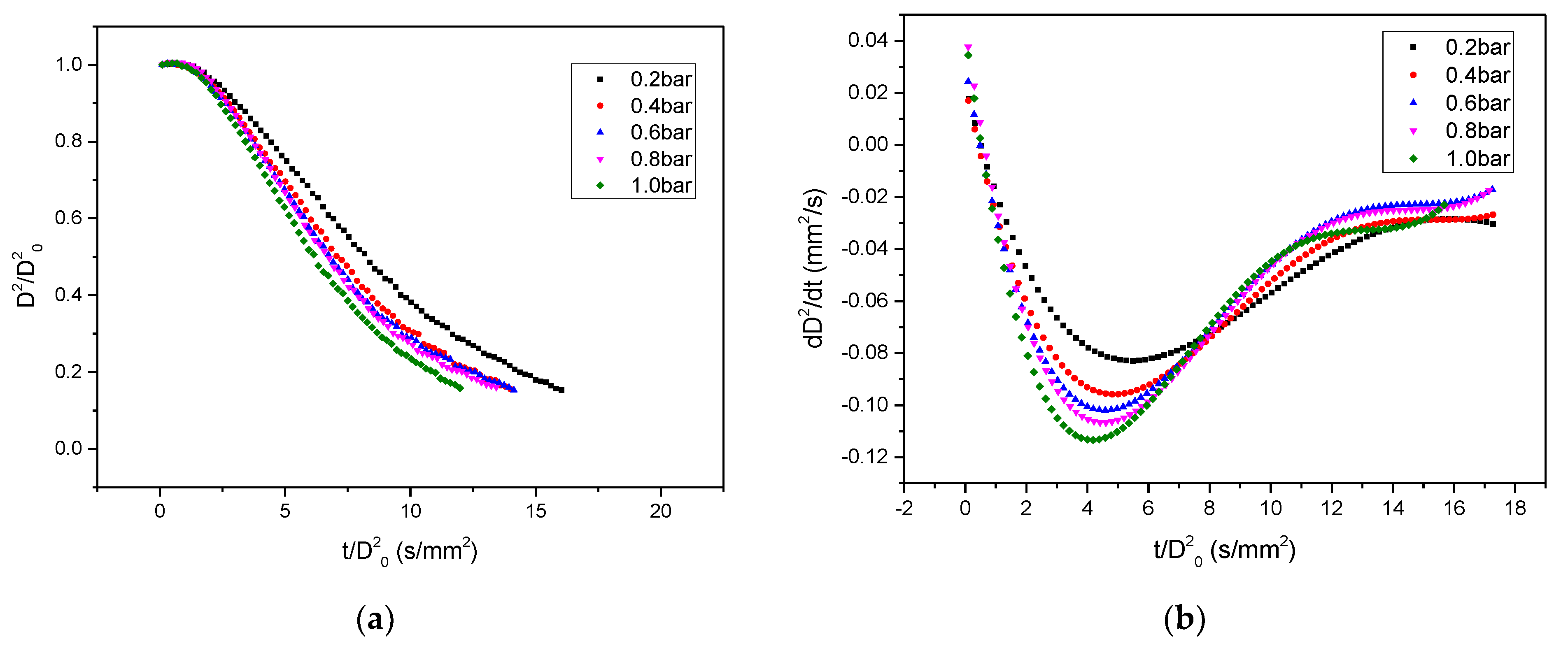
3.2. The Evaporation Rate
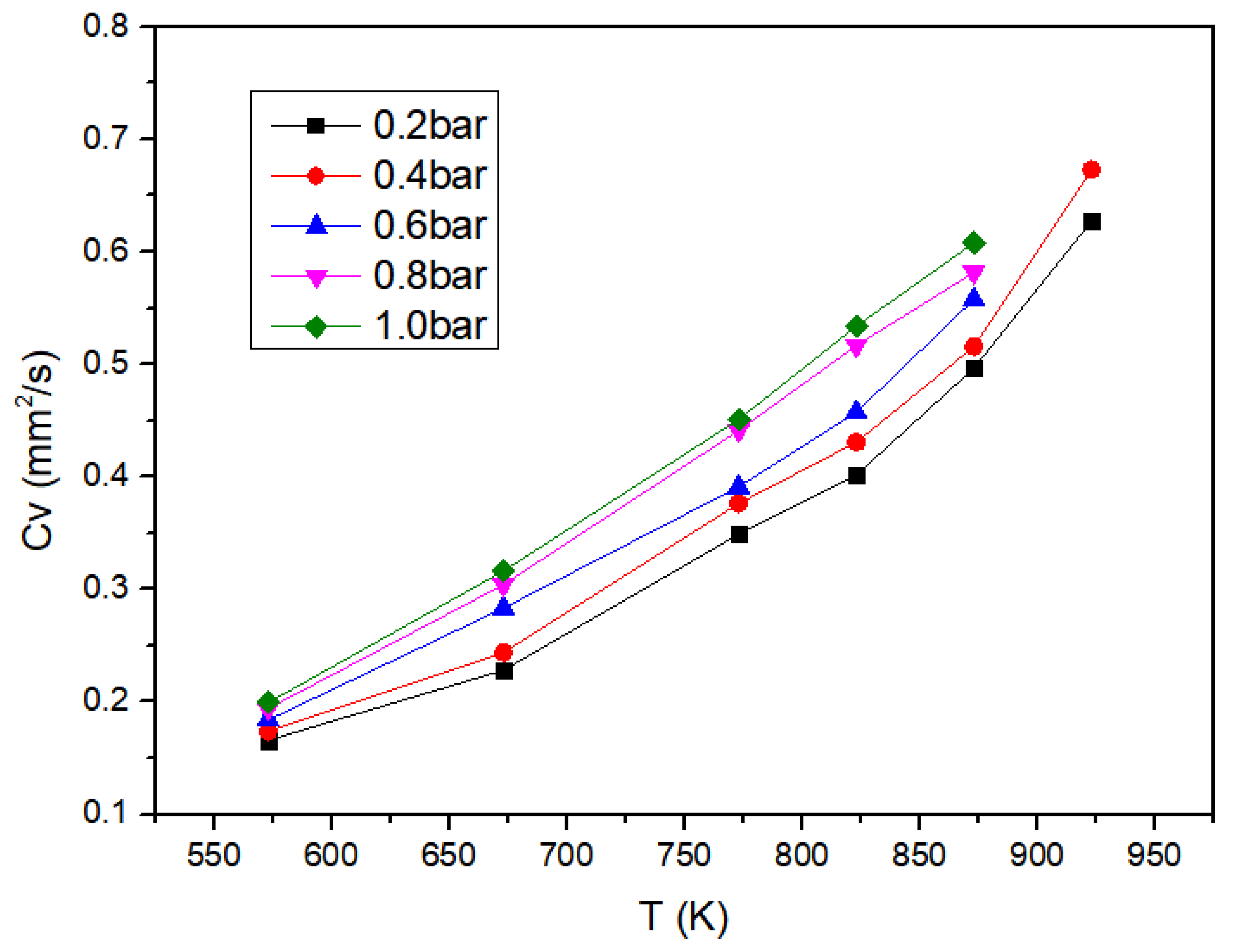
3.2.1. Temperature Influence
3.2.2. Pressure Influence
3.3. Spontaneous Ignition
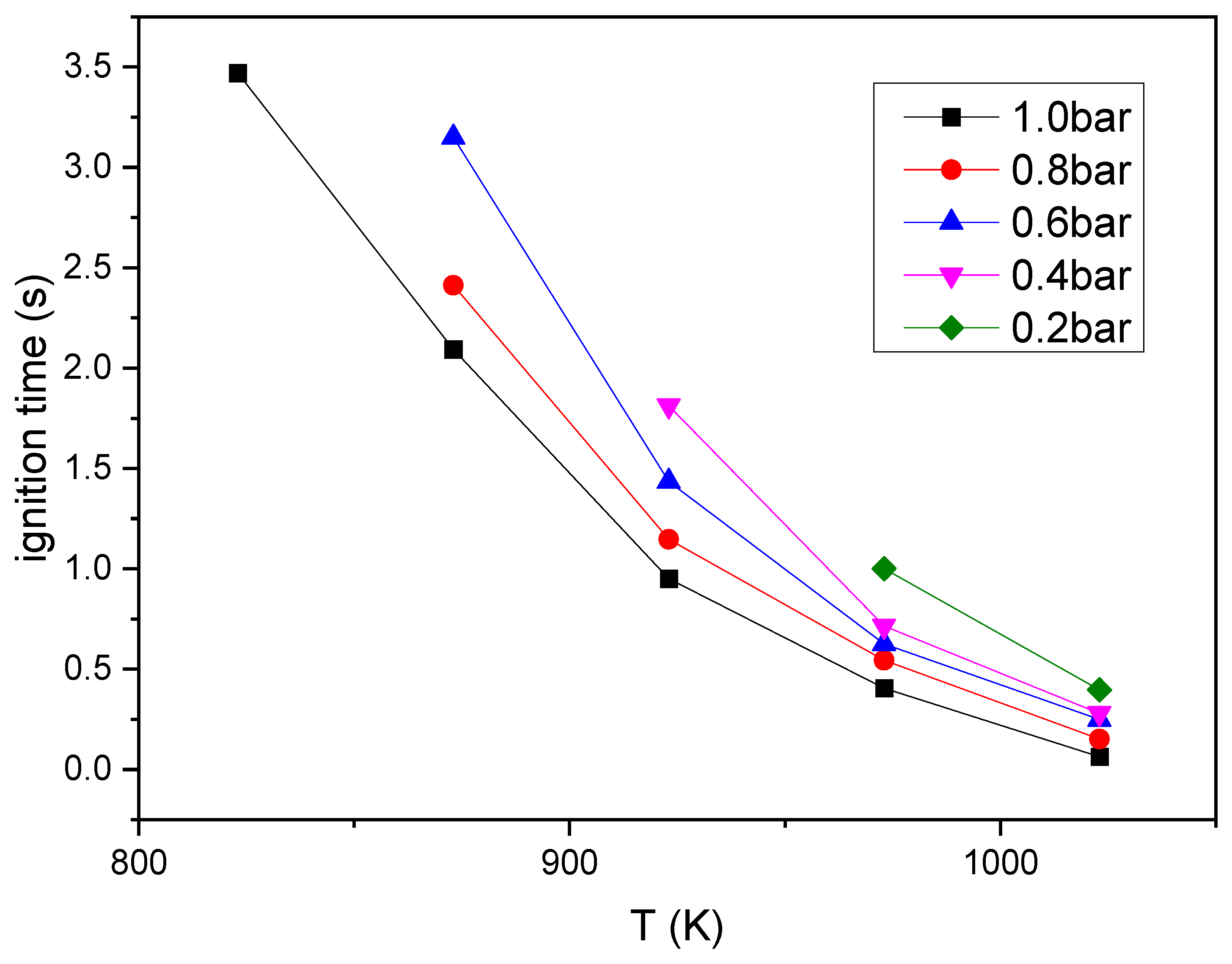
3.3.1. Pressure Influence on Spontaneous Ignition Behavior
3.3.2. Temperature and Pressure Influence on Spontaneous Ignition Delay
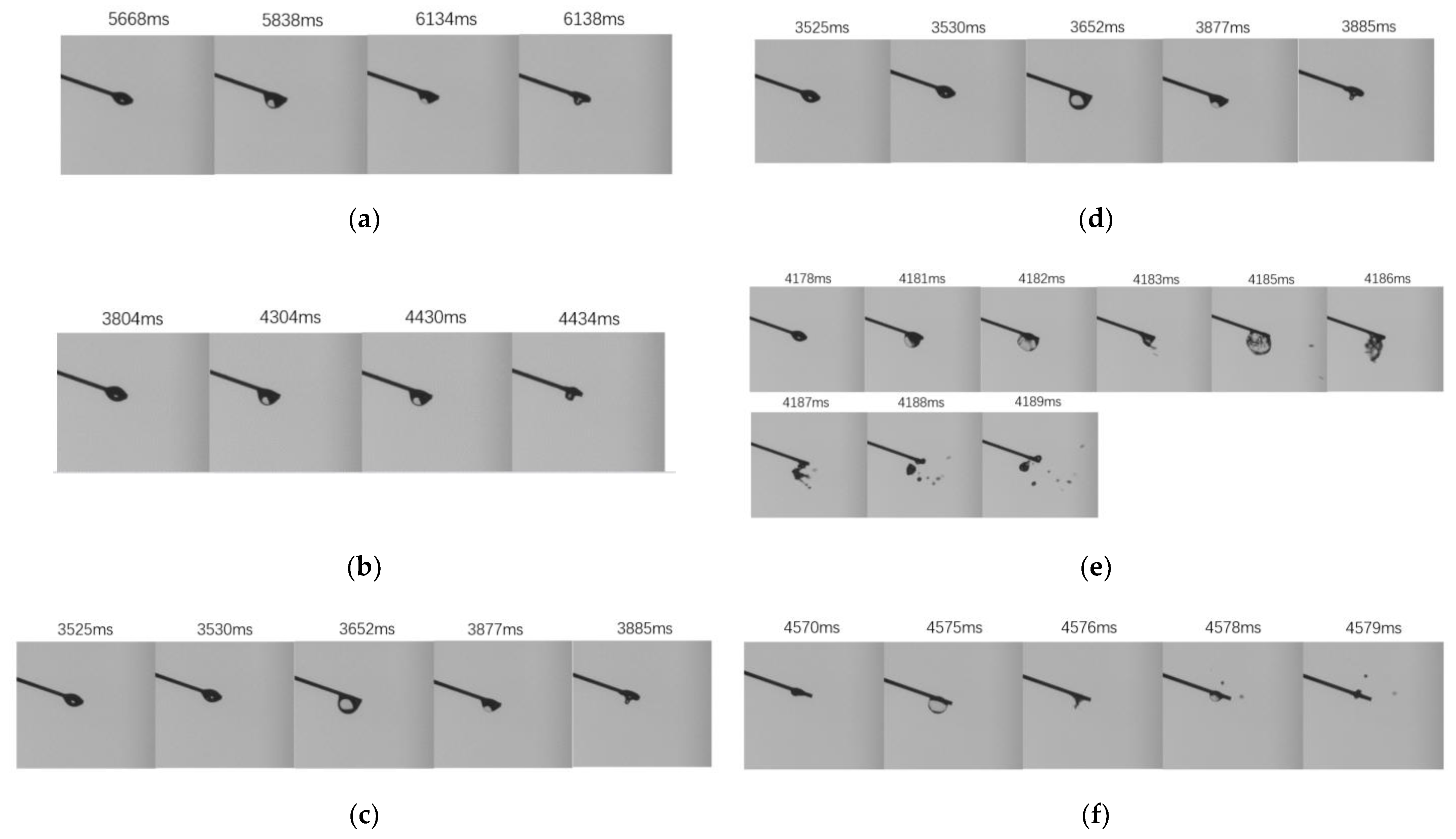
3.4. Micro-Explosion Characteristics
3.4.1. Temperature Influence on Micro-Explosion
3.4.2. Pressure Influence on Micro-Explosion
3.5. Droplet State
4. Conclusions
- Different from other higher temperatures (573–873 K), the normalized D2 significantly deviates from the classical d2-law and the evaporate rate decreases gradually under 473 K and 0.2–1.0 bar. For 473 K, lowering the pressure has been shown to reduce the change in the evaporation rate. This may be due to the fact that the difference in boiling points between the different components of the droplet decreases with decreasing pressure;
- The droplet evaporation rate increases monotonically with increasing temperature and pressure under 573–873 K and 0.2–1.0 bar. Temperature has a greater effect on evaporation rate than pressure. The increase of ambient temperature increases the temperature difference between the environment and the droplet surface, which promotes the heat transfer. The decrease of pressure makes the distance between the gas molecules become larger and the heat transfer between the environment and the droplet surface deteriorates;
