Abstract
Lignin is the second most abundant renewable natural polymer that occurs on Earth, and as such, it should be widely utilised by industries in a variety of applications. However, these applications and possible research seem to be limited or prevented by a variety of factors, mainly the high heterogeneity of lignin. Selective modifications of the structure and of functional groups allow better properties in material applications, whereas the separation of different qualitative lignin groups permits selective application in industry. This review is aimed at modification of the lignin structure, increasing the hydrophobicity of the produced materials, and focusing on several perspective modifications for industrial-scale production of lignin-based polymers, as well as challenges, opportunities, and other important factors to take into consideration.
1. Introduction
Lignin is considered by many to be the second most abundant renewable natural polymer on earth, right after cellulose, where approximations conclude that around 30% of total organic carbon originates from lignin, depending on the calculation [1,2,3]. Occurring naturally in plants as cross-linked aromatic heteropolymer, it has the function of increasing structural strength and aiding in the transport of water through the plant [4,5]. Lignin itself, based on current knowledge, is composed of three monolignols, connected by carbon–carbon or carbon–oxygen–carbon bonds in a complex heterogenous amorphous structure [6,7]. The structure of lignin is one of the main reasons that wide application areas and uses can be assumed, mainly chemical, material, or fuel production [7,8,9]. On the other hand, the high heterogeneity of this structure is one of the main reasons why no high-scale industrial application has been successful worldwide [7,8,10].
Currently, lignin as a resource is considered a by-product of the pulping industry, where, depending on the isolation process from lignocellulosic biomass, it is either burned or sold as lignosulphonate [8,11,12]. The burning of lignin is beneficial to pulp mills because it helps in the generation of heat and energy, as well as the regeneration of cooking chemicals, whereas lignosulphonates are sold on a stable market in relatively low-value applications, such as adhesives, dispersants, emulsion stabilisers, fillers, and others [8,11,13]. There are some small-scale local lignin facilities, but such technologies have not yet seen wide-scale production, however, they show great potential in the produced quality of lignin, even at the cost of great investment and maintenance price [8,11,14,15,16,17].
In recent years, the push for green and renewable industries is observable even in this sector, allowing for research and experimentation with technologies previously deemed unsustainable [17,18,19,20]. Reaching the technological and engineering limits of already existing pulp mills, with the promise of debottlenecking recovery boilers by lignin separation, has allowed for many research coordination groups to be created, wide industrial cooperation, and general solution seeking, for both industrial production as well as for postproduction modification and utilisation [18,21,22,23]. The creation of small-scale pilot plants allows for the production of high purity lignin, however, due to the high operational cost, high investment required, and low demand for products on the global market, high-scale applications seem unsustainable at this time. The main aim of the research and development is thus aimed at separation [16,21,22], fractionation [18,24], modification [8,12,25], and subsequent application to allow for high-scale and high-value applications [8,12,18].
This review is aimed to serve as an overview of lignin separation and modification with the goal of utilising lignin for biopolymer production while considering the hydrophobicity of lignin as a factor.
2. Lignin
As already mentioned, lignin is a rather heterogenous polymer with complex properties based on a variety of factors influencing the mentioned properties and consequently possible applications in industry. In this chapter, the main attention is divided between lignin structure, the processes of extraction from biomass, and their subsequent impact on the functional groups of lignin.
2.1. Origin and Structure of Lignin
A portion of lignin heterogeneity originates in its structure, composed mainly of three monolignols. These derivates of cinnamyl alcohol are p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, as observed in Figure 1. Different plant species have lignin with different ratios of monolignols. It is worth mentioning that monolignols transfer to the corresponding structural units during biosynthesis [4,26].
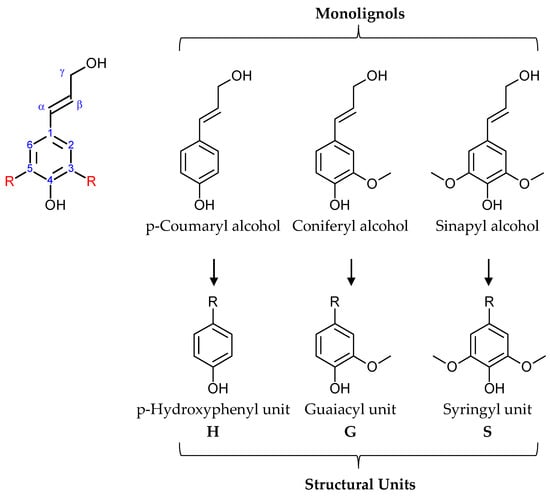
Figure 1.
Structure of monolignols and their corresponding structural units.
Softwood or coniferous plants have a higher content of coniferyl alcohol, whereas hardwood or deciduous plants contain both coniferyl and sinapyl alcohol [25]. Grasses and grass-like plants tend to have all three monomers more equally represented [27]. The structure or bonding of these monomers is also different, based on plant species, where specific distinctions can be observed [4,26]. Softwood trees usually have more carbon–carbon bonds than hardwood trees [25,28]. These diversities make it impossible to determine the uniform structure of lignin. However, trends for specific plants can be observed, like the previously mentioned monolignol or linking ratios [25,27,29].
The most occurring linkages inside the structure of lignin are illustrated in Figure 2.
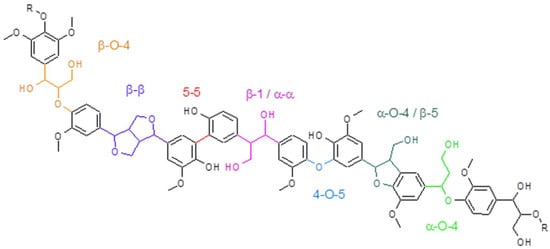
Figure 2.
Representation of different bonds present in the structure of lignin.
Lignin is created in plants during a process called lignification, a dehydrogenation polymerisation mediated by enzymes that results in a complex structure in which different monolignols and different bonds can be observed [4,26]. The formation of lignin occurs inside cell walls in plants, where it functions as a structural component, increasing structural integrity and strength, while serving as a protection against pathogens and insects, having antifungal and antimicrobial functions, thanks to its hydrophobic character, allowing for the transport of water and nutrients through the plant, absorbing UV-radiation and having fire-retardant properties [28,30]. Important heterogeneity can also be observed in the mentioned linkages, as there are seven common linkages found in the lignin structure, whose ratio differs from plant species to plant species [4]. Ether bonds are in general easier to degrade than carbon–carbon bondages, making them interesting for degradation processes and studies [2,6]. Specifically, β-O-4 is the main target during degradation processes, as it is the most abundant linkage of monolignols [2,4,28].
2.2. Isolation and Processing
Isolation of lignin from lignocellulosic biomass is currently conducted by the degradation of lignin, where lignin is degraded over time into smaller fragments of lower molecular weight with modified functional groups, allowing for its dissolution. Alternatively, it is possible to dissolve lignocellulosic biomass with the aim of having little or very mild influence on the structure of lignin, however, this is utilised mainly in an effort to study or measure the amount of lignin [11,31].
All extraction current analytical and industrial methods are influencing the structure and function groups of lignin, changing its thermochemical properties and interactions. Smaller fractions of lignin are soluble in specific solvents that are exploited in the process of extraction, where lignin is transported away from the rest of the biomass, which is usually composed of polysaccharidic compounds [2,32,33].
These separations are classified by different solvents used for extraction, whereas the properties of lignin are influenced by the temperature, pH, and pressure used in this delignification process. There are currently four main processes mentioned below, whereas lignin first is a specific group that requires mentioning [2,33,34]. Major factors, advantages and disadvantages are summarized in Table 1, with addition of two, currently unused, but perspective methods of lignin isolation processes.

Table 1.
Brief overview of lignin isolation processes.
2.2.1. Soda
The soda process is historically one of the oldest delignification processes, consisting of the application of sodium hydroxide solution to biomass. In the current industry, it is widely applied to non-wood biomass, for example, the delignification of sugarcane bagasse, grasses, straw, and other wastes from agriculture production. The benefits are lower molar weight and higher purity of isolated lignin, without sulphur contamination, making it an ideal lignin for catalytic-accelerated degradation [2,33,41].
2.2.2. Sulphite
Sulphite process was the dominant delignification method in industry in the first half of the 20th century, until the introduction of the recovery boiler in industry and the consequent replacement by the kraft process. It uses different cations, for example, sodium, calcium, potassium, magnesium, or ammonium in a wide variety of temperatures and pH, based on the cation selected for the specific process. It is still used, having its advantages and disadvantages [2,41]. One of the main advantages, from the lignin viewpoint, is the production of lignosulphonates as by-products. These are soluble in water, containing sulphur in the form of sulphite ions bonded into a lignin structure [12,41]. They are separated by ultrafiltration, dialysis, electrodialysis, or extraction by amines and are supplied with a relatively stable lignosulphonates market. They have a wide variety of applications, like plasticisers, additives, dispersing agents, viscosity agents, raw materials in dimethyl sulfoxide production, and others [2,3,25,46]. Problematic seems to be the formation of carbon-carbon bonds occurring during these processes, resulting in a wide polydispersity of molar weights of lignosulphonates [11,47].
2.2.3. Kraft (Sulphate)
Kraft process uses a water solution of sodium hydroxide and sodium sulphide with the addition of sodium carbonate and sodium sulphate [11,48]. During delignification, nucleophilic agents influence conjugated centres, carbonyl acids, aromatic parts, and unsaturated parts of lignin [11,33,48]. By the dissolution of ether and ester bonds and the creation of new functional groups in these places, the molecular weight is lowered and lignin becomes soluble in basic conditions of the environment [37,49]. This delignification process is currently the dominant delignification used in pulp production because of the strength properties of the produced fibres and its economic benefits [14]. Lignin is dissolved into a solution called black liquor that is burned, generating heat, and allowing for the regeneration of chemicals for the delignification process [37,50].
The addition of lignin precipitation can be applied using the change of pH of this black liquor. Obtained lignin is usually polluted by sulphur and other impurities, resulting in the need for a purification process [2,11,14,49].
2.2.4. Organosolv
The organosolv process is based on the application of organic solvents like methanol, ethanol, ethylene glycol, acetone, or acetic acid at increased temperature and pressure, resulting in the hydrolytic cleavage of aryl bonds, lowering the molecular weight of lignin, allowing for its dissolution [40,51]. The main disadvantages are the safety of this technology, technological difficulties, economical investment, and sustainability. However, the produced lignin is of high quality and relatively low polydispersity [32,40,51].
2.2.5. Lignin-Centred Fractionation of Biomass
The lignin first approach is a novel concept that prefers the quality and quantity of lignin or treats the entire product spectrum for the separation of lignocellulosic biomass as primary products, unlike the current pulping industry, where the main aim of the process is the quality and quantity of pulp, whereas lignin and other products are considered secondary or waste [52,53]. This allows for the more efficient utilisation of biomass while diminishing the waste streams from refineries. The current suggestion for these technologies and research is aimed mainly at catalytic fractionation, subsequent modification and valorisation, reusable and green catalyst research, selective cleavage of bonds in lignin, as well as efficient energy production [24,52,53,54,55,56,57]. There is also the perspective method of lignin separation by the utilisation of ionic liquids at a pre-treatment stage of delignification [42,58] or the separation of lignin by the enzymatic degradation of lignocellulosic biomass [58,59]. Despite the resulting lignin being of high quality, long operation times, high investment, and operational cost requirements are discouraging factors, preventing industrial application [42,60]. Although showing enormous potential for future use, there are currently no large-scale operations where this technology is used as an efficient and economically sustainable business model, and thus limited attention is devoted to it.
For further understanding, a simplified separation of currently used industrial lignin extraction methods are observable, with their respective lignin products, in Figure 3.
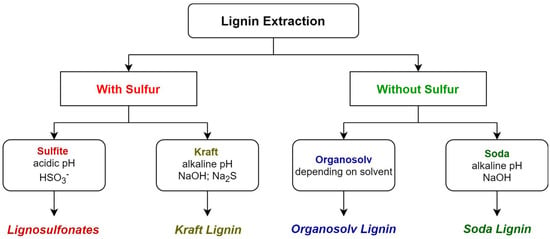
Figure 3.
Summarisation of industrial lignin isolation processes and their respective products.
2.3. Effect of Delignification on Structure
The problematic factor for the industrial utilisation of lignin obtained from currently utilised industrial plants and the delignification process is the changes in the structure of lignin, as well as additional heterogeneity in the structures [11,31,33,61]. During delignification, a cleavage of bonds is expected to occur; while lignin macromolecular weight is lowered, lignin is fractured into smaller particles, and depending on the delignification process, the substitution of groups might occur, which allows for the dissolution of lignin into its surroundings [11,31,34,62]. These changes and effects on the previously heterogenic structures might seem natural, trivial, or insignificant, but are the main factors in consideration for further lignin modification or application, and thus must be mentioned and taken into consideration when working with lignin [11,21,22,50,63]. Observable in Figure 4, are illustrated some major differences between chemical changes occurring during industrial delignification processes. While not all chemical changes can be illustrated due to high heterogeneity of structure, the variation of functional groups is often considered the main reason for alteration of lignin ability to dissolve.
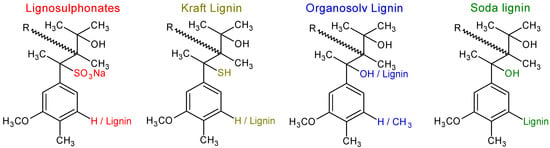
Figure 4.
Predicted changes in the structure of lignin after delignification.
Analyses of lignin properties are therefore a must-do before any prediction about its further use or direct application. The right selection of methods might facilitate the interpretation of results, whereas wrong or very generic methods might decrease the accuracy and make it difficult to interpret results. One of the examples is phenolic hydroxyl group content where the results vary depending on the method used [64,65,66].
Because of this, an improvement in the selection of properties required for screening and application, improvement, and enhancement of the methods applied to obtain these results, and even advancement in the interpretation of these results in general can be observed [12,20,52,67].
