Synthesis and Mechanical Properties of Polyacrylamide Gel Doped with Graphene Oxide
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Experimental Apparatus
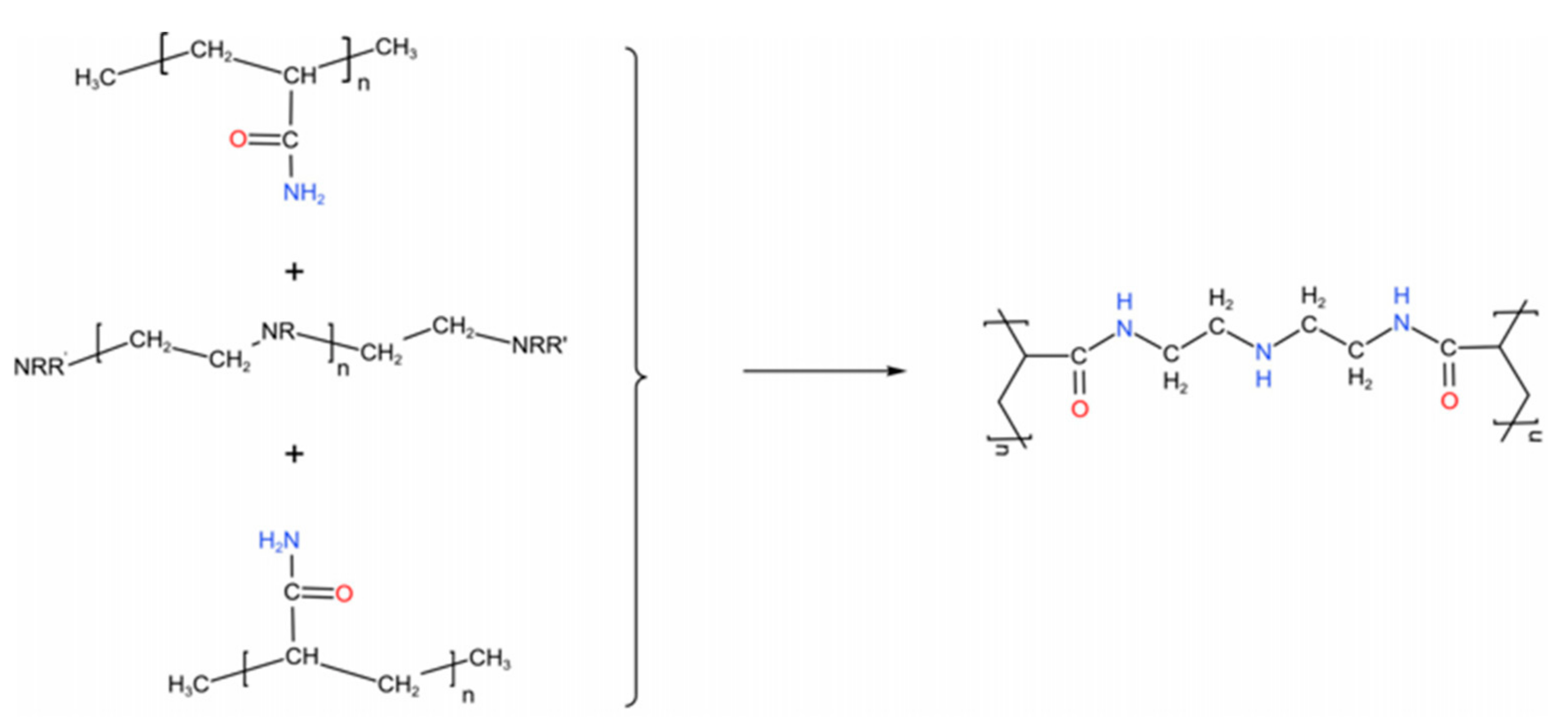
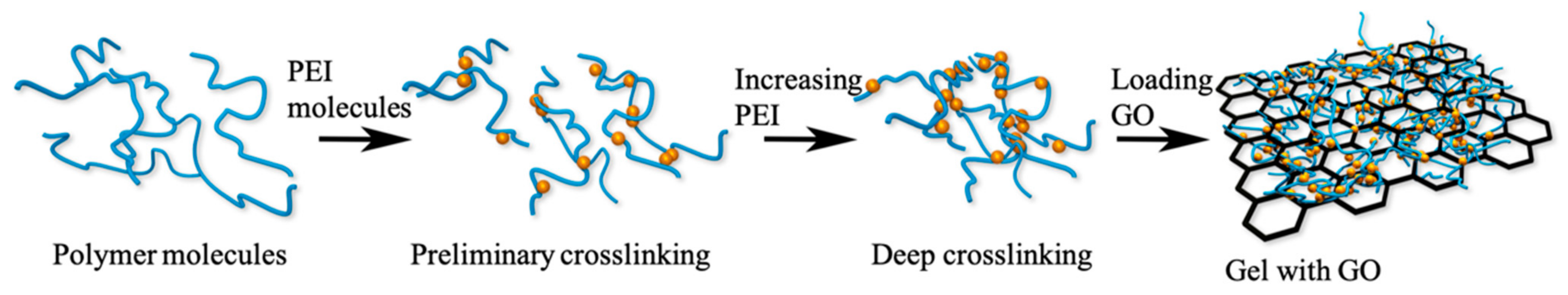
2.3. Preparation of PAM/PEI/GO Hydrogels
2.4. Gel Rheological Property Evaluation Experiment
2.5. Scanning Electron Microscopy
2.6. Transmission Electron Microscopy
2.7. Structure Characterization
2.8. DSC Analysis
3. Results and Discussion
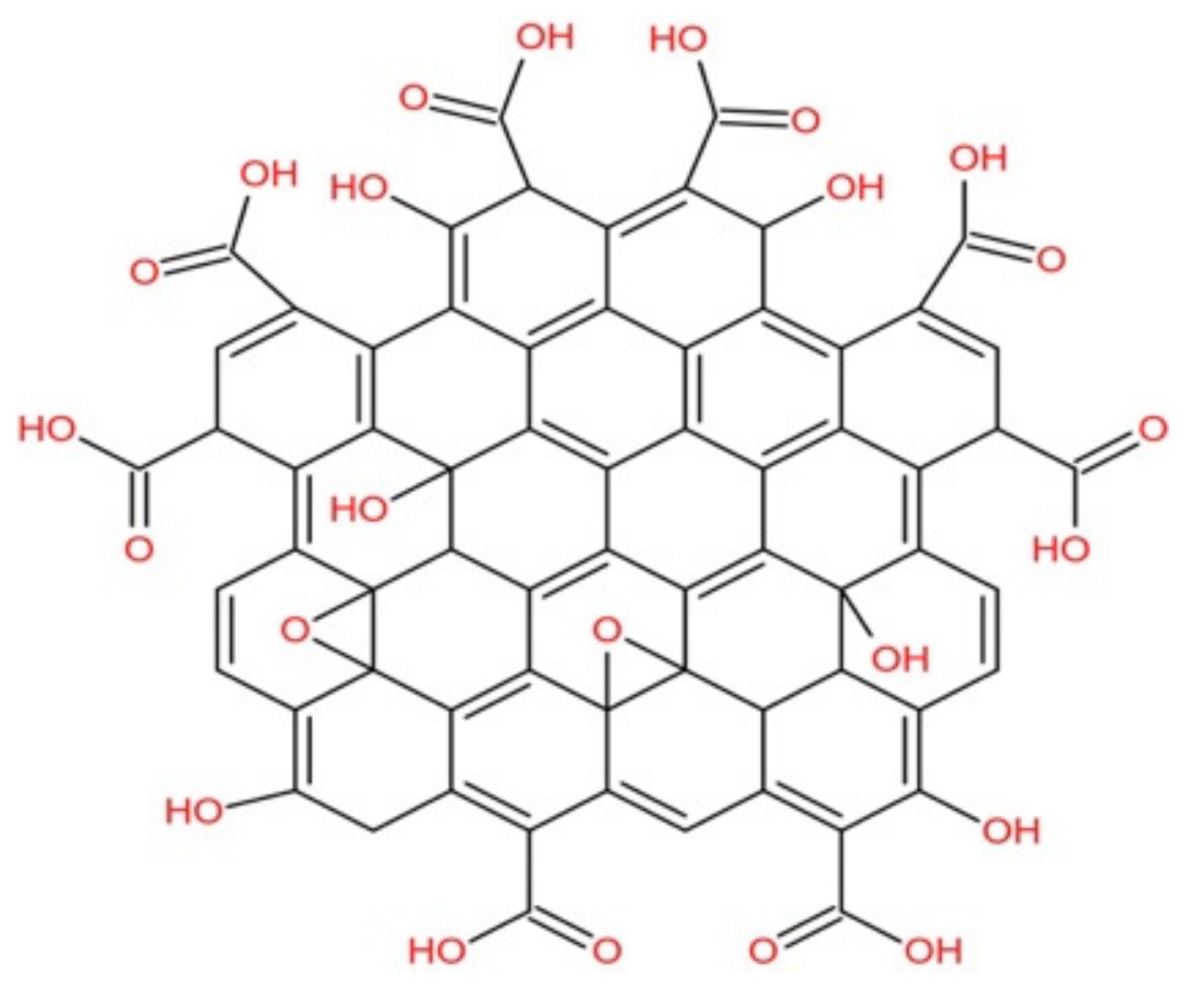
3.1. Structural Characterization
3.2. Rheological Properties of the Gels
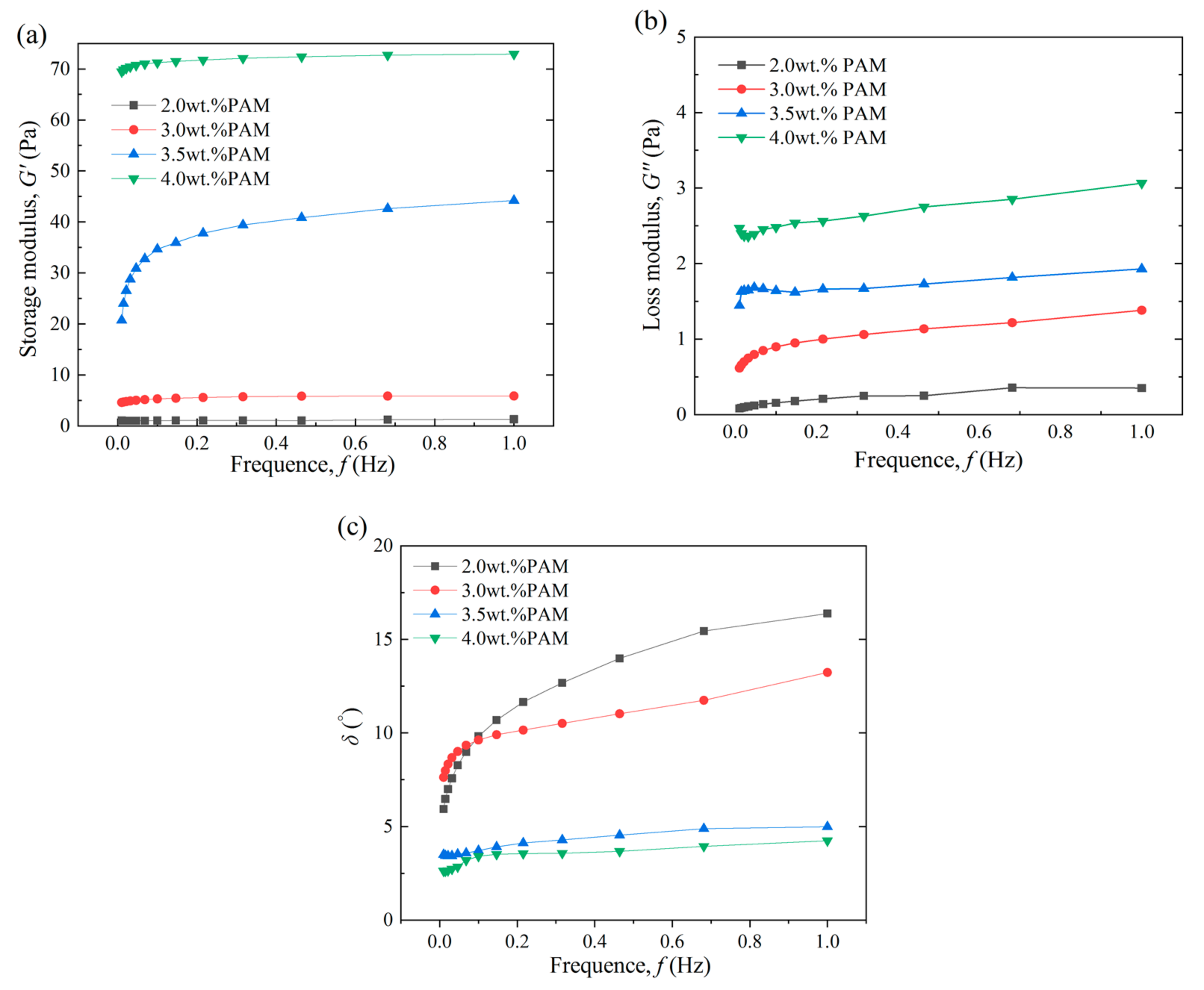
