Raman Spectroscopy of Lignite Gasification Char Morphotypes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Gasification
2.2. Petrographic Analysis
2.3. Raman Spectroscopy
2.4. Statistical Analysis
3. Results and Discussion
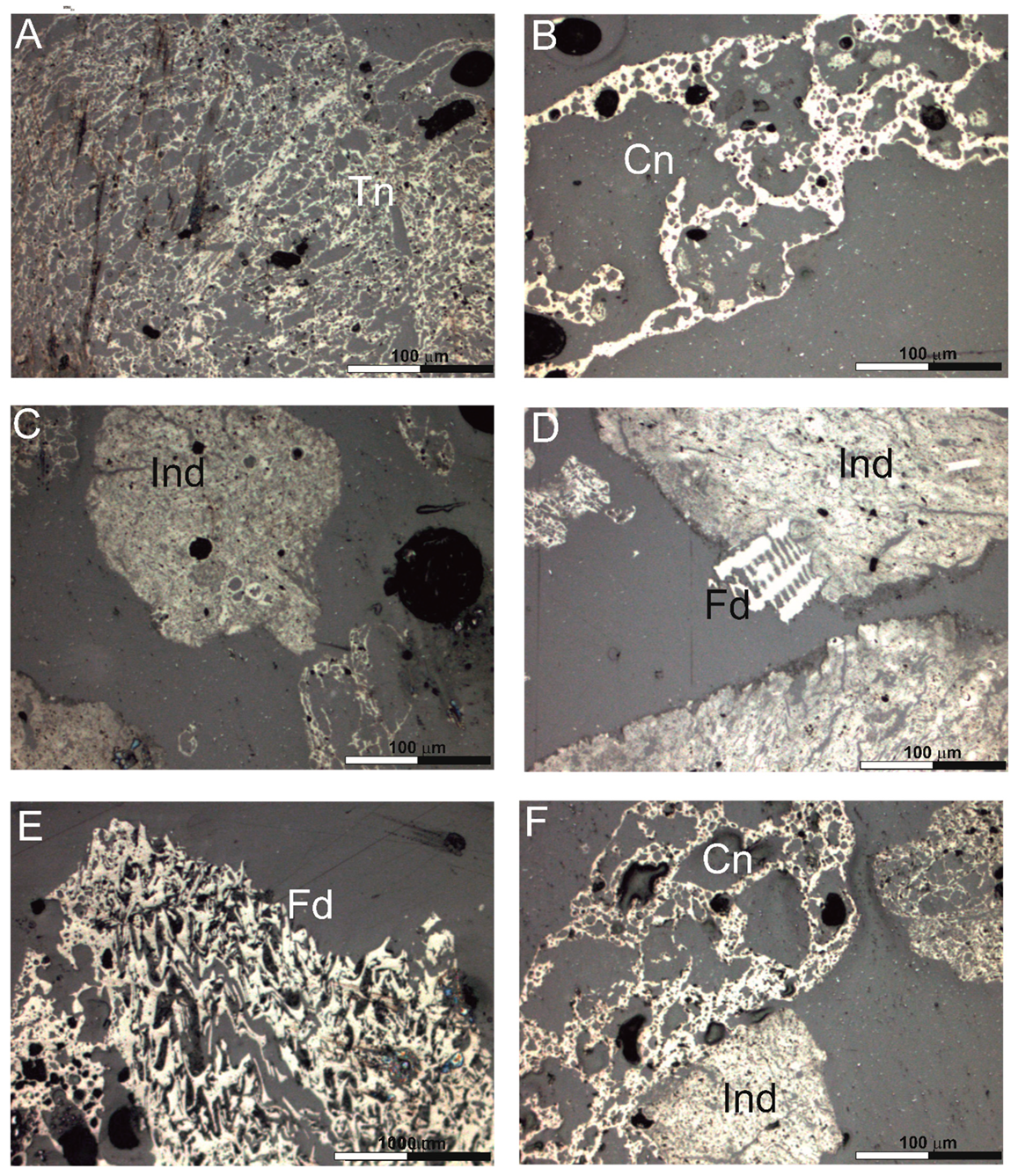
3.1. Petrographic Composition of the Char
3.2. Raman Spectroscopy of the Char Morphotypes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jüntgen, H. Reactivities of carbon to steam and hydrogen and applications to technical gasification processes—A review. Carbon 1981, 19, 167–173. [Google Scholar] [CrossRef]
- Mühlen, H.J.; van Heek, K.H.; Jüntgen, H. Kinetic studies of steam gasification of char in the presence of H2, CO2 and CO. Fuel 1985, 64, 944–949. [Google Scholar] [CrossRef]
- Li, C.Z. Some Recent Advances in the Understanding of the Pyrolysis and Gasification Behaviour of Victorian Brown Coal; Elsevier: Amsterdam, The Netherlands, 2007; Volume 86, pp. 1664–1683. [Google Scholar]
- Wagner, N.J.; Coertzen, M.; Matjie, R.H.; van Dyk, J.C. Chapter 5—Coal Gasification. In Applied Coal Petrology: The Role of Petrology in Coal Utilization; Elsevier: Amsterdam, The Netherlands, 2008; pp. 119–144. [Google Scholar]
- Bell, D.A.; Towler, B.F. Coal Gasification and Its Applications; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Li, T.; Zhang, L.; Dong, L.; Li, C.-Z.Z. Effects of gasification atmosphere and temperature on char structural evolution during the gasification of Collie sub-bituminous coal. Fuel 2014, 117, 1190–1195. [Google Scholar] [CrossRef]
- Radovic, L.R.; Steczko, K.; Walker, P.L.; Jenkins, R.G. Combined effects of inorganic constituents and pyrolysis conditions on the gasification reactivity of coal chars. Fuel Process. Technol. 1985, 10, 311–326. [Google Scholar] [CrossRef]
- Takarada, T.; Tamai, Y.; Tomita, A. Reactivities of 34 coals under steam gasification. Fuel 1985, 64, 1438–1442. [Google Scholar] [CrossRef]
- Van Heek, K.H.; Mühlen, H.-J.J. Aspects of coal properties and constitution important for gasification. Fuel 1985, 64, 1405–1414. [Google Scholar] [CrossRef]
- Hashimoto, K.; Miura, K.; Ueda, T. Correlation of gasification rates of various coals measured by a rapid heating method in a steam atmosphere at relatively low temperatures. Fuel 1986, 65, 1516–1523. [Google Scholar] [CrossRef]
- Kasaoka, S.; Sakata, Y.; Shimada, M. Effects of coal carbonization conditions on rate of steam gasification of char. Fuel 1987, 66, 697–701. [Google Scholar] [CrossRef]
- Miura, K.; Hashimoto, K.; Silveston, P.L. Factors affecting the reactivity of coal chars during gasification, and indices representing reactivity. Fuel 1989, 68, 1461–1475. [Google Scholar] [CrossRef]
- Miura, K.; Makino, M.; Silveston, P.L. Correlation of gasification reactivities with char properties and pyrolysis conditions using low rank Canadian coals. Fuel 1990, 69, 580–589. [Google Scholar] [CrossRef]
- Tomita, A.; Ohtsuka, Y. Gasification and Combustion of Brown Coal. In Advances in the Science of Victorian Brown Coal; Elsevier Science: Amsterdam, The Netherlands, 2004; pp. 223–285. [Google Scholar] [CrossRef]
