Modified Activated Carbon as an Effective Hydrogen Adsorbent
Abstract
:1. Introduction
2. Materials and Methods
- Specific surface area according to Brunauer—Emmet—Teller (BET) methodology.
- Total pore volume for relative pressure p/p0 = 0.99;
- Microporous structure parameters (pores with diameter up to 2 nm) according to Dubinin—Radushkevich, Dubinin—Astakhov and t-plot methods;
- Mesoporous structure parameters (pores with diameters of 2–50 nm)—volume and area distributions of mesopores according to Barrett-Joyner-Halenda (BJH) methodology;
- ISO 9277:2010(E), Determination of the specific surface area of solids by gas adsorption—BET method;
- ISO 15901-2:2006(E), Pore size distribution and porosity of solid materials by mercury porosimetry and gas adsorption—Part 2: Analysis of mesopores and macropores by gas adsorption;
- ISO 15901-3:2007(E), Pore size distribution and porosity of solid materials by mercury porosimetry and gas adsorption—Part 3: Analysis of micropores by gas adsorption;
- NIST 2006, Porosity and Specific Surface Area Measurements for Solid Materials.
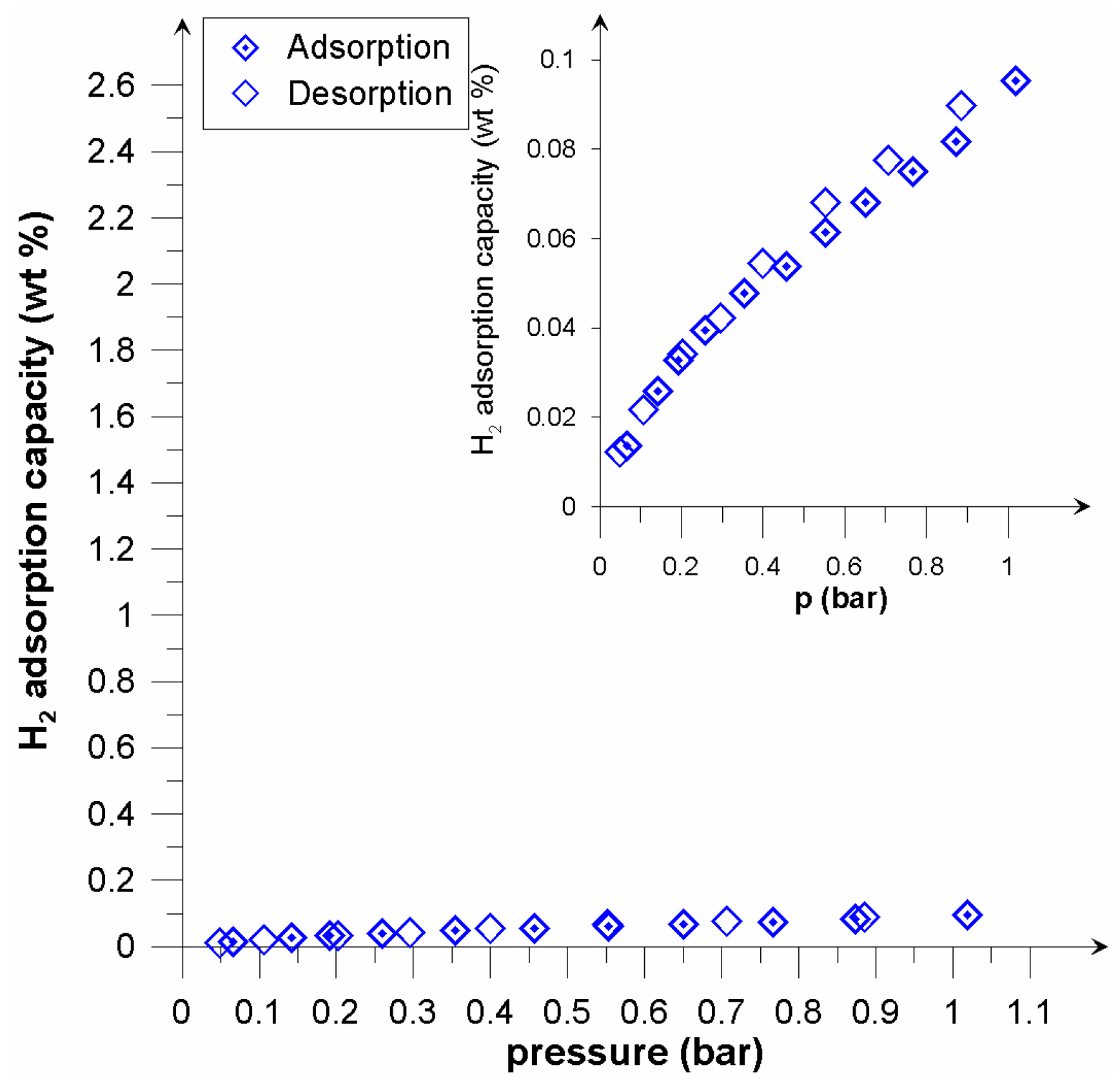
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Broom, D.P. Hydrogen Storage Materials: The Characterisation of Their Storage Properties; Springer: London, UK, 2011; ISBN 9780857292216. [Google Scholar]
- Sherif, S.A.; Goswami, D.Y.; Stefanakos, E.K.; Steinfeld, A. Handbook of Hydrogen Energy; CRC Press: Boca Raton, FL, USA, 2014; ISBN 9781420054477. [Google Scholar]
- Singh, A.K.; Lu, J.; Aga, R.S.; Yakobson, B.I. Hydrogen Storage Capacity of Carbon-Foams: Grand Canonical Monte Carlo Simulations. J. Phys. Chem. C 2011, 115, 2476–2482. [Google Scholar] [CrossRef]