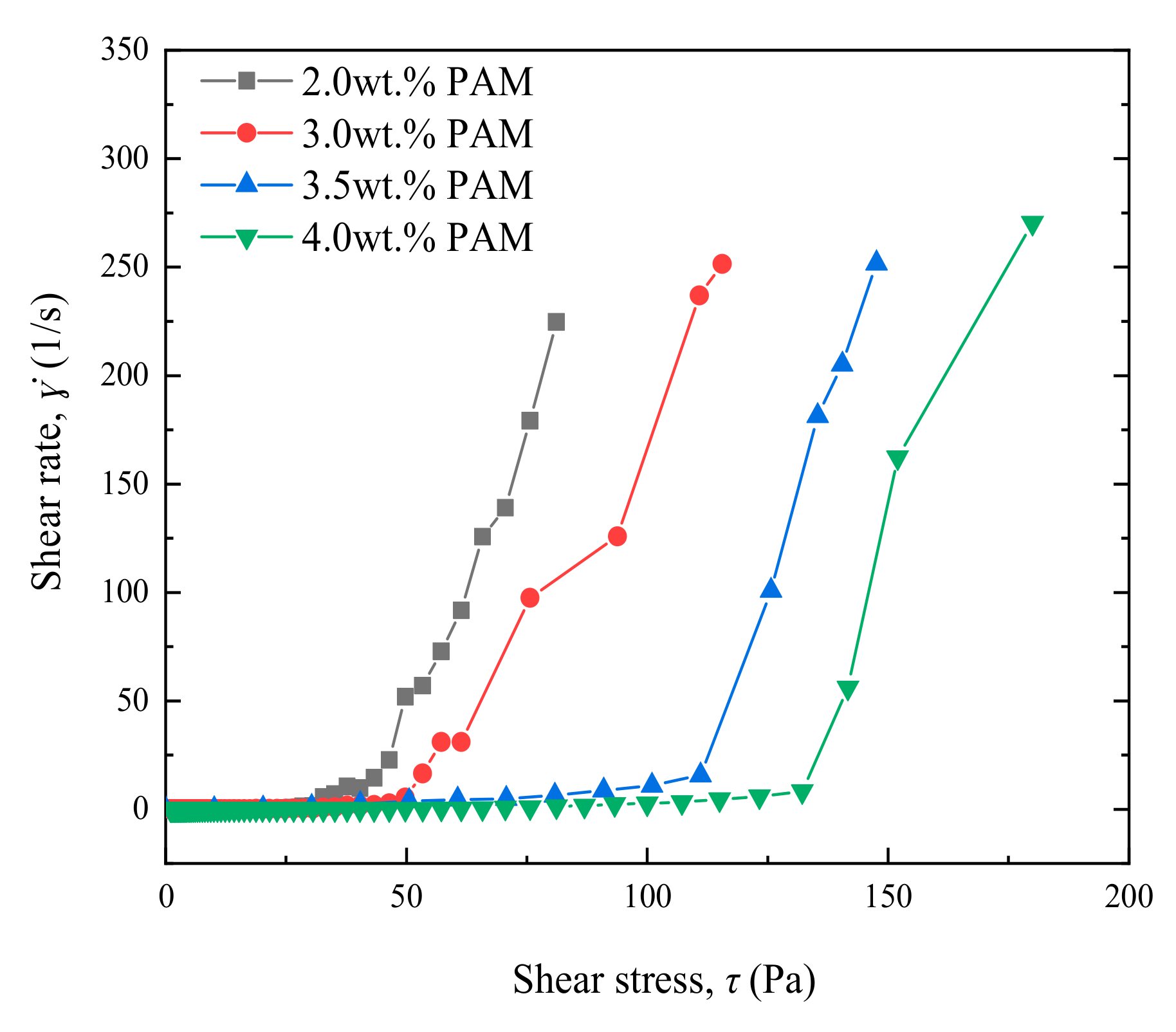
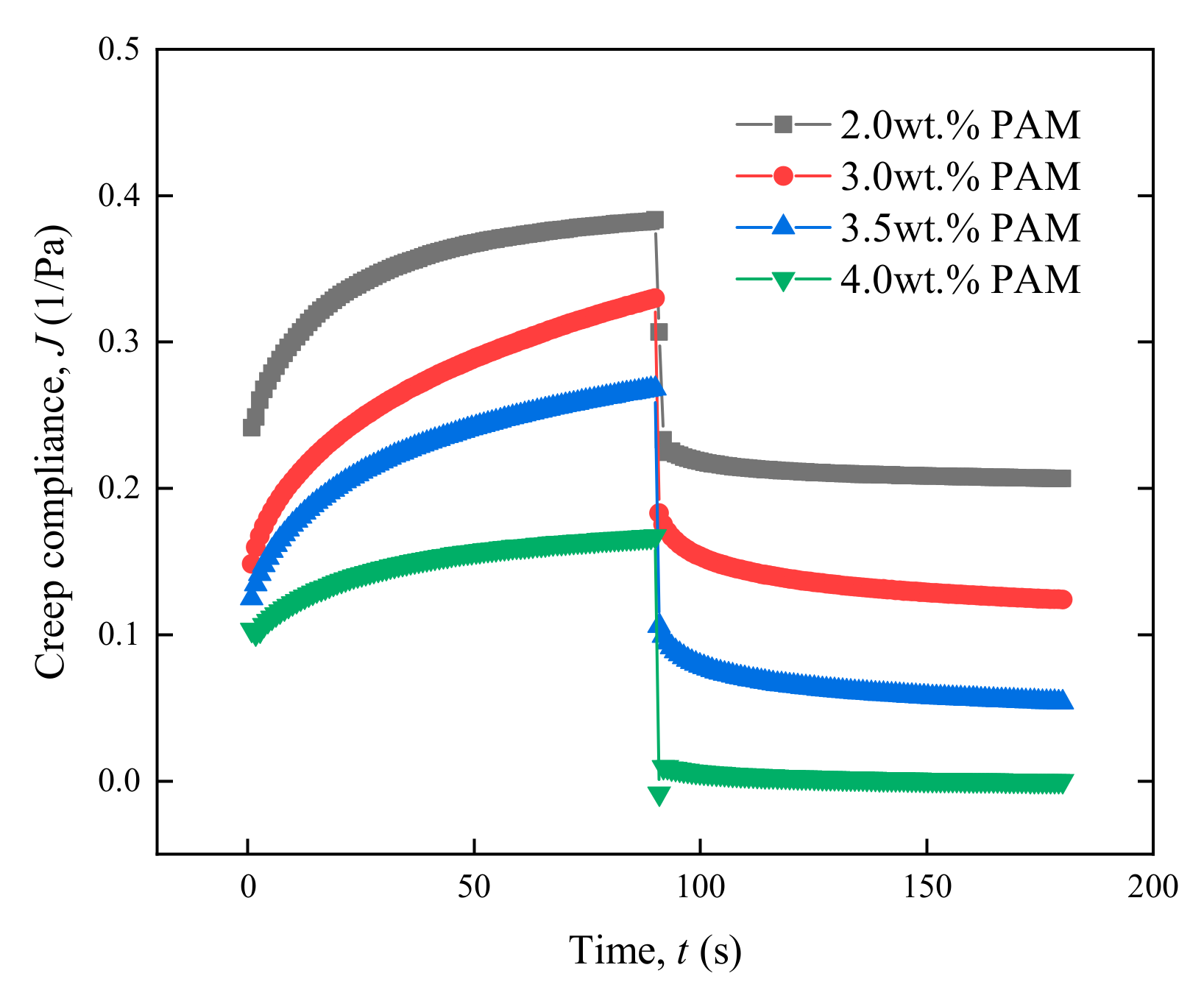
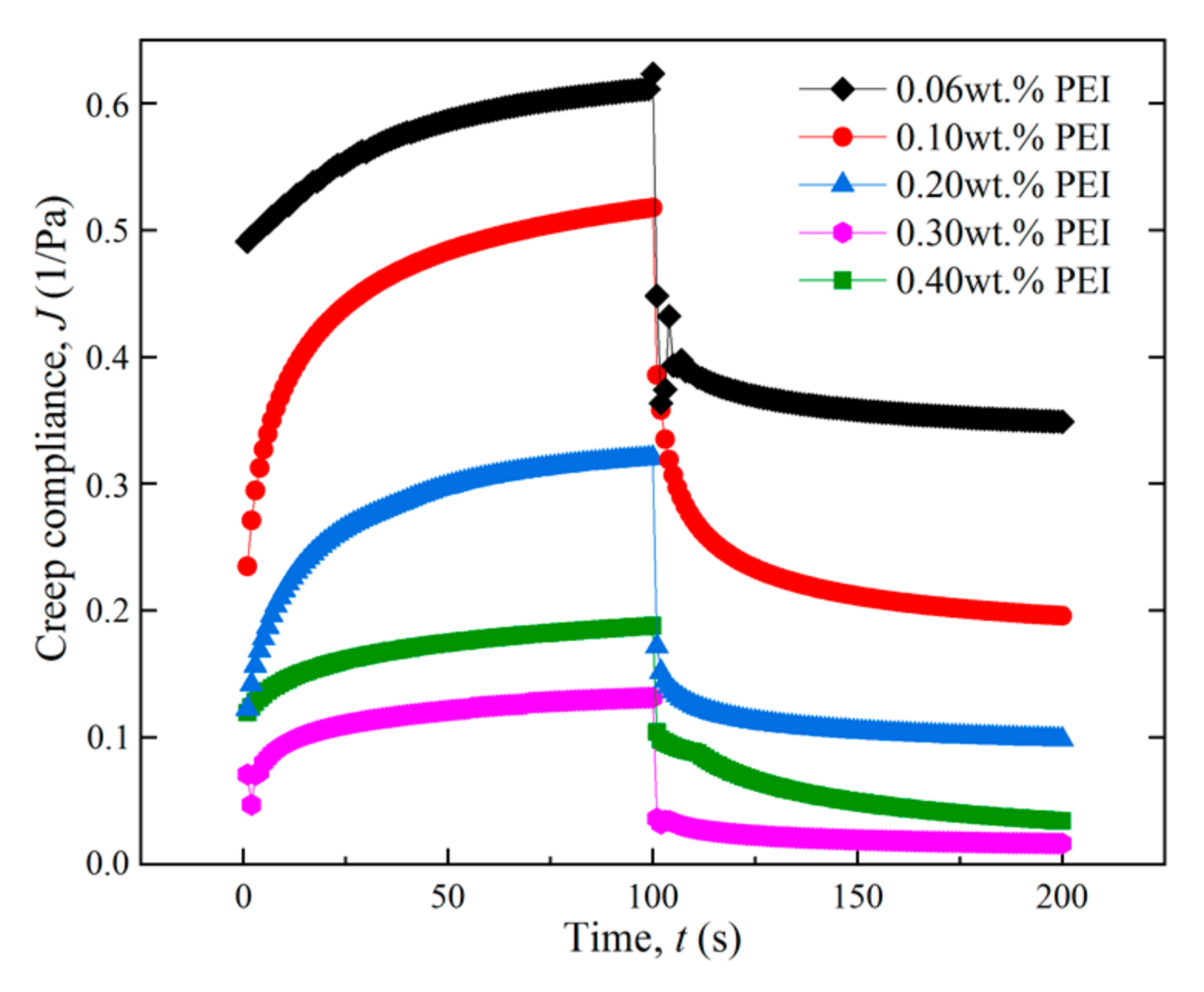
3.2.1. Effect of the Main Agent Concentration
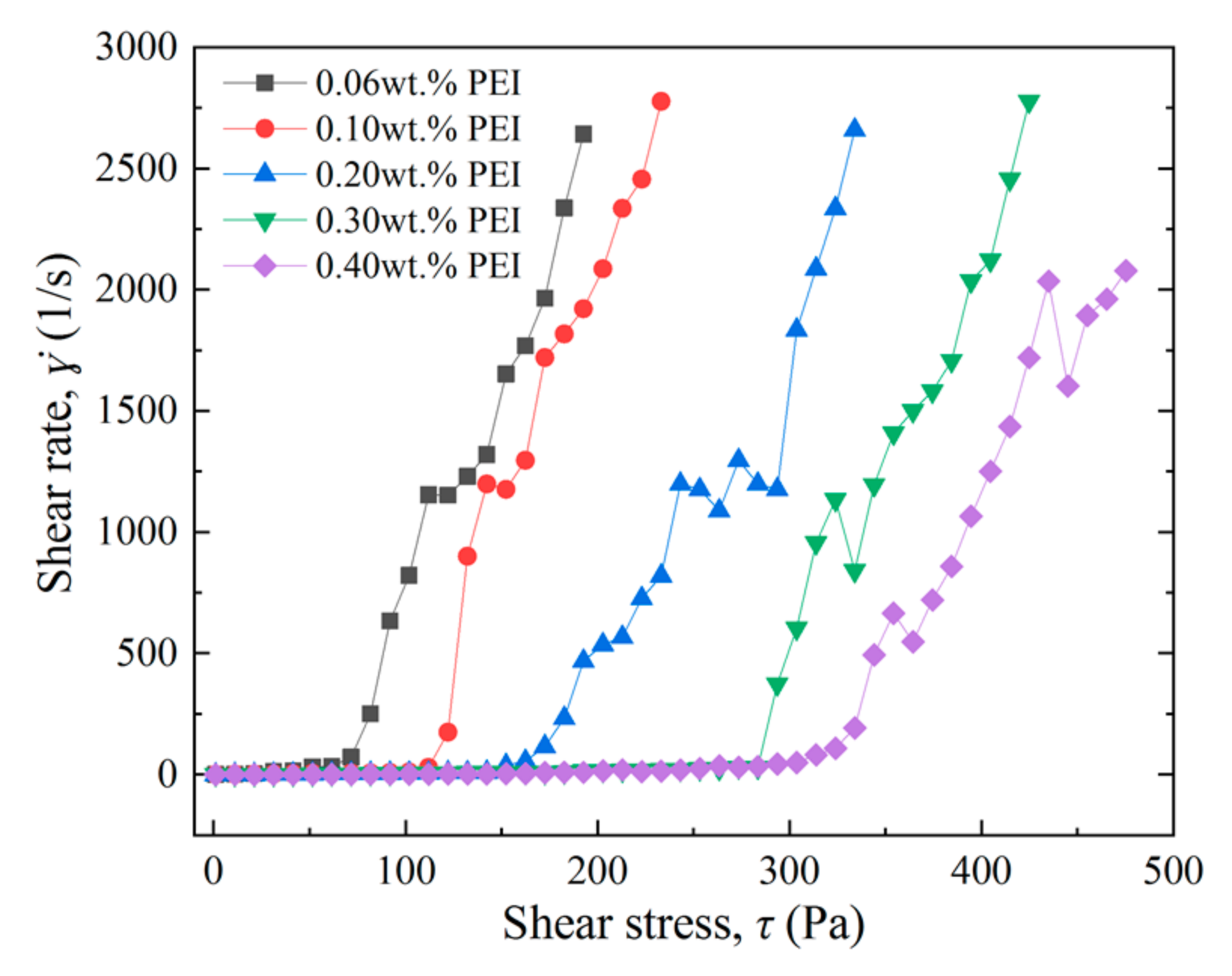
3.2.2. Effect of Cross-Linker Concentration
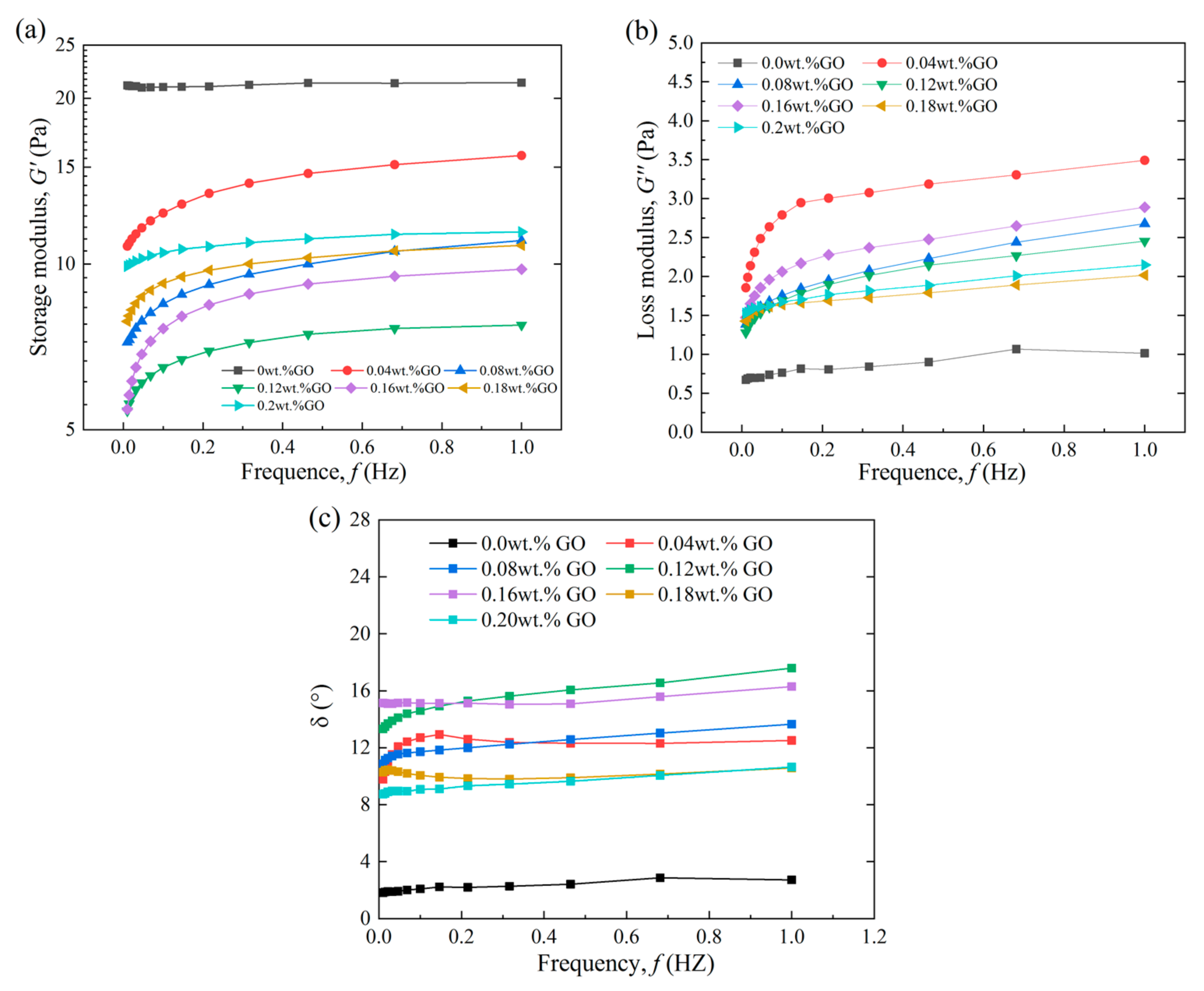
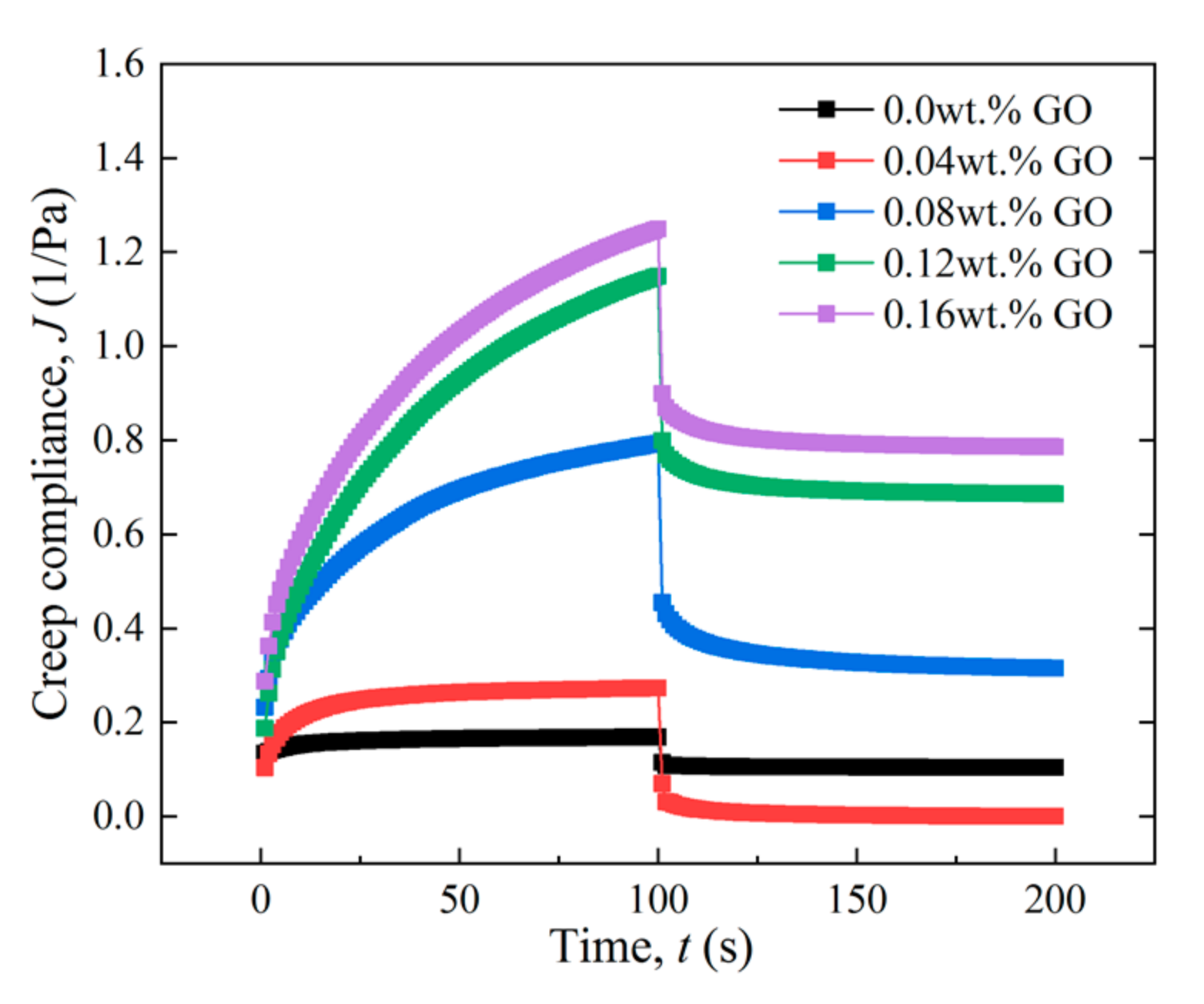
3.2.3. Effect of GO Concentration
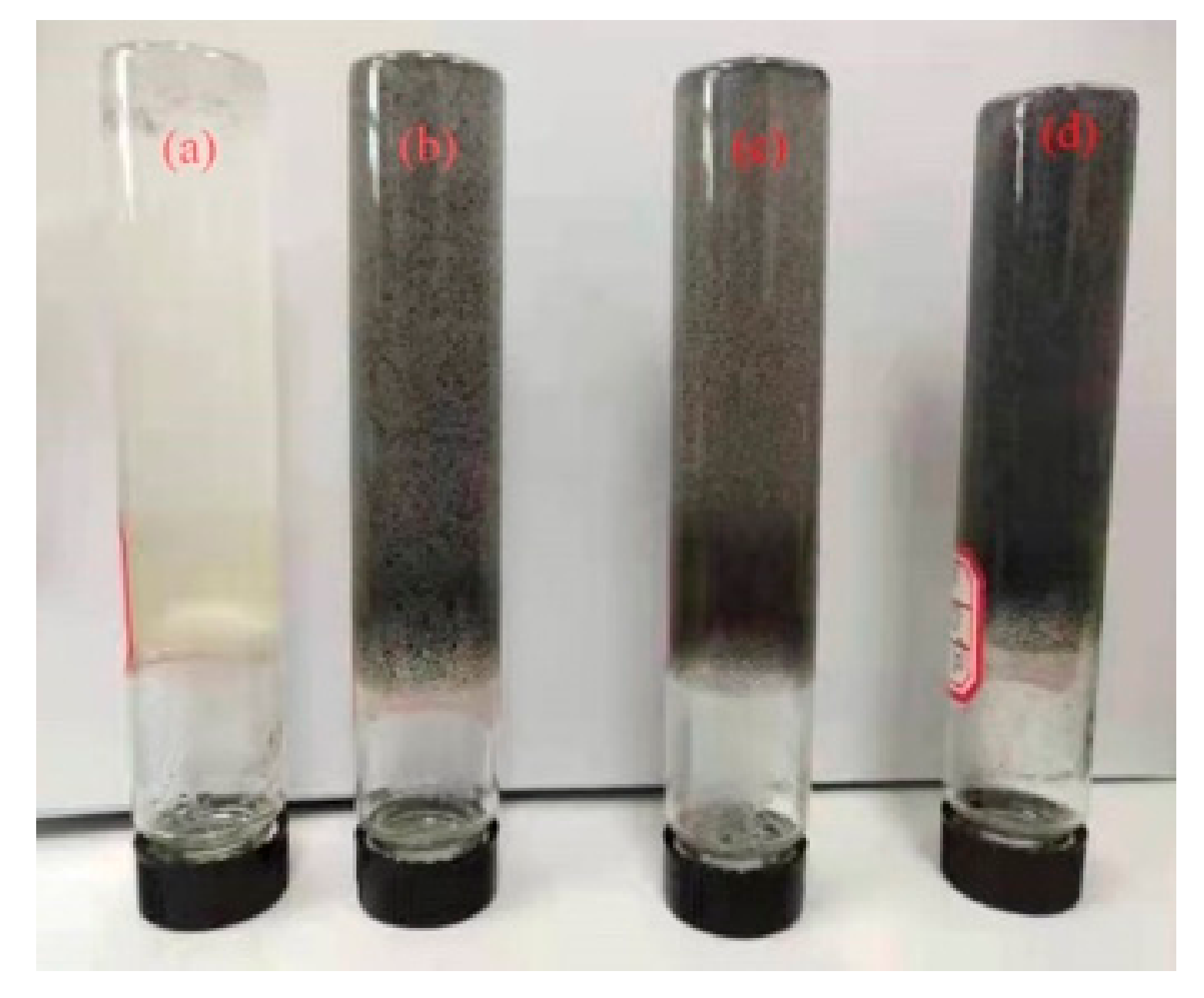
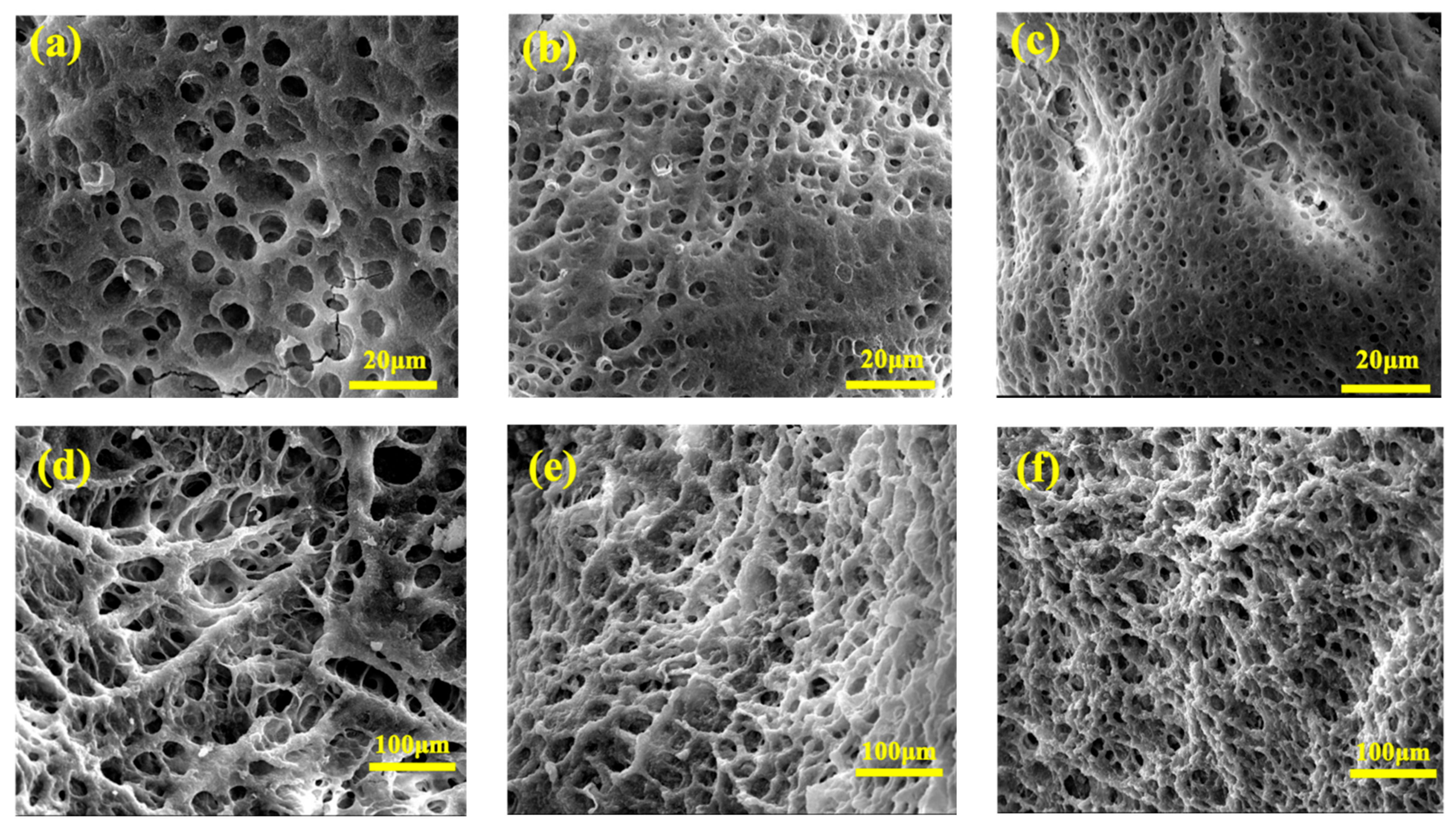
3.3. The Microstructure of the Gels
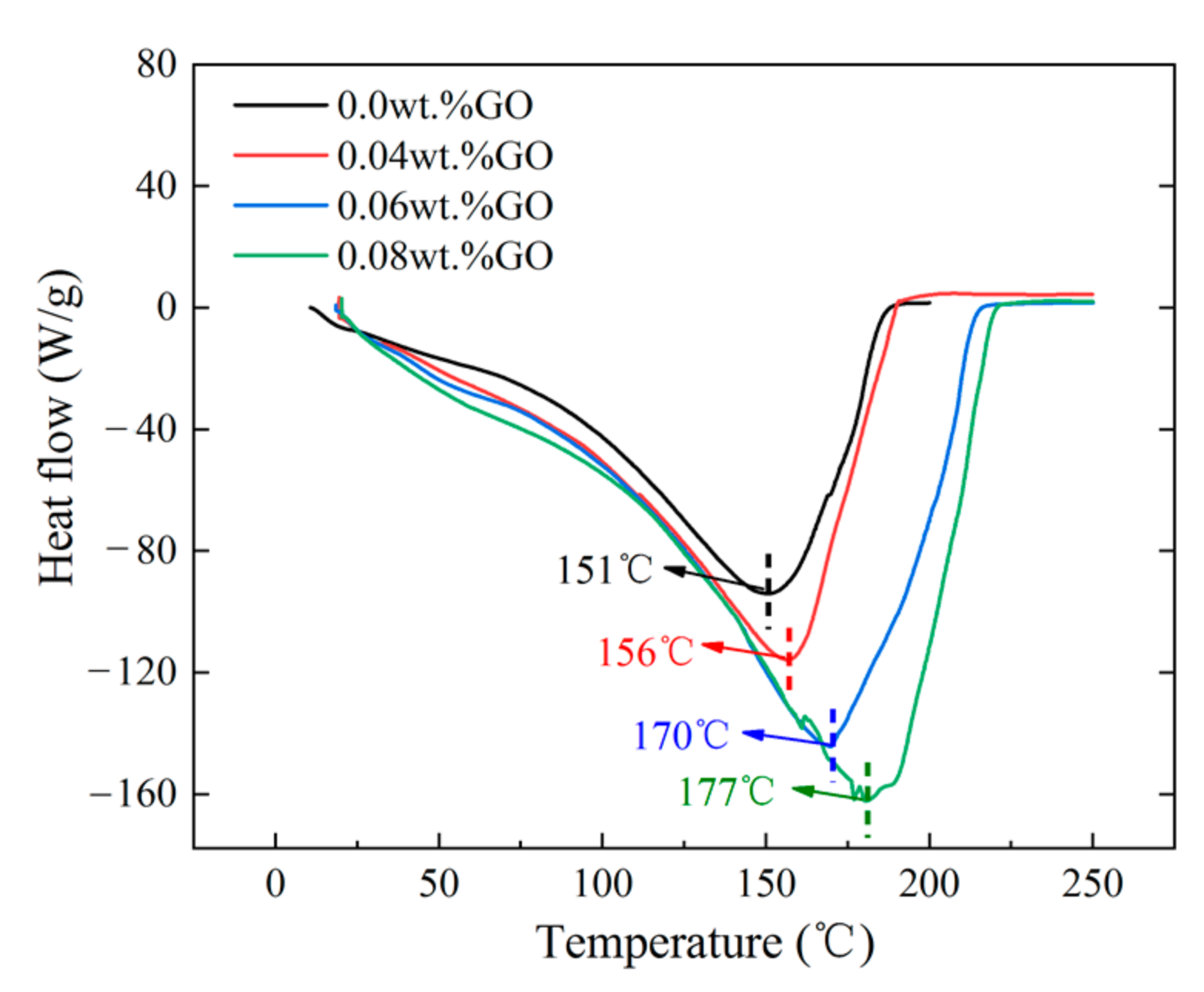
3.4. Thermal Stability of the Gels