- Bielowicz, B. A new technological classification of low-rank coal on the basis of Polish deposits. Fuel 2012, 96, 497–510. [Google Scholar] [CrossRef]
- Bielowicz, B. Petrographic composition of Polish lignite and its possible use in a fluidized bed gasification process. Int. J. Coal Geol. 2013, 116, 236–246. [Google Scholar] [CrossRef]
- Bielowicz, B.; Kasiński, J.R.J.R. The possibility of underground gasification of lignite from Polish deposits. Int. J. Coal Geol. 2014, 131, 304–318. [Google Scholar] [CrossRef]
- Kapusta, K.; Wiatowski, M.; Stańczyk, K. An experimental ex-situ study of the suitability of a high moisture ortho-lignite for underground coal gasification (UCG) process. Fuel 2016, 179, 150–155. [Google Scholar] [CrossRef]
- Xi, J.; Liang, J.; Sheng, X.; Shi, L.; Li, S. Characteristics of lump lignite pyrolysis and the influence of temperature on lignite swelling in underground coal gasification. J. Anal. Appl. Pyrolysis 2016, 117, 228–235. [Google Scholar] [CrossRef]
- Furimsky, E.; Palmer, A.D.D.A.; Kalkreuth, W.D.D.; Cameron, A.R.R.; Kovacik, G. Prediction of coal reactivity during combustion and gasification by using petrographic data. Fuel Process. Technol. 1990, 25, 135–151. [Google Scholar] [CrossRef]
- Duxbury, J. Prediction of coal pyrolysis yields from BS volatile matter and petrographic analyses. Fuel 1997, 76, 1337–1343. [Google Scholar] [CrossRef]
- Sun, Q.; Li, W.; Chen, H.; Li, B. The CO2-gasification and kinetics of Shenmu maceral chars with and without catalyst. Fuel 2004, 83, 1787–1793. [Google Scholar] [CrossRef]
- Wagner, N.J.J.; Matjie, R.H.H.; Slaghuis, J.H.H.; van Heerden, J.H.P.H.P. Characterization of unburned carbon present in coarse gasification ash. Fuel 2008, 87, 683–691. [Google Scholar] [CrossRef]
- Guo, X.; Tang, Y.; Eble, C.F.; Wang, Y.; Li, P. Study on petrographic characteristics of devolatilization char/coke related to coal rank and coal maceral. Int. J. Coal Geol. 2020, 227, 103504. [Google Scholar] [CrossRef]
- Everson, R.C.; Neomagus, H.W.J.P.; Kasaini, H.; Njapha, D. Reaction kinetics of pulverized coal-chars derived from inertinite-rich coal discards: Gasification with carbon dioxide and steam. Fuel 2006, 85, 1076–1082. [Google Scholar] [CrossRef]
- Lester, E.; Alvarez, D.; Borrego, A.G.G.; Valentim, B.; Flores, D.; Clift, D.A.A.; Rosenberg, P.; Kwiecinska, B.; Barranco, R.; Petersen, H.I.I.; et al. The procedure used to develop a coal char classification—Commission III Combustion Working Group of the International Committee for Coal and Organic Petrology. Int. J. Coal Geol. 2010, 81, 333–342. [Google Scholar] [CrossRef]
- Chabalala, V.P.P.; Wagner, N.; Potgieter-Vermaak, S. Investigation into the evolution of char structure using Raman spectroscopy in conjunction with coal petrography; Part 1. Fuel Process. Technol. 2011, 92, 750–756. [Google Scholar] [CrossRef]