- Darkrim Lamari, F.; Levesque, D. Hydrogen Adsorption on Functionalized Graphene. Carbon N. Y. 2011, 49, 5196–5200. [Google Scholar] [CrossRef]
- Ryu, Z.; Rong, H.; Zheng, J.; Wang, M.; Zhang, B. Microstructure and Chemical Analysis of PAN-Based Activated Carbon Fibers Prepared by Different Activation Methods. Carbon N. Y. 2002, 40, 1144–1147. [Google Scholar] [CrossRef]
- Texier-Mandoki, N.; Dentzer, J.; Piquero, T.; Saadallah, S.; David, P.; Vix-Guterl, C. Hydrogen Storage in Activated Carbon Materials: Role of the Nanoporous Texture. Carbon N. Y. 2004, 42, 2744–2747. [Google Scholar] [CrossRef]
- Bhat, V.V.; Contescu, C.I.; Gallego, N.C.; Baker, F.S. Atypical Hydrogen Uptake on Chemically-Activated, Ultramicroporous Carbon. Carbon N. Y. 2010, 48, 1331–1340. [Google Scholar] [CrossRef]
- Xiao, J.; Mei, A.; Tao, W.; Ma, S.; Bénard, P.; Chahine, R. Hydrogen Purification Performance Optimization of Vacuum Pressure Swing Adsorption on Different Activated Carbons. Energies 2021, 14, 2450. [Google Scholar] [CrossRef]
- Ramirez-Vidal, P.; Canevesi, R.L.S.; Sdanghi, G.; Schaefer, S.; Maranzana, G.; Celzard, A.; Fierro, V. A Step Forward in Understanding the Hydrogen Adsorption and Compression on Activated Carbons. ACS Appl. Mater. Interfaces 2021, 13, 12562–12574. [Google Scholar] [CrossRef]
- Ziemiański, P.P.; Derkowski, A.; Szczerba, M.; Guggenheim, S. Smectite Crystallite Swelling Under High Pressure of Methane. J. Phys. Chem. C 2021, 125, 7598–7610. [Google Scholar] [CrossRef]
- Karbownik, M.; Dudzińska, A.; Strzymczok, J. Multi-Parameter Analysis of Gas Losses Occurring during the Determination of Methane-Bearing Capacity in Hard Coal Beds. Energies 2022, 15, 3239. [Google Scholar] [CrossRef]