Observable in Table 2 are the selected properties of some selected isolated lignins as reported in the literature [68,69]. These values depend on the specific conditions under which lignin is obtained and the source of lignin therefore might vary [3,19,25,41]. Additional important properties might include purity properties such as ash, metal, hydrocarbon, or extractives content, structural properties such as the mentioned polydispersity, molar mass distribution, and structure analysis, usually conducted by nuclear magnetic resonance spectroscopy, UV, FTIR, or Raman spectroscopy, and high-pressure liquid chromatography or gas chromatography with a mass spectrometer. Additionally, there are thermal properties analysed by a variety of thermic analysis methods, such as DTA, DSC, TG, TMA, or DMA. The study of electric properties, colloid properties, solubility, and various specific application properties also might be concluded.

Table 2.
Selected properties of isolated lignin.
3. Modification of Lignin
As already mentioned, the advantage of the lignin structure is a relatively easy modification that improves its properties and usability. The application of lignin is possible without chemical modification, for example, as a filler into the polymer matrix, where it enhances flame retardation, acts as an antioxidant, or improves UV stability, however, the application of chemical modification might be beneficial in terms of lignin compatibility with the polymer matrix and widen potential application conditions [20,68,70,71].
The most promising modifications are the degradation of lignin, with the aim of obtaining monomers and chemicals [68,72,73], or chemical modifications of the hydroxyl groups, that are relatively efficient and safe to conduct [12,25], whereas the changes in lignin properties allow for its compatibility with the desired polymer matrix and further applications [7,68]. Observable in Figure 5 are the selected chemical modifications of products, whereas the specific conditions and results for the displayed modifications are described later. Modifications displayed are separated into two main groups, namely the creation of new active sites, marked by green colour, and modification of the hydroxyl group, marked by blue. Rather, a specific change in structure demethylation is marked by a purple colour.
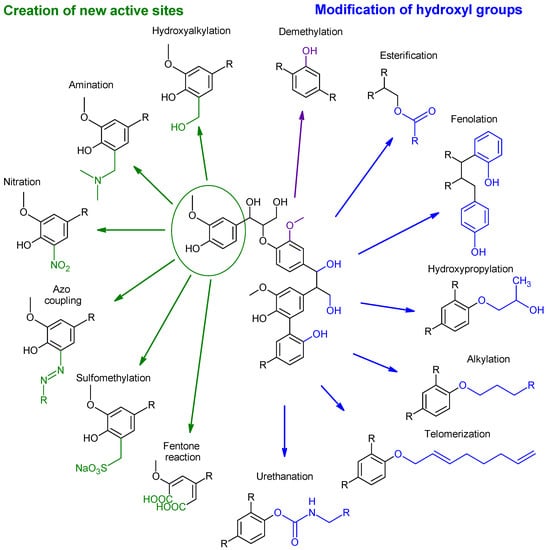
Figure 5.
Simplified schematic of the most promising and studied modifications of lignin.
Not all possible modifications of lignin are in Figure 5, as due to the complexity of the structure of lignin, many possible modifications and chemical reactions are possible, but not all are viable due to low efficiency, high waste product generation, extreme conditions, selectivity of sites, the heterogeneity of the structure, or many other factors [68,74,75,76,77]. Subsequent chapters are divided into the mentioned categories, whether there is the addition of new functional groups or the modification of existing ones.
3.1. Creation of New Active Sites
Lignin already contains a large number and variety of functional groups, such as hydroxyl, methoxyl, carbonyl, and carboxyl in different ratios and placements throughout the structure, depending on many factors [6,12,22,25]. This heterogeneity, preventing high-end applications, can be dodged by creating new functional sites, thus increasing the reactivity of lignin, improving compatibility with the polymer matrix, changing the solubility in solvents, and other additional effects depending on the specific reaction used [23,29,68,78].
The main reactions to introducing new active sites in lignin structures are hydroxyalkylation, amination, nitration, sulphometylation, and sulphonation, whereas reactions such as azo coupling, fentone reaction, halogenation, alkylation or dealkylation, and grafting may be used for modification or as an intermediate stage in modification [25,68,79].
3.1.1. Hydroxyalkylation
The importance and interest in the hydroxyalkylation of lignin are due to the increasing compatibility of lignin in phenol-formaldehyde resins that allows for the replacement of non-renewable phenol by modified lignin [80,81]. This allowed for the creation of many patents, research articles, and even semi-industrial scale implementation of using lignin in phenol-formaldehyde resins for the wood adhesive industry. The reaction of lignin with formaldehyde has been well described and studied for better understanding and the setting of conditions [82,83,84,85]. Usually, it is the reaction of lignin with formaldehyde in a basic environment created by the addition of sodium hydroxide, where formaldehyde can be replaced by other aldehydes, such as glyoxal, furfural, glutaraldehyde, or others if required. This is done due to the lower toxicity and volatility of these aldehydes, compared to formaldehyde [86,87,88].
3.1.2. Amination
The amination of lignin is usually conducted through the Mannich reaction, where lignin is reacted with amines and formaldehyde [89,90,91]. Formed aminated groups that are created can be further ionised and create a positive charge on the structure while in an acidic environment. Products of this reaction are thus cationic surfactants, utilised in asphalt emulgation or as an additive to wood composites, such as a combination of PVC and wood flour. Materials modified by the utilisation of aminated lignin are modified by increased inner interactions and reduced water absorption [91,92,93,94,95,96,97,98].
3.1.3. Nitration
Nitration is usually conducted in a waterless environment where nitration agents react with lignin. Usually, nitric acid with acetanhydride or sulphuric acid is used. The product of this reaction is nitrolignin, in which a distinct colour change can be observed, a change of molar mass, and an increase in nitrogen content can be measured [99,100,101]. This nitrolignin can be applied in the production of polyurethanes, where it interlinks with the structure, increasing the temperature of the glass transition temperature, and increasing tensile strength and stiffness, however, the resistance to the rupture of material decreases [60,101,102,103]. This effect is usually attributed to the increased number of bonds formed during the polymerisation of the polyurethane-lignin structure [103,104,105].
3.1.4. Sulphometylation
Sulphomethylation of lignin introduces methylene sulphonate groups to the aromatic ring inside the lignin structure, mainly at the ortho position [36,59,106]. The reaction is usually performed inside an equimolar mixture of alkali metal sulphite, salt, and formaldehyde in a water-based solution at an increased temperature and alkaline conditions [59,100,107,108]. After modification, lignin is separated by changing the solution pH, or by membrane dialysis filtration. Sulphomethylated lignin has increased tanning capacity, allowing its use in the tanning industry, and has good dispersant properties, being used as a dispersant in dyes, concrete, or as a flocculant in suspensions because of increased hydrophilicity [59,107,108,109,110,111,112,113].
3.1.5. Sulphonation
The sulphonation of lignin occurs by the addition of sulphonated groups to the structure of lignin, usually originating from sulphuric acid or sodium sulphate [100,109,114]. This modification targets primary carbon in the α position, although, if chlorosulphonic acid is utilised, the phenolic ring of lignin is targeted as the reaction centre instead [109,115,116]. Produced sulphonated lignin has increased zeta potential and can be used as a dispersant in the oil industry, paper coating, concrete and cement modifications and production, as well as surfactants, dispersants, or binders. In recent decades, the utilisation of sulphonated lignin can also be observed in some sunscreens due to its UV protection ability [117,118,119,120,121,122].
3.1.6. Other Reactions
Among other less utilised or more specific reactions, whether for safety, renewability, economic, or other reasons, are the Fenton reaction, azo coupling, halogenation, and dealkylation, most of them mentioned later due to their use in grafting polymers or other functional molecules onto lignin.
The azo-coupling reaction is conducted between lignin and diazonium salt that can be modified to a certain degree, allowing for the addition of a macromolecule or polymer chains, such as polyethylene glycol into the structure, modifying the properties of the resulting materials [79,123]. These usually depend on the structure bonded to lignin, but usually consist in the change of solubility under different conditions and in different solvents, as well as the formation of specific colloidal particles that can be further used, or utilising the photochromic effects of azo-modified lignin in photo-responsive materials [79,123,124,125].
The Fenton reaction, usually utilised as one way of metabolic lignin degradation by fungi, is a chemical reaction that opens an aromatic ring inside the lignin structures [126,127]. Depending on the reaction conditions and environment, the reaction is usually a part of the metabolic pathways for degrading lignin into basic chemicals and nutrients and thus can be utilised for chemical production [126,127,128,129,130,131]. From a lignin-based materials perspective, in very mild conditions, this reaction can be utilised with the qualitative aim of increasing reactive sites on lignin, however, this process seems unscalable for large-scale production [126,132,133].
The halogenation of lignin is usually conducted as an intermediate step in the chemical modification of lignin [10,69,79], however, can also be utilised for carbon–carbon bond cleavage, and halogenated functional groups can occur on the lignin structure chemistry of delignification [134]. As already mentioned, these groups are utilised as intermediates in reactions, but also can be used to increase lignin flame retardant properties, or can be formed during waste water treatment with chlorine [105,134,135].
3.2. Modification of Hydroxyl Groups
The structure of lignin contains several hydroxyl groups, either phenolic or aliphatic. Due to intramolecular interactions inside the lignin structure, the reactivity of hydroxyl groups varies, yet phenolic hydroxyl groups are generally more reactive and are significantly affecting the reactivity of lignin [105,136,137,138,139]. Through modification of hydroxyl groups by different functional groups, the creation of polyol derivates of lignin can be achieved [139,140]. The main attention is dedicated to modifications such as alkylation, esterification, etherification, phenolation, urethanisation, or other modifications capable of sufficient modification of properties that allow for successful applications, such as adhesives, surfactants, dispersants, copolymers, fillers, or other.
The potential application of modified lignin will always depend on the modified groups, however, by diminishing the number of hydroxyl groups that are hydrophilic, we would increase the hydrophobic character of the lignin macromolecules, which might lead to increased compatibility with the polymer matrix. This approach seems promising in creating a new generation of lignin bio-based copolymers capable of industrial production and application in the near future [75,105,141].
3.2.1. Alkylation
Although alkylation might be conducted in several places in the lignin structure, the main target of the reaction is usually the oxygen atoms of hydroxyl, carbonyl, or carboxylic groups [68,142,143,144,145]. Lignin can be easily alkylated by nucleophilic substitution of the aromatic hydroxyl groups, while alkyl groups of various lengths are grafted onto the structure of lignin [79,87,146]. This is utilised mainly for grafting alkyl chains in an effort to ameliorate the mechanical and thermal properties of the prepared copolymer materials or change their surface properties. Main applications, therefore, depend on the bonded groups but consist mostly of plasticisers [137], surfactants [147], and copolymers, fillers, or plasticiser in polymer blends [52,138,148].
A well-known dealkylation process of lignin is demethylation, conducted in the presence of melted sulphur in an alkaline environment, during which dimethyl sulphide (DMS) is generated and can further be oxidised, thus producing dimethyl sulphoxide (DMSO) [100,149,150,151]. This method is currently used to produce dimethyl sulphoxide on an industrial scale. The side product is demethylated lignin, which can be used in a mixture with polyethyleneimines for the production of water-resistant adhesives for wood surfaces. Demethylation is also important, because it increases the number of potential hydroxyl groups for modification, thus increasing the reactivity of lignin [100,149,150].
3.2.2. Esterification
Esterification is allowed for the production of esters or polyesters based on lignin due to reactions with hydroxyl groups [152,153]. This modification is possible to realise with a variety of acidic molecules or their anhydrides and chlorides [154]. If bifunctional molecules are utilised, there is a possibility of the creation of polyesters, depending on the conditions and properties of lignin [116,155,156]. Another possibility is the addition of a solvent, such as polyethylene glycol, that dissolves lignin and after modification can act as a copolymer with the prepared polyesters [124,157,158]. Theoretically, it is possible to selectively modify aromatic hydroxyl groups, however, the results proved that esterification of both aromatic and aliphatic hydroxyl groups is unavoidable because of the structural complexity. However, with the selection of the right conditions, one or another might be statistically preferred. The modification of lignin in these cases causes a change of properties, primarily in the increase in molar mass, polydispersity, and changes in thermal properties. Esterified lignin is usually used as a copolymer in polyesters, epoxide resins, polyurethanes, or other such materials, depending on the ester used in the reaction [154,159,160,161,162,163,164,165].
3.2.3. Etherification
The etherification of lignin is a chemical reaction normally realised with the utilisation of alkyl oxides, mainly ethylene and propylene oxide, in polymerisation reactions with epichlorohydrin or in crosslinking reactions with diglycidyl ethers [118,166]. Products of these reactions can be utilised as dispersants or in further polymerisation reactions, creating crosslinked networks [118,143,166].
3.2.4. Phenolation
Phenolation is a modification important for the utilisation of lignin in phenol-formaldehyde resin mixtures. Usually conducted with the utilisation of phenols or phenolic derivates in organic solvents, such as ethanol or methanol, and acidic pH, lignin undergoes condensation reactions with phenols present [130,147,167]. This modification usually increases the number of reactive ortho and para groups, thus increasing the reactivity of lignin. Products are generally used in the substitution of phenol in phenol-formaldehyde resins, or in specific cases, like lignin phenolation by cardanol, can be utilised in polyurethane coatings, improving some thermal and mechanical properties [130,145,150,167,168,169,170,171].
3.2.5. Urethanation
The reaction of lignin hydroxyl group-created urethane with the isocyanate group of another reagent creates urethane bondage [139,172]. Although this reaction is exothermic, lignin acts as a polyol that can be utilised to create polyurethane crosslinked networks with a number of applications in the creation of new more stable polyurethanes with enhanced properties [105,172,173,174,175].
3.3. Grafting of Polymers
As already mentioned in previous chemical modifications, by creating specific reaction conditions, instead of bonding functional groups or molecules onto the structure of lignin, the structure can instead be modified by bonding the polymer to it, or it to the polymer, depending on the environment. This allows for lignin to act as a polymerising agent, creating interlinked networks of polymers, or rather functionalising lignin by creating particles with rather unique colloidal properties [124,176,177]. These effects are however influenced by the lignin content of the reactive groups, the molar mass or rather the size of a macromolecule, and a variety of other properties that depend on the isolation process [79,178]. The utilisation of specific lignin with a smaller size of macromolecules and more approachable hydroxyl groups might be better for the application of modified lignin as colloidal particles, whereas the utilisation of larger and more interlinked structures might be beneficial in the utilisation of polymer blends or act as a stabilisation agent in rubbers [6,75,124,176].