- Oboirien, B.O.; Engelbrecht, A.D.; North, B.C.; Du Cann, V.M.; Falcon, R. Textural properties of chars as determined by petrographic analysis: Comparison between air-blown, oxygen-blown and oxygen-enriched gasification. Fuel 2012, 101, 16–22. [Google Scholar] [CrossRef]
- Bielowicz, B. Petrographic characteristics of lignite gasification chars. Int. J. Coal Geol. 2016, 168, 146–161. [Google Scholar] [CrossRef]
- Bielowicz, B. Change of the petrographic composition of lignite during the ex-situ lignite gasification. Fuel 2017, 206, 219–229. [Google Scholar] [CrossRef]
- Bielowicz, B.; Raszowski, M.; Maciejończyk, N. Petrographic composition of the ex-situ lignite gasification residues. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 1762–1779. [Google Scholar] [CrossRef]
- Guo, X.; Tay, H.L.; Zhang, S.; Li, C.Z. Changes in char structure during the gasification of a Victorian brown coal in steam and oxygen at 800 °C. Energy Fuels 2008, 22, 4034–4038. [Google Scholar] [CrossRef]
- Qi, J.; Fan, C.; Li, S. Characteristics of lignite char derived from oxy-pyrolysis. Fuel 2021, 291, 120261. [Google Scholar] [CrossRef]
- Roberts, M.J.; Everson, R.C.; Neomagus, H.W.J.P.; Van Niekerk, D.; Mathews, J.P.; Branken, D.J. Influence of maceral composition on the structure, properties and behaviour of chars derived from South African coals. Fuel 2015, 142, 9–20. [Google Scholar] [CrossRef]
- Xu, X.Q.; Wang, Y.G.; Chen, Z.D.; Chen, X.J.; Zhang, H.Y.; Bai, L.; Zhang, S. Variations in char structure and reactivity due to the pyrolysis and in-situ gasification using Shengli brown coal. J. Anal. Appl. Pyrolysis 2015, 115, 233–241. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Ding, L.; Gong, Y.; Yu, G. Study on the effect of inherent AAEM on char structure evolution during coal pyrolysis by in-situ Raman and TG. Fuel 2021, 292, 120406. [Google Scholar] [CrossRef]
- Li, L.; Tong, S.; Duan, L.; Zhao, C.; Shi, Z. Effect of CO2 and H2O on lignite char structure and reactivity in a fluidized bed reactor. Fuel Process. Technol. 2021, 211, 106564. [Google Scholar] [CrossRef]
- Liang, C.; Wang, X.; Lyu, Q. Experimental investigation on fluidized modification in gasification of preheated coal using oxygen and steam. Fuel 2021, 304, 121375. [Google Scholar] [CrossRef]
- Sun, J.; Feng, H.; Kou, J.; Jin, H.; Chen, Y.; Guo, L. Experimental investigation on carbon microstructure for coal gasification in supercritical water. Fuel 2021, 306, 121675. [Google Scholar] [CrossRef]
- Bar-Ziv, E.; Zaida, A.; Salatino, P.; Senneca, O. Diagnostics of carbon gasification by raman microprobe spectroscopy. Proc. Combust. Inst. 2000, 28, 2369–2374. [Google Scholar] [CrossRef]
- Li, T.; Zhang, L.; Dong, L.; Zhang, S.; Qiu, P.; Wang, S.; Li, C.-Z.Z. Effects of gasification temperature and atmosphere on char structural evolution and AAEM retention during the gasification of Loy Yang brown coal. Fuel Process. Technol. 2017, 159, 48–54. [Google Scholar] [CrossRef]
