Production and Characterization of Bacterial Cellulose Separators for Nickel-Zinc Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Manufacturing of BC Separator
2.1.1. BC Production
2.1.2. Separator Production
2.2. Separator Characterization
2.2.1. Dimensional Characteristics and Changes upon Electrolyte Uptake
2.2.2. Hydroxide and Zincate Diffusion
2.2.3. XRD Analysis
2.3. Ni-Zn Battery
2.3.1. Cathode Production
2.3.2. Battery Assembly and Electrochemical Cycling
3. Results and Discussion
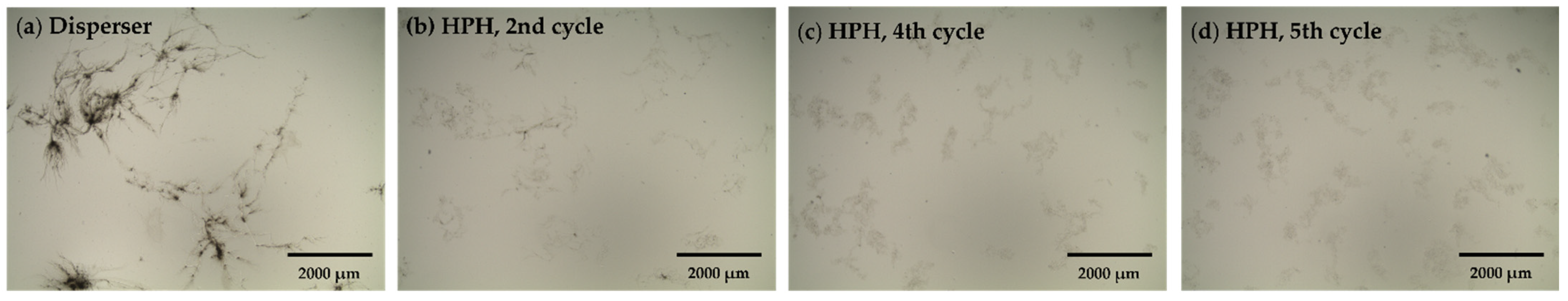
3.1. BC Comminution and the Effect on BC Crystallinity
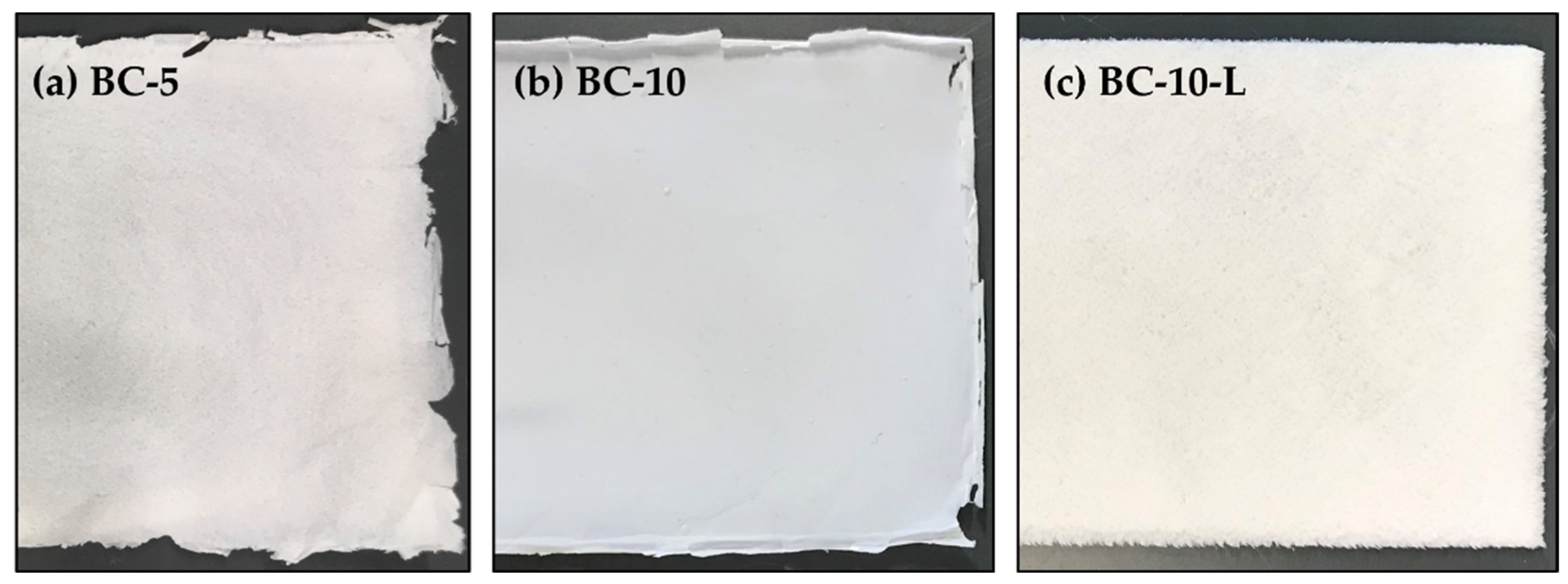
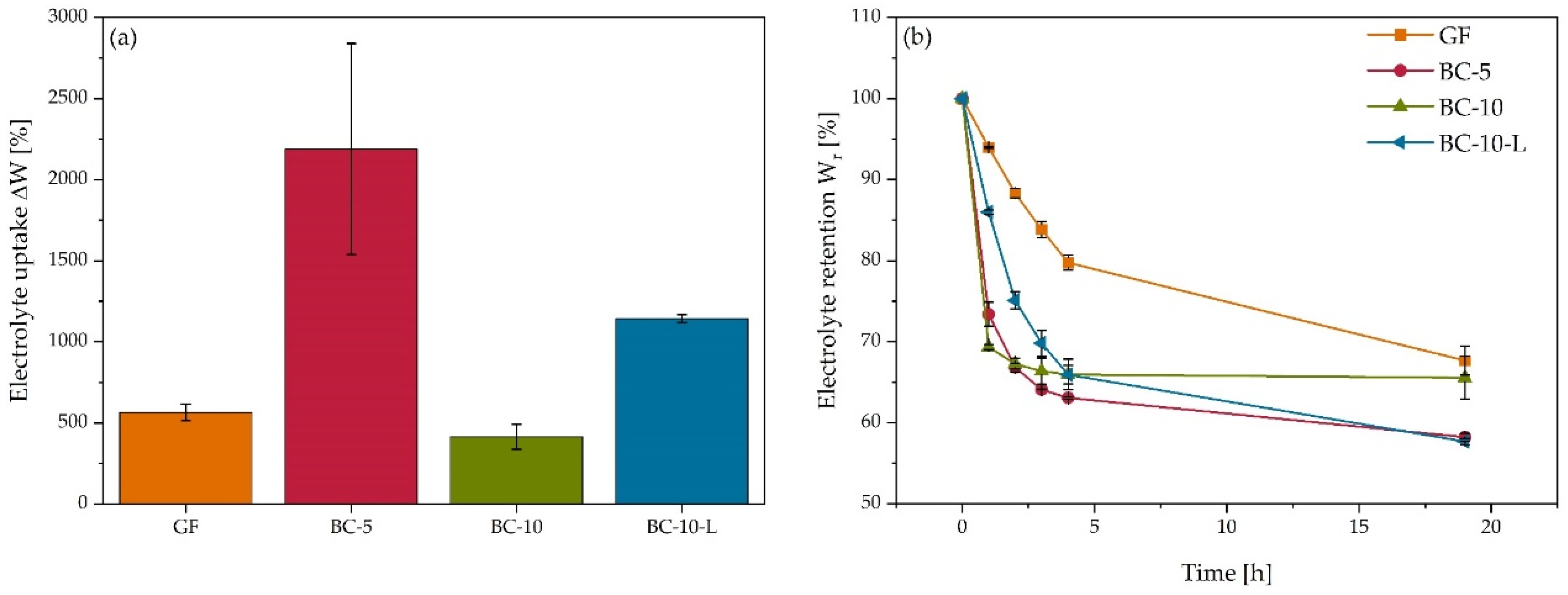
3.2. Dimensional Properties and Interactions with the Electrolyte
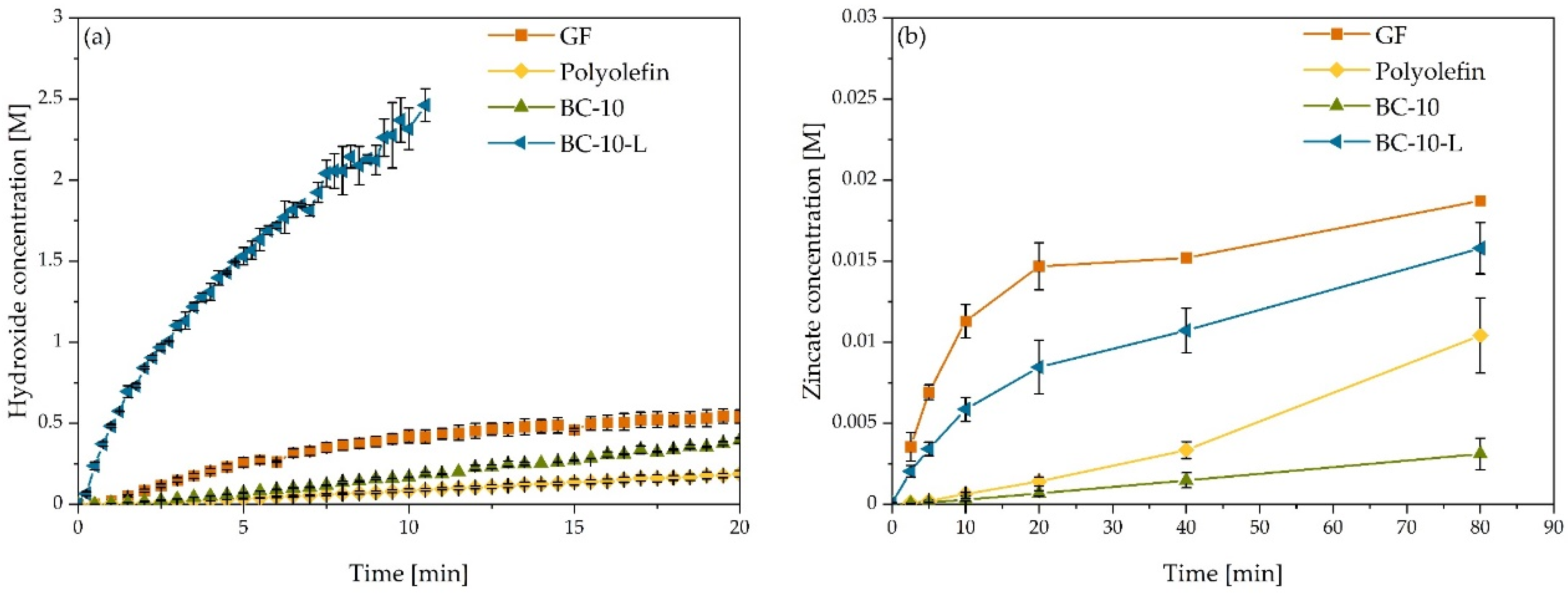
3.3. Seperator Permeability for Hydroxide and Zincate Ions
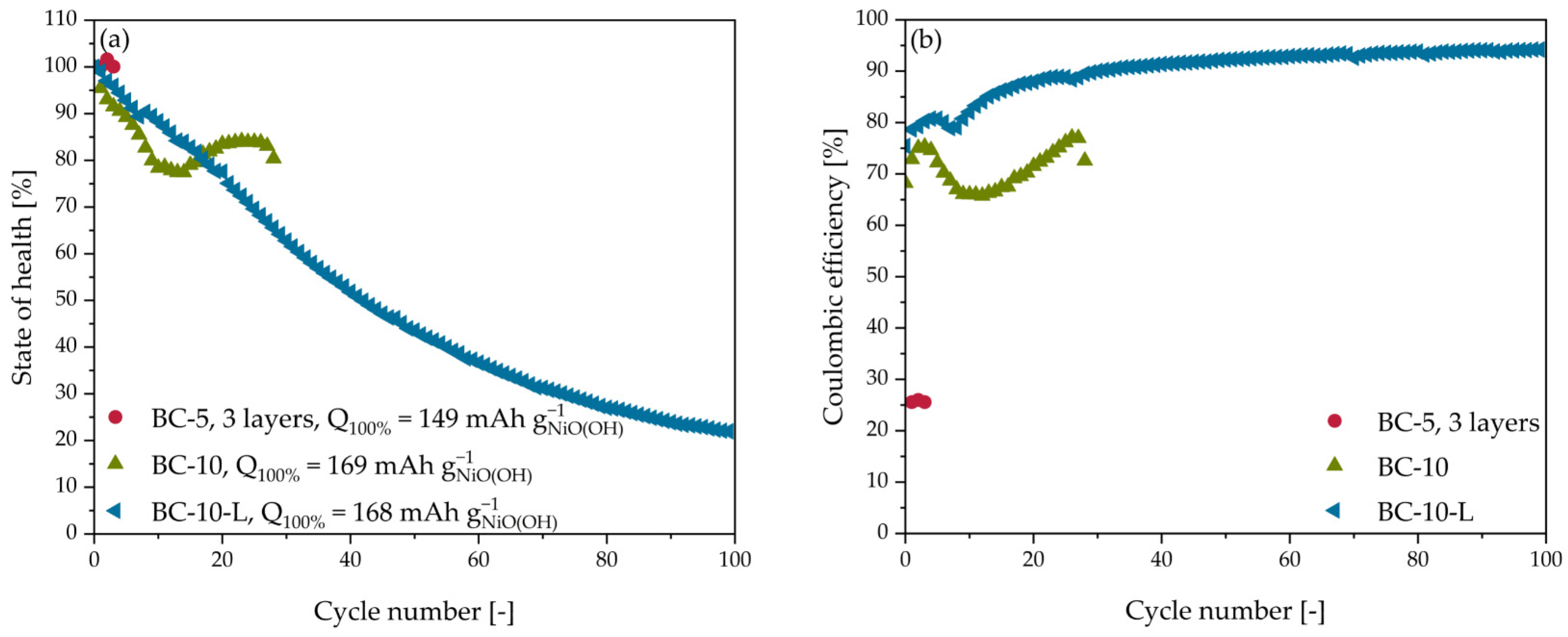
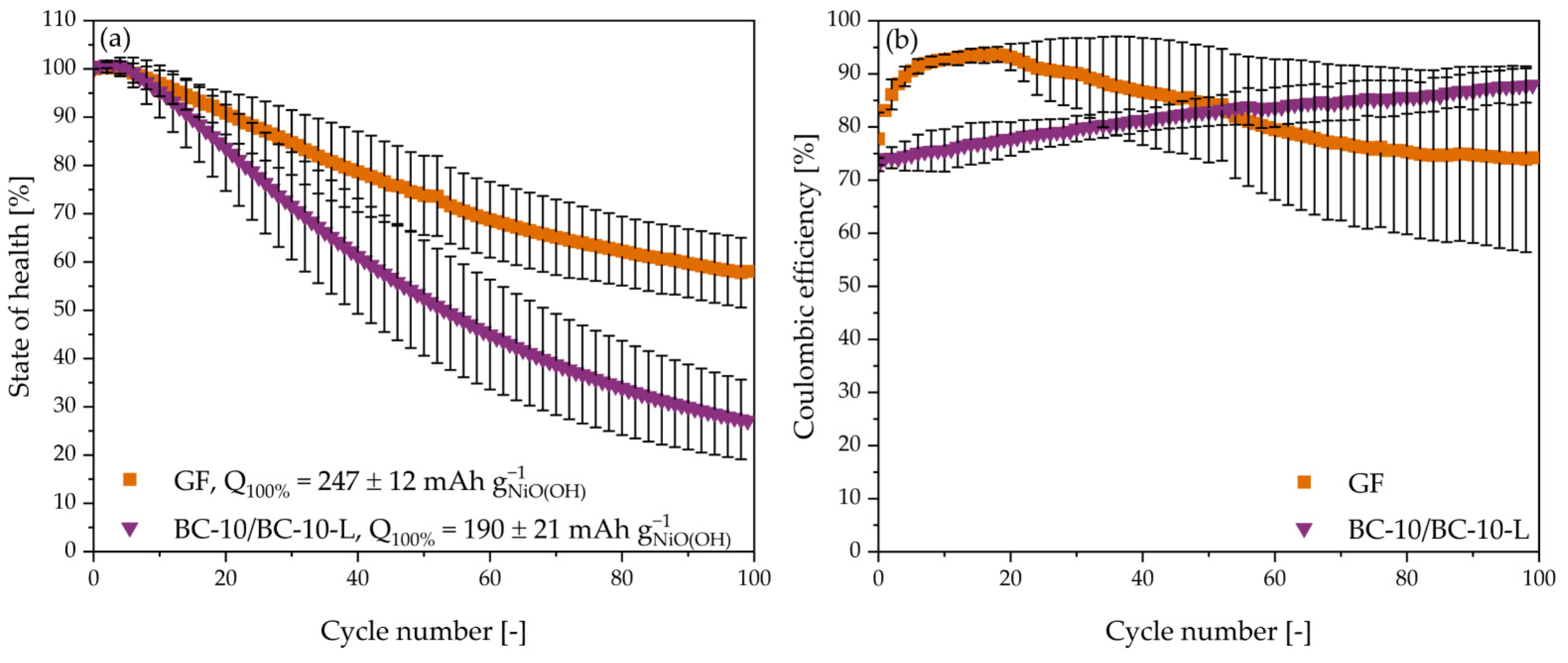
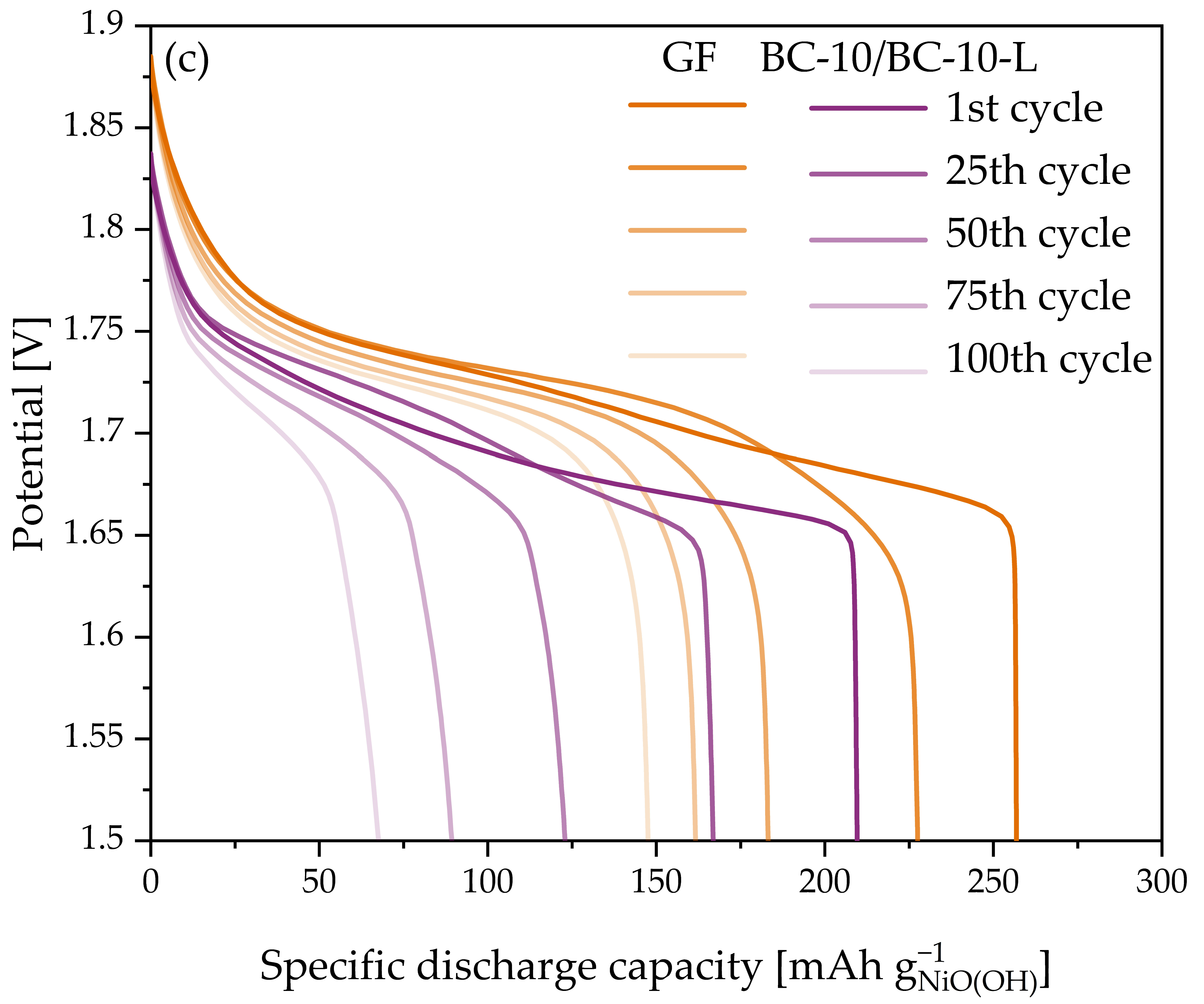
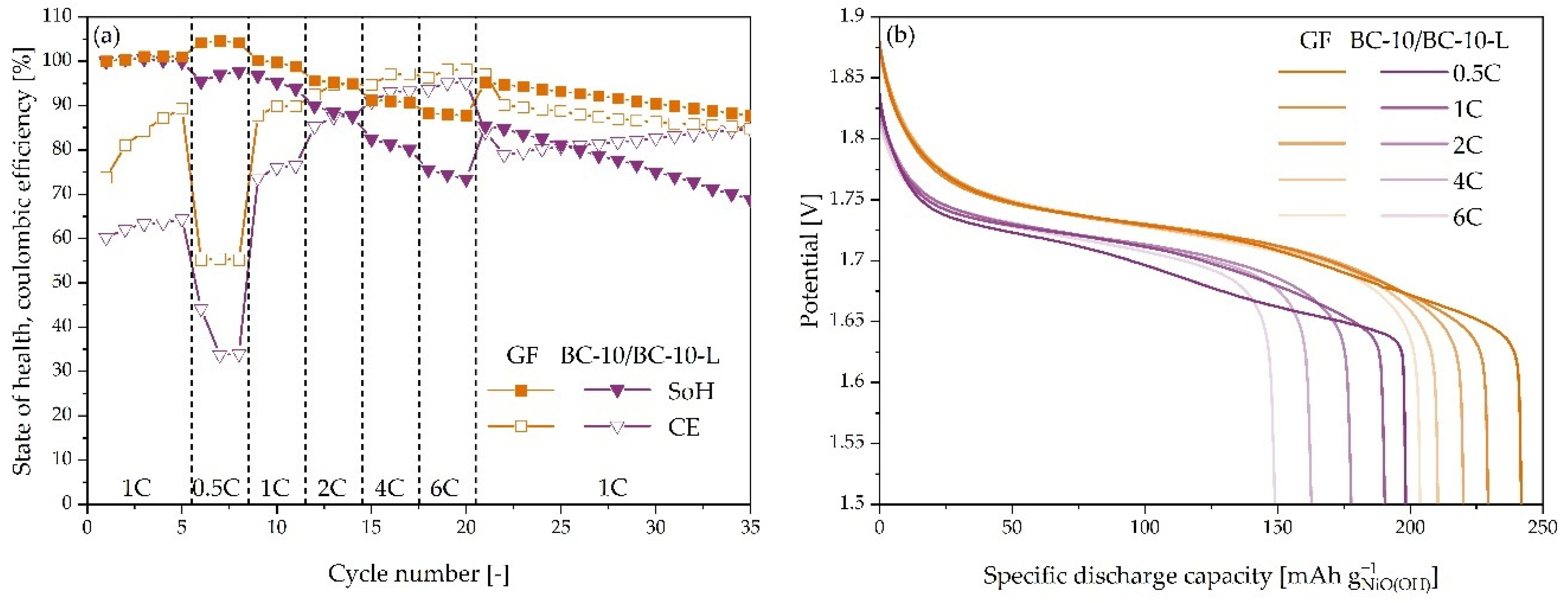
3.4. Ni-Zn Battery Cycling
3.5. C-Rate Dependence
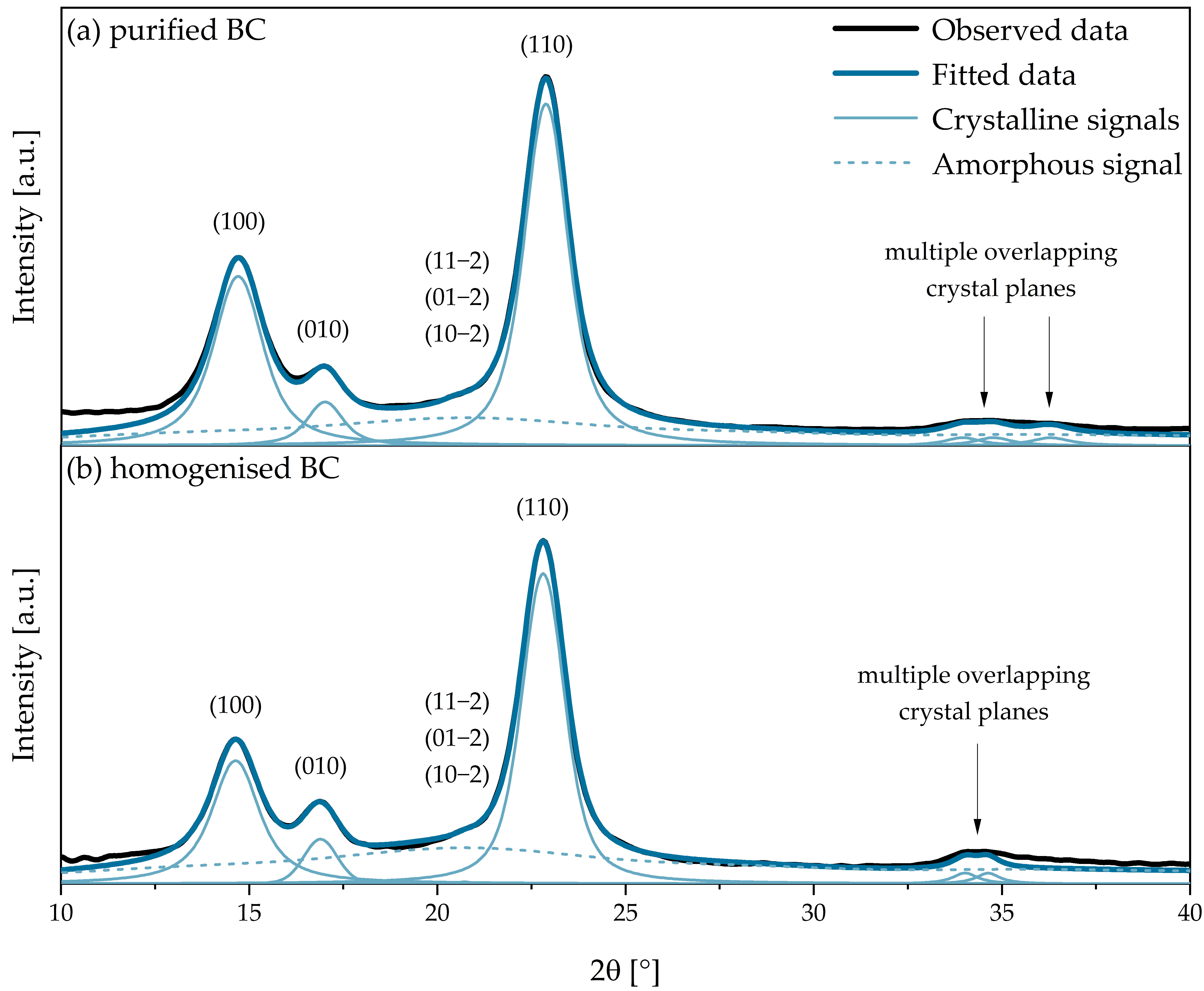
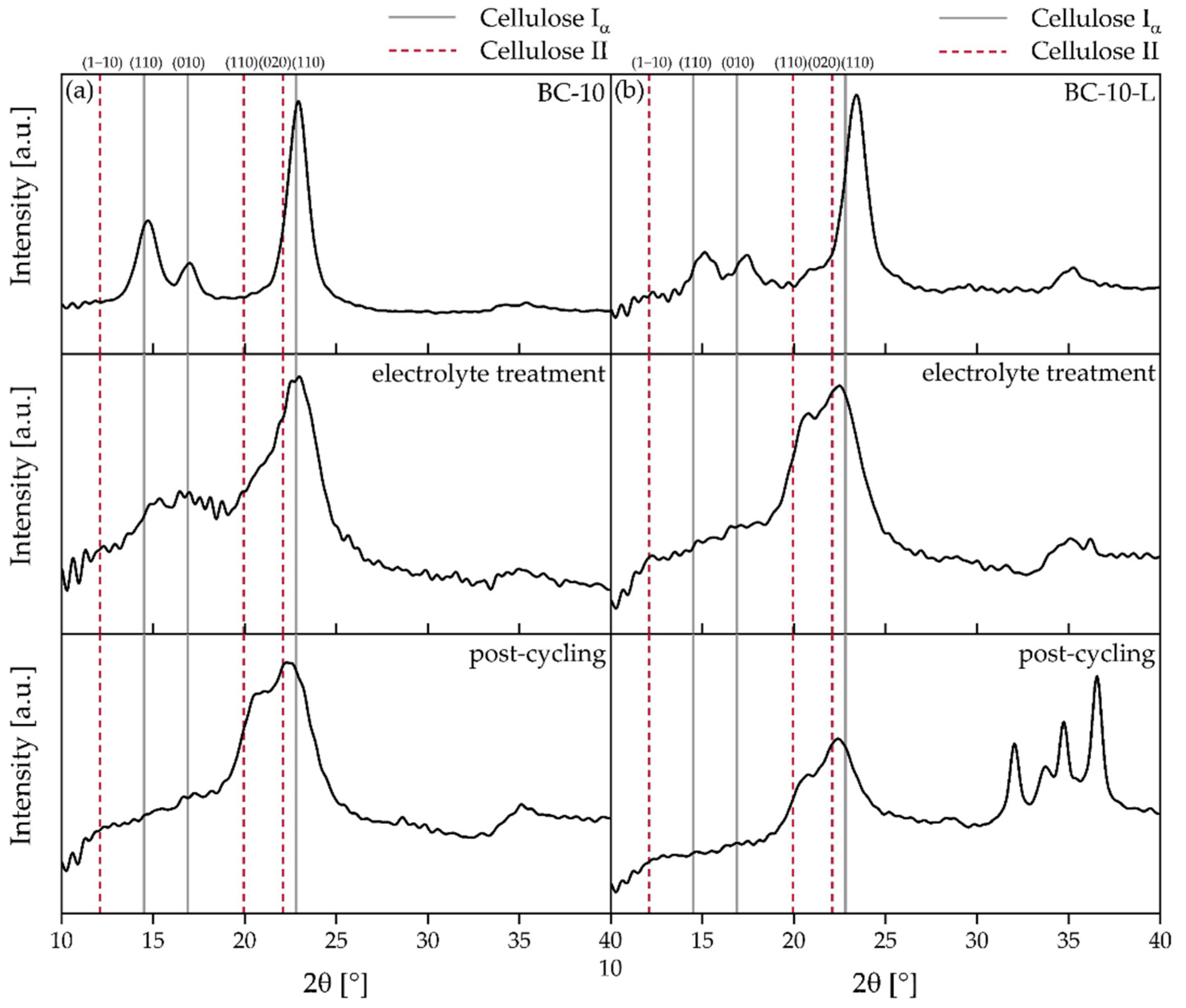
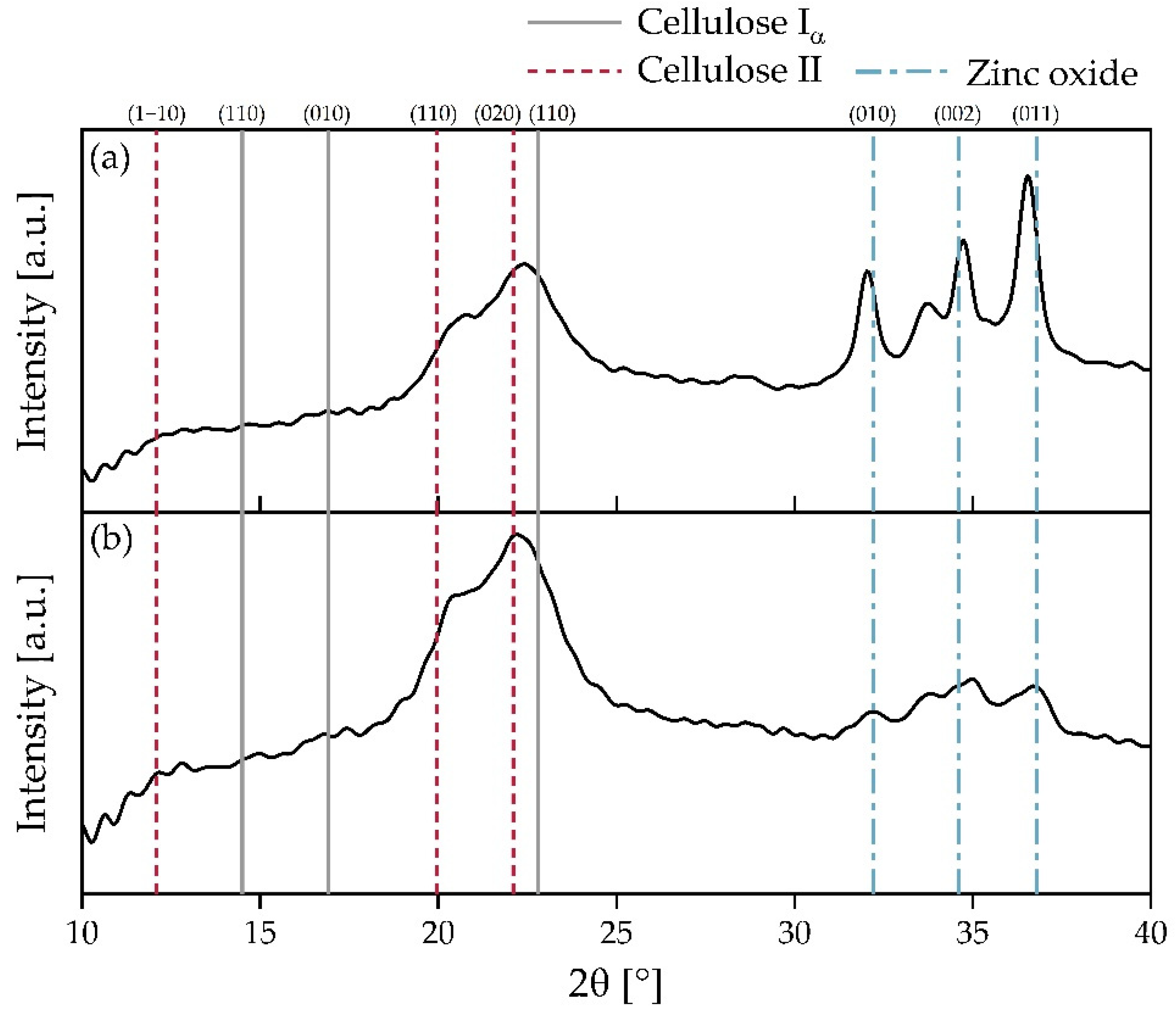
3.6. XRD Analysis of BC Separators
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, J.F.; Chervin, C.N.; Pala, I.R.; Machler, M.; Burz, M.F.; Long, J.W.; Rolison, D.R. Rechargeable Nickel–3D Zinc Batteries: An Energy-Dense, Safer Alternative to Lithium-Ion. Science 2017, 356, 415–418. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.F.; Ko, J.S.; Rolison, D.R.; Long, J.W. Translating Materials-Level Performance into Device-Relevant Metrics for Zinc-Based Batteries. Joule 2018, 2, 2519–2527. [Google Scholar] [CrossRef]
- Fu, J.; Cano, Z.P.; Park, M.G.; Yu, A.; Fowler, M.; Chen, Z. Electrically Rechargeable Zinc-Air Batteries: Progress, Challenges, and Perspectives. Adv. Mater. 2017, 29, 1604685. [Google Scholar] [CrossRef] [PubMed]
- Sabihuddin, S.; Kiprakis, A.; Mueller, M. A Numerical and Graphical Review of Energy Storage Technologies. Energies 2014, 8, 172–216. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Chervin, C.N.; Long, J.W.; Rolison, D.R.; Parker, J.F. Projecting the Specific Energy of Rechargeable Zinc–Air Batteries. ACS Energy Lett. 2020, 5, 3405–3408. [Google Scholar] [CrossRef]
- Kwak, W.-J.; Rosy; Sharon, D.; Xia, C.; Kim, H.; Johnson, L.R.; Bruce, P.G.; Nazar, L.F.; Sun, Y.-K.; Frimer, A.A.; et al. Lithium–Oxygen Batteries and Related Systems: Potential, Status, and Future. Chem. Rev. 2020, 120, 6626–6683. [Google Scholar] [CrossRef]
