Phenyl Vinylsulfonate, a Novel Electrolyte Additive to Improve Electrochemical Performance of Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Theoretical Calculations
2.2. Electrolyte Preparation
2.3. Electrode Preparation and Cell Assembling
2.4. Electrochemical Measurements
2.5. Surface Analyses of the Electrode
3. Results and Discussion
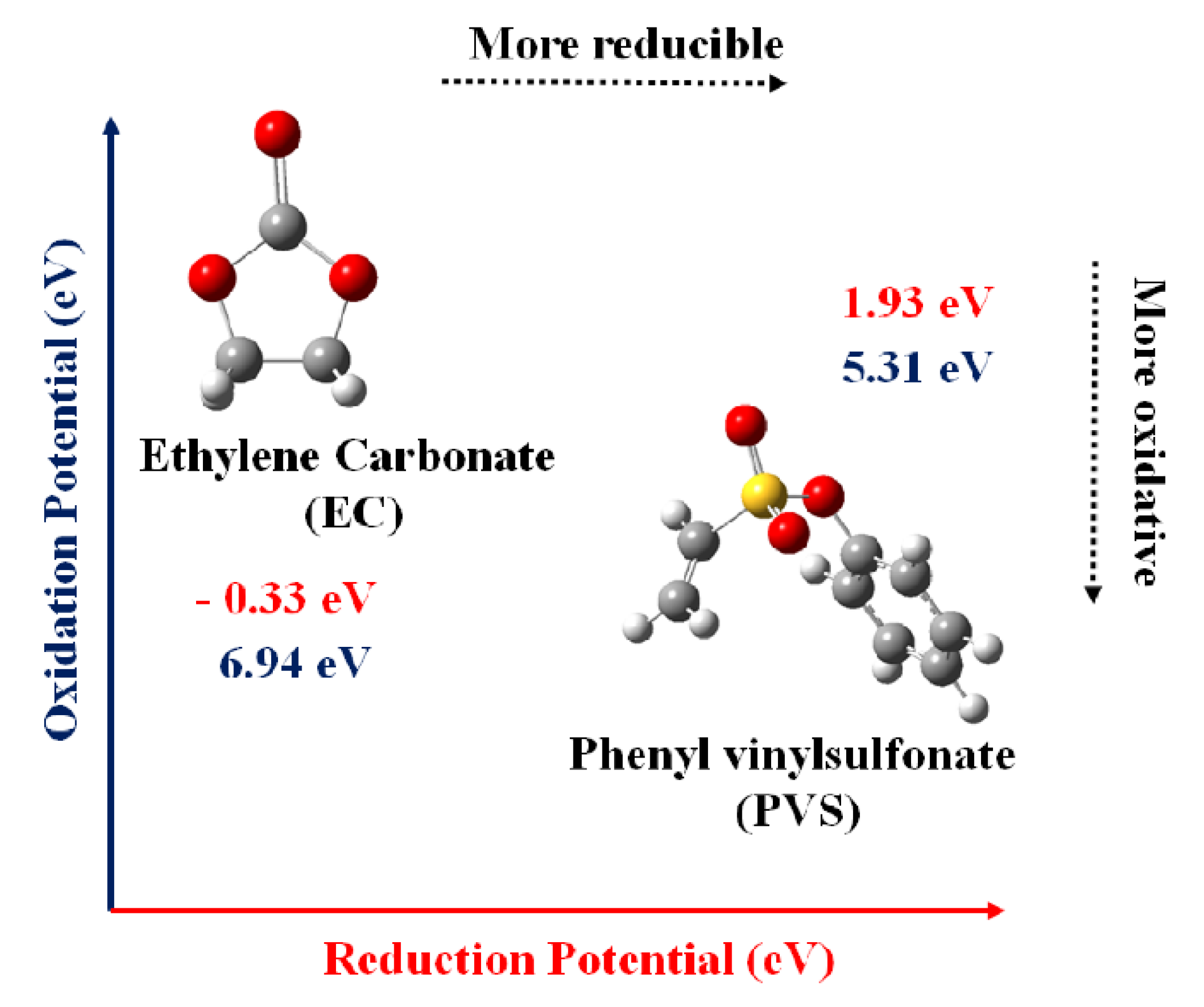
3.1. DFT Calculations
3.2. Electrochemical Study
3.2.1. Charge-Discharge Test
3.2.2. CV Measurements
3.2.3. LSV Measurements
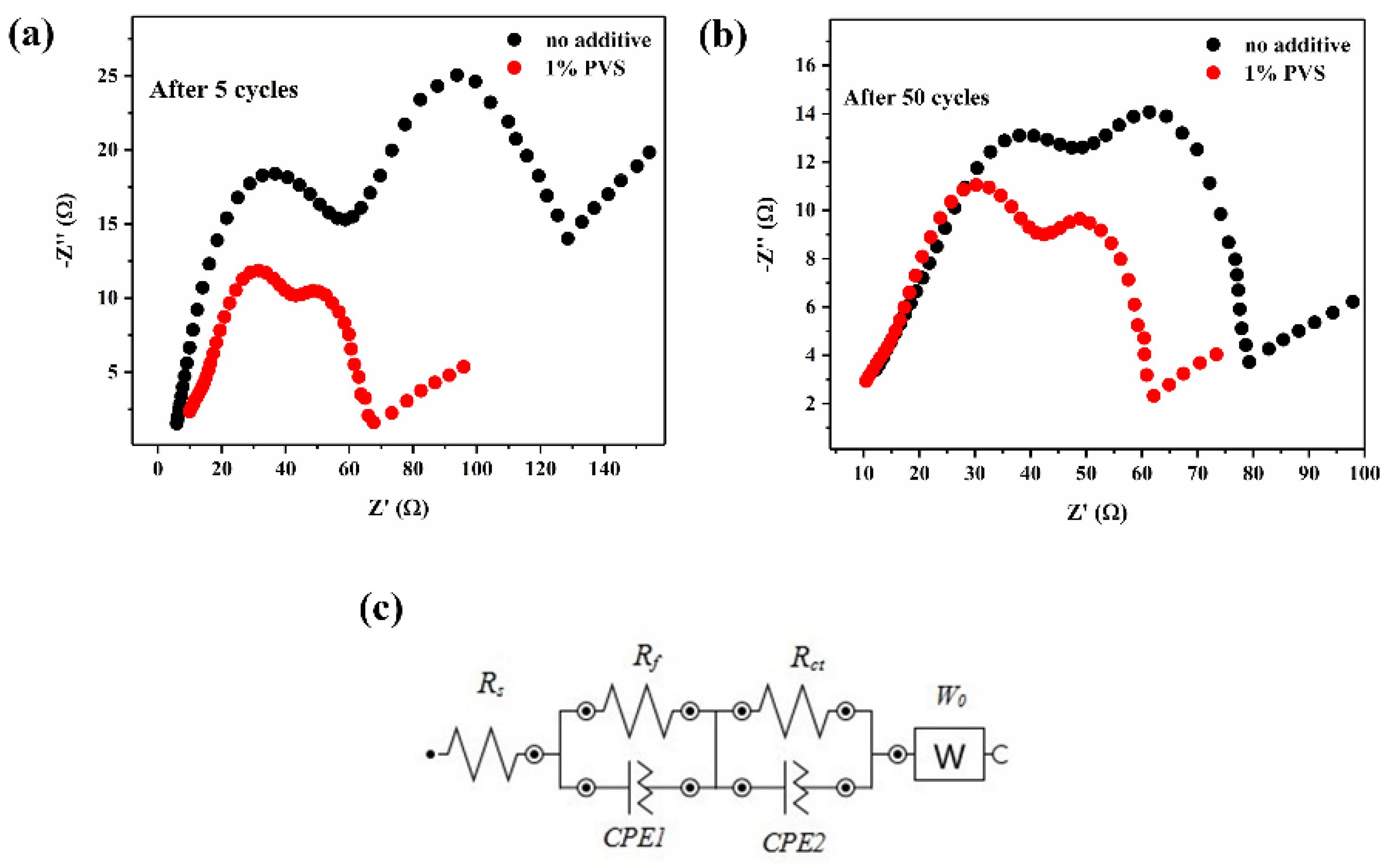
3.2.4. EIS Analysis
3.3. Structural Analyses
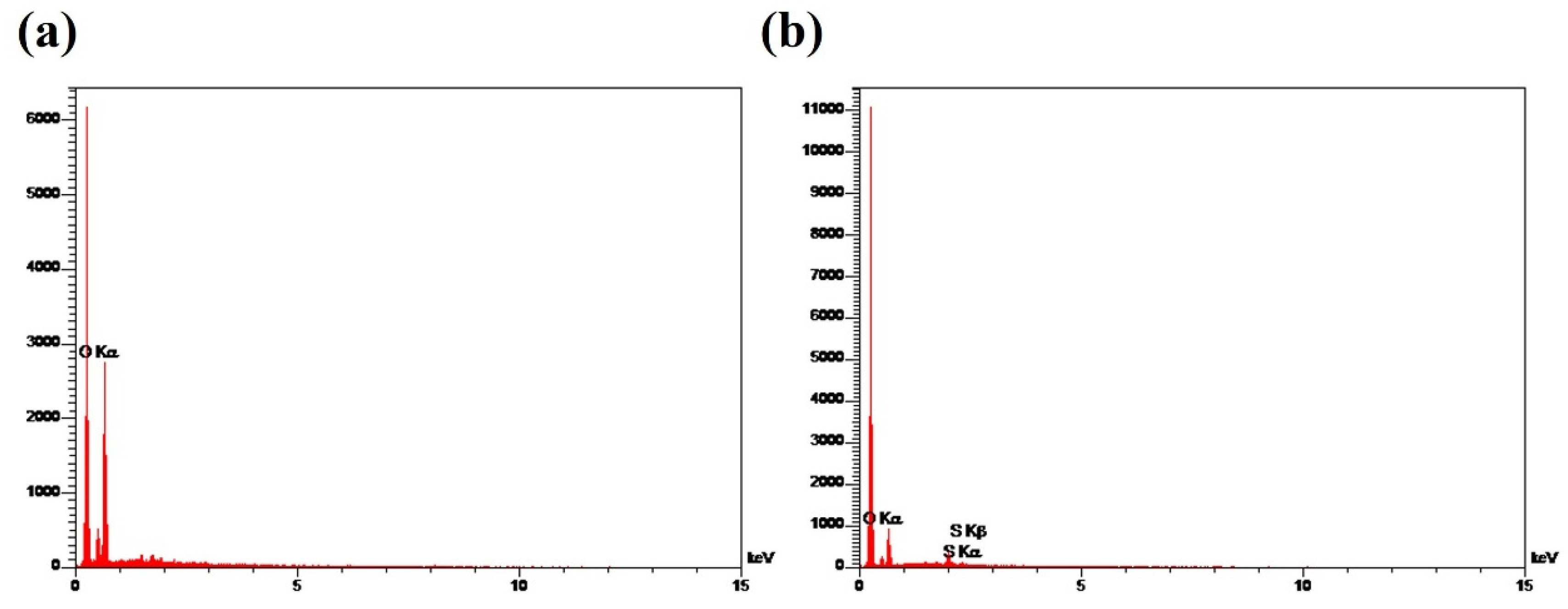
3.3.1. SEM and EDX Analyses
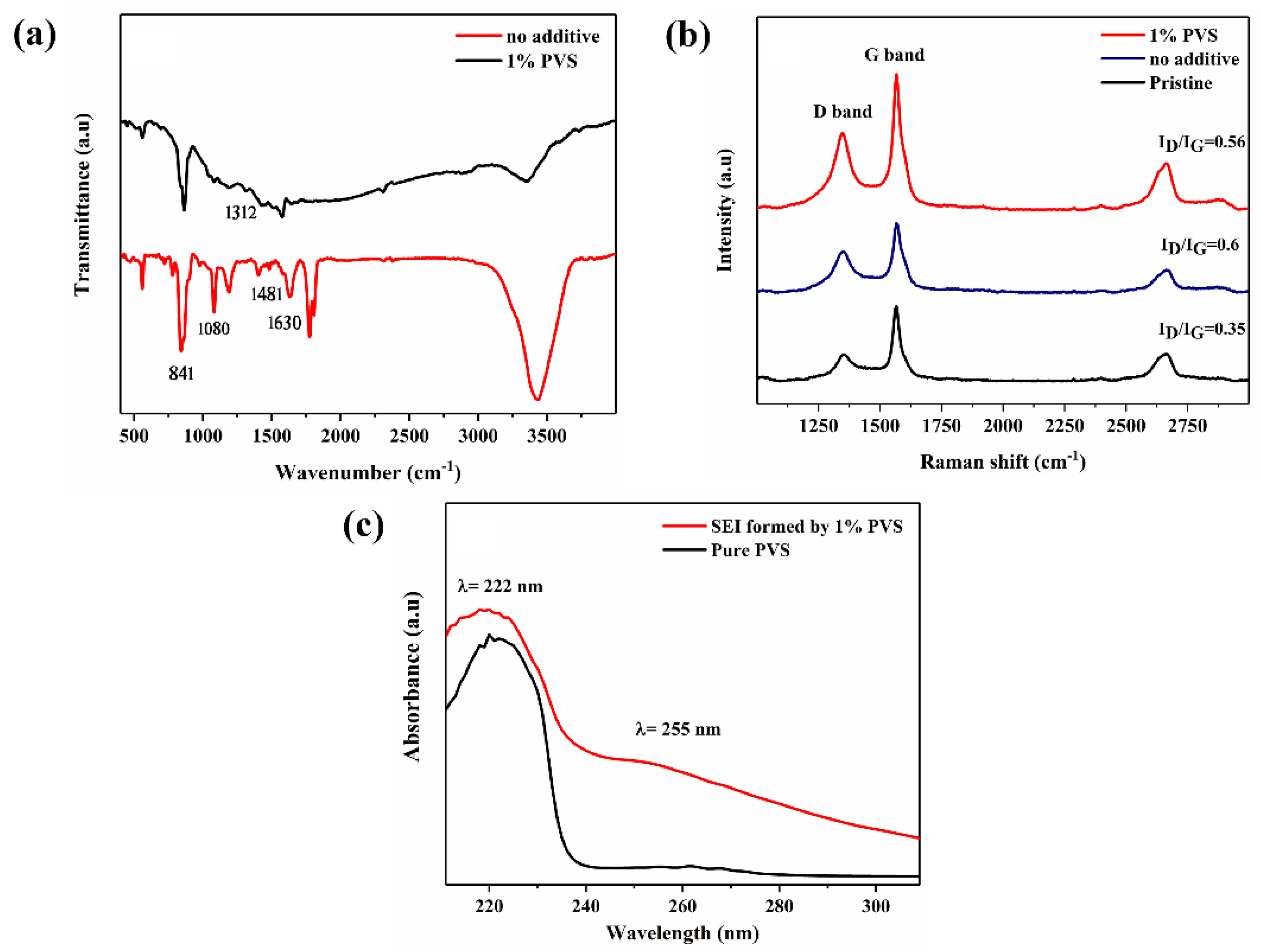
3.3.2. FT-IR Spectroscopy
3.3.3. Raman Spectroscopy
3.3.4. UV-Visible Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perveen, T.; Siddiq, M.; Shahzad, N.; Ihsan, R.; Ahmad, A.; Shahzad, M.I. Prospects in anode materials for sodium ion batteries-A review. Renew. Sustain. Energy Rev. 2020, 119, 109549. [Google Scholar] [CrossRef]
- da Silva Lima, L.; Quartier, M.; Buchmayr, A.; Sanjuan-Delmás, D.; Laget, H.; Corbisier, D.; Mertens, J.; Dewulf, J. Life cycle assessment of lithium-ion batteries and vanadium redox flow batteries-based renewable energy storage systems. Sustain. Energy Technol. Assess. 2021, 46, 101286. [Google Scholar] [CrossRef]
- Xiong, C.; Wang, T.; Zhang, Y.; Li, B.; Han, Q.; Li, D.; Ni, Y. Li–Na metal compounds inserted into porous natural wood as a bifunctional hybrid applied in supercapacitors and electrocatalysis. Int. J. Hydrog. Energy 2022, 47, 2389–2398. [Google Scholar] [CrossRef]