- Jagiełło, J.; Bandosz, T.J.; Schwarz, J.A. Application of Inverse Gas Chromatography at Infinite Dilution to Study the Effects of Oxidation of Activated Carbons. Carbon N. Y. 1992, 30, 63–69. [Google Scholar] [CrossRef]
- Cazorla-Amorós, D.; Alcañiz-Monge, J.; Linares-Solano, A. Characterization of Activated Carbon Fibers by CO2 Adsorption. Langmuir 1996, 12, 2820–2824. [Google Scholar] [CrossRef]
- Salem, M.; Braeuer, P.; Szombathely, M. Thermodynamics of High-Pressure Adsorption of Argon, Nitrogen, and Methane on Microporous Adsorbents. Langmuir 1998, 7463, 3376–3389. [Google Scholar] [CrossRef]
- Park, N.; Choi, K.; Hwang, J.; Kim, D.W.; Kim, D.O.; Ihm, J. Progress on First-Principles-Based Materials Design for Hydrogen Storage. Proc. Natl. Acad. Sci. USA 2012, 109, 19893–19899. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.-H.; Lim, S.; Song, Y.; Ota, Y.; Qiao, W.; Tanaka, A.; Mochida, I. KOH Activation of Carbon Nanofibers. Carbon N. Y. 2004, 42, 1723–1729. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Cazorla-Amorós, D.; Linares-Solano, A. Understanding Chemical Reactions between Carbons and NaOH and KOH: An Insight into the Chemical Activation Mechanism. Carbon N. Y. 2003, 41, 267–275. [Google Scholar] [CrossRef]
- Buczek, B. Adsorption of Methane and Carbon Dioxide on a Potassium Hydroxide-Modified Activated Carbon. Przem. Chem. 2013, 92, 535–537. [Google Scholar]
- Perrin, A.; Celzard, A.; Marêché, J.F.; Furdin, G. Improved Methane Storage Capacities by Sorption on Wet Active Carbons. Carbon 2004, 42, 1243–1249. [Google Scholar] [CrossRef]