- Titscher, P.; Riede, J.-C.; Wiedemann, J.; Kunz, U.; Kwade, A. Multiscale Structured Particle-Based Zinc Anodes in Non-Stirred Alkaline Systems for Zinc-Air Batteries. Energy Technol. 2018, 6, 773–780. [Google Scholar] [CrossRef]
- Stock, D.; Dongmo, S.; Damtew, D.; Stumpp, M.; Konovalova, A.; Henkensmeier, D.; Schlettwein, D.; Schröder, D. Design Strategy for Zinc Anodes with Enhanced Utilization and Retention: Electrodeposited Zinc Oxide on Carbon Mesh Protected by Ionomeric Layers. ACS Appl. Energy Mater. 2018, 1, 5579–5588. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, Y.; Zhang, W.; Xie, G.; Li, Y.; Shen, Q.; Yu, X. Electrochemical Properties of Zn–Al–La Layered Double Hydroxides in Zn–Ni Secondary Batteries. ACS Omega 2021, 6, 35244–35249. [Google Scholar] [CrossRef]
- Fan, X.; Yang, Z.; Long, W.; Zhao, Z.; Yang, B. The Preparation and Electrochemical Performance of In(OH)3-Coated Zn-Al-Hydrotalcite as Anode Material for Zn–Ni Secondary Cell. Electrochim. Acta 2013, 92, 365–370. [Google Scholar] [CrossRef]
- Lim, M.B.; Lambert, T.N.; Ruiz, E.I. Effect of ZnO-Saturated Electrolyte on Rechargeable Alkaline Zinc Batteries at Increased Depth-of-Discharge. J. Electrochem. Soc. 2020, 167, 060508. [Google Scholar] [CrossRef]
- Jain, R.; Adler, T.C.; McLarnon, F.R.; Cairns, E.J. Development of Long-Lived High-Performance Zinc-Calcium/Nickel Oxide Cells. J. Appl. Electrochem. 1992, 22, 1039–1048. [Google Scholar] [CrossRef]
- Wang, N.; Wan, H.; Duan, J.; Wang, X.; Tao, L.; Zhang, J.; Wang, H. A Review of Zinc-Based Battery from Alkaline to Acid. Mater. Today Adv. 2021, 11, 100149. [Google Scholar] [CrossRef]
- Sun, W.; Wang, F.; Zhang, B.; Zhang, M.; Küpers, V.; Ji, X.; Theile, C.; Bieker, P.; Xu, K.; Wang, C.; et al. A Rechargeable Zinc-Air Battery Based on Zinc Peroxide Chemistry. Science 2021, 371, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Cui, S.; Xing, X.; Liu, H.; Yue, X.; Petrova, V.; Lim, H.D.; Chen, R.; Liu, P. Dendrite Suppression Membranes for Rechargeable Zinc Batteries. ACS Appl. Mater. Interfaces 2018, 10, 38928–38935. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Xie, C.; Zhang, H.; Li, X. Inhibition of Zinc Dendrite Growth in Zinc-Based Batteries. ChemSusChem 2018, 11, 3996–4006. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Fan, L.; Shuai, Y.; Zhang, N. Ultrathin and Super-Tough Membrane for Anti-Dendrite Separator in Aqueous Zinc-Ion Batteries. Cell Rep. Phys. Sci. 2022, 3, 100824. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Zhang, X.; Sawangphruk, M.; Qin, J.; Liu, R. A Universal and Facile Approach to Suppress Dendrite Formation for a Zn and Li Metal Anode. J. Mater. Chem. A 2020, 8, 9331–9344. [Google Scholar] [CrossRef]