- The decrease in pressure causes the combustible vapor to be more evenly distributed around the droplet and significantly reduced the formation and aggregation of large soot particles in the process of spontaneous ignition. The decrease of temperature and pressure is obviously detrimental to the successful ignition of droplet and significantly increases the ignition delay time of spontaneous combustion. Ignitions at 0.2 bar are very difficult and required an ambient temperature of at least 973 K, which is about 150 K higher than the minimum ignition temperature at 1.0 bar; and
- A sufficiently high ambient temperature (673 K or higher) is a necessary condition for micro-explosion to occur. The increase of ambient temperature and droplet combustion can effectively promote the occurrence of micro-explosion. Reducing pressure was discovered to reduce the likelihood of micro-explosions at 673, 773 and 823 K, probably due to the boiling point difference among different components of kerosene decreases as the ambient pressure decreases. In addition, the decrease of pressure was discovered to significantly increase the bubble growth rate and droplet breakage intensity. This is probably caused by the fact that pressure reduction can significantly increase the bubble growth rate in both inertial and diffusion-controlled growth stages.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Krishnan, S.; George, P. Solid fuel ramjet combustor design. Prog. Aerosp. Sci. 1998, 34, 219–256. [Google Scholar] [CrossRef]
- Luo, W.; Pan, Y.; Tan, J.; Wang, Z. Experimental investigation on combustion efficiency of the ramjet model at low pressure. J. Propuls. Technol. 2010, 31, 270–275. [Google Scholar] [CrossRef]
- Ghassemi, H.; Baek, S.W.; Khan, Q.S. Experimental Study on Evaporation of Kerosene Droplets at Elevated Pressures and Temperatures. Combust. Sci. Technol. 2006, 178, 1669–1684. [Google Scholar] [CrossRef]
- Khan, Q.S.; Baek, S.W.; Ghassemi, H. On the Autoignition and Combustion Characteristics of Kerosene Droplets at Elevated Pressure and Temperature. Combust. Sci. Technol. 2007, 179, 2437–2451. [Google Scholar] [CrossRef]
- Kim, D.M.; Baek, S.W.; Yoon, J. Ignition characteristics of kerosene droplets with the addition of aluminum nanoparticles at elevated temperature and pressure. Combust. Flame 2016, 173, 106–113. [Google Scholar] [CrossRef]
- Ghassemi, H.; Baek, S.W.; Khan, Q.S. Experimental Study on Binary Droplet Evaporation at Elevated Pressures and Temperatures. Combust. Sci. Technol. 2006, 178, 1031–1053. [Google Scholar] [CrossRef]
- Hashimoto, N.; Nomura, H.; Suzuki, M.; Matsumoto, T.; Nishida, H.; Ozawa, Y. Evaporation characteristics of a palm methyl ester droplet at high ambient temperatures. Fuel 2015, 143, 202–210. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Evaporation characteristics of heptane droplets with the addition of aluminum nanoparticles at elevated temperatures. Combust. Flame 2013, 160, 170–183. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Autoignition and combustion characteristics of kerosene droplets with dilute concentrations of aluminum nanoparticles at elevated temperatures. Combust. Flame 2015, 162, 774–787. [Google Scholar] [CrossRef]