- Baran, P.; Cygankiewicz, J.; Zarȩbska, K. Carbon Dioxide Sorption on Polish Ortholignite Coal in Low and Elevated Pressure. J. CO2 Util. 2013, 3–4, 44–48. [Google Scholar] [CrossRef]
- Baran, P.; Krzak, M.; Zarebska, K.; Szczurowski, J.; Zmuda, W.A. Adsorption of Sulfur(IV) Oxide on Activated Carbon from Pyrolysis of Waste Tires. Przem. Chem. 2016, 95, 1164–1166. [Google Scholar] [CrossRef]
- Fujiwara, M.; Fujio, Y.; Sakurai, H.; Senoh, H.; Kiyobayashi, T. Storage of Molecular Hydrogen into ZSM-5 Zeolite in the Ambient Atmosphere by the Sealing of the Micropore Outlet. Chem. Eng. Process. Process Intensif. 2014, 79, 1–6. [Google Scholar] [CrossRef]
- Masika, E.; Mokaya, R. Preparation of Ultrahigh Surface Area Porous Carbons Templated Using Zeolite 13X for Enhanced Hydrogen Storage. Prog. Nat. Sci. Mater. Int. 2013, 23, 308–316. [Google Scholar] [CrossRef]
- Czosnek, C.; Baran, P.; Grzywacz, P.; Baran, P.; Janik, J.F.; Rózycka, A.; Sitarz, M.; Jeleń, P. Generation of Carbon Nanostructures with Diverse Morphologies by the Catalytic Aerosol-Assisted Vapor-Phase Synthesis Method. Comptes Rendus Chim. 2015, 18, 1198–1204. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Effects of Functionalization, Catenation, and Variation of the Metal Oxide and Organic Linking Units on the Low-Pressure Hydrogen Adsorption Properties of Metal−Organic Frameworks. J. Am. Chem. Soc. 2006, 128, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Panella, B.; Hirscher, M.; Ludescher, B. Low-Temperature Thermal-Desorption Mass Spectroscopy Applied to Investigate the Hydrogen Adsorption on Porous Materials. Microporous Mesoporous Mater. 2007, 103, 230–234. [Google Scholar] [CrossRef]