3.3.1. Grafting From
Grafting polymers from lignin, if simplified, is polymerisation originating from lignin. This is done by modifying the lignin structures with function groups capable of reacting with a monomer, or by the direct reaction of lignin with monomers, usually by radical polymerisation, radical polymerisation with atom transfer, or polymerisation with ring opening [166,177,178].
Radical polymerisation is conducted by creating a radical structure of lignin during initialisation, where hydrogen peroxide or other reagents are applied; however, due to the structure and photochromic properties of lignin, a specific wavelength of light can also be applied. Subsequent polymerisation is uniform in process with a normal polymerisation between monomers. This modification allows for the application of polystyrene, polyacrylic acid, polyvinyl acetate, and other polymers, making lignin subsequently more compatible with polymer blends [139,178]. Disadvantages of this reaction are the homopolymerisation of monomers in the reaction mixture, polymerisation between lignin particles, stabilisation of radicals on lignin due to resonance stabilisation on aromatic rings, and overall, less control over the behaviour and course of polymerisation [139,155,179].
Radical polymerisation with atom transfer solved this problem with slower, but more controlled reactions, where polymer chains of more defined parameters can be generated [156,180].
Polymerisation with ring opening is realised by the reaction between lignin hydroxyl groups and ring structure [25]. An example of these ring structures is propylene oxide, which allows the production of oxypropylated lignin utilised in polyurethane foams [181,182]. Utilisation of another, such as caprolactone, in a mixture with polylactic acid results in a reaction incorporating lignin into the polymer structure, lowering the temperature of glass transition, viscosity, and mechanical properties of the prepared materials, largely depending on the length of the grafted polymers [182,183,184].
3.3.2. Grafting to
During the grafting of polymers to lignin, a reaction occurs between lignin and polymer, resulting in a stable bond between them [124,176]. The advantage over grafting from reactions is that polymerisation is done before the reaction with lignin, thus resulting in better control over the bonded polymers and only changing the number of polymer groups grafted to the lignin structure [155,176]. The disadvantage is that very few changes except for the mentioned number of grafted groups can be influenced, so great attention must be dedicated to the preparation of polymer material [155,177].
3.4. Functionalisation of Lignin
Another group of rather indirect chemical modification methods is the degradation and purification of lignin. Despite that their primary goal is not to change functional groups, they have a substantial effect and are often used as pre-processing procedures in an effort to functionalise lignin further, before application [2,50,63,185].
3.4.1. Purification and Fractionation
Lignin isolated directly from black liquor of a kraft pulp mill by a one-step process is usually of low quality and high heterogeneity, thus requiring further purification, whether as an additional step or preferably, implemented during precipitation [2,23,35]. These technologies usually decrease the quantity of lignin, however, the increase in quality, whether it is purity, lower variety in properties, or creation of fractions dependent on the mentioned properties is a valuable benefit, despite adding an additional technological step.
Purifications currently applied and tested in semi-industrial trials conduct additional washing with acidic solutions, where the most used is sulphuric acid. Despite causing additional degradation and carbonisation of the prepared lignin, there is an observable decrease in ash content, the lower polydispersity of lignin, and other properties based on the conditions used [2,131,185,186]. Current research is aimed at less aggressive purification, mostly the use of ionic liquids, or rather the use of deep eutectic solvents, that would allow for economically and environmentally friendly purification with easy regeneration of solvents [38,42,187]. The overall assumed benefit of these modifications is lowering possible sulphur, nitrogen, and ash contents, while minimal changes to the structure of the lignin macromolecules are made.
3.4.2. Depolymerisation
The depolymerisation of lignin is traditionally separated into categories depending on the conditions during this process. These are usually pyrolysis [25], gasification [188,189], oxidation [190], hydrogenolysis, hydrolysis, and other specific depolymerisation [150,186], such as depolymerisation in supercritical carbon dioxide [191], or enzymatic degradation [127,192]. These are separately well-studied topics, where research is aimed at breaking the lignin structures with simple chemicals, such as benzene or toluene, or even production or mono- or oligomers from the structure of lignin. Applications of these products vary depending on the purity and possibility of separation from the mixture of products. The main application is usually the production of chemicals, fuels, or other simple compounds [12,72,193].
In recent years, specific interest is aimed at mild depolymerisation conditions, where lignin macromolecules could break into larger fragments or could be selectively broken into desired fragments [35,68]. It is important to notice that the fragmentation of lignin, whether it would be monolignols or smaller macromolecular fragments, would open potential reactive sites, and thus, this approach is researched in combination with the previously mentioned chemical modifications of the structure, due to the increase in reactivity, solubility, and accessibility of reactive sites on lignin [35,194,195].
4. Hydrophobicity
Hydrophobic behaviour can be described as the ability to repel water, in contrast to hydrophilic behaviour, where an attraction or affinity to water is observable [196]. However, hydrophobicity of function groups, molecules and surfaces is a rather complex topic. To better understand the challenges of lignin hydrophobic behaviour, basic knowledge of hydrophobic and hydrophilic interactions is required and provided in the next chapter in simplified form, both on a molecular level, as well as for the surface interactions of materials.
4.1. Hydrogen Bond to Hydrophobicity
The polarity of water molecules manifests as the creation of partial charges inside the structure. A partially negative charge on the oxygen of one molecule is thus capable of interactions with a partial charge on the hydrogen of another molecule, creating a hydrogen bond [197]. These hydrogen bonds are responsible for many important properties of water, from high specific heat capacity, the heat of fusion, the heat of vaporisation, thermal conductivity, and boiling and heating points, compared to other similar macromolecules [198]. The creation of these hydrogen bonds is also responsible for relatively high surface tension and capillary forces, whose utilisation can be observed in the transport of water through plants [26].
Hydrogen bonds also play an important role on the macromolecular scale. The creation of multiple partial charges across polymers can lead to the creation of intermolecular and intramolecular hydrogen bonds, further influencing the final structural configuration of the macromolecule and properties of materials [199]. This is important for the correct function of proteins, and DNA, as well as for influencing the structural properties of many natural and synthetic polymers [199,200]. These interactions can be observed in cellulose, allowing for the creation of long polymer chains that interact in between and create microfibrils [201]. These factors may lead to the crystallisation of polymers, further influencing both reactivity and strength properties of polymeric material [199,201]. Similar effects can be observed for synthetic polymer, well-known examples being nylon, polyvinyl alcohol, polyacrylamide, and others. It is worth noting that the properties of these polymers can be significantly influenced by humidity since water is usually interacting with created hydrogen bonds [199].
The mentioned interactions have an important role in the solubility of macromolecules in water or other polar solutions [202]. To fit the scope of this article, a rather simplified explanation is that if enough partially surface charged groups that are accessible to solvent are present and have sufficient charge, dissolution becomes possible. The more partially charged groups present while being accessible to a polar solvent, the main example being water, the more hydrophilic character of the material can be observed [196,202]. Vice versa, if less partially charged groups, capable of hydrogen bonds are present, less hydrophilic, or more hydrophobic character is observed [202].
It is worth noting that not only do these interactions play a role on the material level, but that many superhydrophobic behaviours are achieved by spatial surface modifications, and by the creation of either protrusions or depressions in the microscopic scale on the surface, leading to a reduction in the surface area where material and liquid interacts [196,203]. This effect is best observable on a lotus leaf, which combines both lowered surface area due to protrusions with chemical hydrophobic effects, created by a wax coating of mentioned protrusions [204].
Most used measurements, able to determine the mentioned surface properties or compare two different surfaces, make use of the contact angle between the droplet of liquid and the solid surface [197]. There are different methods to measure said angle, however, the most used is a static contact angle measurement, where a droplet is dropped, and a camera is used to determine the contact angle before the wetting of material or other disturbing factors may have an effect [203,205]. Other possible methods include utilising a sliding angle, advancing angle, or receding angle, depending on the type of apparatus used [197]. The importance of static contact angles resides in the differentiation of hydrophobic and hydrophilic surfaces, where the contact angle lower than 90 degrees is a hydrophilic surface with good wettability, whereas higher than 90 degrees indicates a hydrophobic surface [197,203]. A surface of a material is usually considered superhydrophobic when a static contact angle of water is higher than 150 degrees [203].
These topics come with their own challenges that do not fit the scope of this article, and thus a further study of colloidal and surface sciences is recommended if the reader is interested.
4.2. Hydrophobicity and Hydrophility of Lignin
As previously mentioned, the structure of lignin is rather complex, consisting of many different functional groups with a wide variety of polarity throughout the structure, thus giving lignin the possibility of being either hydrophobic or hydrophilic [10,206]. It can be observed in isolation methods, where lignosulphonates are dissolvable in water, despite their high molecular weight [35], whereas kraft and natron lignin are hydrophobic [75]. It can be concluded that the increased number of functional groups capable of the creation of hydrogen bonds while being accessible to the solvent due to spatial restrictions, makes lignin more hydrophilic [108,207]. It is worth noting that the stabilisation of hydroxyl groups also influences their reactivity and ability to participate in solubilisation [208]. This can be observed between aliphatic hydroxyls and phenolic hydroxyls on lignin, where aliphatic hydroxyls are more reactive, due to the lack of stabilisation [79,208], in contrast to phenolic hydroxyl groups that require a longer time to react [208], or even inhibit enzymatic hydrolysis [209], due to the stabilisation of valence electrons on hydroxyl groups by aromatic ring [139,208].
Thereby, by the modification of existing groups or the creation of new functional groups, a more or less hydrophobic character of lignin is achievable, depending on the character of modified functional groups. This assumption is supported by experimental results of modifications, where after modifying hydroxyl groups on lignin, an increase in hydrophobic behaviour was observed [75,108]. A subsequent increase in compatibility with polymers can be attributed not only to an increase in hydrophobicity, but also to the creation of new functional groups resembling those of the concerned polymer, examples being the application of methylated [210], malleated [77,211], acetylated lignin [212,213] or lignin methacrylate [214]. The resulting increase in thermal and mechanical properties and resistances can be also attributed to the creation of composites and the interaction of lignin with the used polymer [215].
The modification of functional groups by the addition of large non-polar molecules, usually alkyl chains, results in an increase in hydrophobic behaviour, not only due to the nature of these grafted polymers but also due to spherical reasons, where non-modified hydrophobic groups can become inaccessible [216,217]. This effect can be utilised in the creation of thin hydrophobic films, where similar to cell membranes, hydrophilic functional groups bond to surfaces, whereas hydrophobic functional groups create new modified surfaces [87].
5. Discussion
As mentioned previously, the modification of lignin is a rather complicated topic. There are many challenges to overcome that can be separated into two distinct categories, the heterogeneity of lignin and socio-economic factors. The latter can be observed in many articles over the last decades, mentioning a rather complex situation, whether referring to the phrase “you can make anything out of lignin but money” and addressing overall problems [218,219], a rather sceptical opinion about the possibility of lignin utilisation in the future [220], or an effort to make lignin a successful resource by the transformation of the industry and mentality around renewable resources as a whole [138,221,222]. These challenges may not be directly related to modifications of the chemical structure of lignin, however, are an important factor to always have in mind when working with lignin and its derivates.
The heterogeneity of lignin as a material is a problem on its own, from a molecular scale, where lignin can be of different compositions in between fragments, through the industrial separation, where it can change properties over time without changing the conditions of operation, or among different facilities, depending on the source, isolation process, and other. These differences may contribute heavily to the approach of industry and research groups to the valorisation of lignin itself [223,224]. Usually, research is centred around the topic of lignin valorisation, however, often limited by the scope of the subject, without any kind of pre- or post-processing. Another problematic subject is the combination of different technologies and processes with different lignin, where proven efficient and usable in a testing case, heterogeneity in another testing run can diminish the results in both quality and quantity. The realisation of these effects and the influence of heterogeneity on valorisation development has also had a positive effect. A new approach must be adopted and as such, the development has shifted from only large-scale production to mapping, developing small-scale production, and listing the properties of lignin and lignin-related products and processes. One such example is LignoCOST project, where one of the goals is the creation of a database composed of lignin sources, availability, properties, and state-of-the-art analytical methodologies and turnkey methods for industry, as mentioned in their goals and published documents [225,226]. Development of these small-scale high-tech production facilities equipped with knowledge of lignin properties should be able to valorise lignin efficiently, with economic and environmental sustainability [52,227].
Further research thus not only has the aim to modify and search for useful applications of lignin but also for fast information-rich analysis and result interpretation. These analyses and result interpretations are even more relevant in the case of chemically modified lignin, as some previously used analyses might not give accurate or precise results as with unmodified. One such example might be the prediction of properties, such as molar mass, polydispersity, and amount of hydroxyl groups by NIR measurement and subsequent interpolation based on already existing database results, that works for unmodified lignin, but will not be accurate for modified lignin [228,229,230]. The importance of these new fast methods for the determination of a complex array of properties and the creation of wide datasets allows for fast predictions that can be further used and are best efficient in small-scale production. Although sharing these datasets might seem to circumvent this problem and allow for large-scale applications, the lack of proper sorting and division will create further complications in the future due to already mentioned heterogeneity.
All of these mentioned factors are related to the last before directly addressing the chemical modifications of lignin, and that is pre-processing. New emerging technologies, such as the application of deep eutectic liquids, or different methods for purification, sorting, and sequencing lignin based on properties are of great importance, for they allow the production of lignin with specific quality and quantity, required for the creation of biorefineries [54,118,231]. Deep eutectic solvents, a subgroup of ionic liquids, are experiencing a large influx of research attention in the last decades, as can be observed in a sharp increase in articles regarding this topic. They allow for environmentally friendly, relatively cheap, renewable methods of lignin separation and purification [44,154,232]. Although the initial results are showing limited potential in fully replacing currently utilised delignification technologies, the pre-treatment of biomass or the utilisation of deep eutectic solvents to dissolve specific fractions of biomass, or selectively lignin, shows their great potential in future applications in the search for high-quality resource isolation and separation from biomass [45,233,234]. Another important role is played by other isolation, separation, fractionation, and purification technologies, either already developed or further researched, since these allow for the production and research of lignin applications in the present time, opening the market and drawing the attention of society towards these renewable technologies [2,52,224]. Limited worldwide implementations of lignin separation processes, such as WestVaco, LignoBoost, LignoForce, and SLRP, are further highlighting the need for these new separation methods [35,235].