- Xiong, C.; Li, M.; Han, Q.; Zhao, W.; Dai, L.; Ni, Y. Screen printing fabricating patterned and customized full paper-based energy storage devices with excellent photothermal, self-healing, high energy density and good electromagnetic shielding performances. J. Mater. Sci. Technol. 2022, 97, 190–200. [Google Scholar] [CrossRef]
- Zhao, Y.; Fang, L.Z.; Kang, Y.Q.; Wang, L.; Zhou, Y.N.; Liu, X.Y.; Li, T.; Li, Y.X.; Liang, Z.; Zhang, Z.X.; et al. A novel three-step approach to separate cathode components for lithium-ion battery recycling. Rare Met. 2021, 40, 1431–1436. [Google Scholar] [CrossRef]
- Moradi Bilondi, A.; Kermani, M.J.; Heidary, H.; Abdollahzadehsangroudi, M. Investigation of the Effect of Anode Fuel Contaminants on the Performance of Polymer Electrolyte Membrane Fuel Cell. Amirkabir J. Mech. Eng. 2020, 52, 381–398. [Google Scholar]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of lithium-ion batteries in grid-scale energy storage systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Wang, S.; Ren, P.; Takyi-Aninakwa, P.; Jin, S.; Fernandez, C. A Critical Review of Improved Deep Convolutional Neural Network for Multi-Timescale State Prediction of Lithium-Ion Batteries. Energies 2022, 15, 5053. [Google Scholar] [CrossRef]
- Urbańska, W.; Osial, M. Investigation of the Physico-Chemical Properties of the Products Obtained after Mixed Organic-Inorganic Leaching of Spent Li-Ion Batteries. Energies 2020, 13, 6732. [Google Scholar] [CrossRef]