- Javed, I.; Baek, S.W.; Waheed, K. Effects of dense concentrations of aluminum nanoparticles on the evaporation behavior of kerosene droplet at elevated temperatures: The phenomenon of microexplosion. Exp. Therm. Fluid Sci. 2014, 56, 33–44. [Google Scholar] [CrossRef]
- Hiroshi, N.; Yasushige, U. Experimental study on high-pressure droplet evaporation using microgravity conditions. Symp. Combust. 1996, 26, 1267–1273. [Google Scholar]
- Ma, X.; Zhang, F.; Han, K.; Yang, B.; Song, G. Evaporation characteristics of acetone–butanol–ethanol and diesel blends droplets at high ambient temperatures. Fuel 2015, 160, 43–49. [Google Scholar] [CrossRef]
- Moriue, O.; Nishiyama, Y.; Yamaguchi, Y.; Hashimoto, H.; Murase, E. Effects of droplet interaction on spontaneous ignition of an n-decane droplet pair. Proc. Combust. Inst. 2013, 34, 1585–1592. [Google Scholar] [CrossRef]
- Nakaya, S.; Tsue, M.; Imamura, O.; Nishida, S.; Yamashita, K.; Segawa, D.; Kono, M. Effects of Fuel Vapor in Ambience on Spontaneous Ignition of Isolated Fuel Droplet. Combust. Sci. Technol. 2009, 181, 1464–1479. [Google Scholar] [CrossRef]
- Zhu, M.; Ma, Y.; Zhang, Z.; Chan, Y.L.; Zhang, D. Effect of oxygenates addition on the flame characteristics and soot formation during combustion of single droplets of a petroleum diesel in air. Fuel 2015, 150, 88–95. [Google Scholar] [CrossRef]
