Effective Mild Ethanol-Based Organosolv Pre-Treatment for the Selective Valorization of Polysaccharides and Lignin from Agricultural and Forestry Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Ethanol Organosolv Pre-Treatment
2.3. Lignin Precipitation
2.4. Enzymatic Hydrolysis
2.5. Chemical Analysis
3. Results and Discussion
3.1. Biomass Characterisation
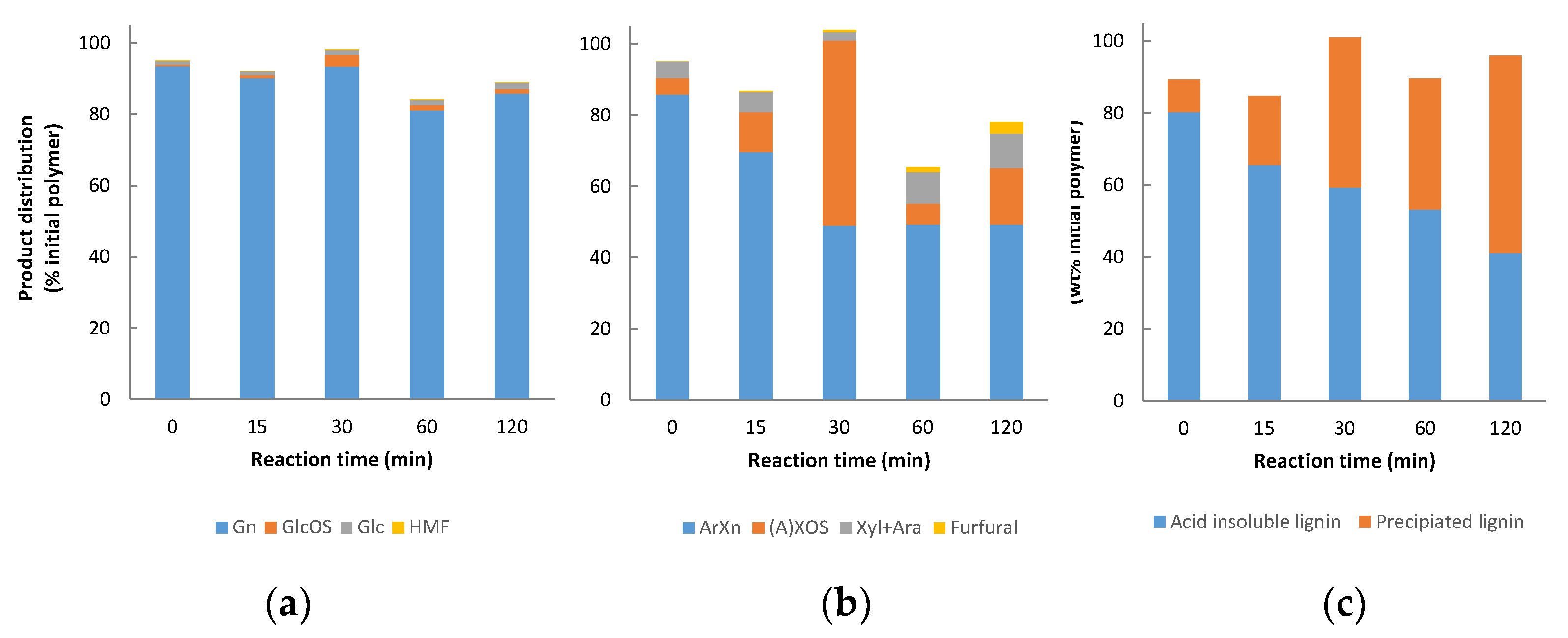
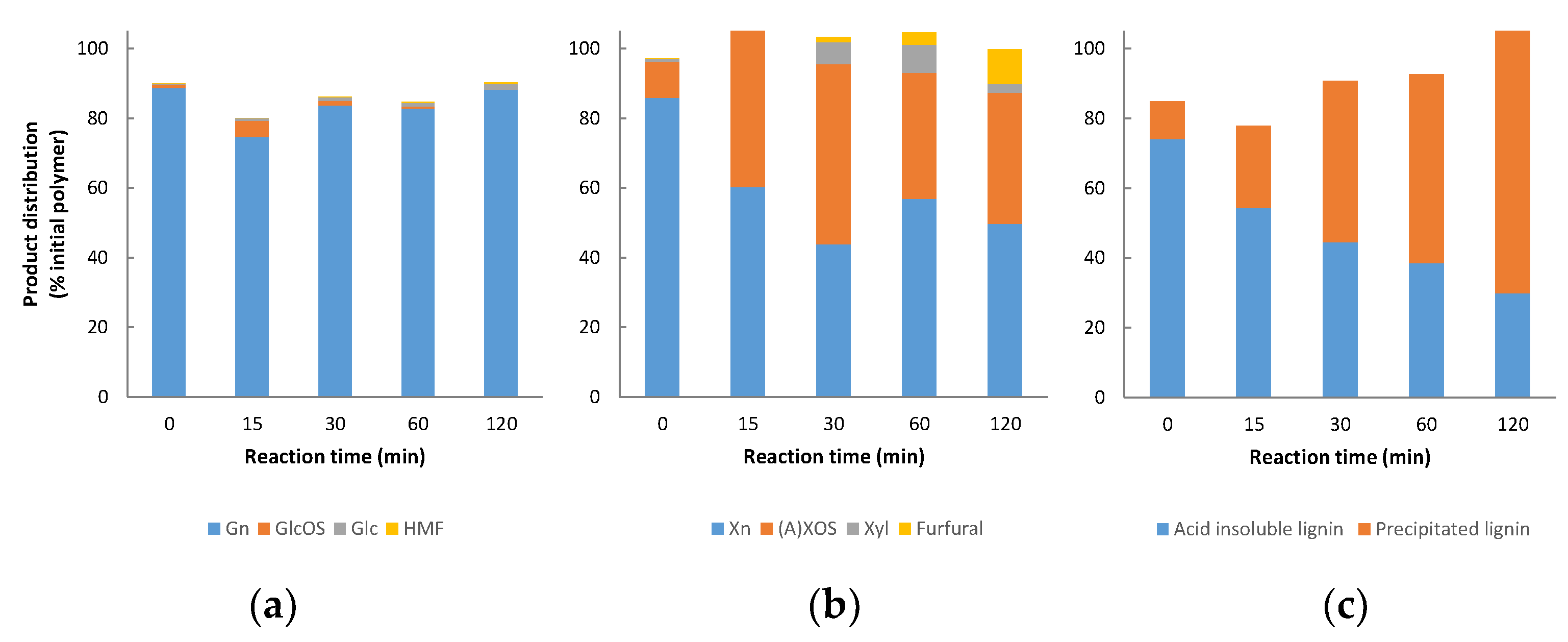
3.2. Non-Catalysed Organosolv Fractionation