- Hu, J.; Yue, M.; Zhang, H.; Yuan, Z.; Li, X. A Boron Nitride Nanosheets Composite Membrane for a Long-Life Zinc-Based Flow Battery. Angew. Chem. Int. Ed. 2020, 59, 6715–6719. [Google Scholar] [CrossRef]
- Yuan, Z.; Liu, X.; Xu, W.; Duan, Y.; Zhang, H.; Li, X. Negatively Charged Nanoporous Membrane for a Dendrite-Free Alkaline Zinc-Based Flow Battery with Long Cycle Life. Nat. Commun. 2018, 9, 3731. [Google Scholar] [CrossRef]
- Lee, H.-J.; Lim, J.-M.; Kim, H.-W.; Jeong, S.-H.; Eom, S.-W.; Hong, Y.T.; Lee, S.-Y. Electrospun Polyetherimide Nanofiber Mat-Reinforced, Permselective Polyvinyl Alcohol Composite Separator Membranes: A Membrane-Driven Step Closer toward Rechargeable Zinc–Air Batteries. J. Memb. Sci. 2016, 499, 526–537. [Google Scholar] [CrossRef]
- Kolesnichenko, I.V.; Arnot, D.J.; Lim, M.B.; Yadav, G.G.; Nyce, M.; Huang, J.; Banerjee, S.; Lambert, T.N. Zincate-Blocking-Functionalized Polysulfone Separators for Secondary Zn–MnO2 Batteries. ACS Appl. Mater. Interfaces 2020, 12, 50406–50417. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yadav, G.G.; Gallaway, J.W.; Wei, X.; Nyce, M.; Banerjee, S. A Calcium Hydroxide Interlayer as a Selective Separator for Rechargeable Alkaline Zn/MnO2 Batteries. Electrochem. Commun. 2017, 81, 136–140. [Google Scholar] [CrossRef]
- Lu, K.; Jiang, T.; Hu, H.; Wu, M. Hydrogel Electrolytes for Quasi-Solid Zinc-Based Batteries. Front. Chem. 2020, 8, 546728. [Google Scholar] [CrossRef]
- Lorca, S.; Santos, F.; Fernández Romero, A.J. A Review of the Use of Gpes in Zinc-Based Batteries. A Step Closer to Wearable Electronic Gadgets and Smart Textiles. Polymers 2020, 12, 2812. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, N.; Zeng, S.; Zhou, S.; Chen, M.; Di, J.; Li, Q. An Adaptive and Stable Bio-Electrolyte for Rechargeable Zn-Ion Batteries. J. Mater. Chem. A 2018, 6, 12237–12243. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Li, X.; Alfred, M.; Li, D.; Huang, F.; Wei, Q. Bacterial Cellulose Hydrogel: A Promising Electrolyte for Flexible Zinc-Air Batteries. J. Power Sources 2021, 482, 228963. [Google Scholar] [CrossRef]