- Mosallanejad, B.; Malek, S.S.; Ershadi, M.; Daryakenari, A.A.; Cao, Q.; Ajdari, F.B.; Ramakrishna, S. Cycling degradation and safety issues in sodium-ion batteries: Promises of electrolyte additives. J. Electroanal. Chem. 2021, 895, 115505. [Google Scholar] [CrossRef]
- Cheng, H.; Shapter, J.G.; Li, Y.; Gao, G. Recent progress of advanced anode materials of lithium-ion batteries. J. Energy Chem. 2021, 57, 451–468. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical challenges and future perspectives of all-solid-state lithium-metal batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing lithium metal batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yang, Y.; Ren, D.; Wang, L.; He, X. Graphite as anode materials: Fundamental mechanism, recent progress and advances. Energy Storage Mater. 2021, 36, 147–170. [Google Scholar] [CrossRef]
- Beheshti, S.H.; Javanbakht, M.; Omidvar, H.; Hosen, M.S.; Hubin, A.; Van Mierlo, J.; Berecibar, M. Development, Retainment and Assessment of the Graphite-Electrolyte Interphase in Li-ion Batteries Regarding the Functionality of SEI-Forming Additives. Iscience 2022, 25, 103862. [Google Scholar] [CrossRef]
- Song, H.-Y.; Jeong, S.-K. Investigating continuous co-intercalation of solvated lithium ions and graphite exfoliation in propylene carbonate-based electrolyte solutions. J. Power Sources 2018, 373, 110–118. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, J.; Yang, L.; Lu, H.; Liu, H.; Zheng, B. Exploring the redox decomposition of ethylene carbonate–propylene carbonate in Li-ion batteries. Mater. Adv. 2021, 2, 1747–1751. [Google Scholar] [CrossRef]