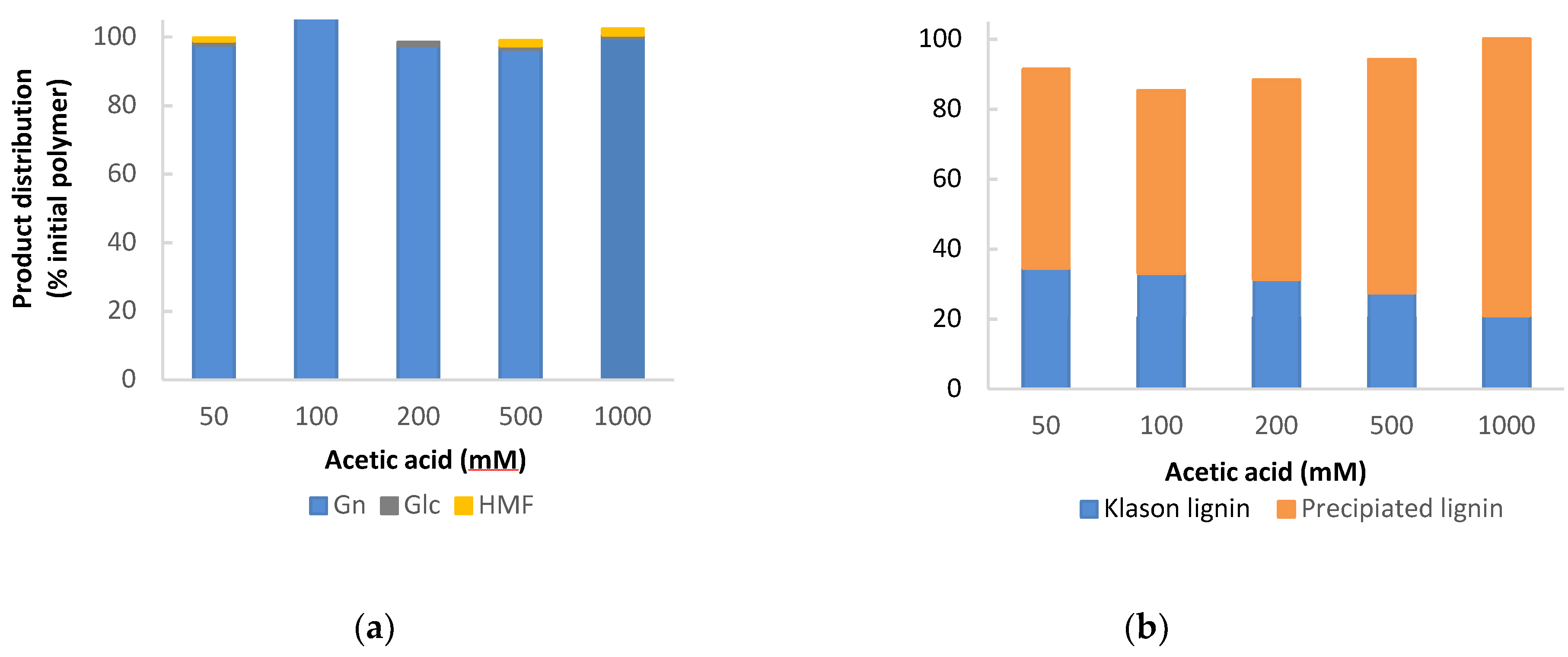
3.3. Acetic Acid Catalysed Organosolv Fractionation
3.4. Sulphuric Acid Catalysed Organosolv Fractionation
3.5. Characterization of Phenolic Compounds
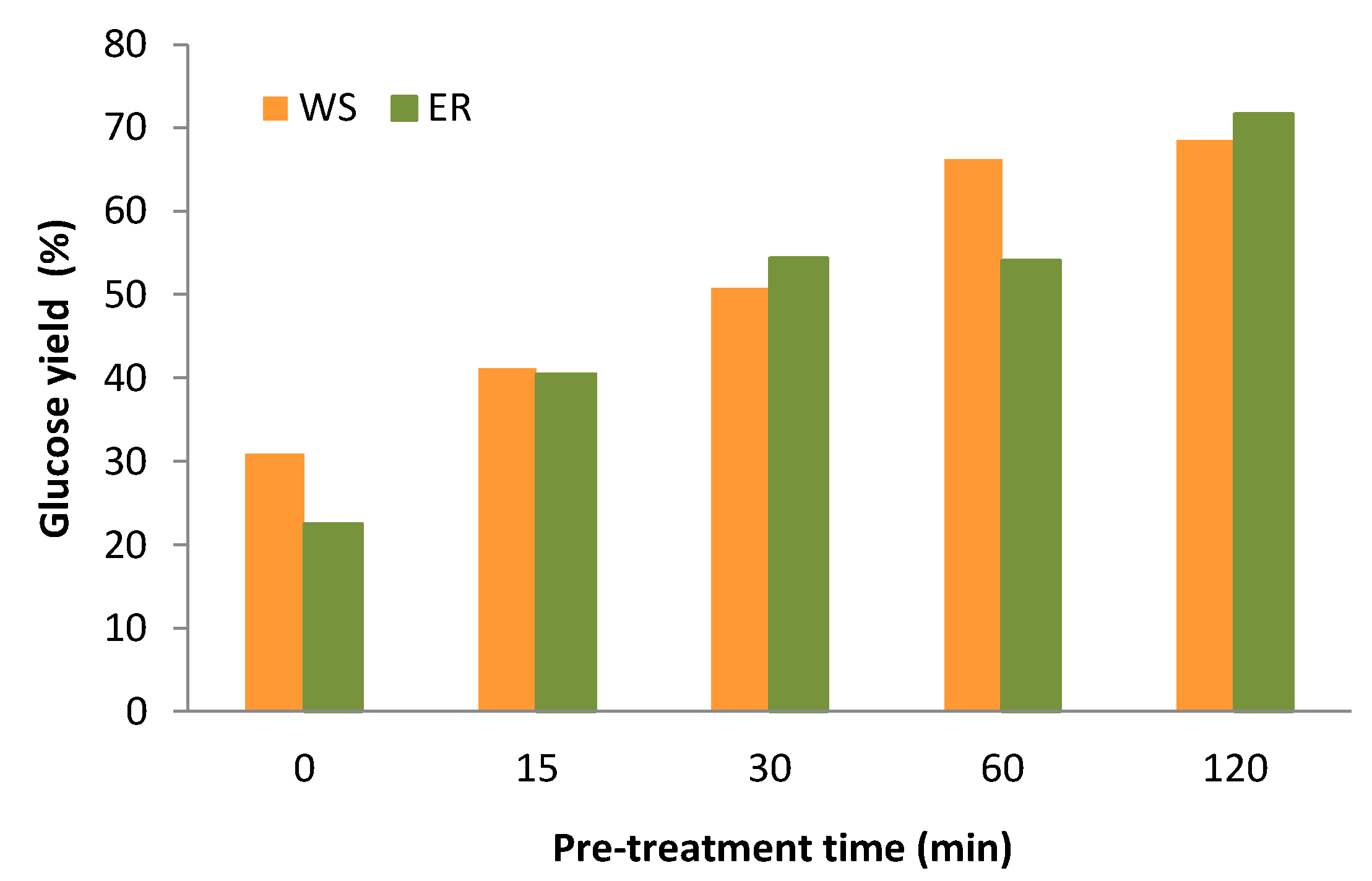
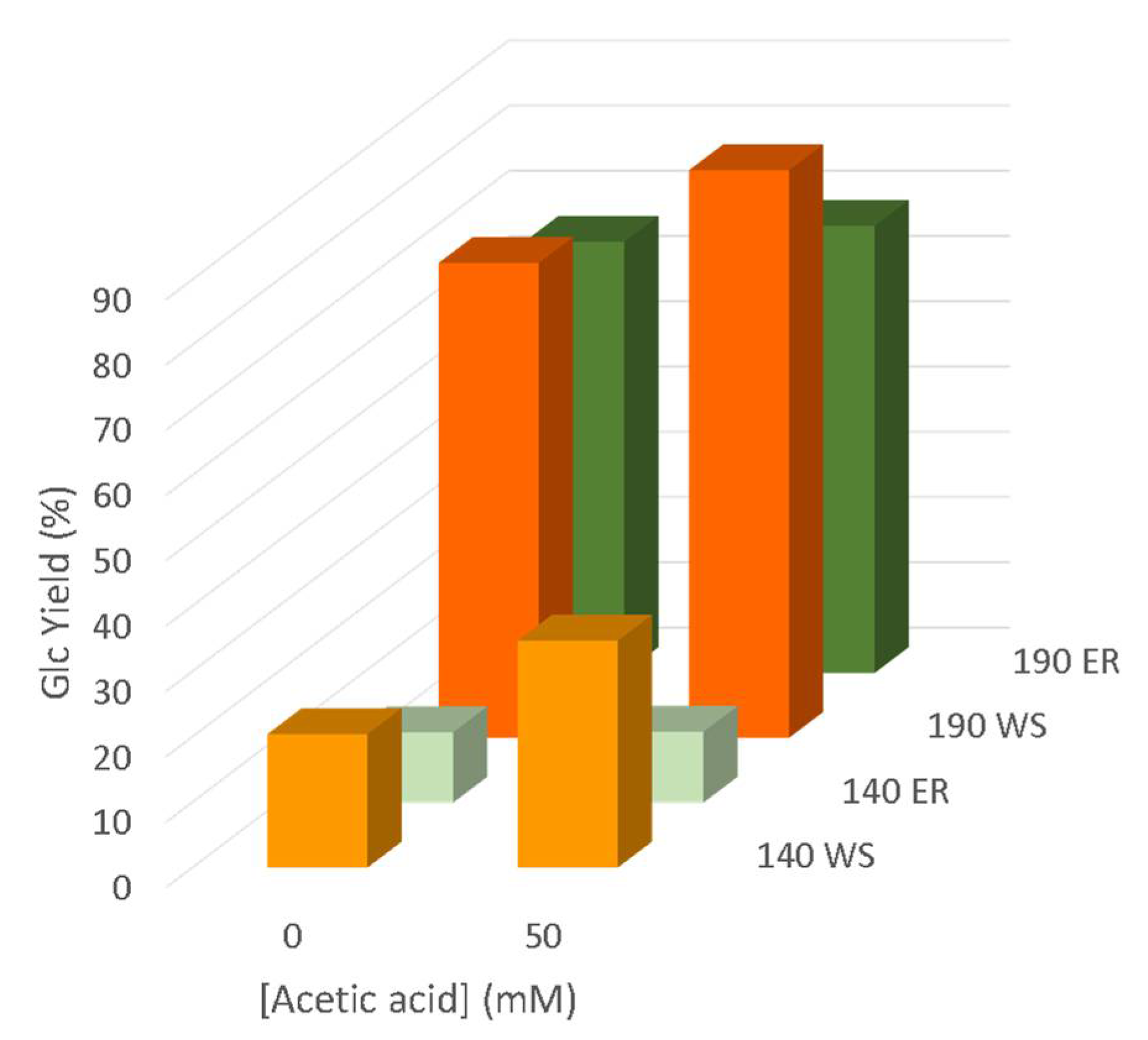
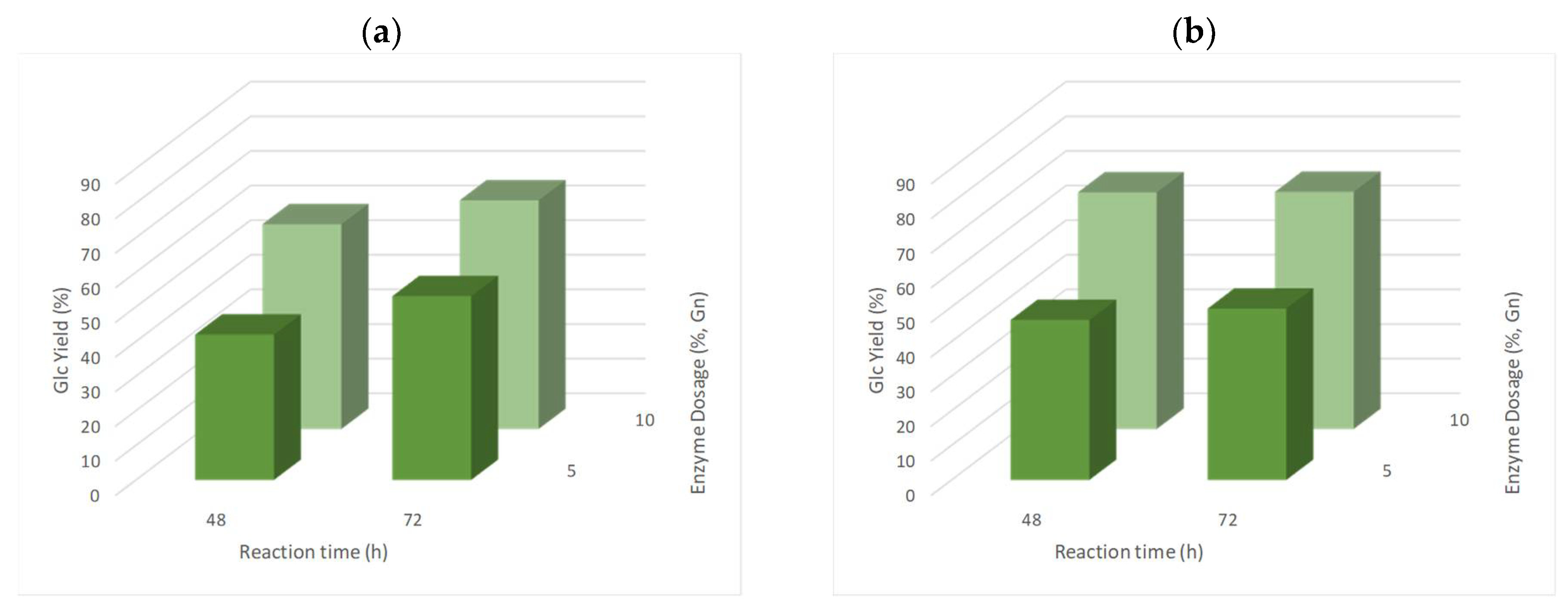
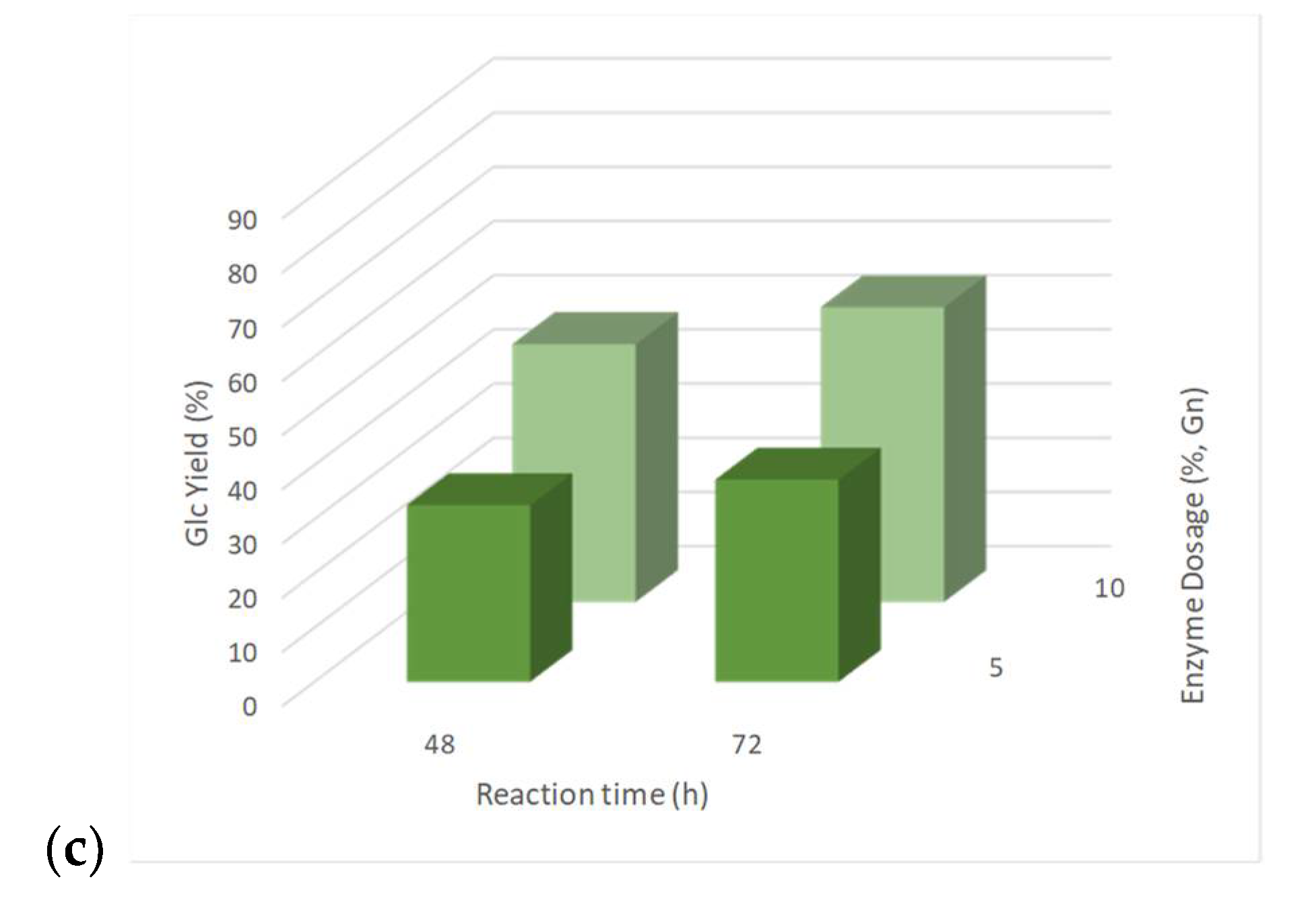
3.6. Enzymatic Saccharification of Pre-Treated Solids
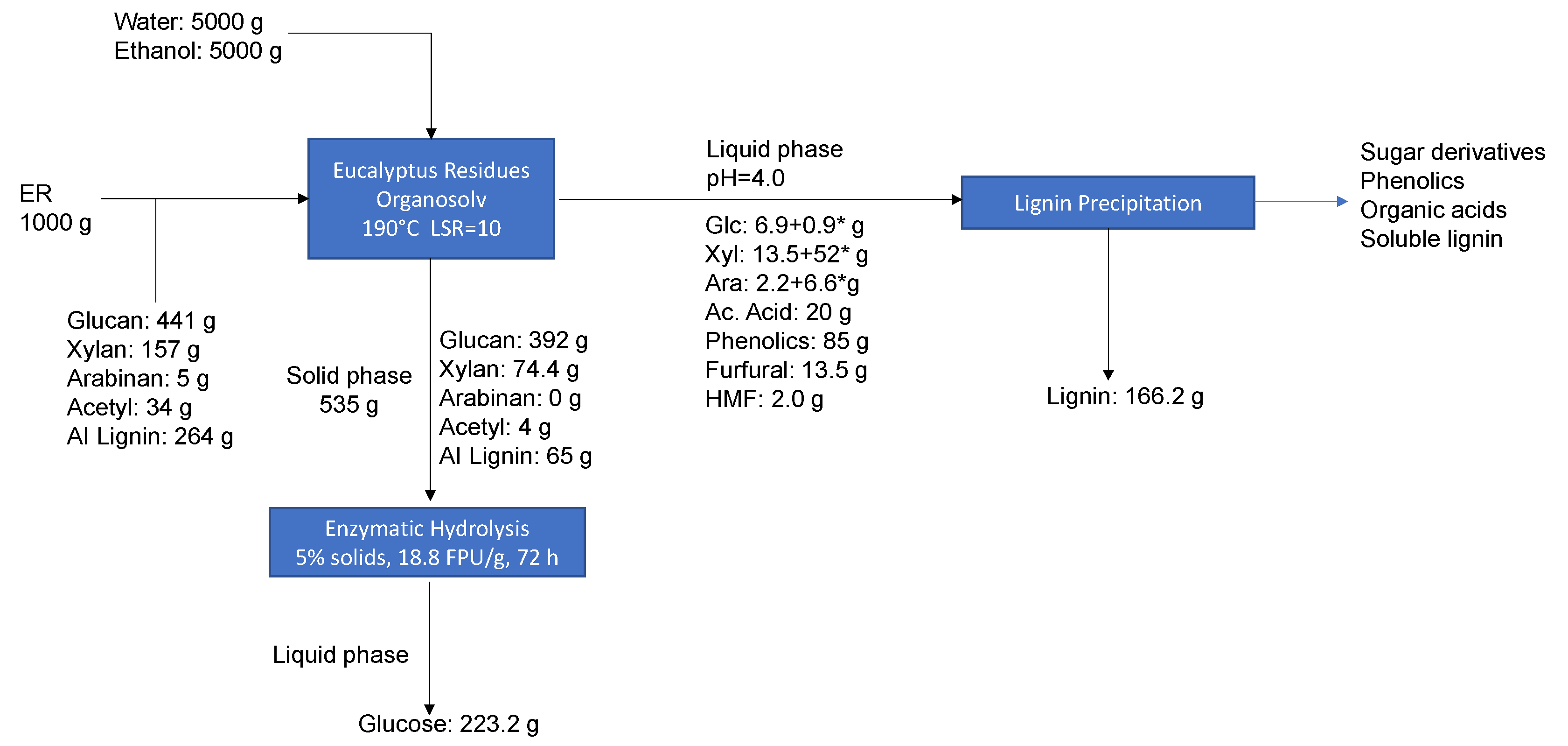