- Zhao, N.; Wu, F.; Xing, Y.; Qu, W.; Chen, N.; Shang, Y.; Yan, M.; Li, Y.; Li, L.; Chen, R. Flexible Hydrogel Electrolyte with Superior Mechanical Properties Based on Poly (Vinyl Alcohol) and Bacterial Cellulose for the Solid-State Zinc–Air Batteries. ACS Appl. Mater. Interfaces 2019, 11, 15537–15542. [Google Scholar] [CrossRef]
- Hopkins, B.J.; Chervin, C.N.; Sassin, M.B.; Long, J.W.; Rolison, D.R.; Parker, J.F. Low-Cost Green Synthesis of Zinc Sponge for Rechargeable, Sustainable Batteries. Sustain. Energy Fuels 2020, 4, 3363–3369. [Google Scholar] [CrossRef]
- Roh, S.H.; Palanisamy, G.; Sadhasivam, T.; Jin, J.E.; Shim, J.Y.; Jung, H.Y. Techno-Economical Feasibility of Biocellulose Membrane along with Polyethylene Film as a Separator for Lead-Acid Batteries. ACS Sustain. Chem. Eng. 2019, 7, 8789–8797. [Google Scholar] [CrossRef]
- Gwon, H.; Park, K.; Chung, S.C.; Kim, R.H.; Kang, J.K.; Ji, S.M.; Kim, N.J.; Lee, S.; Ku, J.H.; Do, E.C.; et al. A Safe and Sustainable Bacterial Cellulose Nanofiber Separator for Lithium Rechargeable Batteries. Proc. Natl. Acad. Sci. USA 2019, 116, 19288–19293. [Google Scholar] [CrossRef]
- Sperotto, G.; Stasiak, L.G.; Godoi, J.P.M.G.; Gabiatti, N.C.; De Souza, S.S. A Review of Culture Media for Bacterial Cellulose Production: Complex, Chemically Defined and Minimal Media Modulations. Cellulose 2021, 28, 2649–2673. [Google Scholar] [CrossRef]
- Huang, C.; Ji, H.; Guo, B.; Luo, L.; Xu, W.; Li, J.; Xu, J. Composite Nanofiber Membranes of Bacterial Cellulose/Halloysite Nanotubes as Lithium Ion Battery Separators. Cellulose 2019, 26, 6669–6681. [Google Scholar] [CrossRef]
- Lewis, H.; Grun, C.; Salkind, A. Cellulosic Separator Applications: New and Improved Separators for Alkaline Rechargeable Cells. J. Power Sources 1997, 65, 29–38. [Google Scholar] [CrossRef]
- Coates, D.; Charkey, A. Electrical Characterization Testing of Sealed Nickel-Zinc Batteries. In Proceedings of the IECEC-97 Proceedings of the Thirty-Second Intersociety Energy Conversion Engineering Conference (Cat. No.97CH6203), Honolulu, HI, USA, 27 July–1 August 1997; IEEE: New York City, NY, USA, 1997; Volume 2, pp. 857–862. [Google Scholar] [CrossRef]
- Arnot, D.J.; Lim, M.B.; Bell, N.S.; Schorr, N.B.; Hill, R.C.; Meyer, A.; Cheng, Y.; Lambert, T.N. High Depth-of-Discharge Zinc Rechargeability Enabled by a Self-Assembled Polymeric Coating. Adv. Energy Mater. 2021, 11, 2101594. [Google Scholar] [CrossRef]
- Hestrin, S.; Schramm, M. Synthesis of Cellulose by Acetobacter xylinum. 2. Preparation of Freeze-Dried Cells Capable of Polymerizing Glucose to Cellulose. Biochem. J. 1954, 58, 345–352. [Google Scholar] [CrossRef]