Chemical modifications of lignin structures themselves are thus colliding with a variety of mentioned problems on the way to being successfully implemented in the industry. These problems and further complications with the limited market demand cannot be solved individually and must be approached as a whole, thus the lengthy descriptions of seemingly unrelated topics. It is worth noting that not only the heterogeneity of lignin is important, but also the heterogeneity of approaching the mentioned topics by the scientific and business communities can differ dramatically.
The search for the best chemical modifications is thus hindered by a number of questions, such as what properties of the modified resources are, what are the applications, what are other products, etc. [54,227,231]. Emerging from the concept of biorefinery, capable of complex processing of biomass, and currently amassing knowledge on the topic of lignin, it is almost inevitable to assume a rather wide spectrum of products, with the possibility of shifting supply depending on the demand inside and outside of such a facility [231,236]. Currently, there is an observable demand from industry in search of more hydrophobic lignin. This can be explained by the assumption of the increased compatibility in copolymers or composites based on lignin [75,237]. More specific modifications are changing the lignin macromolecules with the aim of not only increasing the mentioned hydrophobicity as a result but also implementing more functional groups capable of interacting with another part of the mixture. Examples of this approach are the maleation of lignin with maleic anhydride and a further reaction with polystyrene, where the resulting copolymer is based on polystyrene with grafted lignin macromolecular particles, which results in the increase in thermal stability, solubility, and other mechanical resistances towards external forces [77]. These effects can also be observed in a mixture of low-density polyethylene with maleic anhydride in 3% of weight addition, and lignin in 20% weight addition, where an increase in mechanical properties was observed [211]. Other examples include increased compatibility with polylactic acid where various alkylations and esterifications result in increased mechanical strength and thermal stability, with great influence of utilised lignin purity on the efficiency of the reaction [193,212].
The development and increased compatibility with polylactic acid allow for the utilisation of new manufacturing technologies, such as 3D printing or electrospinning [76,193,238], whereas the desired purity of lignin can be reached by the utilisation of the mentioned deep eutectic solvents [154]. The mentioned electrospinning of lignin is getting relatively increased attention, because of the properties of the prepared materials and the possibilities of preparing filtration membranes, various applications in battery and capacitator production, or biochemical applications [183,239,240,241,242,243,244]. The utilisation of the mentioned membranes is widely applicable in water and air filtration materials, a topic closely related to recent pandemic events, where the ability of lignin to be modified or doped by antiviral particles, such as silver or some bioactive macromolecules, can highly increase its potential [74,137,245,246]. Modification may be beneficial even in the production of carbon nanofibers from lignin, where specific conditions and an understanding of the occurring reactions beneficially affect the resulting quantity and quality of products [221,247]. These modifications by the grafting of compounds on lignin allow for the production of nanoparticles, capable of being modified as bioactive compound carriers, or finding other possible applications such as in the pharmaceutical and medical industry [74,86,148,248], or even in agriculture as a slow fertiliser releasing agent [249]. The utilisation of lignin antioxidative properties is also widely observed, with the modification further increasing the potential to act as a stabilisation agent against oxidisation as well as increasing the material mechanical properties, whether the application is in the medical, pharmaceutical, or material industry [74,138,250]. This antioxidative property in combination with UV light stability is further used in sunscreens, where the modification of active particles by lignin increases the efficiency of the sunscreen, whereas the modification of lignin by alkylation allows for retaining or changing the desired fluid properties of the product [111,250,251].
These advancements in additive manufacturing and interest in bio-renewable polymers are further increasing the interest in lignin and its technology. As mentioned, its potential is currently almost unlimited, due to the low demand for resources, however, with the emerging application of technologies, renewability must be maintained with the clear intention of creating a circular economy [219].
6. Conclusions
The importance of lignin lies in its abundance and relative underutilisation in the current industry. With increased attention to maintaining the renewable factor, lignin can be utilised in a wide variety of applications.
Although lignin in its pure form, after isolation from biomass, lacks high-quality applications as of now, this can be circumvented by the chemical modification of the lignin structure. Depending on the desired composition, mechanical and thermal properties of the produced materials, and the quality of the supplied lignin, it is possible to selectively choose the correct modification. The main aim of these modifications is to increase reactivity and compatibility with different polymers or to change the properties of lignin, such as hydrophobicity, thermal and mechanical properties, or other resistances. Due to the distinct effects of modifications on lignin structure and its further use, similarities are often observed in the increase in both hydrophobicity and compatibility with polymers. Therefore, increased demand for hydrophobic lignin on the market can be observed and explained. A suitable modification will not only increase the hydrophobic character of lignin by diminishing the number of hydrophilic groups on macromolecules but will also change the desired properties by adding specific functional groups that will further react with the environment.
This review is focused on an overview of different lignin structure modifications. Although it tries to cover most pathways of structure modification, due to the complexity of the lignin structure and possible reactions to it, it is impossible to combine all possible reactions together. The main object is focused on the modifications that increase hydrophobicity, and in effect, its compatibility with polymers, or that facilitate the utilisation of modified lignin in material production.
Author Contributions
Conceptualisation, A.L. and A.H.; investigation, A.L.; resources, A.L. and R.N.; data curation, A.L.; writing—original draft preparation, A.L.; writing—review and editing, A.L., A.H. and I.Š.; visualisation, A.L.; supervision, A.H.; project administration, A.H. and M.J.; funding acquisition, I.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This publication was supported by the Operational Program Integrated Infrastructure within the project: Demand-driven research for the sustainable and innovative food, Drive4SIFood 313011V336, co-financed by the European Regional Development Fund. The FFG-Austrian Research Promotion Agency is also acknowledged for their funding of the Projects KraftPell (FFG-Nr. 884529), and by the generous support under the Operational Program Integrated Infrastructure for the project: “Strategic research in the field of SMART monitoring, treatment, and preventive protection against coronavirus (SARS-CoV-2)”, Project no. 313011ASS8, co-financed by the European Regional Development Fund (ERDF). This publication was also partially supported by the Slovak Research and Development Agency under the contract APVV-14-0393.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bar-On, Y.M.; Phillips, R.; Milo, R. The Biomass Distribution on Earth. Proc. Natl. Acad. Sci. USA 2018, 115, 6506–6511. [Google Scholar] [CrossRef] [PubMed]
- Klett, A.S. Purification, Fractionation and Characterization of Lignin from Kraft Black Liquor for Use as a Renewable Biomaterial. Ph.D. Thesis, Clemson University, Clemson, SC, USA, 2017. [Google Scholar]
- Johannes, R.; Gosselink, A. Lignin as a Renewable Aromatic Resource for the Chemical Industry; Wageningen University and Research: Wageningen, The Netherlands, 2011; ISBN 9789461731005. [Google Scholar]
- Tobimatsu, Y.; Schuetz, M. Lignin Polymerization: How Do Plants Manage the Chemistry so Well? Curr. Opin. Biotechnol. 2019, 56, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Structure and Characteristics of Lignin. Lignin Chem. Appl. 2019, 25–50. [CrossRef]
- Huang, J.; Fu, S.; Gan, L. Lignin Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 9780128139417. [Google Scholar]
- Xu, C.; Arancon, R.A.D.; Labidi, J.; Luque, R. Lignin Depolymerisation Strategies: Towards Valuable Chemicals and Fuels. Chem. Soc. Rev. 2014, 43, 7485–7500. [Google Scholar] [CrossRef] [PubMed]
- Berlin, A.; Balakshin, M. Industrial Lignins. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 315–336. ISBN 9780444595614. [Google Scholar]
- Pandey, M.P.; Kim, C.S. Lignin Depolymerization and Conversion: A Review of Thermochemical Methods. Chem. Eng. Technol. 2011, 34, 29–41. [Google Scholar] [CrossRef]
- Wang, H.; Pu, Y.; Ragauskas, A.; Yang, B. From Lignin to Valuable Products–Strategies, Challenges, and Prospects. Bioresour. Technol. 2019, 271, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Gellerstedt, G.L.F.; Henriksson, E.G. Lignins: Major Sources, Structure and Properties. Monomers Polym. Compos. Renew. Resour. 2008, 201–224. [Google Scholar] [CrossRef]
- Kumar, A.; Anushree; Kumar, J.; Bhaskar, T. Utilization of Lignin: A Sustainable and Eco-Friendly Approach. J. Energy Inst. 2020, 93, 235–271. [Google Scholar] [CrossRef]
- Demirbas, A. Higher Heating Values of Lignin Types from Wood and Non-Wood Lignocellulosic Biomasses. Energy Sources Part A Recover. Util. Environ. Eff. 2017, 39, 592–598. [Google Scholar] [CrossRef]
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin Recovery from Spent Alkaline Pulping Liquors Using Acidification, Membrane Separation, and Related Processing Steps: A Review. BioResources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Kavitha, S.; Yukesh Kannah, R.; Poornima Devi, T.; Gunasekaran, M.; Kim, S.-H.H.; Kumar, G. A Review on Biopolymer Production via Lignin Valorization. Bioresour. Technol. 2019, 290, 121790. [Google Scholar] [CrossRef]
- Tomani, P. The Lignoboost Process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Tanzid, M.; Andersson, M.A.; Sun, J.; Stake, J. Microwave Noise Characterization of Graphene Field Effect Transistors. Appl. Phys. Lett. 2014, 104, 013502. [Google Scholar] [CrossRef]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C.C. Global Lignin Supply Overview and Kraft Lignin Potential as an Alternative for Petroleum-Based Polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Chauhan, P.S. Lignin Nanoparticles: Eco-Friendly and Versatile Tool for New Era. Bioresour. Technol. Rep. 2020, 9, 100374. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Lignin Functionalization for the Production of Novel Materials. Trends Chem. 2020, 2, 440–453. [Google Scholar] [CrossRef]
- Holtz, A.; Weidener, D.; Leitner, W.; Klose, H.; Grande, P.M.; Jupke, A. Process Development for Separation of Lignin from OrganoCat Lignocellulose Fractionation Using Antisolvent Precipitation. Sep. Purif. Technol. 2020, 236, 116295. [Google Scholar] [CrossRef]
- da Silva, S.H.F.; Gordobil, O.; Labidi, J. Organic Acids as a Greener Alternative for the Precipitation of Hardwood Kraft Lignins from the Industrial Black Liquor. Int. J. Biol. Macromol. 2020, 142, 583–591. [Google Scholar] [CrossRef]
- Schorr, D.; Diouf, P.N.; Stevanovic, T. Evaluation of Industrial Lignins for Biocomposites Production. Ind. Crops Prod. 2014, 52, 65–73. [Google Scholar] [CrossRef]
- Renders, T.; Van Den Bosch, S.; Koelewijn, S.F.; Schutyser, W.; Sels, B.F. Lignin-First Biomass Fractionation: The Advent of Active Stabilisation Strategies. Energy Environ. Sci. 2017, 10, 1551–1557. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Weng, J.K.; Chapple, C. The Origin and Evolution of Lignin Biosynthesis. New Phytol. 2010, 187, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2014; ISBN 9789400768987. [Google Scholar]
- Novo-Uzal, E.; Pomar, F.; Gómez Ros, L.V.; Espiñeira, J.M.; Ros Barceló, A. Evolutionary History of Lignins. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2012; Volume 61, ISBN 9780124160231. [Google Scholar]
- Ponnusamy, V.K.; Nguyen, D.D.; Dharmaraja, J.; Shobana, S.; Banu, J.R.; Saratale, R.G.; Chang, S.W.; Kumar, G. A Review on Lignin Structure, Pretreatments, Fermentation Reactions and Biorefinery Potential. Bioresour. Technol. 2019, 271, 462–472. [Google Scholar] [CrossRef]
- McCarthy, J.L.; Islam, A. Lignin Chemistry, Technology, and Utilization: A Brief History. ACS Symp. Ser. 1999, 742, 2–66. [Google Scholar] [CrossRef]
- Sundin, J. Precipitation of Kraft Lignin under Alkaline Conditions. Ph.D. Thesis, Royal Institute of Technology, Stockholm, Sweden, 2000. [Google Scholar]
- Manara, P.; Zabaniotou, A.; Vanderghem, C.; Richel, A. Lignin Extraction from Mediterranean Agro-Wastes: Impact of Pretreatment Conditions on Lignin Chemical Structure and Thermal Degradation Behavior. Catal. Today 2014, 223, 25–34. [Google Scholar] [CrossRef]
- Prinsen, P.; Rencoret, J.; Gutiérrez, A.; Liitiä, T.; Tamminen, T.; Colodette, J.L.; Berbis, M.Á.; Jiménez-Barbero, J.; Martínez, Á.T.; Del Río, J.C. Modification of the Lignin Structure during Alkaline Delignification of Eucalyptus Wood by Kraft, Soda-AQ, and Soda-O2 Cooking. Ind. Eng. Chem. Res. 2013, 52, 15702–15712. [Google Scholar] [CrossRef]
- Kalliola, A.; Kuitunen, S.; Liitiä, T.; Rovio, S.; Ohra-Aho, T.; Vuorinen, T.; Tamminen, T. Lignin Oxidation Mechanisms under Oxygen Delignification Conditions. Part 1. Results from Direct Analyses. Holzforschung 2011, 65, 567–574. [Google Scholar] [CrossRef]
- Kienberger, M.; Maitz, S.; Pichler, T.; Demmelmayer, P. Systematic Review on Isolation Processes for Technical Lignin. Processes 2021, 9, 804. [Google Scholar] [CrossRef]
- Aro, T.; Fatehi, P. Production and Application of Lignosulfonates and Sulfonated Lignin. ChemSusChem 2017, 10, 1861–1877. [Google Scholar] [CrossRef]
- Theliander, H. The LignoBoost Process: Solubility of Lignin. In Proceedings of the International Chemical Recovery Conference, Williamsburg, VA, USA, 29 March–1 April 2010; pp. 33–42. [Google Scholar]
- Prado, R.; Erdocia, X.; Serrano, L.; Labidi, J. Lignin Purification with Green Solvents. Cellul. Chem. Technol. 2012, 46, 221–225. [Google Scholar]