- Tay, H.L.; Li, C.Z. Changes in char reactivity and structure during the gasification of a Victorian brown coal: Comparison between gasification in O2 and CO2. In Fuel Processing Technology; Elsevier: Amsterdam, The Netherlands, 2010; Volume 91, pp. 800–804. [Google Scholar]
- Tay, H.-L.L.; Kajitani, S.; Wang, S.; Li, C.-Z.Z. A preliminary Raman spectroscopic perspective for the roles of catalysts during char gasification. Fuel 2014, 121, 165–172. [Google Scholar] [CrossRef]
- Zhang, S.; Min, Z.; Tay, H.-L.L.; Asadullah, M.; Li, C.-Z.Z. Effects of volatile–char interactions on the evolution of char structure during the gasification of Victorian brown coal in steam. Fuel 2011, 90, 1529–1535. [Google Scholar] [CrossRef]
- Li, X.; Hayashi, J.-I.; Li, C.-Z. FT-Raman spectroscopic study of the evolution of char structure during the pyrolysis of a Victorian brown coal. Fuel 2006, 85, 1700–1707. [Google Scholar] [CrossRef]
- Zhang, X.P.; Zhang, C.; Tan, P.; Li, X.; Fang, Q.Y.; Chen, G. Effects of hydrothermal upgrading on the physicochemical structure and gasification characteristics of Zhundong coal. Fuel Process. Technol. 2018, 172, 200–208. [Google Scholar] [CrossRef]
- Qi, X.; Guo, X.; Xue, L.; Zheng, C. Effect of iron on Shenfu coal char structure and its influence on gasification reactivity. J. Anal. Appl. Pyrolysis 2014, 110, 401–407. [Google Scholar] [CrossRef]
- Guedes, A.; Valentim, B.; Prieto, A.C.C.; Noronha, F. Raman spectroscopy of coal macerals and fluidized bed char morphotypes. Fuel 2012, 97, 443–449. [Google Scholar] [CrossRef]
- Bielowicz, B.; Morga, R. Micro-Raman Spectroscopy of Selected Macerals of the Huminite Group: An Example from the Szczerców Lignite Deposit (Central Poland). Energies 2021, 14, 281. [Google Scholar] [CrossRef]
- Chmielniak, T.; Sobolewski, A.; Tomaszewicz, G. CO2-Enhanced coal gasification. Experience of the Institute for Chemical Processing of Coal. Przem. Chem. 2015, 94, 442–448. [Google Scholar]
- ISO 589:2008; Hard Coal—Determination of Total Moisture. Available online: https://www.iso.org/standard/45370.html (accessed on 28 October 2021).
- ISO 1171; Solid Mineral Fuels—Determination of Ash. 2010. Available online: https://www.iso.org/standard/55944.html (accessed on 28 October 2021).
- ISO 19579:2006; Solid Mineral Fuels—Determination of Sulfur by IR Spectrometry. Available online: https://www.iso.org/standard/39113.html (accessed on 28 October 2021).
- ISO 1928:2020; Coal and Coke—Determination of Gross Calorific Value. Available online: https://www.iso.org/standard/75883.html (accessed on 28 October 2021).
- ISO 562:2010; Hard coal and Coke—Determination of Volatile Matter. Available online: https://www.iso.org/standard/55943.html (accessed on 17 June 2020).
- ISO 29541:2010; Solid mineral fuels—Determination of Total Carbon, Hydrogen and Nitrogen Content—Instrumental Method. Available online: https://www.iso.org/standard/45546.html (accessed on 28 October 2021).
- ISO 7404-2; Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples. 2009. Available online: https://www.iso.org/standard/42798.html (accessed on 28 October 2021).