- Mohite, B.V.; Kamalja, K.K.; Patil, S.V. Statistical Optimization of Culture Conditions for Enhanced Bacterial Cellulose Production by Gluconoacetobacter hansenii NCIM 2529. Cellulose 2012, 19, 1655–1666. [Google Scholar] [CrossRef]
- Song, Z.; Ding, J.; Liu, B.; Liu, X.; Han, X.; Deng, Y.; Hu, W.; Zhong, C. A Rechargeable Zn–Air Battery with High Energy Efficiency and Long Life Enabled by a Highly Water-Retentive Gel Electrolyte with Reaction Modifier. Adv. Mater. 2020, 32, 1908127. [Google Scholar] [CrossRef]
- Abbasi, A.; Hosseini, S.; Somwangthanaroj, A.; Mohamad, A.A.; Kheawhom, S. Poly (2,6-Dimethyl-1,4-Phenylene Oxide)-Based Hydroxide Exchange Separator Membranes for Zinc–Air Battery. Int. J. Mol. Sci. 2019, 20, 3678. [Google Scholar] [CrossRef]
- Fang, L.; Catchmark, J.M. Structure Characterization of Native Cellulose during Dehydration and Rehydration. Cellulose 2014, 21, 3951–3963. [Google Scholar] [CrossRef]
- Yao, W.; Weng, Y.; Catchmark, J.M. Improved Cellulose X-Ray Diffraction Analysis Using Fourier Series Modeling. Cellulose 2020, 27, 5563–5579. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugiyama, J.; Chanzy, H.; Langan, P. Crystal Structure and Hydrogen Bonding System in Cellulose Iα from Synchrotron X-Ray and Neutron Fiber Diffraction. J. Am. Chem. Soc. 2003, 125, 14300–14306. [Google Scholar] [CrossRef]
- French, A.D. Idealized Powder Diffraction Patterns for Cellulose Polymorphs. Cellulose 2014, 21, 885–896. [Google Scholar] [CrossRef]
- Wada, M.; Okano, T.; Sugiyama, J. Allomorphs of Native Crystalline Cellulose I Evaluated by Two Equatoriald-Spacings. J. Wood Sci. 2001, 47, 124–128. [Google Scholar] [CrossRef]
- Fu, G.R.; Hu, Z.A.; Xie, L.J.; Jin, X.Q.; Xie, Y.L.; Wang, Y.X.; Zhang, Z.Y.; Yang, Y.Y.; Wu, H.Y. Electrodeposition of Nickel Hydroxide Films on Nickel Foil and Its Electrochemical Performances for Supercapacitor. Int. J. Electrochem. Sci. 2009, 4, 1052–1062. [Google Scholar]
- Corrigan, D.A.; Bendert, R.M. Effect of Coprecipitated Metal Ions on the Electrochemistry of Nickel Hydroxide Thin Films: Cyclic Voltammetry in 1M KOH. J. Electrochem. Soc. 1989, 136, 723–728. [Google Scholar] [CrossRef]
- Zhao, D.; Zhou, W.; Li, H. Effects of Deposition Potential and Anneal Temperature on the Hexagonal Nanoporous Nickel Hydroxide Films. Chem. Mater. 2007, 19, 3882–3891. [Google Scholar] [CrossRef]