- El Mansouri, N.E.; Salvadó, J. Structural Characterization of Technical Lignins for the Production of Adhesives: Application to Lignosulfonate, Kraft, Soda-Anthraquinone, Organosolv and Ethanol Process Lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Carvajal, J.C.; Gómez, Á.; Cardona, C.A. Comparison of Lignin Extraction Processes: Economic and Environmental Assessment. Bioresour. Technol. 2016, 214, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Naron, D.R.; Collard, F.X.; Tyhoda, L.; Görgens, J.F. Characterisation of Lignins from Different Sources by Appropriate Analytical Methods: Introducing Thermogravimetric Analysis-Thermal Desorption-Gas Chromatography–Mass Spectroscopy. Ind. Crops Prod. 2017, 101, 61–74. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Moreno, A.D.; Alvira, P.; Ibarra, D.; Tomás-pejó, E. Production of Platform Chemicals from Sustainable Resources; Springer: Singapore, 2017; pp. 375–410. ISBN 978-981-10-4171-6. [Google Scholar]
- Gellerstedt, G.; Majtnerova, A.; Zhang, L. Towards a New Concept of Lignin Condensation in Kraft Pulping. Initial Results. Comptes Rendus. Biol. 2004, 327, 817–826. [Google Scholar] [CrossRef]
- Singh, S.K. Ionic Liquids and Lignin Interaction: An Overview. Bioresour. Technol. Rep. 2022, 17, 100958. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards Lignin-Based Functional Materials in a Sustainable World. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Bhattacharya, P.K.; Todi, R.K.; Tiwari, M.; Bhattacharjee, C.; Bhattacharjee, S.; Datta, S. Studies on Ultrafiltration of Spent Sulfite Liquor Using Various Membranes for the Recovery of Lignosulphonates. Desalination 2005, 174, 287–297. [Google Scholar] [CrossRef]
- Zhu, W.; Westman, G.; Theliander, H. Investigation and Characterization of Lignin Precipitation in the Lignoboost Process. J. Wood Chem. Technol. 2014, 34, 77–97. [Google Scholar] [CrossRef]
- Helander, M.; Theliander, H.; Lawoko, M.; Henriksson, G.; Zhang, L.; Lindström, M.E. Fractionation of Technical Lignin: Molecular Mass and PH Effects. BioResources 2013, 8, 2270–2282. [Google Scholar] [CrossRef]
- Minu, K.; Jiby, K.K.; Kishore, V.V.N. Isolation and Purification of Lignin and Silica from the Black Liquor Generated during the Production of Bioethanol from Rice Straw. Biomass Bioenergy 2012, 39, 210–217. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, D. Chemical and Thermal Characteristics of Lignins Isolated from Siam Weed Stem by Acetic Acid and Formic Acid Delignification. Ind. Crops Prod. 2010, 32, 284–291. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Barta, K.; Beckham, G.T.; Luterbacher, J.S.; Ralph, J.; Rinaldi, R.; Román-Leshkov, Y.; Samec, J.S.M.; Sels, B.F.; Wang, F. Guidelines for Performing Lignin-First Biorefining. Energy Environ. Sci. 2021, 14, 262–292. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar Energy-Driven Lignin-First Approach to Full Utilization of Lignocellulosic Biomass under Mild Conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Huang, Y.; Duan, Y.; Qiu, S.; Wang, M.; Ju, C.; Cao, H.; Fang, Y.; Tan, T. Lignin-First Biorefinery: A Reusable Catalyst for Lignin Depolymerization and Application of Lignin Oil to Jet Fuel Aromatics and Polyurethane Feedstock. Sustain. Energy Fuels 2018, 2, 637–647. [Google Scholar] [CrossRef]
- Cao, Z.; Dierks, M.; Clough, M.T.; Daltro de Castro, I.B.; Rinaldi, R. A Convergent Approach for a Deep Converting Lignin-First Biorefinery Rendering High-Energy-Density Drop-in Fuels. Joule 2018, 2, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive Catalytic Fractionation: State of the Art of the Lignin-First Biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, J.; Korányi, T.I.; Boot, M.D.; Hensen, E.J.M. Effective Release of Lignin Fragments from Lignocellulose by Lewis Acid Metal Triflates in the Lignin-First Approach. ChemSusChem 2016, 9, 3262–3267. [Google Scholar] [CrossRef]
- Bhalla, A.; Cai, C.M.; Xu, F.; Singh, S.K.; Bansal, N.; Phongpreecha, T.; Dutta, T.; Foster, C.E.; Kumar, R.; Simmons, B.A.; et al. Performance of Three Delignifying Pretreatments on Hardwoods: Hydrolysis Yields, Comprehensive Mass Balances, and Lignin Properties. Biotechnol. Biofuels 2019, 12, 213. [Google Scholar] [CrossRef]
- Ying, W.; Yang, J.; Zhang, J. In-Situ Modification of Lignin in Alkaline-Pretreated Sugarcane Bagasse by Sulfomethylation and Carboxymethylation to Improve the Enzymatic Hydrolysis Efficiency. Ind. Crops Prod. 2022, 182, 114863. [Google Scholar] [CrossRef]
- Park, G.W.; Gong, G.; Joo, J.C.; Song, J.; Lee, J.; Lee, J.P.; Kim, H.T.; Ryu, M.H.; Sirohi, R.; Zhuang, X.; et al. Recent Progress and Challenges in Biological Degradation and Biotechnological Valorization of Lignin as an Emerging Source of Bioenergy: A State-of-the-Art Review. Renew. Sustain. Energy Rev. 2022, 157, 112025. [Google Scholar] [CrossRef]
- Bujanovic, B.; Ralph, S.A.; Reiner, R.S.; Atalla, R.H. Erratum: Lignin Modification in the Initial Phase of Softwood Kraft Pulp Delignification with Polyoxometalates (POMs) (Holzforschung 61, (492–498)). Holzforschung 2007, 61, 731. [Google Scholar] [CrossRef]
- Dos Santos, P.S.B.; Da Silva, S.H.F.; Erdociaa, X.; Gatto, D.A.; Labidi, J. Characterization of Kraft Lignin Precipitated with Different Alcohols. Chem. Eng. Trans. 2015, 43, 469–474. [Google Scholar] [CrossRef]
- Bouxin, F.P.; David Jackson, S.; Jarvis, M.C. Isolation of High Quality Lignin as a By-Product from Ammonia Percolation Pretreatment of Poplar Wood. Bioresour. Technol. 2014, 162, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Gärtner, A.; Gellerstedt, G.; Tamminen, T. Determination of Phenolic Hydroxyl Groups in Residual Lignin Using a Modified UV-Method. Nord. Pulp Pap. Res. J. 1999, 14, 163–170. [Google Scholar] [CrossRef]
- Jablonský, M.; Kočiš, J.; Ház, A.; Šima, J. Characterization and Comparison by UV Spectroscopy of Precipitated Lignins and Commercial Lignosulfonates. Cellul. Chem. Technol. 2015, 49, 267–274. [Google Scholar]
- Goldmann, W.M.; Ahola, J.; Mankinen, O.; Kantola, A.M.; Komulainen, S.; Telkki, V.V.; Tanskanen, J. Determination of Phenolic Hydroxyl Groups in Technical Lignins by Ionization Difference Ultraviolet Spectrophotometry (∆ε-IDUS Method). Period. Polytech. Chem. Eng. 2017, 61, 93–101. [Google Scholar] [CrossRef]
- Zhang, H.; Fu, S.; Chen, Y. Basic Understanding of the Color Distinction of Lignin and the Proper Selection of Lignin in Color-Depended Utilizations. Int. J. Biol. Macromol. 2020, 147, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Fatehi, P. Technical Lignin and Its Potential Modification Routes: A Mini-Review. Ind. Crops Prod. 2020, 154, 112732. [Google Scholar] [CrossRef]
- Matsushita, Y. Conversion of Technical Lignins to Functional Materials with Retained Polymeric Properties. J. Wood Sci. 2015, 61, 230–250. [Google Scholar] [CrossRef]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Md Tahir, P.; Halis, R. Lignin-Based Copolymer Adhesives for Composite Wood Panels—A Review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Ha, J.-M.; Hwang, K.-R.; Kim, Y.-M.; Jae, J.; Kim, K.H.; Lee, H.W.; Kim, J.-Y.; Park, Y.-K. Recent Progress in the Thermal and Catalytic Conversion of Lignin. Renew. Sustain. Energy Rev. 2019, 111, 422–441. [Google Scholar] [CrossRef]
- Daniel, D.; Khachatryan, L.; Astete, C.; Asatryan, R.; Marculescu, C.; Boldor, D. Sulfur Contaminations Inhibit Depolymerization of Kraft Lignin. Bioresour. Technol. Rep. 2019, 8, 100341. [Google Scholar] [CrossRef]
- Nan, N.; Hu, W.; Wang, J. Lignin-Based Porous Biomaterials for Medical and Pharmaceutical Applications. Biomedicines 2022, 10, 747. [Google Scholar] [CrossRef]
- Shorey, R.; Gupta, A.; Mekonnen, T.H. Hydrophobic Modification of Lignin for Rubber Composites. Ind. Crops Prod. 2021, 174, 114189. [Google Scholar] [CrossRef]
- Attia, A.A.M.; Abas, K.M.; Ahmed Nada, A.A.; Shouman, M.A.H.; Šišková, A.O.; Mosnáček, J. Fabrication, Modification, and Characterization of Lignin-Based Electrospun Fibers Derived from Distinctive Biomass Sources. Polymers 2021, 13, 2277. [Google Scholar] [CrossRef]
- Lisperguer, J.; Nuñez, C.; Perez-Guerrero, P. Structure and Thermal Properties of Maleated Lignin-Recycled Polystyrene Composites. J. Chil. Chem. Soc. 2013, 58, 1937–1940. [Google Scholar] [CrossRef]
- Londoño Zuluaga, C.; Du, J.; Chang, H.-M.; Jameel, H.; Gonzalez, R.W. Lignin Modifications and Perspectives towards Applications of Phenolic Foams: A Review. BioResources 2018, 13, 9158–9179. [Google Scholar] [CrossRef]
- Eraghi Kazzaz, A.; Hosseinpour Feizi, Z.; Fatehi, P. Grafting Strategies for Hydroxy Groups of Lignin for Producing Materials. Green Chem. 2019, 21, 5714–5752. [Google Scholar] [CrossRef]
- Effendi, A.; Gerhauser, H.; Bridgwater, A.V. Production of Renewable Phenolic Resins by Thermochemical Conversion of Biomass: A Review. Renew. Sustain. Energy Rev. 2008, 12, 2092–2116. [Google Scholar] [CrossRef]
- Çetin, N.S.; Özmen, N. Use of Organosolv Lignin in Phenol-Formaldehyde Resins for Particleboard Production: I. Organosolv Lignin Modified Resins. Int. J. Adhes. Adhes. 2002, 22, 477–480. [Google Scholar] [CrossRef]
- Siddiqui, H. Production of Lignin-Based Phenolic Resin Using De-Polymerized Kraft Lignin and Process Optimization. Master’s Thesis, The University of Western Ontario, London, ON, USA, 2013. [Google Scholar]
- Çetin, N.S.; Özmen, N. Use of Organosolv Lignin in Phenol-Formaldehyde Resins for Particleboard Production: II. Particleboard Production and Properties. Int. J. Adhes. Adhes. 2002, 22, 481–486. [Google Scholar] [CrossRef]
- Kalami, S.; Arefmanesh, M.; Master, E.; Nejad, M. Replacing 100% of Phenol in Phenolic Adhesive Formulations with Lignin. J. Appl. Polym. Sci. 2017, 134, 45124. [Google Scholar] [CrossRef]
- da Silva, C.G.; Grelier, S.; Pichavant, F.; Frollini, E.; Castellan, A. Adding Value to Lignins Isolated from Sugarcane Bagasse and Miscanthus. Ind. Crops Prod. 2013, 42, 87–95. [Google Scholar] [CrossRef]
- Verdini, F.; Gaudino, E.C.; Canova, E.; Tabasso, S.; Behbahani, P.J.; Cravotto, G. Lignin as a Natural Carrier for the Efficient Delivery of Bioactive Compounds: From Waste to Health. Molecules 2022, 27, 3598. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, M.; Liu, G.; Wang, J.; Chen, Y.; Zhang, Z. Alkylated Lignin with Graft Copolymerization for Enhancing Toughness of PLA. J. Mater. Sci. 2022, 57, 8687–8700. [Google Scholar] [CrossRef]
- Hofmann, K.; Glasser, W.G. Engineering Plastics from Lignin. 21.1 Synthesis and Properties of Epoxidized Lignin- Poly (Propylene Oxide) Copolymers. J. Wood Chem. Technol. 1993, 13, 73–95. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and Characterization of Aminated Lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef]
- Du, X.; Li, J.; Lindström, M.E. Modification of Industrial Softwood Kraft Lignin Using Mannich Reaction with and without Phenolation Pretreatment. Ind. Crops Prod. 2014, 52, 729–735. [Google Scholar] [CrossRef]
- Rong, Y.; Ji, N.; Yu, Z.; Diao, X.; Li, H.; Lei, Y.; Lu, X.; Fukuoka, A. Lignin Amination Valorization: Heterogeneous Catalytic Synthesis of Aniline and Benzylamine from Lignin-Derived Chemicals. Green Chem. 2021, 23, 6761–6788. [Google Scholar] [CrossRef]
- Ntakirutimana, S.; Xu, T.; Liu, H.; Cui, J.-Q.; Zong, Q.-J.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. Amine-Based Pretreatments for Lignocellulose Fractionation and Lignin Valorization: A Review. Green Chem. 2022, 24, 5460–5478. [Google Scholar] [CrossRef]
- Ott, M.W.; Dietz, C.; Trosien, S.; Mehlhase, S.; Bitsch, M.J.; Nau, M.; Meckel, T.; Geissler, A.; Siegert, G.; Huong, J.; et al. Co-Curing of Epoxy Resins with Aminated Lignins: Insights into the Role of Lignin Homo Crosslinking during Lignin Amination on the Elastic Properties. Holzforschung 2021, 75, 390–398. [Google Scholar] [CrossRef]
- Chen, J.; An, L.; Bae, J.H.; Heo, J.W.; Han, S.Y.; Kim, Y.S. Green and Facile Synthesis of Aminated Lignin-Silver Complex and Its Antibacterial Activity. Ind. Crops Prod. 2021, 173, 114102. [Google Scholar] [CrossRef]
- Chen, J.; An, L.; Heo, J.W.; Bae, J.H.; Jeong, H.; Kim, Y.S. Utilization of Aminated Lignin as an Adsorbent to Remove Cationic and Anionic Dyes from Aqueous Solutions. J. Wood Chem. Technol. 2022, 42, 114–124. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of Lignin in Preparation of Slow-Release Fertilizer: Current Status and Future Perspectives. Ind. Crops Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, L.; Huang, M.; Mersal, G.A.M.; Ibrahim, M.M.; El-Bahy, Z.M.; Shi, X.; Jiang, Q. Aminated Lignin by Ultrasonic Method with Enhanced Arsenic (V) Adsorption from Polluted Water. Adv. Compos. Hybrid Mater. 2022, 5, 1044–1053. [Google Scholar] [CrossRef]
- Dong, R.; Yang, Z.; Fu, Y.; Chen, Z.; Hu, Y.; Zhou, Y.; Qin, H. Aminated Lignin Chelated Metal Derived Bifunctional Electrocatalyst with High Catalytic Performance. Appl. Surf. Sci. 2022, 580, 152205. [Google Scholar] [CrossRef]
- Graglia, M.; Pampel, J.; Hantke, T.; Fellinger, T.P.; Esposito, D. Nitro Lignin-Derived Nitrogen-Doped Carbon as an Efficient and Sustainable Electrocatalyst for Oxygen Reduction. ACS Nano 2016, 10, 4364–4371. [Google Scholar] [CrossRef]
- Suota, M.J.; Kochepka, D.M.; Ganter Moura, M.G.; Pirich, C.L.; Matos, M.; Magalhães, W.L.E.; Ramos, L.P. Lignin Functionalization Strategies and the Potential Applications of Its Derivatives—A Review. BioResources 2021, 16, 6471–6511. [Google Scholar] [CrossRef]
- Ahmad, Z.; Paleologou, M.; Xu, C.C. Oxidative Depolymerization of Lignin Using Nitric Acid under Ambient Conditions. Ind. Crops Prod. 2021, 170, 113757. [Google Scholar] [CrossRef]
- Chen, W.-J.; Zhao, C.-X.; Li, B.-Q.; Yuan, T.-Q.; Zhang, Q. Lignin-Derived Materials and Their Applications in Rechargeable Batteries. Green Chem. 2022, 24, 565–584. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L. Effects of NCO/OH Molar Ratio on Structure and Properties of Graft-Interpenetrating Polymer Networks from Polyurethane and Nitrolignin. Polymer 2002, 43, 2287–2294. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, J. Effects of Hard-Segment Compositions on Properties of Polyurethane-Nitrolignin Films. J. Appl. Polym. Sci. 2001, 81, 3251–3259. [Google Scholar] [CrossRef]
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-Based Polyurethane: Recent Advances and Future Perspectives. Macromol. Rapid Commun. 2021, 42, e2000492. [Google Scholar] [CrossRef]
- Madzhidova, V.E.; Dalimova, G.N.; Abduazimov, K.A. Sulfomethylation of Lignins. Chem. Nat. Compd. 1998, 34, 179–181. [Google Scholar] [CrossRef]
- Abdulkhani, A.; Khorasani, Z.; Hamzeh, Y.; Momenbeik, F.; Zadeh, Z.E.; Sun, F.; Madadi, M.; Zhang, X.M. Valorization of Bagasse Alkali Lignin to Water-Soluble Derivatives through Chemical Modification. 2022. Available online: https://link.springer.com/article/10.1007/s13399-022-02935-x (accessed on 10 July 2022).