- Haregewoin, A.M.; Wotango, A.S.; Hwang, B.-J. Electrolyte additives for lithium ion battery electrodes: Progress and per-spectives. Energy Environ. Sci. 2016, 9, 1955–1988. [Google Scholar] [CrossRef]
- Zhang, S.S. A review on electrolyte additives for lithium-ion batteries. J. Power Sources 2006, 162, 1379–1394. [Google Scholar] [CrossRef]
- Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, S.H.; Javanbakht, M.; Omidvar, H.; Behi, H.; Zhu, X.; Mamme, M.H.; Hubin, A.; Van Mierlo, J.; Berecibar, M. Effects of structural substituents on the electrochemical decomposition of carbonyl derivatives and formation of the solid–electrolyte interphase in lithium-ion batteries. Energies 2021, 14, 7352. [Google Scholar] [CrossRef]
- Lee, J.-T.; Lin, Y.-W.; Jan, Y.-S. Allyl ethyl carbonate as an additive for lithium-ion battery electrolytes. J. Power Sources 2004, 132, 244–248. [Google Scholar] [CrossRef]
- Wagner, R.; Brox, S.; Kasnatscheew, J.; Gallus, D.R.; Amereller, M.; Cekic-Laskovic, I.; Winter, M. Vinyl sulfones as SEI-forming additives in propylene carbonate based electrolytes for lithium-ion batteries. Electrochem. Commun. 2014, 40, 80–83. [Google Scholar] [CrossRef]