- Mortimer, R.J.; Sialvi, M.Z.; Varley, T.S.; Wilcox, G.D. An in Situ Colorimetric Measurement Study of Electrochromism in the Thin-Film Nickel Hydroxide/Oxyhydroxide System. J. Solid State Electrochem. 2014, 18, 3359–3367. [Google Scholar] [CrossRef]
- Streinz, C.C.; Hartman, A.P.; Motupally, S.; Weidner, J.W. The Effect of Current and Nickel Nitrate Concentration on the Deposition of Nickel Hydroxide Films. J. Electrochem. Soc. 1995, 142, 1084–1089. [Google Scholar] [CrossRef]
- Cheng, X.; Zhou, L.; Lu, Y.; Xu, W.; Zhang, P.; Lu, X. Facile Activation of Commercial Ni Foil as Robust Cathode for Advanced Rechargeable Ni-Zn Battery. Electrochim. Acta 2018, 263, 311–317. [Google Scholar] [CrossRef]
- Li, L.; Jiang, L.; Qing, Y.; Zeng, Y.; Zhang, Z.; Xiao, L.; Lu, X.; Wu, Y. Manipulating Nickel Oxides in Naturally Derived Cellulose Nanofiber Networks as Robust Cathodes for High-Performance Ni–Zn Batteries. J. Mater. Chem. A 2020, 8, 565–572. [Google Scholar] [CrossRef]
- Bonnick, P.; Dahn, J.R. A Simple Coin Cell Design for Testing Rechargeable Zinc-Air or Alkaline Battery Systems. J. Electrochem. Soc. 2012, 159, A981–A989. [Google Scholar] [CrossRef]
- Martins, D.; de Carvalho Ferreira, D.; Gama, M.; Dourado, F. Dry Bacterial Cellulose and Carboxymethyl Cellulose Formulations with Interfacial-Active Performance: Processing Conditions and Redispersion. Cellulose 2020, 27, 6505–6520. [Google Scholar] [CrossRef]
- Suryanto, H.; Muhajir, M.; Darnanto Susilo, B.; Rohmat Aji Pradana, Y.; Wahyu Wijaya, H.; Saad Ansari, A.; Yanuhar, U. Nanofibrillation of Bacterial Cellulose Using High-Pressure Homogenization and Its Films Characteristics. J. Renew. Mater. 2021, 9, 1717–1728. [Google Scholar] [CrossRef]
- Vazquez, A.; Foresti, M.L.; Cerrutti, P.; Galvagno, M. Bacterial Cellulose from Simple and Low Cost Production Media by Gluconacetobacter xylinus. J. Polym. Environ. 2013, 21, 545–554. [Google Scholar] [CrossRef]
- Czaja, W.; Romanovicz, D.; Brown, R.M. Structural Investigations of Microbial Cellulose Produced in Stationary and Agitated Culture. Cellulose 2004, 11, 403–411. [Google Scholar] [CrossRef]
- Ruan, C.; Zhu, Y.; Zhou, X.; Abidi, N.; Hu, Y.; Catchmark, J.M. Effect of Cellulose Crystallinity on Bacterial Cellulose Assembly. Cellulose 2016, 23, 3417–3427. [Google Scholar] [CrossRef]