3.7. Mass Balances
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Girio, F.M.; Fonseca, C.; Carvalheiro, F.; Duarte, L.C.; Marques, S.; Bogel-Lukasik, R. Hemicelluloses for fuel ethanol: A review. Bioresour. Technol. 2010, 101, 4775–4800. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, F.; Duarte, L.C.; Gírio, F.; Moniz, P. Hydrothermal/Liquid Hot Water pretreatment (Autohydrolysis): A multipurpose process for biomass upgrading. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Mussatto, S.I., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 315–347. [Google Scholar] [CrossRef]
- Ma, C.; Kim, T.H.; Liu, K.; Ma, M.G.; Choi, S.E.; Si, C. Multifunctional lignin-based composite materials for emerging applications. Front. Bioeng. Biotechnol. 2021, 9, 708976. [Google Scholar] [CrossRef] [PubMed]
- Gosselink, R.J.A. Lignin as a Renewable Aromatic Resource for the Chemical Industry. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2011. [Google Scholar]
- Mastrolitti, S.; Borsella, E.; Giuliano, A.; Petrone, M.T.; Bari, I.D.; Gosselink, R.; Erven, G.V.; Annevelink, E.; Triantafyllidis, K.S.; Stichnothe, H. Sustainable Lignin Valorization: Technical Lignin, Processes and Market Development; IEA TASK 42. 2021. Available online: https://task42.ieabioenergy.com/wp-content/uploads/sites/10/2021/11/Sustainable-Lignin-Valorization_rev26-01-2022.pdf (accessed on 30 June 2022).