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| PAM | Polyacrylamide |
| PEI | Polyethyleneimine |
| GO | Graphene oxide |
| TEM | Transmission electron microscopy |
| SEM | Scanning electron microscopy |
| DSC | Differential scanning calorimetry |
| XRD | X-ray diffraction |
| FT-IR | Infrared spectrum |
| G′ | Elastic modulus or storage modulus |
| G″ | Viscous modulus or loss modulus |
| Phase angle | |
| tan | Damping factor |
References
- Fan, T.-Y.; Song, X.-M.; Wu, S.-H.; Li, Q.-Y.; Wang, B.-H.; Li, X.-B.; Li, H.; Liu, H.-L. A mathematical model and numerical simulation of waterflood induced dynamic fractures of low permeability reservoirs. Pet. Explor. Dev. 2015, 42, 541–547. [Google Scholar] [CrossRef]
- Han, Y.-J.; Zhou, C.-C.; Yu, J.; Li, C.-L.; Hu, F.-L.; Xu, H.-J. Experimental investigation on the effect of wettability on rock-electricity response in sandstone reservoirs. Fuel 2019, 239, 1246–1257. [Google Scholar] [CrossRef]
- Chu, H.-Q.; Han, W.-W.; Cao, W.-J.; Gu, M.-Y.; Xu, G.-J. Effect of methane addition to ethylene on the morphology and size distribution of soot in a laminar co-flow diffusion flame. Energy 2019, 166, 392–400. [Google Scholar] [CrossRef]