- Paek, N.H.; Ri, S.H.; Jong, H.C.; Guo, Z.Y. Study on Production of Cation Asphalt Emulsifier for Micro-Surfacing by Using Sulfomethylated Lignin. 2022. Available online: https://www.sciencedirect.com/science/article/pii/S2046043022000053?via%3Dihub (accessed on 10 July 2022).
- Büyükdere, B.K.; Ünlü, C.H.; Atıcı, O.G. Synthesis of Surface Active Agents from Natural Waste Phenolics. Tenside Surfactants Deterg. 2022, 59, 192–203. [Google Scholar] [CrossRef]
- Kanwar, V.S.; Sharma, S.K.; Prakasam, C. 3rd International Conference on Innovative Technologies for Clean and Sustainable Development; Springer: Berlin/Heidelberg, Germany, 2020; Volume 32, ISBN 9783030939359. [Google Scholar]
- Hong, N. Pickering Emulsions Stabilized by an Alkyl Chain-Bridged Lignin-Based Polymer without Additives and Organic Solvents. J. Agric. Food Chem. 2022, 70, 1196–1202. [Google Scholar] [CrossRef]
- Bertella, S.; Bernardes Figueirêdo, M.; De Angelis, G.; Mourez, M.; Bourmaud, C.; Amstad, E.; Luterbacher, J.S. Extraction and Surfactant Properties of Glyoxylic Acid-Functionalized Lignin. ChemSusChem 2022, 15, e202200270. [Google Scholar] [CrossRef]
- Li, M.; Yuan, Y.; Zhu, Y.; Jiang, B.; Wu, W.; Wu, S.; Jin, Y. Comparison of Sulfomethylated Lignin from Poplar and Masson Pine on Cellulase Adsorption and the Enzymatic Hydrolysis of Wheat Straw. Bioresour. Technol. 2022, 343, 126142. [Google Scholar] [CrossRef]
- Ouyang, X.; Ke, L.; Qiu, X.; Guo, Y.; Pang, Y. Sulfonation of Alkali Lignin and Its Potential Use in Dispersant for Cement. J. Dispers. Sci. Technol. 2009, 30, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Qiu, M.; Tang, Y.; Yang, J.; Shen, F.; Qi, X.; Yu, Y. Synthesis of Sulfonated Lignin-Derived Ordered Mesoporous Carbon for Catalytic Production of Furfural from Xylose. Int. J. Biol. Macromol. 2021, 187, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, W.; Kong, F.; Fatehi, P. One-Pot Preparation of Zwitterion-Type Lignin Polymers. Int. J. Biol. Macromol. 2019, 140, 429–440. [Google Scholar] [CrossRef]
- Puumala, L.S.; Fatehi, P. Dispersion Performance of Hydroxypropyl Sulfonated Lignin in Aluminum Oxide Suspension. Sep. Purif. Technol. 2021, 276, 119247. [Google Scholar] [CrossRef]
- Xiao, X.; Jiang, J.; Wang, Y.; Wang, B.; Yuan, T.Q.; Shi, Q.; Liao, X.; Shi, B.; Sun, R.C. Microwave-Assisted Sulfonation of Lignin for the Fabrication of a High-Performance Dye Dispersant. ACS Sustain. Chem. Eng. 2021, 9, 9053–9061. [Google Scholar] [CrossRef]
- Varão, L.H.R.; Silva, T.A.L.; Zamora, H.D.Z.; de Morais, L.C.; Pasquini, D. Synthesis of Methyl Biodiesel by Esterification Using Magnetic Nanoparticles Coated with Sulfonated Lignin. 2022. Available online: https://link.springer.com/article/10.1007/s13399-021-02214-1 (accessed on 10 July 2022).
- Yuan, Z.; Shang, X.; Fang, J.; Li, H. A Simple Method for Preparation of Lignin/TiO2 Nanocomposites by Sulfonation Degree Regulation and Their Application in Polyurethane Films. Int. J. Biol. Macromol. 2022, 198, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Liu, M.; Lin, L.; Pu, X.; Ge, C.; Zhang, T.; Liu, G. Sulfonated Lignin Modified with Silane Coupling Agent as Biodegradable Shale Inhibitor in Water-Based Drilling Fluid. J. Pet. Sci. Eng. 2022, 208, 109618. [Google Scholar] [CrossRef]
- Liu, Y.; Mao, X.; Wu, H.; Wang, X.; Shi, B.; Fan, C.; Kong, Y.; Jiang, Z. Sulfonated Lignin Intercalated Graphene Oxide Membranes for Efficient Proton Conduction. J. Membr. Sci. 2022, 644, 120126. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Shen, J.; Wu, B.; He, Y. Azo Coupling Reaction Induced Macromolecular Self-Assembly in Aqueous Solution. ACS Macro Lett. 2018, 7, 437–441. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Liang, R.; Wu, B.; He, Y. Synthesis and Characterization of Water-Soluble PEGylated Lignin-Based Polymers by Macromolecular Azo Coupling Reaction. Chin. Chem. Lett. 2018, 29, 143–146. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, Y.; Qian, Y.; Zhang, W.; Qiu, X. Preparation of Photoresponsive Azo Polymers Based on Lignin, a Renewable Biomass Resource. ACS Sustain. Chem. Eng. 2015, 3, 1111–1116. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Gupta, P.; Patidar, R.; Srivastava, V.C. Microbial Peroxide Producing Cell Mediated Lignin Valorization. Int. J. Biol. Macromol. 2022, 202, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xie, S.; Pu, Y.; Zhang, R.; Huang, F.; Ragauskas, A.J.; Yuan, J.S. Synergistic Enzymatic and Microbial Lignin Conversion. Green Chem. 2016, 18, 1306–1312. [Google Scholar] [CrossRef]
- Bajpai, P. Pretreatment of Lignocellulosic Biomass for Biofuel Production; Springer: Singapore, 2016. [Google Scholar] [CrossRef]
- Li, C.; Chen, C.; Wu, X.; Tsang, C.-W.; Mou, J.; Yan, J.; Liu, Y.; Lin, C.S.K. Recent Advancement in Lignin Biorefinery: With Special Focus on Enzymatic Degradation and Valorization. Bioresour. Technol. 2019, 291, 121898. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jiang, X.; Lin, J.; Zhao, G.; Chang, H.M.; Jameel, H. Reactivity Improvement by Phenolation of Wheat Straw Lignin Isolated from a Biorefinery Process. New J. Chem. 2019, 43, 2238–2246. [Google Scholar] [CrossRef]
- Tolbert, A.; Akinosho, H.; Khunsupat, R.; Naskar, A.K.; Ragauskas, A.J. Characterization and Analysis of the Molecular Weight of Lignin for Biorefining Studies. Biofuels Bioprod. Biorefining 2014, 8, 836–856. [Google Scholar] [CrossRef]
- Li, F.; Zhao, H.; Shao, R.; Zhang, X.; Yu, H. Enhanced Fenton Reaction for Xenobiotic Compounds and Lignin Degradation Fueled by Quinone Redox Cycling by Lytic Polysaccharide Monooxygenases. J. Agric. Food Chem. 2021, 69, 7104–7114. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Z.; Ge, M.; Liu, B.; Chen, S.; Zhang, D.; Gao, L. Converting Lignin into Long-Chain Fatty Acids with the Electro-Fenton Reaction. GCB Bioenergy 2021, 13, 1290–1302. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Z.; Liu, H.; Wu, T.; Han, B. Dehydroxyalkylative Halogenation of C(Aryl)-C Bonds of Aryl Alcohols. Chem. Commun. 2020, 56, 7120–7123. [Google Scholar] [CrossRef]
- Milstead, R.P.; Remucal, C.K. Molecular-Level Insights into the Formation of Traditional and Novel Halogenated Disinfection Byproducts. ACS EST Water 2021, 1, 1966–1974. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Synthesis, Thermal Properties, Rheological and Mechanical Behaviors of Lignins-Grafted-Poly(ε-Caprolactone). Polymer 2013, 54, 3882–3890. [Google Scholar] [CrossRef]
- Zhou, S.-J.J.; Wang, H.-M.M.; Xiong, S.-J.J.; Sun, J.-M.M.; Wang, Y.-Y.Y.; Yu, S.; Sun, Z.; Wen, J.-L.L.; Yuan, T.-Q.Q. Technical Lignin Valorization in Biodegradable Polyester-Based Plastics (BPPs). ACS Sustain. Chem. Eng. 2021, 9, 12017–12042. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X.; Shi, Y. A Review on Lignin Antioxidants: Their Sources, Isolations, Antioxidant Activities and Various Applications. Int. J. Biol. Macromol. 2022, 210, 716–741. [Google Scholar] [CrossRef] [PubMed]
- Rubens, M.; Van Wesemael, M.; Feghali, E.; Lufungula, L.L.; Blockhuys, F.; Vanbroekhoven, K.; Eevers, W.; Vendamme, R. Exploring the Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Lignin Hydrogenolysis Oil towards Urethane Bond Formation. Ind. Crops Prod. 2022, 180, 114703. [Google Scholar] [CrossRef]
- Faris, A.H.; Rahim, A.A.; Ibrahim, M.N.M.; Alkurdi, A.M.; Shah, I. Combination of Lignin Polyol-Tannin Adhesives and Polyethylenimine for the Preparation of Green Water-Resistant Adhesives. J. Appl. Polym. Sci. 2016, 133, 43437. [Google Scholar] [CrossRef]
- Cao, M.; Hu, Y.; Cheng, W.; Huan, S.; Bai, T.; Niu, Z.; Zhao, Y.; Yue, G.; Zhao, Y.; Han, G. Lignin-Based Multi-Scale Cellular Aerogels Assembled from Co-Electrospun Nanofibers for Oil/Water Separation and Energy Storage. Chem. Eng. J. 2022, 436, 135233. [Google Scholar] [CrossRef]
- Sakai, H.; Kuroda, K.; Muroyama, S.; Tsukegi, T.; Kakuchi, R.; Takada, K.; Hata, A.; Kojima, R.; Ogoshi, T.; Omichi, M.; et al. Alkylated Alkali Lignin for Compatibilizing Agents of Carbon Fiber-Reinforced Plastics with Polypropylene. Polym. J. 2018, 50, 281–284. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Matsakas, L.; Rova, U.; Christakopoulos, P. Melt Stable Functionalized Organosolv and Kraft Lignin Thermoplastic. Processes 2020, 8, 1108. [Google Scholar] [CrossRef]
- Wang, S.; Bai, J.; Innocent, M.T.; Wang, Q.; Xiang, H.; Tang, J.; Zhu, M. Lignin-Based Carbon Fibers: Formation, Modification and Potential Applications. Green Energy Environ. 2021, 7, 578–605. [Google Scholar] [CrossRef]
- Karthäuser, J.; Biziks, V.; Mai, C.; Militz, H. Lignin and Lignin-Derived Compounds for Wood Applications—A Review. Molecules 2021, 26, 2533. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, Z.; Ji, X.; Ma, H.; Yang, G.; He, M.; Dai, L.; Xu, T.; Si, C. Alkylation Modification for Lignin Color Reduction and Molecular Weight Adjustment. Int. J. Biol. Macromol. 2022, 201, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chen, S.; Wang, W.; Deng, T.; Wang, H. Empowering Alkali Lignin with High Performance in Pickering Emulsion by Selective Phenolation for the Protection and Controlled-Release of Agrochemical. J. Clean. Prod. 2022, 339, 130769. [Google Scholar] [CrossRef]
- Sugiarto, S.; Leow, Y.; Tan, C.L.; Wang, G.; Kai, D. How Far Is Lignin from Being a Biomedical Material? Bioact. Mater. 2022, 8, 71–94. [Google Scholar] [CrossRef]
- Liu, Y.; Li, K. Preparation and Characterization of Demethylated Lignin-Polyethylenimine Adhesives. J. Adhes. 2006, 82, 593–605. [Google Scholar] [CrossRef]
- Zhen, X.; Li, H.; Xu, Z.; Wang, Q.; Xu, J.; Zhu, S.; Wang, Z.; Yuan, Z. Demethylation, Phenolation, and Depolymerization of Lignin for the Synthesis of Lignin-Based Epoxy Resin via a One-Pot Strategy. Ind. Crops Prod. 2021, 173, 114135. [Google Scholar] [CrossRef]
- Gao, C.; Li, M.M.; Zhu, C.; Hu, Y.; Shen, T.; Li, M.M.; Ji, X.; Lyu, G.; Zhuang, W. One-Pot Depolymerization, Demethylation and Phenolation of Lignin Catalyzed by HBr under Microwave Irradiation for Phenolic Foam Preparation. Compos. Part B Eng. 2021, 205, 108530. [Google Scholar] [CrossRef]
- Sivasankarapillai, G.; McDonald, A.G.; Li, H. Lignin Valorization by Forming Toughened Lignin-Co-Polymers: Development of Hyperbranched Prepolymers for Cross-Linking. Biomass Bioenergy 2012, 47, 99–108. [Google Scholar] [CrossRef]
- Sivasankarapillai, G.; McDonald, A.G. Synthesis and Properties of Lignin-Highly Branched Poly (Ester-Amine) Polymeric Systems. Biomass Bioenergy 2011, 35, 919–931. [Google Scholar] [CrossRef]
- Park, C.W.; Han, S.Y.; Bandi, R.; Dadigala, R.; Lee, E.A.; Kim, J.K.; Cindradewi, A.W.; Kwon, G.J.; Lee, S.H. Esterification of Lignin Isolated by Deep Eutectic Solvent Using Fatty Acid Chloride, and Its Composite Film with Poly(Lactic Acid). Polymers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Chemical Modification of Lignin. Lignin Chem. Appl. 2019, 51–78. [CrossRef]