- Komaba, S.; Itabashi, T.; Tatsuya Ohtsuka, H.; Groult, N.; Kumagai, N.; Kaplan, B.; Yashiro, H. Impact of 2-vinylpyridine as electrolyte additive on surface and electrochemistry of graphite for C/LiMn2O4 Li-ion Cells. J. Electrochem. Soc. 2005, 152, A937. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Mosallanejad, B. Phthalimide Derivatives: New Promising Additives for Functional Electrolyte in Lithium-ion Batteries. Chem. Methodol. 2019, 3, 261–275. [Google Scholar]
- Li, X.; Yin, Z.; Li, X.; Wang, C. Ethylene sulfate as film formation additive to improve the compatibility of graphite electrode for lithium-ion battery. Ionics 2014, 20, 795–801. [Google Scholar] [CrossRef]
- Li, B.; Xu, M.; Li, B.; Liu, Y.; Yang, L.; Li, W.; Hu, S. Properties of solid electrolyte interphase formed by prop-1-ene-1,3-sultone on graphite anode of Li-ion batteries. Electrochim. Acta 2013, 105, 1–6. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, C.; Qiu, C.; Ma, X. Vinyl ethylene carbonate as an additive to ionic liquid electrolyte for lithium ion batteries. J. Appl. Electrochem. 2009, 39, 2597–2603. [Google Scholar] [CrossRef]
- Wang, R.; Li, X.; Wang, Z.; Zhang, H. Electrochemical analysis graphite/electrolyte interface in lithium-ion batteries: P-Toluenesulfonyl isocyanate as electrolyte additive. Nano Energy 2017, 34, 131–140. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Dolocan, A.; Cui, Z.; Xin, S.; Xue, L.; Xu, H.; Park, K.; Goodenough, J.B. Garnet electrolyte with an ultralow interfacial resistance for Li-metal batteries. J. Am. Chem. Soc. 2018, 140, 6448–6455. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Li, X.; Wang, Z.; Guo, H.; Wang, J. Compatibility of graphite with 1,3-(1,1,2,2-Tetrafluoroethoxy) propane and fluoroethylene carbonate as cosolvents for nonaqueous electrolyte in lithium-ion batteries. J. Phys. Chem. C 2014, 118, 6586–6593. [Google Scholar] [CrossRef]