- Califano, V.; Calabria, R.; Massoli, P. Experimental evaluation of the effect of emulsion stability on micro-explosion phenomena for water-in-oil emulsions. Fuel 2014, 117, 87–94. [Google Scholar] [CrossRef]
- Han, K.; Zhao, C.; Fu, G.; Zhang, F.; Pang, S.; Li, Y. Evaporation characteristics of dual component droplet of benzyl azides-hexadecane mixtures at elevated temperatures. Fuel 2015, 157, 270–278. [Google Scholar] [CrossRef]
- Ooi, J.B.; Ismail, H.M.; Swamy, V.; Wang, X.; Swain, A.K.; Rajanren, J.R. Graphite Oxide Nanoparticle as a Diesel Fuel Additive for Cleaner Emissions and Lower Fuel Consumption. Energy Fuels 2016, 30, 1341–1353. [Google Scholar] [CrossRef]
- Sazhin, S.S. Advanced models of fuel droplet heating and evaporation. Prog. Energy Combust. Sci. 2006, 32, 162–214. [Google Scholar] [CrossRef]
- Bykov, V.; Goldfarb, I.; Gol’dshtein, V.; Greenberg, J.B. Thermal explosion in a hot gas mixture with fuel droplets: A two reactant model. Combust. Theory Model. 2007, 6, 339–359. [Google Scholar] [CrossRef]
- Nguyen, D.; Soria, J.; Honnery, D. Efficiency of the lumped parameter concept and the role of liquid properties in modelling microdroplet evaporation. Fuel 2016, 166, 86–95. [Google Scholar] [CrossRef]
- Olguin, H.; Gutheil, E. Influence of evaporation on spray flamelet structures. Combust. Flame 2014, 161, 987–996. [Google Scholar] [CrossRef]
- Bertoli, C.; Na Migliaccio, M. A finite conductivity model for diesel spray evaporation computations. Int. J. Heat Fluid Flow 1999, 20, 552–561. [Google Scholar] [CrossRef]