- Robinson, J.M.; Burgess, C.E.; Bently, M.A.; Brasher, C.D.; Horne, B.O.; Lillard, D.M.; Macias, J.M.; Mandal, H.D.; Mills, S.C.; O’Hara, K.D.; et al. The use of catalytic hydrogenation to intercept carbohydrates in a dilute acid hydrolysis of biomass to effect a clean separation from lignin. Biomass Bioenerg. 2004, 26, 473–483. [Google Scholar] [CrossRef]
- Martins, M.M.; Carvalheiro, F.; Girio, F. An overview of lignin pathways of valorization: From isolation to refining and conversion into value-added products. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Zhang, Z.; Harrison, M.D.; Rackemann, D.W.; Doherty, W.O.S.; O’Hara, I.M. Organosolv pretreatment of plant biomass for enhanced enzymatic saccharification. Green Chem. 2016, 18, 360–381. [Google Scholar] [CrossRef] [Green Version]
- Huijgen, W.J.J.; Smit, A.T.; Reith, J.H.; den Uil, H. Catalytic organosolv fractionation of willow wood and wheat straw as pretreatment for enzymatic cellulose hydrolysis. J. Chem. Technol. Biotechnol. 2011, 86, 1428–1438. [Google Scholar] [CrossRef] [Green Version]
- Vilcocq, L.; Crepet, A.; Jame, P.; Carvalheiro, F.; Duarte, L.C. Combination of autohydrolysis and catalytic hydrolysis of biomass for the production of hemicellulose oligosaccharides and sugars. Reactions 2022, 3, 30–46. [Google Scholar] [CrossRef]
- Santibanez, L.; Henriquez, C.; Corro-Tejeda, R.; Bernal, S.; Armijo, B.; Salazar, O. Xylooligosaccharides from lignocellulosic biomass: A comprehensive review. Carbohydr. Polym. 2021, 251, 117118. [Google Scholar] [CrossRef]
- Moniz, P.; Ho, A.L.; Duarte, L.C.; Kolida, S.; Rastall, R.A.; Pereira, H.; Carvalheiro, F. Assessment of the bifidogenic effect of substituted xylo-oligosaccharides obtained from corn straw. Carbohydr. Polym. 2016, 136, 466–473. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Reith, J.H.; den Uil, H. Pretreatment and fractionation of wheat straw by an acetone-based organosolv process. Ind. Eng. Chem. Res. 2010, 49, 10132–10140. [Google Scholar] [CrossRef] [Green Version]
- Romaní, A.; Ruiz, H.A.; Teixeira, J.A.; Domingues, L. Valorization of Eucalyptus wood by glycerol-organosolv pretreatment within the biorefinery concept: An integrated and intensified approach. Renew. Energy 2016, 95, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Viola, E.; Zimbardi, F.; Morgana, M.; Cerone, N.; Valerio, V.; Romanelli, A. Optimized organosolv pretreatment of biomass residues using 2-Methyltetrahydrofuran and n-Butanol. Processes 2021, 9, 2051. [Google Scholar] [CrossRef]
- Wildschut, J.; Smit, A.T.; Reith, J.H.; Huijgen, W.J. Ethanol-based organosolv fractionation of wheat straw for the production of lignin and enzymatically digestible cellulose. Bioresour. Technol. 2013, 135, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Technol. 2016, 199, 21–33. [Google Scholar] [CrossRef]
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. Biotechnol. 2009, 82, 815–827. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012.