- Rajan, K.; Mann, J.K.; English, E.; Harper, D.P.; Carrier, D.J.; Rials, T.G.; Labbé, N.; Chmely, S.C. Sustainable Hydrogels Based on Lignin-Methacrylate Copolymers with Enhanced Water Retention and Tunable Material Properties. Biomacromolecules 2018, 19, 2665–2672. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of Lignin by Polyethyleneglycol and Glycerol. Bioresour. Technol. 2011, 102, 3581–3583. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhou, M.; Wang, S.; Lou, H.; Yang, D.; Qiu, X. Synthesis, Structure, and Dispersion Property of a Novel Lignin-Based Polyoxyethylene Ether from Kraft Lignin and Poly(Ethylene Glycol). ACS Sustain. Chem. Eng. 2014, 2, 1902–1909. [Google Scholar] [CrossRef]
- Orebom, A.; Di Francesco, D.; Shakari, P.; Samec, J.S.M.; Pierrou, C. Thermal and Mechanical Properties of Esterified Lignin in Various Polymer Blends. Molecules 2021, 26, 3219. [Google Scholar] [CrossRef]
- Adjaoud, A.; Dieden, R.; Verge, P. Sustainable Esterification of a Soda Lignin with Phloretic Acid. Polymers 2021, 13, 637. [Google Scholar] [CrossRef]
- Saffian, H.A.; Yamaguchi, M.; Ariffin, H.; Abdan, K.; Kassim, N.K.; Lee, S.H.; Lee, C.H.; Shafi, A.R.; Alias, A.H. Thermal, Physical and Mechanical Properties of Poly(Butylene Succinate)/Kenaf Core Fibers Composites Reinforced with Esterified Lignin. Polymers 2021, 13, 2359. [Google Scholar] [CrossRef]
- Shorey, R.; Mekonnen, T.H. Sustainable Paper Coating with Enhanced Barrier Properties Based on Esterified Lignin and PBAT Blend. Int. J. Biol. Macromol. 2022, 209, 472–484. [Google Scholar] [CrossRef]
- Jedrzejczyk, M.A.; Van den Bosch, S.; Van Aelst, J.; Van Aelst, K.; Kouris, P.D.; Moalin, M.; Haenen, G.R.M.M.; Boot, M.D.; Hensen, E.J.M.; Lagrain, B.; et al. Lignin-Based Additives for Improved Thermo-Oxidative Stability of Biolubricants. ACS Sustain. Chem. Eng. 2021, 9, 12548–12559. [Google Scholar] [CrossRef]
- Liu, L.Y.; Chen, S.; Ji, L.; Jang, S.K.; Renneckar, S. One-Pot Route to Convert Technical Lignin into Versatile Lignin Esters for Tailored Bioplastics and Sustainable Materials. Green Chem. 2021, 23, 4567–4579. [Google Scholar] [CrossRef]
- Anugwom, I.; Lahtela, V.; Hedenström, M.; Kiljunen, S.; Kärki, T.; Kallioinen-Mänttäri, M. Esterified Lignin from Construction and Demolition Waste (CDW) as a Versatile Additive for Polylactic-Acid (PLA) Composites—The Effect of Artificial Weathering on Its Performance. Glob. Chall. 2022, 6, 2100137. [Google Scholar] [CrossRef]
- Dong, C.; Meng, X.; Leu, S.Y.; Xu, L.; Wu, Z.; Cravotto, G.; Fang, Z. Enhancing α-Etherification of Lignin in Eucalyptus Diol Pretreatment to Improve Lignin Monomer Production. Ind. Crops Prod. 2022, 185, 115130. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, J.; Du, X.; Hu, Z.; Chang, H.M.; Jameel, H. Phenolation to Improve Lignin Reactivity toward Thermosets Application. ACS Sustain. Chem. Eng. 2018, 6, 5504–5512. [Google Scholar] [CrossRef]
- Zhang, H.N.; Ren, H.; Zhai, H.M. Analysis of Phenolation Potential of Spruce Kraft Lignin and Construction of Its Molecular Structure Model. Ind. Crops Prod. 2021, 167, 113506. [Google Scholar] [CrossRef]
- Liang, D.; Zhu, X.; Dai, P.; Lu, X.; Guo, H.; Que, H.; Wang, D.; He, T.; Xu, C.; Robin, H.M.; et al. Preparation of a Novel Lignin-Based Flame Retardant for Epoxy Resin. Mater. Chem. Phys. 2021, 259, 124101. [Google Scholar] [CrossRef]
- Bertella, S.; Luterbacher, J.S. Simultaneous Extraction and Controlled Chemical Functionalization of Hardwood Lignin for Improved Phenolation. Green Chem. 2021, 23, 3459–3467. [Google Scholar] [CrossRef]
- Maree, C.; Görgens, J.F.; Tyhoda, L. Lignin Phenol Formaldehyde Resins Synthesised Using South African Spent Pulping Liquor. Waste Biomass Valorization 2022, 13, 3489–3507. [Google Scholar] [CrossRef]
- Cao, H.; Liu, R.; Li, B.; Wu, Y.; Wang, K.; Yang, Y.; Li, A.; Zhuang, Y.; Cai, D.; Qin, P. Biobased Rigid Polyurethane Foam Using Gradient Acid Precipitated Lignin from the Black Liquor: Revealing the Relationship between Lignin Structural Features and Polyurethane Performances. Ind. Crops Prod. 2022, 177, 114480. [Google Scholar] [CrossRef]
- Arefmanesh, M.; Nikafshar, S.; Master, E.R.; Nejad, M. From Acetone Fractionation to Lignin-Based Phenolic and Polyurethane Resins. Ind. Crops Prod. 2022, 178, 114604. [Google Scholar] [CrossRef]
- Henry, C.; Gondaliya, A.; Thies, M.; Nejad, M. Studying the Suitability of Nineteen Lignins as Partial Polyol Replacement in Rigid Polyurethane/Polyisocyanurate Foam. Molecules 2022, 27, 2535. [Google Scholar] [CrossRef]
- Borrero-López, A.M.; Valencia, C.; Ibarra, D.; Ballesteros, I.; Franco, J.M. Lignin-Enriched Residues from Bioethanol Production: Chemical Characterization, Isocyanate Functionalization and Oil Structuring Properties. Int. J. Biol. Macromol. 2022, 195, 412–423. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, S.; Gibril, M.E.; Kong, F. The Copolymer of Polyvinyl Acetate Containing Lignin-Vinyl Acetate Monomer: Synthesis and Characterization. Eur. Polym. J. 2020, 123, 109411. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Lokupitiya, H.N.; Tang, C. Lignin Biopolymers in the Age of Controlled Polymerization. Polymers 2019, 11, 1176. [Google Scholar] [CrossRef]
- Panesar, S.S.; Jacob, S.; Misra, M.; Mohanty, A.K. Functionalization of Lignin: Fundamental Studies on Aqueous Graft Copolymerization with Vinyl Acetate. Ind. Crops Prod. 2013, 46, 191–196. [Google Scholar] [CrossRef]
- Jung, H.Y.; Lee, J.S.; Han, H.T.; Jung, J.; Eom, K.; Lee, J.T. Lignin-Based Materials for Sustainable Rechargeable Batteries. Polymers 2022, 14, 673. [Google Scholar] [CrossRef] [PubMed]
- Goliszek, M.; Podkościelna, B.; Sevastyanova, O.; Fila, K.; Chabros, A.; Pączkowski, P. Investigation of Accelerated Aging of Lignin-Containing Polymer Materials. Int. J. Biol. Macromol. 2019, 123, 910–922. [Google Scholar] [CrossRef] [PubMed]
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization Study of Lignin Oxypropylation in View of the Preparation of Polyurethane Rigid Foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589. [Google Scholar] [CrossRef]
- Nadji, H.; Bruzzèse, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of Lignins and Preparation of Rigid Polyurethane Foams from the Ensuing Polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016. [Google Scholar] [CrossRef]
- Kai, D.; Jiang, S.; Low, Z.W.; Loh, X.J. Engineering Highly Stretchable Lignin-Based Electrospun Nanofibers for Potential Biomedical Applications. J. Mater. Chem. B 2015, 3, 6194–6204. [Google Scholar] [CrossRef]
- Chen, P.; Zhou, W.; Yang, Z.; Mai, Z.; Huang, Z.; Bie, Y.; Wu, S.; Dong, X.; Fu, X.; Ko, F.; et al. Electrospun Nanofibrous Membrane with Antibacterial and Antiviral Properties Decorated with Myoporum Bontioides Extract and Silver-Doped Carbon Nitride Nanoparticles for Medical Masks Application. SSRN Electron. J. 2022, 298, 121565. [Google Scholar] [CrossRef]
- Fitzgerald, C.L.; Thies, M.C. Continuous Recovery of High-Purity Kraft Lignin from Black Liquor via Simultaneous, Liquid-Phase Acidification and Purification. Ind. Crops Prod. 2022, 184, 115084. [Google Scholar] [CrossRef]
- Roy, P.S.; Garnier, G.; Allais, F.; Saito, K. Effective Lignin Utilization Strategy: Major Depolymerization Technologies, Purification Process and Production of Valuable Material. Chem. Lett. 2021, 50, 1123–1130. [Google Scholar] [CrossRef]
- Hasanov, I.; Shanmugam, S.; Kikas, T. Extraction and Isolation of Lignin from Ash Tree (Fraxinus Exselsior) with Protic Ionic Liquids (PILs). Chemosphere 2022, 290, 133297. [Google Scholar] [CrossRef] [PubMed]
- Resende, F.L.P.; Fraley, S.A.; Berger, M.J.; Savage, P.E. Noncatalytic Gasification of Lignin in Supercritical Water. Energy Fuels 2008, 22, 1328–1334. [Google Scholar] [CrossRef]
- Salimi, M.; Nejati, B.; Karimi, A.; Tavasoli, A. Hydrothermal Gasification Performance of Iranian Rice Straw in Supercritical Water Media for Hydrogen-Rich Gas Production. BioResources 2016, 11, 6362–6377. [Google Scholar] [CrossRef][Green Version]
- Cai, Z.; Long, J.; Li, Y.; Ye, L.; Yin, B.; France, L.J.; Dong, J.; Zheng, L.; He, H.; Liu, S.; et al. Selective Production of Diethyl Maleate via Oxidative Cleavage of Lignin Aromatic Unit. Chem 2019, 5, 2365–2377. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; Teunissen, W.; van Dam, J.E.G.; de Jong, E.; Gellerstedt, G.; Scott, E.L.; Sanders, J.P.M. Lignin Depolymerisation in Supercritical Carbon Dioxide/Acetone/Water Fluid for the Production of Aromatic Chemicals. Bioresour. Technol. 2012, 106, 173–177. [Google Scholar] [CrossRef]
- Schoenherr, S.; Ebrahimi, M.; Czermak, P. Lignin Degradation Processes and the Purification of Valuable Products. In Lignin—Trends and Applications; IntechOpen: London, UK, 2018; 308p. [Google Scholar] [CrossRef]
- Mimini, V.; Sykacek, E.; Hashim, S.N.A.; Holzweber, J.; Hettegger, H.; Fackler, K.; Potthast, A.; Mundigler, N.; Rosenau, T. Compatibility of Kraft Lignin, Organosolv Lignin and Lignosulfonate with PLA in 3D Printing. J. Wood Chem. Technol. 2019, 39, 14–30. [Google Scholar] [CrossRef]
- Ponnuchamy, V.; Sandak, J.; Sandak, A. Revealing of Supercritical Water Gasification Process of Lignin by Reactive Force Field Molecular Dynamics Simulations. Processes 2021, 9, 714. [Google Scholar] [CrossRef]
- Gieseking, B.; Jäck, B.; Preis, E.; Jung, S.; Forster, M.; Scherf, U.; Deibel, C.; Dyakonov, V. Excitation Dynamics in Low Band Gap Donor-Acceptor Copolymers and Blends. Polym. Polym. Compos. 2012, 16, 101–113. [Google Scholar] [CrossRef]
- Law, K.Y. Water-Surface Interactions and Definitions for Hydrophilicity, Hydrophobicity and Superhydrophobicity. Pure Appl. Chem. 2015, 87, 759–765. [Google Scholar] [CrossRef]
- Law, K.-Y. Definitions for Hydrophilicity, Hydrophobicity, and Superhydrophobicity: Getting the Basics Right. J. Phys. Chem. Lett. 2014, 5, 686–688. [Google Scholar] [CrossRef] [PubMed]
- Stokely, K.; Mazza, M.G.; Stanley, H.E.; Franzese, G. Effect of Hydrogen Bond Cooperativity on the Behavior of Water. Proc. Natl. Acad. Sci. USA 2010, 107, 1301–1306. [Google Scholar] [CrossRef] [PubMed]
- Gilormini, P.; Verdu, J. On the Role of Hydrogen Bonding on Water Absorption in Polymers. Polymer 2018, 142, 164–169. [Google Scholar] [CrossRef]
- He, Y.; Zhu, B.; Inoue, Y. Hydrogen Bonds in Polymer Blends. Prog. Polym. Sci. 2004, 29, 1021–1051. [Google Scholar] [CrossRef]
- Ago, M.; Okajima, K.; Jakes, J.E.; Park, S.; Rojas, O.J. Lignin-Based Electrospun Nanofibers Reinforced with Cellulose Nanocrystals. Biomacromolecules 2012, 13, 918–926. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J. Hydrophilic Polymers. Polymers 2019, 11, 693. [Google Scholar] [CrossRef]
- Kung, C.H.; Sow, P.K.; Zahiri, B.; Mérida, W. Assessment and Interpretation of Surface Wettability Based on Sessile Droplet Contact Angle Measurement: Challenges and Opportunities. Adv. Mater. Interfaces 2019, 6, 1–27. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Sui, T.; Li, A.; Chen, D. Investigation on Hydrophobicity of Lotus Leaf: Experiment and Theory. Plant Sci. 2009, 176, 687–695. [Google Scholar] [CrossRef]
- Geils, J.; Patzelt, G.; Kesel, A. The Larger the Contact Angle, the Lower the Adhesion? 2019. Available online: https://www.researchgate.net/profile/Judith_Geils/publication/330556275_The_larger_the_contact_angle_the_lower_the_adhesion/links/5c4831e1299bf12be3dcac4f/The-larger-the-contact-angle-the-lower-the-adhesion.pdf (accessed on 19 July 2022).