- Lünsdorf, N.K. Raman spectroscopy of dispersed vitrinite—Methodical aspects and correlation with reflectance. Int. J. Coal Geol. 2016, 153, 75–86. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Sheng, C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity. Fuel 2007, 86, 2316–2324. [Google Scholar] [CrossRef]
- Morga, R. Micro-Raman spectroscopy of carbonized semifusinite and fusinite. Int. J. Coal Geol. 2011, 87, 253–267. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Liu, Z.; Ding, H.; Huang, X.; Zheng, C. Study on the evolution of the char structure during hydrogasification process using Raman spectroscopy. Fuel 2015, 157, 97–106. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Nemanich, R.J.; Solin, S.A. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B 1979, 20, 392–401. [Google Scholar] [CrossRef]
- Green, P.D.; Johnson, C.A.; Thomas, K.M. Applications of laser Raman microprobe spectroscopy to the characterization of coals and cokes. Fuel 1983, 62, 1013–1023. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J.M.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Beyssac, O.; Goffé, B.; Petitet, J.P.; Froigneux, E.; Moreau, M.; Rouzaud, J.N. On the characterization of disordered and heterogeneous carbonaceous materials by Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2003, 59, 2267–2276. [Google Scholar] [CrossRef]
- Rouzaud, J.N.; Oberlin, A.; Beny-Bassez, C. Carbon films: Structure and microtexture (optical and electron microscopy, Raman spectroscopy). Thin Solid Film. 1983, 105, 75–96. [Google Scholar] [CrossRef]
- Beny-Bassez, C.; Rouzaud, J.N.; Bassez, C.; Rouzaud, J.N. Characterization of carbonaceous materials by correlated electron and optical microscopy and Raman microspectrometry. Scanning Electron Microsc. 1985, 1, 119–132. [Google Scholar]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef] [Green Version]
- Livneh, T.; Bar-Ziv, E.; Senneca, O.; Salatino, P. Evolution of Reactivity of Highly Porous Chars from Raman Microscopy. Combust. Sci. Technol. 2000, 153, 65–82. [Google Scholar] [CrossRef]
- Zaida, A.; Bar-Ziv, E.; Radovic, L.R.; Lee, Y.-J. Further development of Raman Microprobe spectroscopy for characterization of char reactivity. Proc. Combust. Inst. 2007, 31, 1881–1887. [Google Scholar] [CrossRef]
- Borrego, A.G.; Alvarez, D.; Menéndez, R. Effects of Inertinite Content in Coal on Char Structure and Combustion. Energy Fuels 1997, 11, 702–708. [Google Scholar] [CrossRef]
- Lünsdorf, N.K.; Dunkl, I.; Schmidt, B.C.; Rantitsch, G.; von Eynatten, H. Towards a Higher Comparability of Geothermometric Data Obtained by Raman Spectroscopy of Carbonaceous Material. Part 2: A Revised Geothermometer. Geostand. Geoanal. Res. 2017, 41, 593–612. [Google Scholar] [CrossRef]
- Morga, R. Changes of Semifusinite and Fusinite Microstructure during Carbonization Inferred from the Raman Spectroscopy Examination; Monograph. In Polish, English abstract; Silesian University of Technology Publishing House: Gliwice, Poland, 2013. [Google Scholar]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef] [Green Version]
- Dillon, R.O.; Woollam, J.A.; Katkanant, V. Use of Raman scattering to investigate disorder and crystallite formation in as-deposited and annealed carbon films. Phys. Rev. B 1984, 29, 3482. [Google Scholar] [CrossRef]
- Prawer, S.; Ninio, F.; Blanchonette, I. Raman spectroscopic investigation of ion-beam-irradiated glassy carbon. J. Appl. Phys. 1998, 68, 2361. [Google Scholar] [CrossRef]