- Yang, H.; Hu, L.; Chen, C. Synthesis and plugging behavior of fluorescent polymer microspheres as a kind of conformance control agent in reservoirs. RSC Adv. 2018, 8, 10478–10488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Kang, W.; Yu, Y.; Yin, X.; Wang, P.; Zhang, X. A new approach to evaluate the particle growth and sedimentation of dispersed polymer microsphere profile control system based on multiple light scattering. Powder Technol. 2017, 315, 477–485. [Google Scholar] [CrossRef]
- Yang, H.; Shao, S.; Zhu, T.; Chen, C.; Liu, S.; Zhou, B.; Hou, X.; Zhang, Y.; Kang, W. Shear resistance performance of low elastic polymer microspheres used for conformance control treatment. J. Ind. Eng. Chem. 2019, 79, 295–306. [Google Scholar] [CrossRef]
- Bai, B.; Zhou, J.; Yin, M. A comprehensive review of polyacrylamide polymer gels for conformance control. Pet. Explor. Dev. 2015, 42, 525–532. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Guo, J.-X.; Gu, Y.; Zhao, Q.; Yang, R.-J.; Yang, Y.-Q. Polyacrylamide gel formed by Cr(III) and phenolic resin for water control in high-temperature reservoirs. J. Pet. Sci. Eng. 2020, 194, 107423. [Google Scholar] [CrossRef]
- Wu, Q.; Ge, J.; Ding, L.; Wei, K.; Liu, Y. Identification and characterization of a proper gel system for large-volume conformance control treatments in a fractured tight reservoir: From lab to field. J. Pet. Sci. Eng. 2021, 198, 108119. [Google Scholar] [CrossRef]
- Sun, F.; Lin, M.; Dong, Z.; Zhu, D.; Wang, S.L.; Yang, J. Effect of composition of HPAM/chromium(III) acetate gels on delayed gelation time. J. Dispers. Sci. Technol. 2015, 37, 753–759. [Google Scholar] [CrossRef]
- Guan, H.; Berkland, C.; Moradi-Araghi, A.; Liang, J.T.; Christian, T.M.; Needham, R.B.; Cheng, M. Delayed Gelation of Polymers. U.S. Patent US201514847734, 8 September 2015. Available online: http://www.freepatentsonline.com/y2016/0137906.html (accessed on 18 May 2016).