- Xu, M.Q.; Li, W.S.; Zuo, X.X.; Liu, J.S.; Xu, X. Performance improvement of lithium ion battery using PC as a solvent component and BS as an SEI forming additive. J. Power Sources 2007, 174, 705–710. [Google Scholar] [CrossRef]
- Hu, Y.; Kong, W.; Li, H.; Huang, X.; Chen, L. Experimental and theoretical studies on reduction mechanism of vinyl ethylene carbonate on graphite anode for lithium ion batteries. Electrochem. Commun. 2004, 6, 126–131. [Google Scholar] [CrossRef]
- Ota, H.; Kominato, A.; Chun, W.-J.; Yasukawa, E.; Kasuya, S. Effect of cyclic phosphate additive in non-flammable electrolyte. J. Power Sources 2003, 119, 393–398. [Google Scholar] [CrossRef]
- Choi, N.-S.; Profatilova, I.A.; Kim, S.-S.; Song, E.-H. Thermal reactions of lithiated graphite anode in LiPF6-based electrolyte. Thermochim. Acta 2008, 480, 10–14. [Google Scholar] [CrossRef]
- Wang, L.; Menakath, A.; Han, F.; Wang, Y.; Zavalij, P.Y.; Gaskell, K.J.; Borodin, O.; Iuga, D.; Brown, S.P.; Wang, C.; et al. Identifying the components of the solid–electrolyte interphase in Li-ion batteries. Nat. Chem. 2019, 11, 789–796. [Google Scholar] [CrossRef]
- Xing, M.L.; Li, W. Interphases between electrolytes and anodes in Li-ion battery. In Electrolytes for Lithium and Lithium-Ion Batteries; Jow, T.R., Xu, K., Borodin, O., Ue, M., Eds.; Springer: New York, NY, USA, 2014; pp. 227–277. [Google Scholar]