- Abramzon, B.; Sirignano, W.A. Droplet vaporization model for spray combustion calculations. Int. J. Heat Mass Transf. 1989, 32, 1605–1618. [Google Scholar] [CrossRef]
- Barry, D.A.; Parlange, J.Y. Recirculation within a fluid sphere at moderate Reynolds numbers. J. Fluid Mech. 2002, 465, 293–300. [Google Scholar] [CrossRef]
- Chiang, C.H.; Raju, M.S.; Sirignano, W.A. Numerical analysis of convecting, vaporizing fuel droplet with variable properties. Int. J. Heat Mass Transf. 1991, 35, 1307–1324. [Google Scholar] [CrossRef]
- Law, C.K. Asymptotic theory for ignition and extinction in droplet burning. Combust. Flame 1975, 24, 89–98. [Google Scholar] [CrossRef]
- Law, C.K. Theory of thermal ignition in fuel droplet burning. Combust. Flame 1978, 31, 285–296. [Google Scholar] [CrossRef]
- Ivanov, V.M.; Nefedov, P.I. Experimental investigation of the combustion process of natural and emulsified liquid fuels. Tr. Goryachikh Iskopayemykh 1962, 19, 35–45. [Google Scholar]
- Wang, C.H.; Li, X.Q.; Law, C.K. Combustion and microexplosion of freely falling multicomponent droplets. Combust. Flame 1984, 56, 175–797. [Google Scholar] [CrossRef]
- Mikami, M.; Yagi, T.; Kojima, N. Occurrence probability of microexplosion in droplet combustion of miscible binary fuels. Symp. Combust. 1998, 27, 1933–1941. [Google Scholar] [CrossRef]
- Avulapati, M.M.; Megaritis, T.; Xia, J.; Ganippa, L. Experimental understanding on the dynamics of micro-explosion and puffing in ternary emulsion droplets. Fuel 2019, 239, 1284–1292. [Google Scholar] [CrossRef]