- Hasankhani, G.M.; Madani, M.; Esmaeilzadeh, F.; Mowla, D.; Daryasafar, A. An experimental investigation of polyacrylamide and sulfonated polyacrylamides based gels crosslinked with cr(III)-acetate for water shutoff in fractured oil reservoirs. J. Dispers. Sci. Technol. 2018, 39, 1780–1789. [Google Scholar] [CrossRef]
- Xie, B.; Ma, J.; Wang, Y.; Tchameni, A.P.; Luo, M.; Wen, J. Enhanced hydrophobically modified polyacrylamide gel for lost circulation treatment in high temperature drilling. J. Mol. Liq. 2021, 325, 115155. [Google Scholar] [CrossRef]
- Chen, J.; Ao, Y.; Lin, T.; Yang, X.; Peng, J.; Huang, W. High-toughness polyacrylamide gel containing hydrophobic crosslinking and its double network gel. Polymer 2016, 87, 73–80. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, H.; Wu, P.; Huang, X.; Wang, X. The preparation of MgO nanopowders synthesized via an improved polyacrylamide gel method. RSC Adv. 2019, 9, 14893–14898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sengupta, B.; Sharma, V.P.; Udayabhanu, G. A Study of the Effect of the Concentration of Constituents on the Characteristics of a Cross-Linked Polyacrylamide Gel. Pet. Sci. Technol. 2012, 30, 1865–1881. [Google Scholar] [CrossRef]
- Xu, J.; Finkenstadt, V.L. Rheological properties of reactive extrusion modified waxy starch and waxy starch-polyacrylamide copolymer gels. Starch-Stärke 2013, 65, 984–990. [Google Scholar] [CrossRef]
- Shen, J.; Li, K.; Muhammad, Y. Removal of Cu(II) ions from simulated wastewater using bagasse pith grafted polyacrylamide copolymer. Chem. Eng. Res. Des. 2020, 164, 361–372. [Google Scholar] [CrossRef]
- Punyodom, W. Novel Poly(Methylenelactide-g-L-Lactide) Graft Copolymers Synthesized by a Combination of Vinyl Addition and Ring-Opening Polymerizations. Polymers 2021, 13, 3374. [Google Scholar] [CrossRef]
- Farjood, M.; Zanjanchi, M.A. A new synthesis methodology for SiO2 gel-based nanostructures and their application for elimination of dye pollutants. New J. Chem. 2020, 44, 5386–5395. [Google Scholar] [CrossRef]
- Aqcheli, F.; Salehi, M.B.; Pahlevani, H.; Taghikhani, V. Rheological properties and the micromodel investigation of nanosilica gel-reinforced preformed particle gels developed for improved oil recovery. J. Pet. Sci. Eng. 2020, 192, 107258. [Google Scholar] [CrossRef]
- Li, Q.; Yu, X.; Wang, L. Nano-silica hybrid polyacrylamide/polyethylenimine gel for enhanced oil recovery at harsh conditions. Colloids and Surfaces A. Physicochem. Eng. Asp. 2022, 633, 127898. [Google Scholar] [CrossRef]
- Shamlooh, M.; Hamza, A.; Hussein, I.A.; Nasser, M.S.; Magzoub, M.; Salehi, S. Investigation of the Rheological Properties of Nanosilica-Reinforced Polyacrylamide/Polyethyleneimine Gels for Wellbore Strengthening at High Reservoir Temperatures. Energy Fuels 2019, 33, 6829–6836. [Google Scholar] [CrossRef]
- Piao, Y.-Z.; Chen, B.-Q. Synthesis and mechanical properties of double cross-linked gelatin-graphene oxide hydrogels. Int. J. Biol. Macromol. 2017, 101, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Tarashi, S.; Nazockdast, H.; Sodeifian, G. A comparative study on microstructure, physical-mechanical properties, and self-healing performance of two differently synthesized nanocomposite double network hydrogels based on κ-car/PAm/GO. Polymer 2020, 188, 122138. [Google Scholar] [CrossRef]
- Bashir, S.; Hasan, K.; Hina, M. Conducting polymer/graphene hydrogel electrodes based aqueous smart Supercapacitors: A review and future prospects. J. Electroanal. Chem. 2021, 898, 115626. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, K.; Deng, R.; Ren, X.; Wu, C.; Li, J. Tunable stiffness of graphene oxide/polyacrylamide composite scaffolds regulates cytoskeleton assembly. Chem. Sci. 2019, 9, 6516–6522. [Google Scholar] [CrossRef] [Green Version]
- Dong, Y.; Wu, W.; Liang, L.; Tao, S.; Meng, X.J.N.E.; Technology, P. Preparation and Adsorption Properties of Polyacrylamide/Graphene Oxide Composite Aerogel. J. Biomater. Tissue Eng. 2016, 19, 627–636. [Google Scholar] [CrossRef]
- Fathinejad, H.; Souri, A. Study of swelling behavior of hydrogel nanocomposite based on polydopamine-graphene oxide–polyacrylamide. Fuller. Nanotub. Car. Nanostruct. 2021, 30, 503–507. [Google Scholar] [CrossRef]
- Yan, X.-Q.; Yang, J.; Chen, F. Mechanical properties of gelatin/polyacrylamide/graphene oxide nanocomposite double-network hydrogels. Composites Science and Technology Compos. Sci. Technol. 2018, 163, 81–88. [Google Scholar] [CrossRef]
- Wang, B.; Lin, M.; Guo, J.; Wang, D.; Xu, F.; Li, M. Plugging properties and profile control effects of crosslinked polyacrylamide microspheres. J. Appl. Polym. Sci. 2016, 133, 30. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Hummers, W.-S.; Offeman, R.-E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Xu, J.-J.; Sun, Z.-H.; Li, C.-Z.; Pan, C.-X. Preparation of graphene and TiO2 layer by layer composite with highly photocatalytic efficiency, Prog. Nat. Sci. 2011, 21, 467–471. [Google Scholar] [CrossRef] [Green Version]
- Meiqin, L.I.N.; Xianxian, W.E.I.; Zhaoxia, D.O.N.G.; Zihao, Y.A.N.G.; Feifei, S.U.N. Rheological Properties of Starch Graft Polyacrylamide Gel. Acta Pet. Sin. 2020, 36, 801–811. [Google Scholar] [CrossRef]






| Samples | Concentrations (wt.%) | Samples | Concentrations (wt.%) | Samples | Concentrations (wt.%) |
|---|---|---|---|---|---|
| PAM | 2 | GO | 0.01 | Cross-linker PEI | 0.2 |
| 3 | 0.02 | 0.3 | |||
| 4 | 0.04 | 0.4 | |||
| 5 | 0.08 | 0.5 | |||
| 6 | 0.16 | 0.6 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.-P.; Cao, J.-J.; Jiang, W.-B.; Yang, Y.-Q.; Zhu, B.-Y.; Liu, X.-Y.; Wu, Y.; Sun, X.; Essouma, A.F.B.E.; Liu, J.; et al. Synthesis and Mechanical Properties of Polyacrylamide Gel Doped with Graphene Oxide. Energies 2022, 15, 5714. https://doi.org/10.3390/en15155714
Zhang H-P, Cao J-J, Jiang W-B, Yang Y-Q, Zhu B-Y, Liu X-Y, Wu Y, Sun X, Essouma AFBE, Liu J, et al. Synthesis and Mechanical Properties of Polyacrylamide Gel Doped with Graphene Oxide. Energies. 2022; 15(15):5714. https://doi.org/10.3390/en15155714
Chicago/Turabian StyleZhang, Hai-Peng, Jing-Jing Cao, Wen-Bing Jiang, Yu-Qi Yang, Bo-Yuan Zhu, Xiao-Yu Liu, Yang Wu, Xin Sun, Ariane Felicite Bibiche Essouma Essouma, Jian Liu, and et al. 2022. "Synthesis and Mechanical Properties of Polyacrylamide Gel Doped with Graphene Oxide" Energies 15, no. 15: 5714. https://doi.org/10.3390/en15155714
APA StyleZhang, H.-P., Cao, J.-J., Jiang, W.-B., Yang, Y.-Q., Zhu, B.-Y., Liu, X.-Y., Wu, Y., Sun, X., Essouma, A. F. B. E., Liu, J., & Xing, T.-Y. (2022). Synthesis and Mechanical Properties of Polyacrylamide Gel Doped with Graphene Oxide. Energies, 15(15), 5714. https://doi.org/10.3390/en15155714