- Faria-Tischer, P.C.S.; Tischer, C.A.; Heux, L.; Le Denmat, S.; Picart, C.; Sierakowski, M.-R.; Putaux, J.-L. Preparation of Cellulose II and IIII Films by Allomorphic Conversion of Bacterial Cellulose I Pellicles. Mater. Sci. Eng. C 2015, 51, 167–173. [Google Scholar] [CrossRef]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose Crystallinity Index: Measurement Techniques and Their Impact on Interpreting Cellulase Performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef]
- Watanabe, K.; Tabuchi, M.; Morinaga, Y.; Yoshinaga, F. Structural Features and Properties of Bacterial Cellulose Produced in Agitated Culture. Cellulose 1998, 5, 187–200. [Google Scholar] [CrossRef]
- Kaur, G.J.; Orsat, V.; Singh, A. An Overview of Different Homogenizers, Their Working Mechanisms and Impact on Processing of Fruits and Vegetables. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Horii, F.; Odani, H. Structural Changes of Native Cellulose Crystals Induced by Annealing in Aqueous Alkaline and Acidic Solutions at High Temperatures. Macromolecules 1989, 22, 4130–4132. [Google Scholar] [CrossRef]
- Shibazaki, H.; Kuga, S.; Onabe, F.; Usuda, M. Bacterial Cellulose Membrane as Separation Medium. J. Appl. Polym. Sci. 1993, 50, 965–969. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling Behaviors of pH- and Salt-Responsive Cellulose-Based Hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Fält, S.; Wågberg, L.; Vesterlind, E.-L. Swelling of Model Films of Cellulose Having Different Charge Densities and Comparison to the Swelling Behavior of Corresponding Fibers. Langmuir 2003, 19, 7895–7903. [Google Scholar] [CrossRef]
- Ioelovich, M.Y. Models of Supramolecular Structure and Properties of Cellulose. Polym. Sci. Ser. A 2016, 58, 925–943. [Google Scholar] [CrossRef]
- Ottesen, V.; Syverud, K. Swelling of Individual Cellulose Nanofibrils in Water, Role of Crystallinity: An AFM Study. Cellulose 2021, 28, 19–29. [Google Scholar] [CrossRef]
- Nakano, T. Modeling of the Morphological Change of Cellulose Microfibrils Caused with Aqueous NaOH Solution: The Longitudinal Contraction and Laterally Swelling during Decrystallization. J. Mol. Model. 2017, 23, 129. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Teklay Gebreslassie, G.; Heon Choi, N.; Milian, D.; Martin, V.; Fischer, P.; Tübke, J.; El Kissi, N.; Donten, M.L.; Alloin, F.; et al. Pristine and Modified Porous Membranes for Zinc Slurry–Air Flow Battery. Molecules 2021, 26, 4062. [Google Scholar] [CrossRef]
- EL Cell GmbH Separator ECC1-01-0012-C/L. Available online: https://el-cell.com/products/test-cells/standard-test-cells/ecc-std/#1492776437255-39005db0-359d (accessed on 11 May 2022).