- McEnaney, B. Estimation of the Dimensions of Micropores in Active Carbons Using the Dubinin-Radushkevich Equation. Carbon N. Y. 1987, 25, 69–75. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH Activation of Carbon-Based Materials for Energy Storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High Hydrogen Storage Capacity of Porous Carbons Prepared by Using Activated Carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef]
- Geng, Z.; Zhang, C.; Wang, D.; Zhou, X.; Cai, M. Pore Size Effects of Nanoporous Carbons with Ultra-High Surface Area on High-Pressure Hydrogen Storage. J. Energy Chem. 2015, 24, 1–8. [Google Scholar] [CrossRef]
- Minoda, A.; Oshima, S.; Iki, H.; Akiba, E. Synthesis of KOH-Activated Porous Carbon Materials and Study of Hydrogen Adsorption. J. Alloys Compd. 2013, 580, S301–S304. [Google Scholar] [CrossRef]
- Panella, B.; Hirscher, M.; Roth, S. Hydrogen Adsorption in Different Carbon Nanostructures. Carbon N. Y. 2005, 43, 2209–2214. [Google Scholar] [CrossRef]
- Rzepka, M.; Lamp, P.; de la Casa-Lillo, M.A. Physisorption of Hydrogen on Microporous Carbon and Carbon Nanotubes. J. Phys. Chem. B 1998, 102, 10894–10898. [Google Scholar] [CrossRef]
- Wang, Q.; Johnson, J.K. Molecular Simulation of Hydrogen Adsorption in Single-Walled Carbon Nanotubes and Idealized Carbon Slit Pores. J. Chem. Phys. 1998, 110, 577–586. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Portet, C.; Osswald, S.; Simmons, J.M.; Yildirim, T.; Laudisio, G.; Fischer, J.E. Importance of Pore Size in High-Pressure Hydrogen Storage by Porous Carbons. Int. J. Hydrog. Energy 2009, 34, 6314–6319. [Google Scholar] [CrossRef]
- Akasaka, H.; Takahata, T.; Toda, I.; Ono, H.; Ohshio, S.; Himeno, S.; Kokubu, T.; Saitoh, H. Hydrogen Storage Ability of Porous Carbon Material Fabricated from Coffee Bean Wastes. Int. J. Hydrog. Energy 2011, 36, 580–585. [Google Scholar] [CrossRef]