- Zickler, G.A.; Smarsly, B.; Gierlinger, N.; Peterlik, H.; Paris, O. A reconsideration of the relationship between the crystallite size La of carbons determined by X-ray diffraction and Raman spectroscopy. Carbon 2006, 44, 3239–3246. [Google Scholar] [CrossRef]
- Johnson, C.A.; Patrick, J.W.; Mark Thomas, K. Characterization of coal chars by Raman spectroscopy, X-ray diffraction and reflectance measurements. Fuel 1986, 65, 1284–1290. [Google Scholar] [CrossRef]
- Lu, L.; Sahajwalla, V.; Harris, D. Characteristics of chars prepared from various pulverized coals at different temperatures using drop-tube furnace. Energy Fuels 2000, 14, 869–876. [Google Scholar] [CrossRef]
- Sharma, A.; Kyotani, T.; Tomita, A. Quantitative evaluation of structural transformations in raw coals on heat-treatment using HRTEM technique. Fuel 2001, 80, 1467–1473. [Google Scholar] [CrossRef]
- Feng, B.; Bhatia, S.K.; Barry, J.C. Structural ordering of coal char during heat treatment and its impact on reactivity. Carbon 2002, 40, 481–496. [Google Scholar] [CrossRef]
- Feng, B.; Bhatia, S.K.; Barry, J.C. Variation of the Crystalline Structure of Coal Char during Gasification. Energy Fuels 2003, 17, 744–754. [Google Scholar] [CrossRef]
- Matsuoka, K.; Akahane, T.; Aso, H.; Sharma, A.; Tomita, A. The size of polyaromatic layer of coal char estimated from elemental analysis data. Fuel 2008, 87, 539–545. [Google Scholar] [CrossRef]
- Everson, R.C.; Okolo, G.N.; Neomagus, H.W.J.P.; Dos Santos, J.M. X-ray diffraction parameters and reaction rate modeling for gasification and combustion of chars derived from inertinite-rich coals. Fuel 2013, 109, 148–156. [Google Scholar] [CrossRef]
- Morga, R.; Jelonek, I.; Kruszewska, K.; Szulik, W. Relationships between quality of coals, resulting cokes, and micro-Raman spectral characteristics of these cokes. Int. J. Coal Geol. 2015, 144, 130–137. [Google Scholar] [CrossRef]
- Yu, J.; Lucas, J.A.; Wall, T.F. Formation of the structure of chars during devolatilization of pulverized coal and its thermoproperties: A review. Prog. Energy Combust. Sci. 2007, 33, 135–170. [Google Scholar] [CrossRef]
- Sýkorová, I.; Pickel, W.; Christanis, K.; Wolf, M.; Taylor, G.H.; Flores, D. Classification of huminite—ICCP System 1994. Int. J. Coal Geol. 2005, 62, 85–106. [Google Scholar] [CrossRef]
- Russell, N.J. Gelification of Victorian tertiary soft brown coal wood. I. Relationship between chemical composition and microscopic appearance and variation in the degree of gelification. Int. J. Coal Geol. 1984, 4, 99–118. [Google Scholar] [CrossRef]
- Wagner, M. The character of the IR absorption in the spectral rang 1700–1500 cm−1 of some macerals of the huminite group of brown coal. Bull. L’Academie Pol. Des Sci. 1981, 29, 321–330. [Google Scholar]
- Drobniak, A.; Mastalerz, M. Chemical evolution of Miocene wood: Example from the Belchatow brown coal deposit, central Poland. Int. J. Coal Geol. 2006, 66, 157–178. [Google Scholar] [CrossRef]
- Mastalerz, M.; Hower, J.C.; Taulbee, D.N. Variations in chemistry of macerals as refl ected by micro-scale analysis of a Spanish coal. Geol. Acta 2013, 11, 483–493. [Google Scholar] [CrossRef]
- Kalaitzidis, S.; Georgakopoulos, A.; Christanis, K.; Iordanidis, A. Early coalification features as approached by solid state 13C CP/MAS NMR spectroscopy. Geochim. Cosmochim. Acta 2006, 70, 947–959. [Google Scholar] [CrossRef]
- Taylor, G.H.; Teichmuller, M.; Davis, A.; Diessel, C.F.K.; Littke, R.; Robert, P. Organic Petrology: A New Handbook Incorporating Some Revised Parts of Stach’s Textbook of Coal Petrology; Gebrüder Borntraeger: Berlin, Germany, 1998; ISBN 9783443010362. [Google Scholar]