- Kim, H.W.; Lim, J.M.; Lee, H.J.; Eom, S.W.; Hong, Y.T.; Lee, S.Y. Artificially Engineered, Bicontinuous Anion-Conducting/-Repelling Polymeric Phases as a Selective Ion Transport Channel for Rechargeable Zinc-Air Battery Separator Membranes. J. Mater. Chem. A 2016, 4, 3711–3720. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z. Battery Separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhang, J.; Song, X.; Zarrin, H.; Tian, X.; Qiao, J.; Rasen, L.; Li, K.; Chen, Z. A Flexible Solid-State Electrolyte for Wide-Scale Integration of Rechargeable Zinc–Air Batteries. Energy Environ. Sci. 2016, 9, 663–670. [Google Scholar] [CrossRef]
- Tsehaye, M.T.; Alloin, F.; Iojoiu, C.; Tufa, R.A.; Aili, D.; Fischer, P.; Velizarov, S. Membranes for Zinc-Air Batteries: Recent Progress, Challenges and Perspectives. J. Power Sources 2020, 475, 228689. [Google Scholar] [CrossRef]
- Konovalova, A.; Stock, D.; Schröder, S.; Park, H.S.; Jang, J.H.; Kim, H.-J.; Han, J.; Schröder, D.; Henkensmeier, D. Partially Methylated Polybenzimidazoles as Coating for Alkaline Zinc Anodes. J. Memb. Sci. 2020, 610, 118254. [Google Scholar] [CrossRef]
- Dongmo, S.; Stock, D.; Alexander Kreissl, J.J.; Groß, M.; Weixler, S.; Hagen, M.; Miyazaki, K.; Abe, T.; Schröder, D. Implications of Testing a Zinc-Oxygen Battery with Zinc Foil Anode Revealed by Operando Gas Analysis. ACS Omega 2020, 5, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, K. Observation of Water Consumption in Zn–Air Secondary Batteries. J. Electrochem. Sci. Technol. 2019, 10, 381–386. [Google Scholar] [CrossRef]
- Turney, D.E.; Gallaway, J.W.; Yadav, G.G.; Ramirez, R.; Nyce, M.; Banerjee, S.; Chen-Wiegart, Y.K.; Wang, J.; D’Ambrose, M.J.; Kolhekar, S.; et al. Rechargeable Zinc Alkaline Anodes for Long-Cycle Energy Storage. Chem. Mater. 2017, 29, 4819–4832. [Google Scholar] [CrossRef]
- Bockelmann, M.; Becker, M.; Reining, L.; Kunz, U.; Turek, T. Passivation of Zinc Anodes in Alkaline Electrolyte: Part I. Determination of the Starting Point of Passive Film Formation. J. Electrochem. Soc. 2018, 165, A3048–A3055. [Google Scholar] [CrossRef]
- Hu, P.; Wang, T.; Zhao, J.; Zhang, C.; Ma, J.; Du, H.; Wang, X.; Cui, G. Ultrafast Alkaline Ni/Zn Battery Based on Ni-Foam-Supported Ni3S2 Nanosheets. ACS Appl. Mater. Interfaces 2015, 7, 26396–26399. [Google Scholar] [CrossRef]
- Jian, Y.; Wang, D.; Huang, M.; Jia, H.-L.; Sun, J.; Song, X.; Guan, M. Facile Synthesis of Ni(OH)2/Carbon Nanofiber Composites for Improving NiZn Battery Cycling Life. ACS Sustain. Chem. Eng. 2017, 5, 6827–6834. [Google Scholar] [CrossRef]
- Li, M.; Pu, Y.; Yoo, C.G.; Gjersing, E.; Decker, S.R.; Doeppke, C.; Shollenberger, T.; Tschaplinski, T.J.; Engle, N.L.; Sykes, R.W.; et al. Study of Traits and Recalcitrance Reduction of Field-Grown COMT down-Regulated Switchgrass. Biotechnol. Biofuels 2017, 10, 12. [Google Scholar] [CrossRef]
- Schröder, D.; Sinai Borker, N.N.; König, M.; Krewer, U. Performance of Zinc Air Batteries with Added K2CO3 in the Alkaline Electrolyte. J. Appl. Electrochem. 2015, 45, 427–437. [Google Scholar] [CrossRef]
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H. High Electrochemical and Mechanical Performance of Zinc Conducting-Based Gel Polymer Electrolytes. Sci. Rep. 2021, 11, 13268. [Google Scholar] [CrossRef] [PubMed]
- Suryanto, H.; Marsyahyo, E.; Irawan, Y.S.; Soenoko, R. Effect of Alkali Treatment on Crystalline Structure of Cellulose Fiber from Mendong (Fimbristylis globulosa) Straw. Key Eng. Mater. 2013, 594–595, 720–724. [Google Scholar] [CrossRef]
| Separator | Thickness, Dry [mm] | Thickness, Wet [mm] | Area Shrinkage ΔA [%] | Swelling Degree ΔV [%] |
|---|---|---|---|---|
| Glass-fiber | 1.55 [71] | - | - | - |
| Polyolefin | 0.025 | - | - | - |
| BC-5 | 0.023 ± 0.005 | 0.062 ± 0.013 | 39.3 ± 1.7 | 63.5 ± 59.0 |
| BC-10 | 0.045 ± 0.005 | 0.109 ± 0.013 | 39.3 ± 5.6 | 45.3 ± 27.0 |
| BC-10-L | 1.54 ± 0.15 | 0.64 ± 0.19 | 58.1 ± 3.4 | −82.4 ± 9.5 |
| Separator | DOH− · 10−9 [m2 s−1] | DZn(OH)42− · 10−9 [m2 s−1] | Selectivity [-] |
|---|---|---|---|
| Glass-fiber | 63.2 ± 5.0 | 13.2 ± 3.4 | 4.8 ± 1.3 |
| Polyolefin | 0.17 ± 0.02 | 0.018 ± 0.003 | 9.5 ± 2.0 |
| BC-10 | 1.6 ± 0.2 | 0.036 ± 0.012 | 45.1 ± 16.5 |
| BC-10-L | 125.1 ± 37.2 | 3.0 ± 1.0 | 41.2 ± 17.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heydorn, R.L.; Niebusch, J.; Lammers, D.; Görke, M.; Garnweitner, G.; Dohnt, K.; Krull, R. Production and Characterization of Bacterial Cellulose Separators for Nickel-Zinc Batteries. Energies 2022, 15, 5727. https://doi.org/10.3390/en15155727
Heydorn RL, Niebusch J, Lammers D, Görke M, Garnweitner G, Dohnt K, Krull R. Production and Characterization of Bacterial Cellulose Separators for Nickel-Zinc Batteries. Energies. 2022; 15(15):5727. https://doi.org/10.3390/en15155727
Chicago/Turabian StyleHeydorn, Raymond Leopold, Jana Niebusch, David Lammers, Marion Görke, Georg Garnweitner, Katrin Dohnt, and Rainer Krull. 2022. "Production and Characterization of Bacterial Cellulose Separators for Nickel-Zinc Batteries" Energies 15, no. 15: 5727. https://doi.org/10.3390/en15155727
APA StyleHeydorn, R. L., Niebusch, J., Lammers, D., Görke, M., Garnweitner, G., Dohnt, K., & Krull, R. (2022). Production and Characterization of Bacterial Cellulose Separators for Nickel-Zinc Batteries. Energies, 15(15), 5727. https://doi.org/10.3390/en15155727