- Fierro, V.; Szczurek, A.; Zlotea, C.; Marêché, J.F.; Izquierdo, M.T.; Albiniak, A.; Latroche, M.; Furdin, G.; Celzard, A. Experimental Evidence of an Upper Limit for Hydrogen Storage at 77 K on Activated Carbons. Carbon N. Y. 2010, 48, 1902–1911. [Google Scholar] [CrossRef]
- Rowlandson, J.L.; Coombs OBrien, J.; Edler, K.J.; Tian, M.; Ting, V.P. Application of Experimental Design to Hydrogen Storage: Optimisation of Lignin-Derived Carbons. C J. Carbon Res. 2019, 5, 82. [Google Scholar] [CrossRef]
- Harjanto, S.; Noviana, L.N.; Diniati, M.; Yunior, S.W.; Nasruddin. Hydrogen Adsorption Capacity Reduction of Activated Carbon Produced from Indonesia Low Rank Coal by Pelletizing. Sains Malays. 2015, 5, 747–752. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, L.; Chen, T.; Li, Z.; Zhang, Z.; Fan, M. Activated Carbons from Oil Palm Shell for Hydrogen Storage. IOP Conf. Ser. Mater. Sci. Eng. 2018, 368, 12031. [Google Scholar] [CrossRef]
- Alfadlil, B.R.; Knowles, G.P.; Parsa, M.R.; Subagyono, R.R.D.J.N.; Daniel; Chaffee, A.L. Carbon Monolith from Victorian Brown Coal for Hydrogen Storage. J. Phys. Conf. Ser. 2019, 1277, 12024. [Google Scholar] [CrossRef]
- Toprak, A. Production and Characterization of Microporous Activated Carbon from Cherry Laurel (Prunus laurocrasus L.) Stone: Application of H2 and CH4 Adsorption. Biomass Convers. Biorefinery 2020, 10, 977–986. [Google Scholar] [CrossRef]