- Wadher, S.J.; Puranik, M.P.; Karande, N.A.; Yeole, P.G. Synthesis and biological evaluation of Schiff base of dapsone and their derivative as antimicrobial agents. Int. J. PharmTech Res. 2009, 1, 22–33. [Google Scholar]
- Puro, L.; Mänttäri, M.; Pihlajamäki, A.; Nyström, M. Characterization of modified nanofiltration membranes by octanoic acid permeation and FTIR analysis. Chem. Eng. Res. Des. 2006, 84, 87–96. [Google Scholar] [CrossRef]
- Markervich, E.; Salitra, G.; Levi, M.D.; Aurbach, D. Capacity fading of lithiated graphite electrodes studied by a combination of electroanalytical methods, Raman spectroscopy and SEM. J. Power Sources 2005, 146, 146–150. [Google Scholar] [CrossRef]
- Sethuraman, V.A.; Hardwick, L.J.; Srinivasan, V.; Kostecki, R. Surface structural disordering in graphite upon lithium intercalation/deintercalation. J. Power Sources 2010, 195, 3655–3660. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Lin, H.; Wang, X.; Xu, M.; Wang, Y.; Xing, L.; Li, W. Performance improvement of phenyl acetate as propylene carbonate-based electrolyte additive for lithium ion battery by fluorine-substituting. J. Power Sources 2014, 267, 182–187. [Google Scholar] [CrossRef]
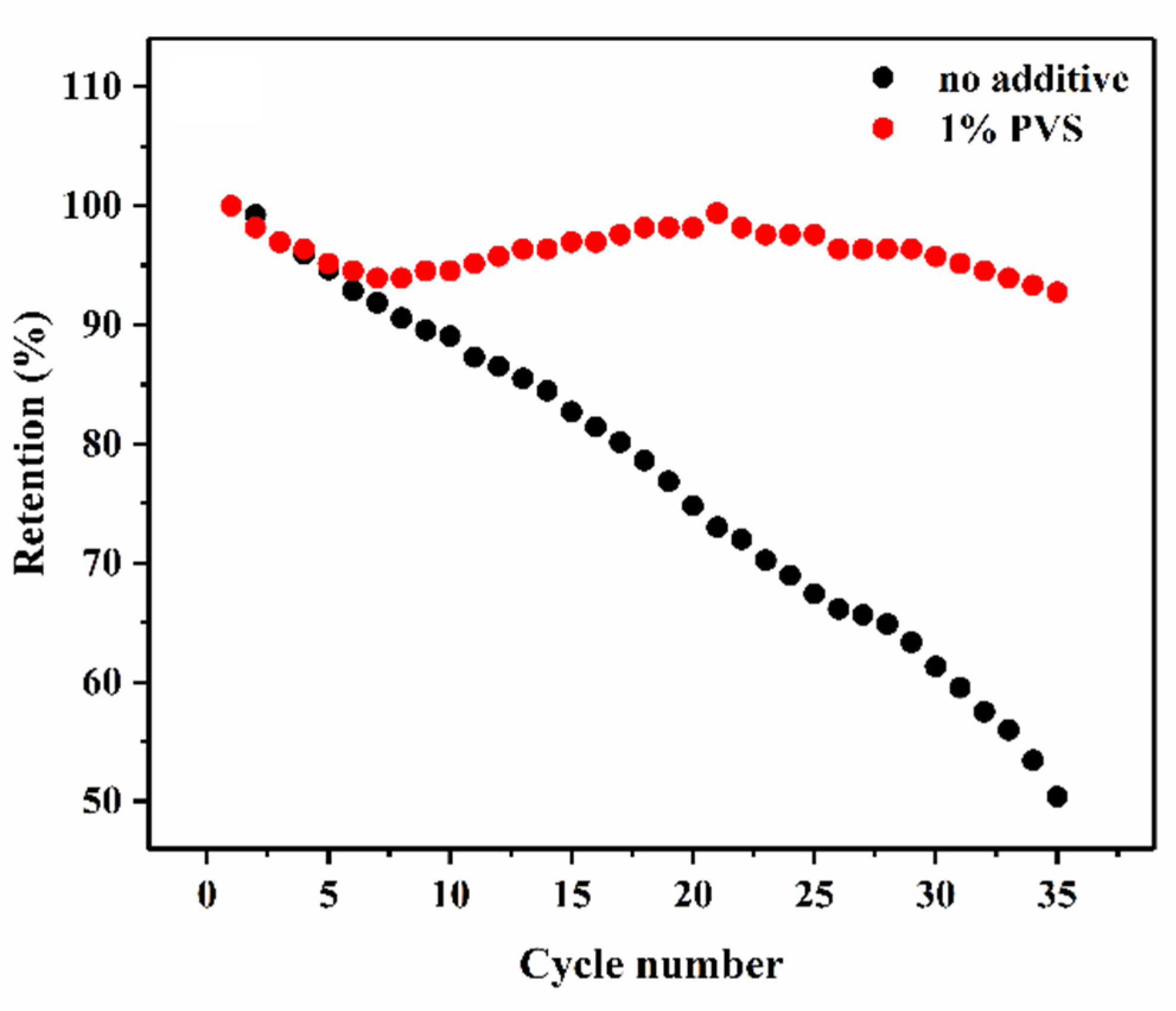
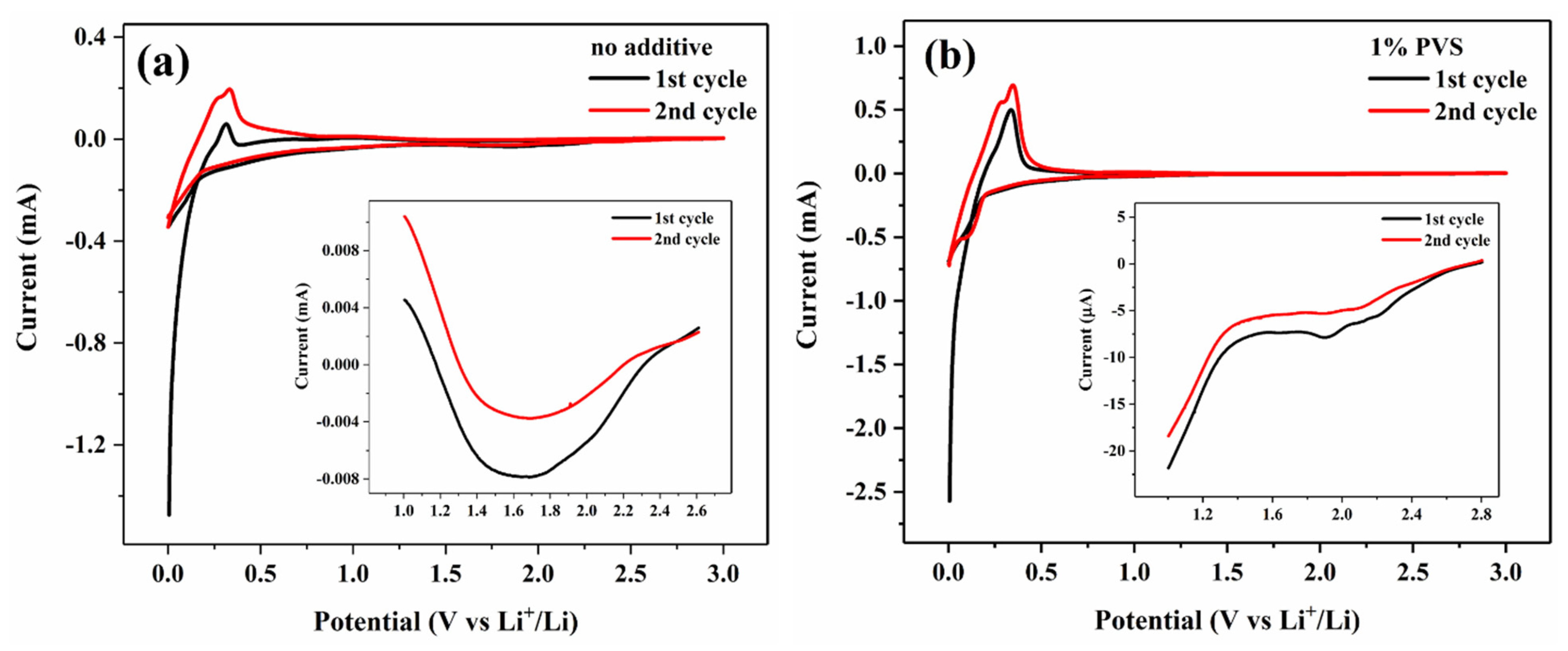
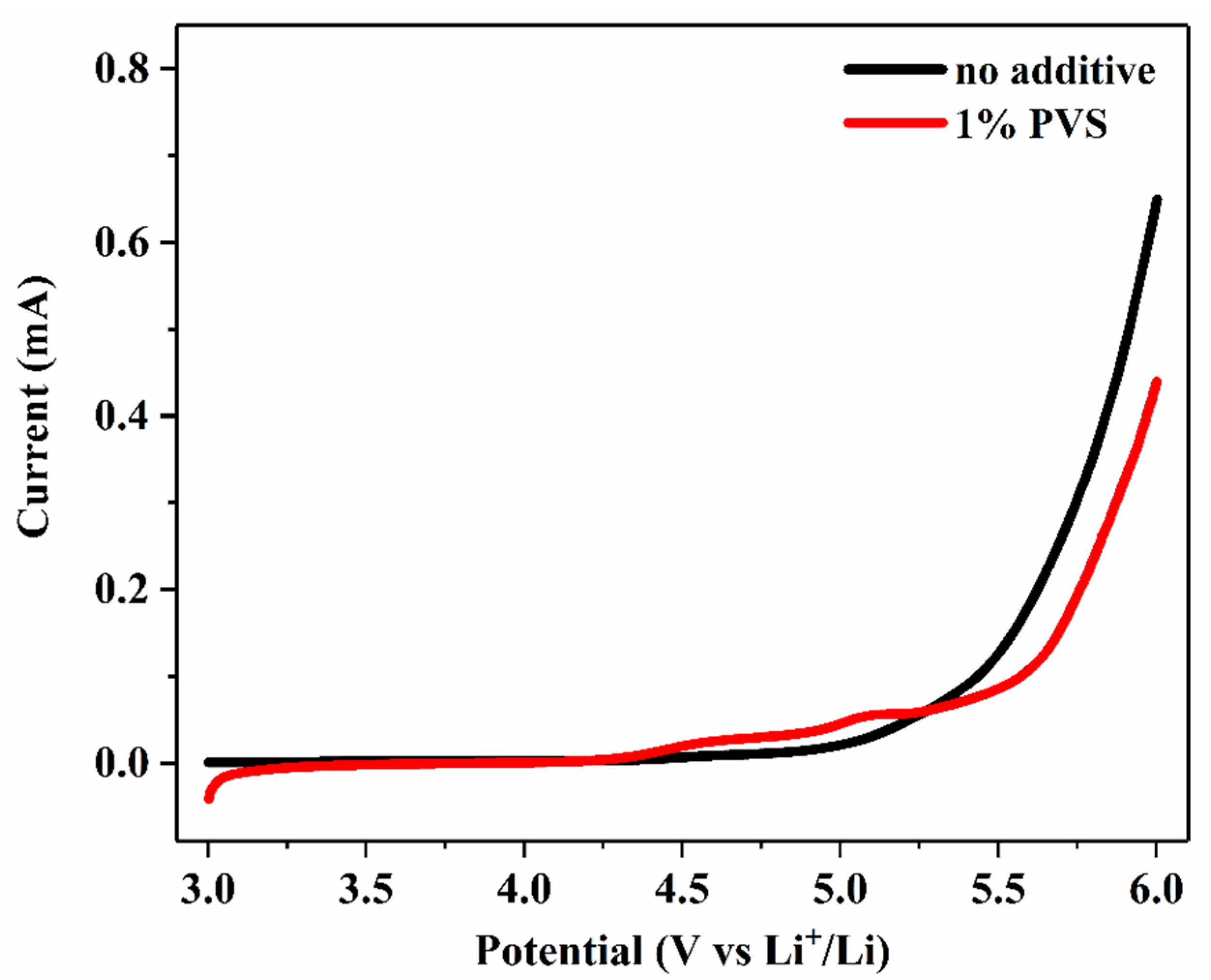

| Samples | Discharge Capacity (mAh g−1) | Charge Capacity (mAh g−1) | Coulombic Efficiency (%) |
|---|---|---|---|