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL/TP-510-42619; National Renewable Energy Laboratory: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.J.; Sluiter, J.; Templeton, D. Determination of Ash in Biomass; NREL/TP-510-42622; NREL: Golden, CO, USA, 2008.
- Hames, B.; Scarlata, C.; Sluiter, A. Determination of Protein Content in Biomass; NREL: Golden, CO, USA, 2008.
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.J.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples; NREL/TP-510-42623; NREL: Golden, CO, USA, 2008.
- Roseiro, L.B.; Tavares, C.S.; Roseiro, J.C.; Rauter, A.P. Antioxidants from aqueous decoction of carob pods biomass (Ceretonia siliqua L.): Optimisation using response surface methodology and phenolic profile by capillary electrophoresis. Ind. Crops Prod. 2013, 44, 119–126. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Chemical characterization of barks from Picea abies and Pinus sylvestris after fractioning into different particle sizes. Ind. Crops Prod. 2012, 36, 395–400. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Silva-Fernandes, T.; Duarte, L.C.; Girio, F.M. Wheat straw autohydrolysis: Process optimization and products characterization. Appl. Biochem. Biotechnol. 2009, 153, 84–93. [Google Scholar] [CrossRef]
- Khan, T.S.; Mubeen, U. Wheat straw: A pragmatic overview. Curr. Res. J. Biol. Sci. 2012, 4, 673–675. [Google Scholar]
- Miranda, I.; Pereira, H. The variation of chemical composition and pulping yield with age and growth factors in young Eucalyptus globulus. Wood Fiber Sci. 2002, 34, 140–145. [Google Scholar]
- Bruun, S.; Jensen, J.W.; Magid, J.; Lindedam, J.; Engelsen, S.B. Prediction of the degradability and ash content of wheat straw from different cultivars using near infrared spectroscopy. Ind. Crops Prod. 2010, 31, 321–326. [Google Scholar] [CrossRef]
- Monção, M.; Hruzová, K.; Rova, U.; Matsakas, L.; Christakopoulos, P. Organosolv fractionation of birch sawdust: Establishing a lignin-first biorefinery. Molecules 2021, 26, 6754. [Google Scholar] [CrossRef]
- Lopes, T.F.; Carvalheiro, F.; Duarte, L.C.; Girio, F.; Quintero, J.A.; Aroca, G. Techno-economic and life-cycle assessments of small-scale biorefineries for isobutene and xylo-oligosaccharides production: A comparative study in Portugal and Chile. Biofuels Bioprod. Biorefin. 2019, 13, 1321–1332. [Google Scholar] [CrossRef]
- Silva-Fernandes, T.; Duarte, L.C.; Carvalheiro, F.; Loureiro-Dias, M.C.; Fonseca, C.; Girio, F. Hydrothermal pretreatment of several lignocellulosic mixtures containing wheat straw and two hardwood residues available in Southern Europe. Bioresour. Technol. 2015, 183, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Smit, A.T.; Huijgen, W.J.J.; Grisel, R.J.H. Process for the Treatment of Lignocellulosic Biomass. Patent WO 2014/126471, 21 August 2014. [Google Scholar]
- Wang, T.-P.; Li, H.; Yuan, J.-M.; Li, W.-X.; Li, K.; Huang, Y.-B.; Xiao, L.-P.; Lu, Q. Structures and pyrolytic characteristics of organosolv lignins from typical softwood, hardwood and herbaceous biomass. Ind. Crops Prod. 2021, 171, 113912. [Google Scholar] [CrossRef]