- Zhou, X.F.; Lu, X.J. Structural Characterization of Kraft Lignin for Its Green Utilization. Wood Res. 2014, 59, 583–592. [Google Scholar]
- Li, B.; You, S.; Qi, W.; Wang, Y.; Su, R.; He, Z. Structure-Tunable Assembly of Lignin Sub-Micro Spheres by Modifying the Amphiphilic Interfaces of Lignin via n-Alkane. Eur. Polym. J. 2020, 126, 109539. [Google Scholar] [CrossRef]
- Antonino, L.D.; Gouveia, J.R.; de Sousa Júnior, R.R.; Garcia, G.E.S.; Gobbo, L.C.; Tavares, L.B.; dos Santos, D.J. Reactivity of Aliphatic and Phenolic Hydroxyl Groups in Kraft Lignin towards 4,4′ MDI. Molecules 2021, 26, 2131. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, X. Correlation between Lignin Physicochemical Properties and Inhibition to Enzymatic Hydrolysis of Cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Methylation of Softwood Kraft Lignin with Dimethyl Carbonate. Green Chem. 2015, 17, 1077–1087. [Google Scholar] [CrossRef]
- Diop, A.; Mijiyawa, F.; Koffi, D.; Kokta, B.V.; Montplaisir, D. Study of Lignin Dispersion in Low-Density Polyethylene. J. Thermoplast. Compos. Mater. 2015, 28, 1662–1674. [Google Scholar] [CrossRef]
- Gao, Y.; Qu, W.; Liu, Y.; Hu, H.; Cochran, E.; Bai, X. Agricultural Residue-derived Lignin as the Filler of Polylactic Acid Composites and the Effect of Lignin Purity on the Composite Performance. J. Appl. Polym. Sci. 2019, 136, 47915. [Google Scholar] [CrossRef]
- Gordobil, O.; Delucis, R.; Egüés, I.; Labidi, J. Kraft Lignin as Filler in PLA to Improve Ductility and Thermal Properties. Ind. Crops Prod. 2015, 72, 46–53. [Google Scholar] [CrossRef]
- Hajirahimkhan, S.; Xu, C.C.; Ragogna, P.J. Ultraviolet Curable Coatings of Modified Lignin. ACS Sustain. Chem. Eng. 2018, 6, 14685–14694. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Zhu, Z.; Fu, S. High-Value Utilization of Hydroxymethylated Lignin in Polyurethane Adhesives. Int. J. Biol. Macromol. 2020, 152, 775–785. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W.; Knozowska, K. Hydrophobic Ceramic Membranes for Water Desalination. Appl. Sci. 2017, 7, 402. [Google Scholar] [CrossRef]
- Kudanga, T.; Prasetyo, E.N.; Sipilä, J.; Guebitz, G.M.; Nyanhongo, G.S. Reactivity of Long Chain Alkylamines to Lignin Moieties: Implications on Hydrophobicity of Lignocellulose Materials. J. Biotechnol. 2010, 149, 81–87. [Google Scholar] [CrossRef]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Wenger, J.; Haas, V.; Stern, T. Why Can We Make Anything from Lignin Except Money? Towards a Broader Economic Perspective in Lignin Research. Curr. For. Rep. 2020, 6, 294–308. [Google Scholar] [CrossRef]
- Hemmilä, V.; Trischler, J.; Sandberg, D. Lignin—An Adhesive Raw Materials of the Future or a Waste of Research Energy? In Northern European Network for Wood Science and Engineering, Proceedings of the WSE–9th Meeting, Hannover, Germany, 11–12 September 2013; Leibniz Universität: Hannover, Germany, 2013; pp. 98–103. [Google Scholar]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of Lignin in Polymer and Composite Systems for Advanced Engineering Applications—A Review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Vives, M.B.; Thuvander, J.; Arkell, A.; Lipnizki, F. Low-Molecular-Weight Lignin Recovery with Nanofiltration in the Kraft Pulping Process. Membranes 2022, 12, 310. [Google Scholar] [CrossRef]
- Wong, S.S.; Shu, R.; Zhang, J.; Liu, H.; Yan, N. Downstream Processing of Lignin Derived Feedstock into End Products. Chem. Soc. Rev. 2020, 49, 5510–5560. [Google Scholar] [CrossRef]
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Development of ‘Lignin-First’ Approaches for the Valorization of Lignocellulosic Biomass. Molecules 2020, 25, 2815. [Google Scholar] [CrossRef]
- Budnyak, T.M.; Slabon, A.; Sipponen, M.H. Lignin–Inorganic Interfaces: Chemistry and Applications from Adsorbents to Catalysts and Energy Storage Materials. ChemSusChem 2020, 13, 4344–4355. [Google Scholar] [CrossRef]
- José Borges Gomes, F.; de Souza, R.E.; Brito, E.O.; Costa Lelis, R.C. A Review on Lignin Sources and Uses. J. Appl. Biotechnol. Bioeng. 2020, 7, 100–105. [Google Scholar] [CrossRef]
- Chaves, A.V.; Waghorn, G.C.; Tavendale, M.H. A Simplified Method for Lignin Measurement in a Range of Forage Species. In Proceedings of the New Zealand Grassland Association; New Zealand Grassland Association: Dunedin, New Zealand, 2002; pp. 129–133. [Google Scholar] [CrossRef]
- Poke, F.S.; Wright, J.K.; Raymond, C.A. Predicting Extractives and Lignin Contents in Eucalyptus Globulus Using Near Infrared Reflectance Analysis. J. Wood Chem. Technol. 2005, 24, 55–67. [Google Scholar] [CrossRef]
- Wu, X.; Li, G.; Liu, X.; He, F. Rapid Non-destructive Analysis of Lignin Using NIR Spectroscopy and Chemo-metrics. Food Energy Secur. 2021, 10, e289. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Hoefnagels, R.; Vural-Gürsel, I.; Gosselink, R.; Junginger, M. Review of Life Cycle Assessments of Lignin and Derived Products: Lessons Learned. Sci. Total Environ. 2021, 770, 144656. [Google Scholar] [CrossRef] [PubMed]
- Jablonsky, M.; Haz, A.; Majova, V. Assessing the Opportunities for Applying Deep Eutectic Solvents for Fractionation of Beech Wood and Wheat Straw. Cellulose 2019, 26, 7675–7684. [Google Scholar] [CrossRef]
- Ghareh Bagh, F.S.; Ray, S.; Seth, R. Optimizing Lignin Extraction from Kraft Black Liquor Using Protic Ionic Liquids. Biomass Bioenergy 2021, 154, 106249. [Google Scholar] [CrossRef]
- Szalaty, T.J.; Klapiszewski, Ł.; Jesionowski, T. Recent Developments in Modification of Lignin Using Ionic Liquids for the Fabrication of Advanced Materials–A Review. J. Mol. Liq. 2020, 301, 112417. [Google Scholar] [CrossRef]
- Sahoo, S.; Seydibeyoĝlu, M.Ö.; Mohanty, A.K.; Misra, M. Characterization of Industrial Lignins for Their Utilization in Future Value Added Applications. Biomass Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Wang, M.; Dessie, W.; Li, H. Chemically Modified Lignin: Correlation between Structure and Biodegradability. J. Renew. Mater. 2021, 9, 2119–2128. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Collet, C.; Gaugler, M.; Lloyd-Jones, G. Integrating Softwood Biorefinery Lignin into Polyhydroxybutyrate Composites and Application in 3D Printing. Mater. Today Commun. 2019, 19, 286–296. [Google Scholar] [CrossRef]
- Tanase-Opedal, M.; Espinosa, E.; Rodríguez, A.; Chinga-Carrasco, G. Lignin: A Biopolymer from Forestry Biomass for Biocomposites and 3D Printing. Materials 2019, 12, 3006. [Google Scholar] [CrossRef]
- Kumar, M.; Hietala, M.; Oksman, K. Lignin-Based Electrospun Carbon Nanofibers. Front. Mater. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, J.; Dong, R.; Fu, Y.; Zhou, J.; Qin, H. High-Performance Electrospun Carbon Fiber Derived from Lignin and Metal Composite. Ionics 2022, 28, 1119–1127. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, H.; Cao, Q.; Zheng, H.; Xu, D.; Guo, H.; Wang, S.; Li, Y.; Zhou, J. Electrospun Lignin-Based Carbon Nanofibers as Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2020, 8, 12831–12841. [Google Scholar] [CrossRef]
- Roman, J.; Neri, W.; Derré, A.; Poulin, P. Electrospun Lignin-Based Twisted Carbon Nanofibers for Potential Microelectrodes Applications. Carbon 2019, 145, 556–564. [Google Scholar] [CrossRef]
- Mikeš, P.; Baker, D.A.; Uhlin, A.; Lukáš, D.; Kuželová-Košťáková, E.; Vidrich, A.; Valtera, J.; Kopřivová, B.; Asatiani, N.; Salmén, L.; et al. The Mass Production of Lignin Fibres by Means of Needleless Electrospinning. J. Polym. Environ. 2021, 29, 2164–2173. [Google Scholar] [CrossRef]
- Svinterikos, E.; Zuburtikudis, I.; Al-Marzouqi, M. Electrospun Lignin-Derived Carbon Micro- and Nanofibers: A Review on Precursors, Properties, and Applications. ACS Sustain. Chem. Eng. 2020, 8, 13868–13893. [Google Scholar] [CrossRef]
- Rivière, G.N.; Korpi, A.; Sipponen, M.H.; Zou, T.; Kostiainen, M.A.; Österberg, M. Agglomeration of Viruses by Cationic Lignin Particles for Facilitated Water Purification. ACS Sustain. Chem. Eng. 2020, 8, 4167–4177. [Google Scholar] [CrossRef]
- Hoyo, J.; Ivanova, K.; Torrent-Burgues, J.; Tzanov, T. Interaction of Silver-Lignin Nanoparticles with Mammalian Mimetic Membranes. Front. Bioeng. Biotechnol. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Dalton, N.; Lynch, R.P.; Collins, M.N.; Culebras, M. Thermoelectric Properties of Electrospun Carbon Nanofibres Derived from Lignin. Int. J. Biol. Macromol. 2019, 121, 472–479. [Google Scholar] [CrossRef]
- Huerta, R.R.; Silva, E.K.; Ekaette, I.; El-Bialy, T.; Saldaña, M.D.A. High-Intensity Ultrasound-Assisted Formation of Cellulose Nanofiber Scaffold with Low and High Lignin Content and Their Cytocompatibility with Gingival Fibroblast Cells. Ultrason. Sonochem. 2020, 64, 104759. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, Structure, and Applications of Therapeutic and Amino Acid-Based Deep Eutectic Solvents: An Overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Pereira, A.D.E.S.; Luiz de Oliveira, J.; Maira Savassa, S.; Barbara Rogério, C.; Araujo de Medeiros, G.; Fraceto, L.F. Lignin Nanoparticles: New Insights for a Sustainable Agriculture. J. Clean. Prod. 2022, 345, 131145. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Guo, J.; Xu, F.; Li, C.; Puglia, D.; Kenny, J.; Ma, P. Enhancing the Radical Scavenging Activity and UV Resistance of Lignin Nanoparticles via Surface Mannich Amination toward a Biobased Antioxidant. Biomacromolecules 2021, 22, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Tran, T.M.T.; Choi, J.W.; Won, K. Lignin for White Natural Sunscreens. Int. J. Biol. Macromol. 2019, 122, 549–554. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).