- Shaw, B.D. Studies of influences of liquid-phase species diffusion on spherically symmetric combustion of miscible binary droplets. Combust. Flame 1990, 81, 277–288. [Google Scholar] [CrossRef]
- Terry, B.C.; Gunduz, I.E.; Pfeil, M.A.; Sippel, T.R.; Son, S.F. A mechanism for shattering microexplosions and dispersive boiling phenomena in aluminum–lithium alloy based solid propellant. Proc. Combust. Inst. 2017, 36, 2309–2316. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, R.; Wang, Z.; Xu, S.; Huang, S.; Ma, Y. Experimental study on puffing characteristics of biodiesel-butanol droplet. Fuel 2017, 191, 454–462. [Google Scholar] [CrossRef]
- Rao, D.C.K.; Karmakar, S.; Som, S.K. Puffing and Micro-Explosion Behavior in Combustion of Butanol/Jet A-1 and Acetone-Butanol-Ethanol (A-B-E)/Jet A-1 Fuel Droplets. Combust. Sci. Technol. 2017, 189, 1796–1812. [Google Scholar] [CrossRef]
- Shang, W.; Yang, S.; Xuan, T.; He, Z.; Cao, J. Experimental Studies on Combustion and Microexplosion Characteristics of N-Alkane Droplets. Energy Fuels 2020, 34, 16613–16623. [Google Scholar] [CrossRef]
- Wang, C.H.; Law, C.K. Microexplosion of fuel droplets under high pressure. Combust. Flame 1985, 59, 53–62. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; He, Y.; Xia, J.; Zhang, J.; Zhao, H.; Cen, K. Ignition, puffing and sooting characteristics of kerosene droplet combustion under sub-atmospheric pressure. Fuel 2021, 285, 119182. [Google Scholar] [CrossRef]
- NIST (National Institute of Standards and Technology) Web Database. Available online: https://webbook.nist.gov/chemistry/ (accessed on 1 September 2022).