| no additive | 481.4 | 333.6 | 69.3 |
| 1% PVS | 328.4 | 256.9 | 80.9 |
| 2% PVS | 278.1 | 196.4 | 70.6 |
| 3% PVS | 287.2 | 153.1 | 53.3 |
| 4% PVS | 147.7 | 50.3 | 34.1 |
| 5% PVS | 76.9 | 22.4 | 29.2 |
| Sample | After 5 Cycles | After 50 Cycles | ||||
|---|---|---|---|---|---|---|
| Rs (Ω) | Rf (Ω) | Rct (Ω) | Rs (Ω) | Rf (Ω) | Rct (Ω) | |
| No Additive | 5.88 | 52.87 | 68.09 | 12.24 | 35.15 | 34.6 |
| 1% PVS | 9.91 | 33.29 | 24.44 | 10.44 | 31.99 | 19.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosallanejad, B.; Javanbakht, M.; Shariatinia, Z.; Akrami, M. Phenyl Vinylsulfonate, a Novel Electrolyte Additive to Improve Electrochemical Performance of Lithium-Ion Batteries. Energies 2022, 15, 6205. https://doi.org/10.3390/en15176205
Mosallanejad B, Javanbakht M, Shariatinia Z, Akrami M. Phenyl Vinylsulfonate, a Novel Electrolyte Additive to Improve Electrochemical Performance of Lithium-Ion Batteries. Energies. 2022; 15(17):6205. https://doi.org/10.3390/en15176205
Chicago/Turabian StyleMosallanejad, Behrooz, Mehran Javanbakht, Zahra Shariatinia, and Mohammad Akrami. 2022. "Phenyl Vinylsulfonate, a Novel Electrolyte Additive to Improve Electrochemical Performance of Lithium-Ion Batteries" Energies 15, no. 17: 6205. https://doi.org/10.3390/en15176205
APA StyleMosallanejad, B., Javanbakht, M., Shariatinia, Z., & Akrami, M. (2022). Phenyl Vinylsulfonate, a Novel Electrolyte Additive to Improve Electrochemical Performance of Lithium-Ion Batteries. Energies, 15(17), 6205. https://doi.org/10.3390/en15176205