- Williams, N.E.; Oba, O.A.; Aydinlik, N.P. Modification, Production, and Methods of KOH-Activated Carbon. ChemBioEng Rev. 2022, 9, 164–189. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Czekajło, Ł.; Sibera, D.; Moszyński, D.; Sreńscek-Nazzal, J.; Morawski, A.W.; Wrobel, R.J.; Michalkiewicz, B.; Arabczyk, W.; Narkiewicz, U. Surface Characteristics of KOH-Treated Commercial Carbons Applied for CO2 Adsorption. Adsorpt. Sci. Technol. 2017, 36, 478–492. [Google Scholar] [CrossRef]
- Dai, W.; Liu, Y.; Su, W.; Hu, G.; Deng, G.; Hu, X. Preparation and CO2 Sorption of a High Surface Area Activated Carbon Obtained from the KOH Activation of Finger Citron Residue. Adsorpt. Sci. Technol. 2012, 30, 183–191. [Google Scholar] [CrossRef]
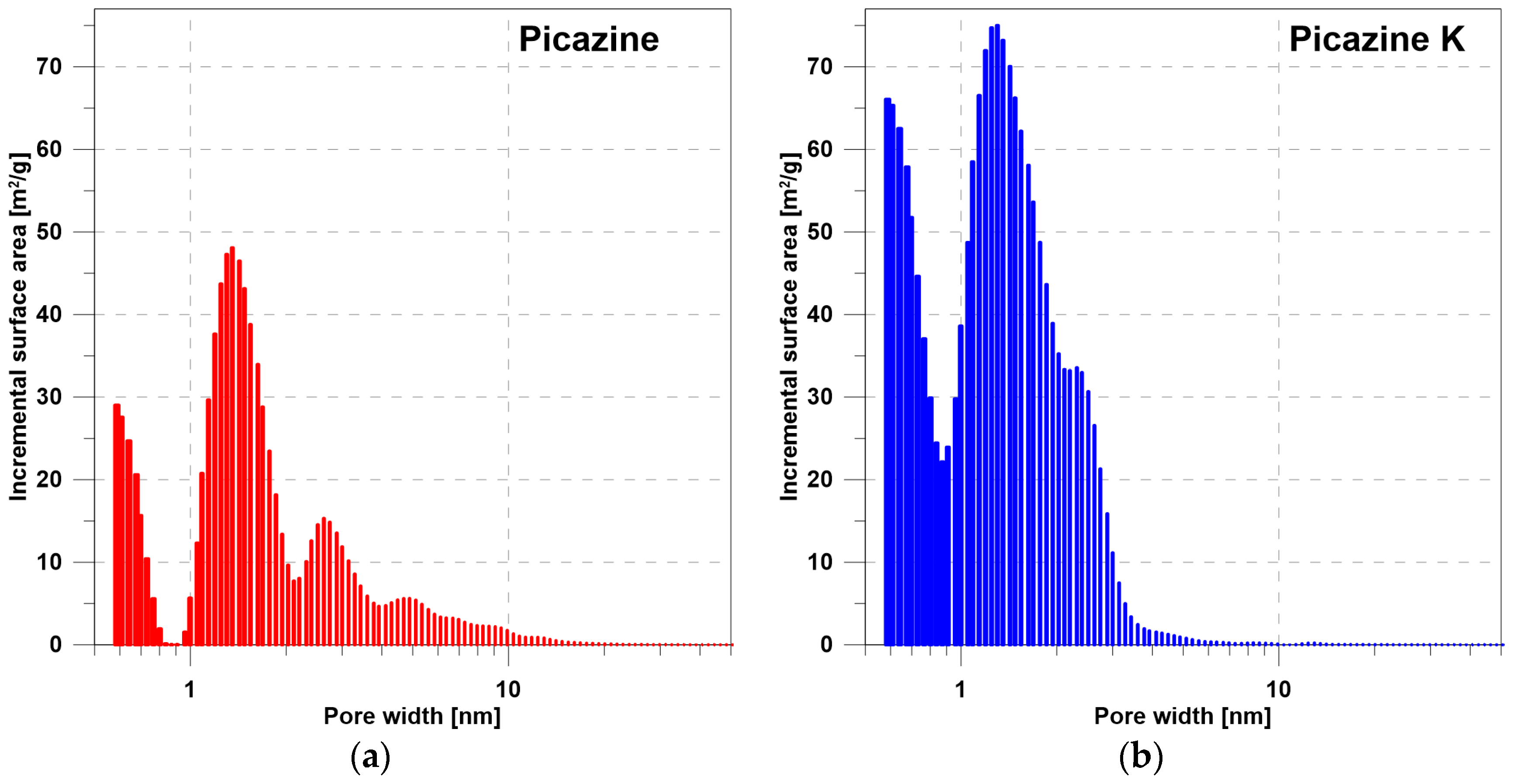


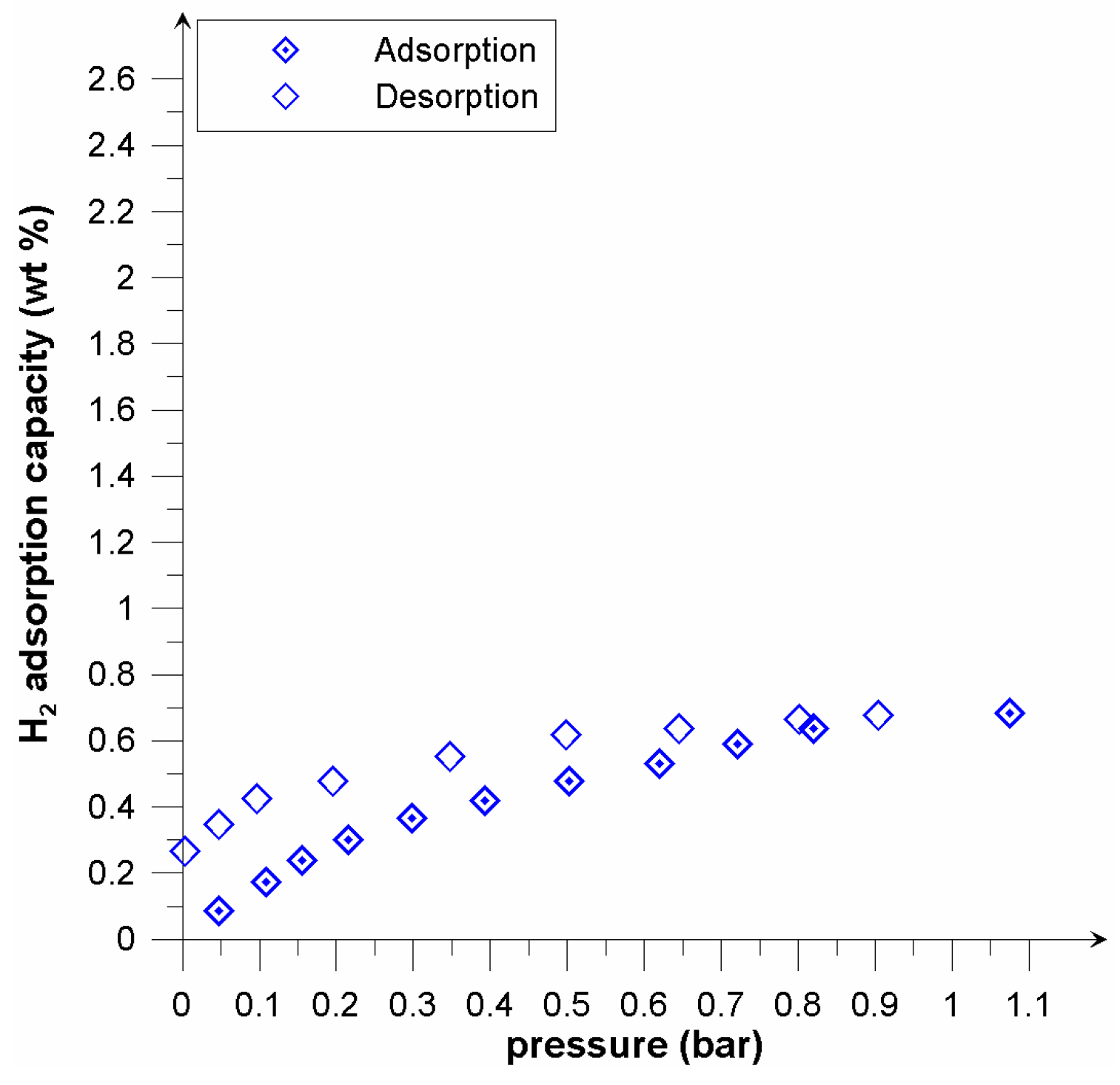
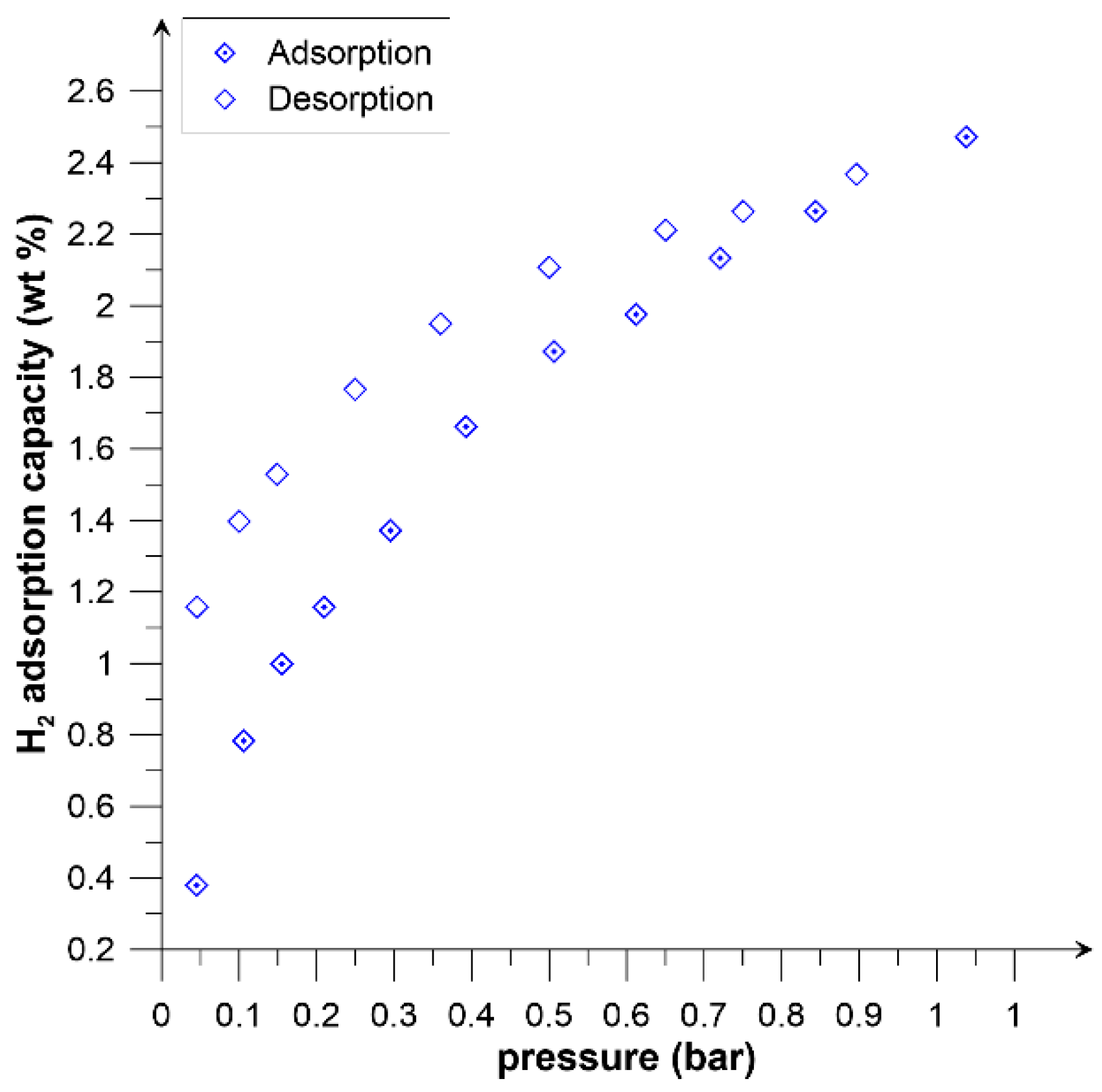
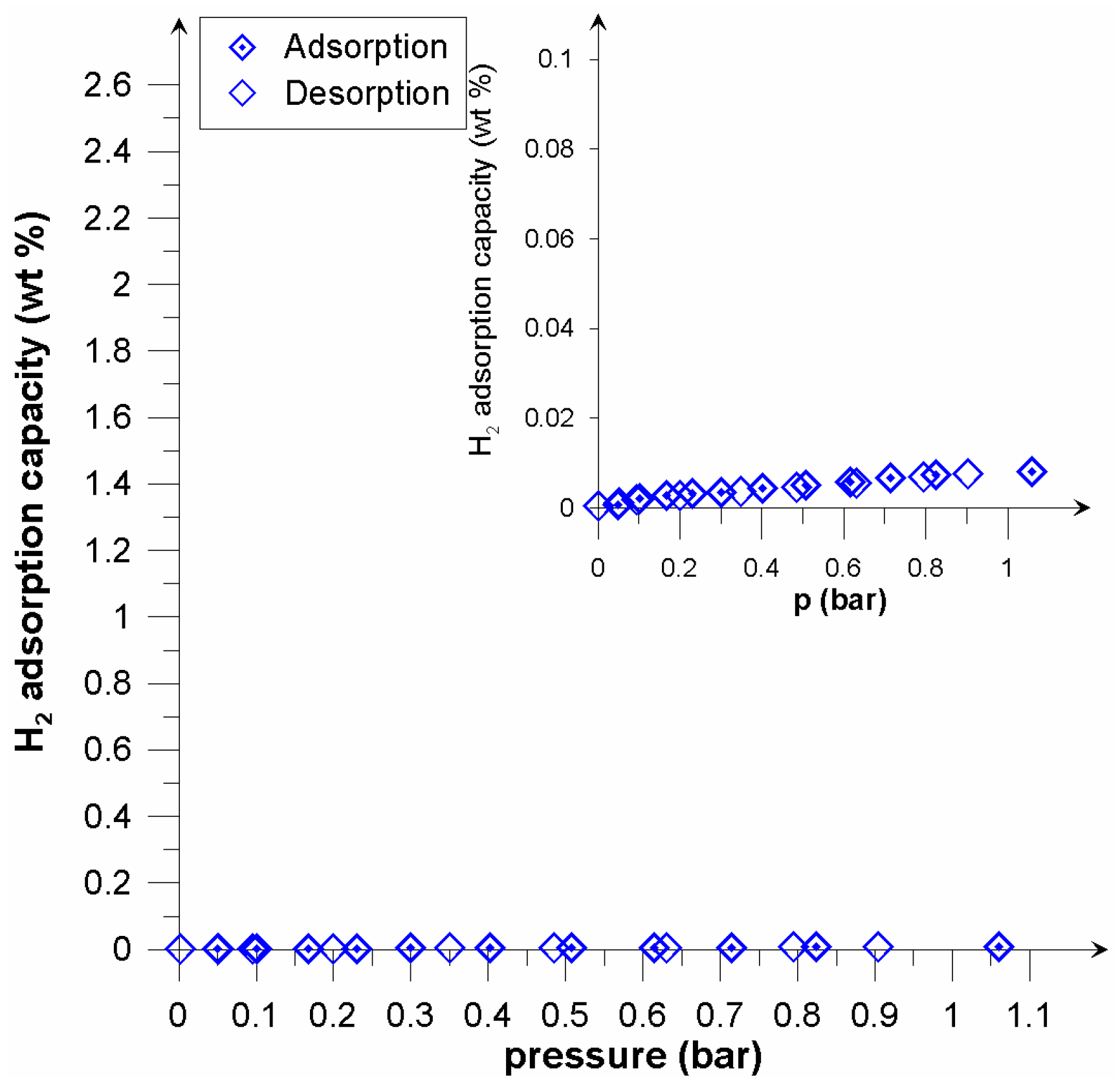
| Properties | Picazine | Picazine K |
|---|---|---|
| Bulk density (n), g/cm3 | 0.204 | 0.120 |
| Apparent density (p), g/cm3 | 0.450 | 0.325 |
| Real density ((r), g/cm3 | 1.777 | 2.669 |
| Total porosity (εc), cm3/cm3 | 0.885 | 0.631 |
| Volume of pores (V), cm3/cm3 | 1.660 | 2.702 |
| Parameter | Picazine | Picazine K |
|---|---|---|
| Specific surface area calculated using the BET method, , m2/g | 1462 | 2939 |
| Total volume of pores for p/p0 = 0.99, , cm3/g | 1.024 | 1.488 |
| Parameters of texture of micropores by Dubinin and Radushkevich (DR) | ||
| Surface of micropores, , m2/g | 1392 | 2817 |
| Volume of micropores, , cm3/g | 0.494 | 1.001 |
| Adsorption energy in micropores, , kJ/mol | 16.22 | 17.77 |
| Parameters of texture of micropores by Dubinin and Astakhov (DA) | ||
| Surface of micropores, , m2/g | 1127 | 2229 |
| Volume of micropores, , cm3/g | 0.500 | 0.938 |
| Adsorption energy in micropores, , kJ/mol | 16.11 | 18.49 |