- Sontag, E.; Süss, M. Beispiele petrologischer Untersuchungen zur Klärung rohstoffabhängiger verfahrenstechnischer Probleme der Braunkphlenveredlung. Bergbautechnik 1969, 19, 255–260, 376–381. [Google Scholar]
- Jones, R.B.B.; McCourt, C.B.B.; Morley, C.; King, K. Maceral and rank influences on the morphology of coal char. Fuel 1985, 64, 1460–1467. [Google Scholar] [CrossRef]
- Bailey, J.G.G.; Tate, A.; Diessel, C.F.K.F.K.; Wall, T.F.F. A char morphology system with applications to coal combustion. Fuel 1990, 69, 225–239. [Google Scholar] [CrossRef]
- Maroto-Valer, M.M.; Taulbee, D.N.; Andrésen, J.M.; Hower, J.C.; Snape, C.E. The Role of Semifusinite in Plasticity Development for a Coking Coal. Energy Fuels 1998, 12, 1040–1046. [Google Scholar] [CrossRef]



| Parameter | Norm | Lignite |
|---|---|---|
| As received | ||
| Total moisture content Mtar [%] | ISO 589:2008 [51] | 50.3 |
| Ash content Aar [%] | ISO 1171:2010 [52] | 11.6 |
| Total sulfur content Star [%] | ISO 19579:2006 [53] | 0.46 |
| Net Calorific Value NCVar [kJ/kg] | ISO 1928:2020 [54] | 8159 |
| Air-dried | ||
| Moisture content Mad [%] | ISO 589:2008 [51] | 8.5 |
| Ash content Aad [%] | ISO 1171:2010 [52] | 20.1 |
| Volatile matter content Vad [%] | ISO 562:2010 [55] | 41.13 |
| Net Calorific Value NCVad [kJ/kg] | ISO 1928:2020 [54] | 17,075 |
| Carbon content Ctad [%] | ISO 29541:2010 [56] | 46.40 |
| Hydrogen content Htad [%] | ISO 29541:2010 [56] | 3.75 |
| Nitrogen content Ntad [%] | ISO 29541:2010 [56] | 0.52 |
| Total sulphur content Stad [%] | ISO 19579:2006 [53] | 0.84 |
| Dry basis | ||
| Ash content Adb [%] | ISO 1171:2010 [52] | 23.2 |
| Total sulphur content Stdb [%] | ISO 19579:2006 [53] | 0.92 |
| Volatile matter content Vdaf [%] | ISO 562:2010 [55] | 58.59 |
| Gross Calorific Value GCVdaf [kJ/kg] | ISO 1928:2020 [54] | 18,099 |
| Component | Content [% vol.] | ||
|---|---|---|---|
| Macerals | Huminite | Textinite | 11.7 |
| Ulminite | 12.1 | ||
| Attrinite | 29.9 | ||
| Densinite | 23.9 | ||
| Corpohuminite | 1.6 | ||
| Gelinite | 1.5 | ||
| Liptinite | Sporinite | 0.6 | |
| Cutinite | 0.1 | ||
| Resinite | 1.1 | ||
| Suberinite | 0.2 | ||
| Alginite | 0.1 | ||
| Liptodetrinite | 3.6 | ||
| Inertinite | Fusinite | 1.0 | |
| Semifusinite | 0.2 | ||
| Funginite | 0.1 | ||
| Micrinite | 0.0 | ||
| Inertodetrinite | 1.8 | ||
| Minerals | Pyrite | 0.8 | |
| Carbonates | 0.5 | ||
| Quartz + Clays | 9.2 |
| Parameter | Value |
|---|---|
| Gasification temperature [°C] | 856–952 |
| Carbon flow rate [kg/h] | 83–130 |
| Air flow rate [m3/h] [m3/h] | 40–120 |
| CO2 flow rate [m3/h] | 0–65 |
| O2 flow rate [m3/h] | 0–19 |
| Hydrogen [% vol.] | 9.1–13.2 |
| Methane [% vol.] | 2.3–4.6 |
| Carbon monoxide [% vol.] | 12.3–20.1 |
| Parameter | Norm | Char |
|---|---|---|
| As received | ||
| Total moisture content Mtar [%] | ISO 589:2008 [51] | 0.4 |
| Ash content Aar [%] | ISO 1171:2010 [52] | 29.1 |
| Total sulphur content Star [%] | ISO 19579:2006 [53] | 1.18 |
| Net Calorific Value NCVar [kJ/kg] | ISO 1928:2020 [54] | 22,337 |
| Air-dried | ||
| Moisture content Mad [%] | ISO 589:2008 [51] | 1.6 |
| Ash content Aad [%] | ISO 1171:2010 [52] | 28.7 |
| Volatile matter content Vad [%] | ISO 562:2010 [55] | 7.8 |
| Net Calorific Value NCVad [kJ/kg] | ISO 1928:2020 [54] | 22,039 |
| Carbon content Ctad [%] | ISO 29541:2010 [56] | 64.7 |
| Hydrogen content Htad [%] | ISO 29541:2010 [56] | 0.88 |