- Turns, S. An Introduction to Combustion; International Editions: Singapore, 1996. [Google Scholar]
- Spalding, D. The combustion of liquid fuels. Proc. Combust. Inst. 1953, 4, 847–864. [Google Scholar] [CrossRef]
- Lefebvre, A. Atomization and Sprays; CRC Press: Boca Raton, FL, USA, 1989. [Google Scholar]
- Raslavičius, L.; Markšaitis, D. Research into three-component biodiesel fuels combustion process using a single droplet technique. Transport 2007, 22, 312–315. [Google Scholar] [CrossRef]
- El-Wakil, M.M.; Abdou, M.I. The Self Ignition of Fuel Drops in Heated Air Streams. Fuel 1966, 45, 177. [Google Scholar]
- Lasheras, J.C.; Yap, L.T.; Dryer, F.L. Effect of the ambient pressure on the explosive burning of emulsified and multicomponent fuel droplets. Symp. Combust. 1985, 20, 1761–1772. [Google Scholar] [CrossRef]
- Scriven, L.E. On the dynamics of phase growth. Chem. Eng. Sci. 1959, 10, 1–13. [Google Scholar] [CrossRef]
- Van Stralen, S.J.D. The growth rate of vapour bubbles in superheated pure liquids and binary mixtures: Part I: Theory. Int. J. Heat Mass Transf. 1968, 11, 1467–1489. [Google Scholar] [CrossRef]





| Chemical Name | Chemical Formula | Boiling Point/K (1 bar) | Mass Fraction/% |
|---|---|---|---|
| Nonadecane | C19H40 | 602.9 | 12.61 |
| Hexane,2,5-dimethyl- | C8H18 | 382.1 ± 0.9 | 5.44 |
| Cyclohexane,1,3-dimethyl-,cis- | C8H16 | 393 ± 1 | 5.27 |
| Heptane,3-methyl- | C8H18 | 392 ± 1 | 4.21 |
| Pentadecane | C15H32 | 540 ± 20 | 4.17 |
| Hexadecanoic acid, methyl ester | C17H34O2 | 690 | 3.23 |
| Undecane,5-methyl- | C12H26 | 480.9 | 3.12 |
| Cyclohexane,1,3,5-trimethyl- | C9H18 | 411 ± 3 | 2.42 |
| Octane,3-methyl- | C9H20 | 417 ± 1 | 2.36 |
| Tetradecane | C15H32 | 523 ± 10 | 2.33 |
| 1-Hexene,2,5,5-trimethyl- | C9H18 | 405 ± 7 | 2.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhang, H.; He, Y.; Zhu, Y.; Wang, Z. Evaporation, Autoignition and Micro-Explosion Characteristics of RP-3 Kerosene Droplets under Sub-Atmospheric Pressure and Elevated Temperature. Energies 2022, 15, 7172. https://doi.org/10.3390/en15197172
Huang J, Zhang H, He Y, Zhu Y, Wang Z. Evaporation, Autoignition and Micro-Explosion Characteristics of RP-3 Kerosene Droplets under Sub-Atmospheric Pressure and Elevated Temperature. Energies. 2022; 15(19):7172. https://doi.org/10.3390/en15197172
Chicago/Turabian StyleHuang, Jie, Hongtao Zhang, Yong He, Yanqun Zhu, and Zhihua Wang. 2022. "Evaporation, Autoignition and Micro-Explosion Characteristics of RP-3 Kerosene Droplets under Sub-Atmospheric Pressure and Elevated Temperature" Energies 15, no. 19: 7172. https://doi.org/10.3390/en15197172
APA StyleHuang, J., Zhang, H., He, Y., Zhu, Y., & Wang, Z. (2022). Evaporation, Autoignition and Micro-Explosion Characteristics of RP-3 Kerosene Droplets under Sub-Atmospheric Pressure and Elevated Temperature. Energies, 15(19), 7172. https://doi.org/10.3390/en15197172