| Mean diameter of pores, , nm | 1.78 | 1.68 |
| Dominant diameter of pores, , nm | 1.60 | 1.54 |
| Type of Adsorbent | SBET (m2/g) | H2 Uptake (wt. %) | Storage Conditions | Additional Information | Ref. |
|---|---|---|---|---|---|
| AC from coffee beans | 2070 | 0.6 | 120 bar, 298 K 40 bar, 77 K | KOH activated | [36] |
| 2070 | 0.4 | ||||
| AC from anthracite | 1149 | 3.2 | 40 bar, 77 K | KOH/NaOH activated | [37] |
| 2029 | 4.9 | ||||
| 2849 | 6.0 | ||||
| 3220 | 5.7 | ||||
| 1308 | 2.9 | ||||
| 2451 | 5.8 | ||||
| 3073 | 5.7 | ||||
| AC from pine | 1055 | 1.61 | 1 bar, 77 K | CO2 activated | [38] |
| 1409 | 1.93 | ||||
| AC from low rank coal | 640 | 0.29 | 40 bar, 77 K | KOH activated | [39] |
| AC from oil palm shell | 3503 | 6.7 2.86 | 40 bar, 77 K 1 bar; 77 K | KOH activated | [40] |
| Carbon monolith from lignite | 973 | 1.28 | 60 bar, 293 K | CO2 activated | [41] |
| AC from stone of cherry laurel | 1624 | 2.9 | 1 bar, 77 K | KOH activated | [42] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, P.; Buczek, B.; Zarębska, K. Modified Activated Carbon as an Effective Hydrogen Adsorbent. Energies 2022, 15, 6122. https://doi.org/10.3390/en15176122
Baran P, Buczek B, Zarębska K. Modified Activated Carbon as an Effective Hydrogen Adsorbent. Energies. 2022; 15(17):6122. https://doi.org/10.3390/en15176122
Chicago/Turabian StyleBaran, Paweł, Bronisław Buczek, and Katarzyna Zarębska. 2022. "Modified Activated Carbon as an Effective Hydrogen Adsorbent" Energies 15, no. 17: 6122. https://doi.org/10.3390/en15176122
APA StyleBaran, P., Buczek, B., & Zarębska, K. (2022). Modified Activated Carbon as an Effective Hydrogen Adsorbent. Energies, 15(17), 6122. https://doi.org/10.3390/en15176122