| Nitrogen content Ntad [%] | ISO 29541:2010 [56] | 1.01 |
| Total sulphur content Stad [%] | ISO 19579:2006 [53] | 1.17 |
| Dry basis | ||
| Ash content Adb [%] | ISO 1171:2010 [52] | 29.2 |
| Total sulphur content Stdb [%] | ISO 19579:2006 [53] | 1.19 |
| Volatile matter content Vdaf [%] | ISO 562:2010 [55] | 8.0 |
| Gross Calorific Value GCVdaf [kJ/kg] | ISO 1928:2020 [54] | 22,270 |
| Component | Content [% vol.] |
|---|---|
| Tenuisphere | 1.92 |
| Crassisphere | 1.92 |
| Tenuinetwork | 12.64 |
| Crassinetwork | 31.59 |
| Mixed Porous | 4.26 |
| Mixed Dense | 2.34 |
| Inertoid | 35.44 |
| Fusinoid/Solid | 1.79 |
| Mineroid | 8.10 |
| Morphotype | D2 ω [cm−1] | G ω [cm−1] | G FWHM [cm−1] | D3 ω [cm−1] | D1 ω [cm−1] | D1 FWHM [cm−1] | D4 ω [cm−1] |
|---|---|---|---|---|---|---|---|
| Tn | 1611.4 (2.2) | 1584.6 (2.8) | 79.3 (5.5) | 1510.6 (11.1) | 1340.4 (2.7) | 200.3 (11.6) | 1200.3 (12.0) |
| Cn | 1612.2 (2.2) | 1585.9 (3.4) | 80.2 (5.8) | 1509.9 (15.0) | 1344.3 (2.7) | 201.2 (8.2) | 1202.4 (10.5) |
| Ind | 1612.3 (1.9) | 1585.8 (3.3) | 75.0 (4.5) | 1523.4 (7.8) | 1341.3 (3.8) | 207.9 (11.3) | 1188.3 (9.0) |
| Fd | 1613.6 (1.1) | 1588.4 (1.7) | 71.3 (4.0) | 1525.1 (6.5) | 1341.1 (1.1) | 195.1 (10.9) | 1179.8 (7.9) |
| Morphotype | ID1/IG | AD3/AALL | AD1/AALL | AD4/AALL |
|---|---|---|---|---|
| Tn | 1.83 (0.21) | 0.09 (0.02) | 0.58 (0.05) | 0.09 (0.03) |
| Cn | 1.85 (0.26) | 0.10 (0.02) | 0.56 (0.05) | 0.10 (0.03) |
| Ind | 2.08 (0.18) | 0.10 (0.01) | 0.64 (0.04) | 0.04 (0.03) |
| Fd | 1.99 (0.12) | 0.09 (0.01) | 0.64 (0.02) | 0.05 (0.01) |
| Morphotype | G Band Position | |||
|---|---|---|---|---|
| Fusinoid | Inertoid | Tenuinetwork | Crassinetwork | |
| Fusinoid | 0.02234 | 0.00042 | 0.03551 | |
| Inertoid | 0.48539 | 0.99801 | ||
| Tenuinetwork | 0.14469 | |||
| Crassinetwork | ||||
| Morphotype | G Band FWHM | |||
|---|---|---|---|---|
| Fusinoid | Inertoid | Tenuinetwork | Crassinetwork | |
| Fusinoid | 0.07111 | 0.00014 | 0.00014 | |
| Inertoid | 0.01064 | 0.00229 | ||
| Tenuinetwork | 0.92636 | |||
| Crassinetwork | ||||
| Morphotype | ID1/IG Ratio | |||
|---|---|---|---|---|
| Fusinoid | Inertoid | Tenuinetwork | Crassinetwork | |
| Fusinoid | 0.49077 | 0.02942 | 0.07602 | |
| Inertoid | 0.00021 | 0.00073 | ||
| Tenuinetwork | 0.97940 | |||
| Crassinetwork | ||||
| Morphotype | AD1/AALL Ratio | |||
|---|---|---|---|---|
| Fusinoid | Inertoid | Tenuinetwork | Crassinetwork | |
| Fusinoid | 0.99982 | 0.00015 | 0.00014 | |
| Inertoid | 0.00014 | 0.00014 | ||
| Tenuinetwork | 0.39791 | |||
| Crassinetwork | ||||
| Morphotype | AD4/AALL Ratio | |||
|---|---|---|---|---|
| Fusinoid | Inertoid | Tenuinetwork | Crassinetwork | |
| Fusinoid | 0.62918 | 0.00031 | 0.00014 | |
| Inertoid | 0.00014 | 0.00014 | ||
| Tenuinetwork | 0.47441 | |||
| Crassinetwork | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morga, R.; Bielowicz, B. Raman Spectroscopy of Lignite Gasification Char Morphotypes. Energies 2022, 15, 6057. https://doi.org/10.3390/en15166057
Morga R, Bielowicz B. Raman Spectroscopy of Lignite Gasification Char Morphotypes. Energies. 2022; 15(16):6057. https://doi.org/10.3390/en15166057
Chicago/Turabian StyleMorga, Rafał, and Barbara Bielowicz. 2022. "Raman Spectroscopy of Lignite Gasification Char Morphotypes" Energies 15, no. 16: 6057. https://doi.org/10.3390/en15166057
APA StyleMorga, R., & Bielowicz, B. (2022). Raman Spectroscopy of Lignite Gasification Char Morphotypes. Energies, 15(16), 6057. https://doi.org/10.3390/en15166057