- Zhou, J.-M.; Ibrahim, R.K. Tricin—A potential multifunctional nutraceutical. Phytochem. Rev. 2009, 9, 413–424. [Google Scholar] [CrossRef]
- Xu, C.; Liao, B.; Shi, W. Organosolv pretreatment of pine sawdust for bio-ethanol production. In Pretreatment Techniques for Biofuels and Biorefineries; Fang, Z., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 435–457. [Google Scholar] [CrossRef]
- Wilkinson, S.; Smart, K.A.; James, S.; Cook, D.J. Maximising high solid loading enzymatic saccharification yield from acid-catalysed hydrothermally-pretreated brewers spent grain. Biofuel Res. J. 2016, 3, 417–429. [Google Scholar] [CrossRef] [Green Version]
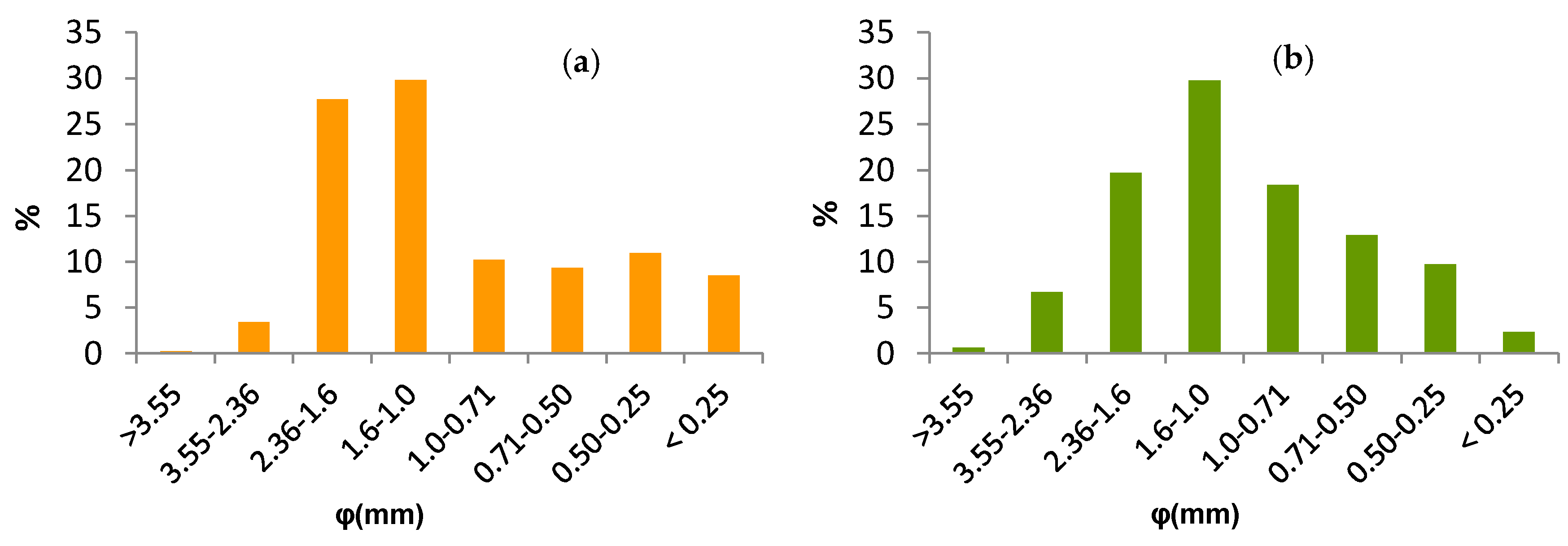
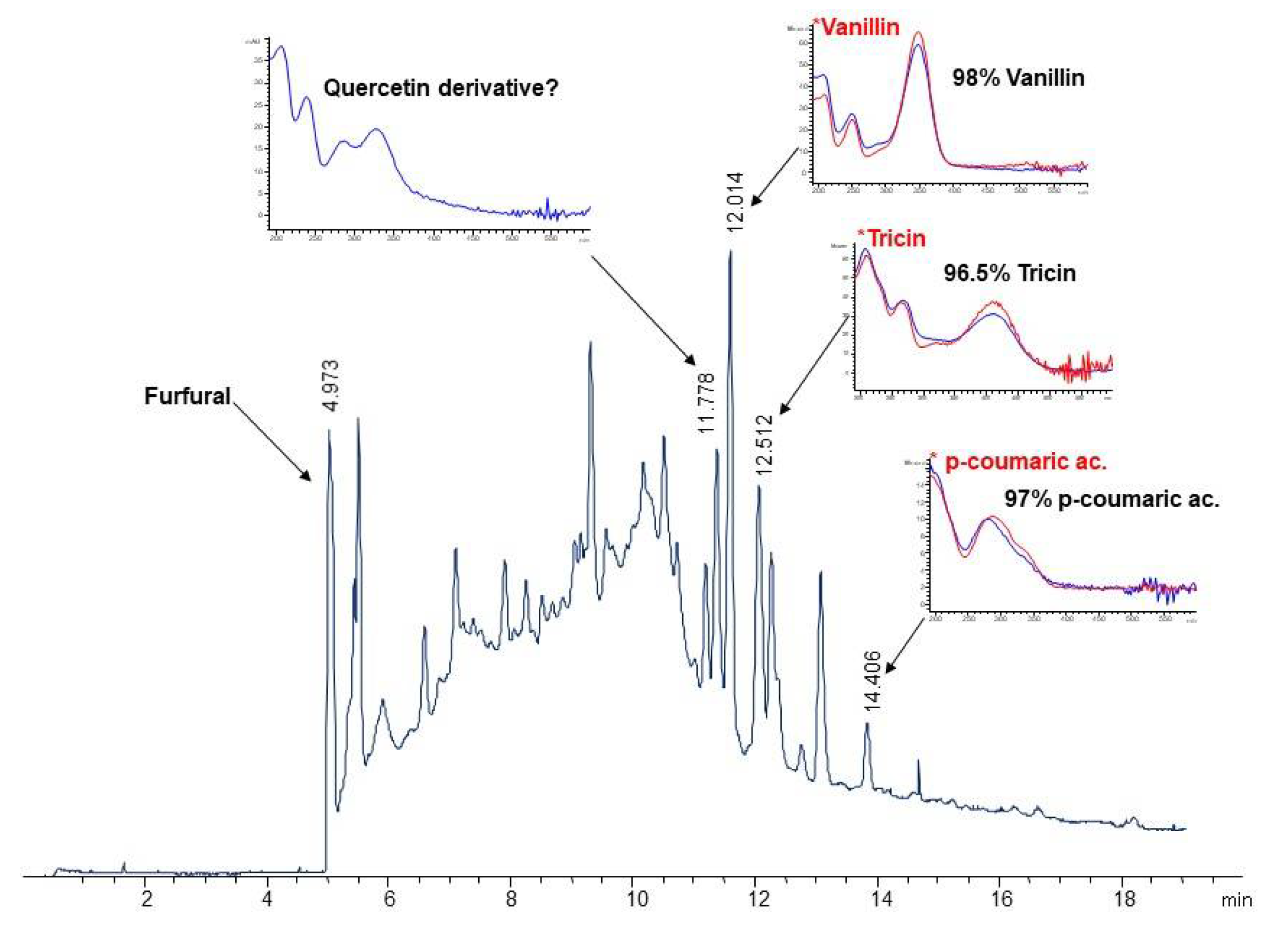





| Component | Wheat Straw (WS) | Eucalyptus Residues (ER) |
|---|---|---|
| Glucan | 35.9 | 44.1 |
| Hemicellulose | 26.7 | 19.6 |
| Xylan | 22.1 | 15.7 |
| Arabinosyl groups | 2.0 | 0.5 |
| Acetyl groups | 2.6 | 3.4 |
| Lignin | 16.7 | 33.8 |
| Acid-insoluble | 15.5 | 26.4 |
| Acid-soluble | 1.2 | 7.4 |
| Ash | 11.4 | 0.99 |
| Extractives | ||
| Water | 9.4 | 3.3 |
| Water (not ash) | 5.1 | 0.17 |
| Ethanol | 1.4 | 1.5 |
| Time (min) | pH | Solid Yield (%) | Glucan Solub. (%) | Xylan Solub. (%) | Delignification (%) | |
|---|---|---|---|---|---|---|
| WS | 0 | 5.6 | 86.4 | 6.6 | 9.1 | 19.8 |
| 15 | 5.2 | 78.8 | 9.8 | 28.0 | 34.3 | |
| 30 | 5.1 | 74.9 | 6.7 | 48.8 | 40.7 | |
| 60 | 4.9 | 66.7 | 18.9 | 46.0 | 46.8 | |
| 120 | 4.5 | 62.8 | 14.2 | 44.6 | 59.0 | |
| ER | 0 | 5.0 | 87.6 | 11.3 | 14.1 | 26.1 |
| 15 | 4.5 | 68.9 | 25.4 | 39.8 | 45.8 | |
| 30 | 4.0 | 68.6 | 22.3 | 59.3 | 58.8 | |
| 60 | 4.1 | 63.7 | 17.8 | 47.3 | 64.4 | |
| 120 | 4.0 | 58.4 | 12.5 | 51.5 | 70.9 |
| Acetic Acid (mM) | pH | Solid Yield (%) | Glucan Solub. (%) | Xylan Solub. (%) | Delignification (%) | |
|---|---|---|---|---|---|---|
| WS | 50 | 4.46 | 59.4 | 7.6 | 60.0 | 61.8 |
| 100 | 4.43 | 59.0 | 14.2 | 68.5 | 58.8 | |
| 200 | 4.38 | 57.2 | 17.7 | 65.2 | 64.4 | |
| 500 | 4.07 | 56.7 | 21.4 | 65.2 | 61.2 | |
| 1000 | 3.93 | 55.1 | 12.7 | 65.2 | 55.0 | |
| ER | 50 | 3.91 | 55.9 | 2.24 | 67.0 | 65.4 |
| 100 | 3.93 | 56.3 | 0 | 100 | 66.9 | |
| 200 | 3.87 | 53.8 | 2.51 | 100 | 68.6 | |
| 500 | 3.70 | 52.6 | 3.50 | 100 | 72.5 | |
| 1000 | 3.57 | 51.1 | 0.23 | 100 | 79.0 |
| Catalyst | pH | Solid Yield (%) | Glucan Solub. (%) | Xylan Solub. (%) | Delignification (%) | |
|---|---|---|---|---|---|---|
| WS | - | 5.30 | 84.6 | n.a | n.a. | n.a. |
| CH3COOH | 5.12 | 89.5 | 19.6 | 13.6 | 12.0 | |
| H2SO4 | 2.00 | 54.4 | 0 | 71.1 | 72.4 | |
| ER | - | 4.60 | 92.5 | 6.6 | 0 | 14.1 |
| CH3COOH | 4.17 | 91.6 | 7.2 | 0 | 20.3 | |
| H2SO4 | 1.70 | 49.8 | 5.5 | 100 | 74.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalheiro, F.; Duarte, L.C.; Pires, F.; Van-Dúnem, V.; Sanfins, L.; Roseiro, L.B.; Gírio, F. Effective Mild Ethanol-Based Organosolv Pre-Treatment for the Selective Valorization of Polysaccharides and Lignin from Agricultural and Forestry Residues. Energies 2022, 15, 5654. https://doi.org/10.3390/en15155654
Carvalheiro F, Duarte LC, Pires F, Van-Dúnem V, Sanfins L, Roseiro LB, Gírio F. Effective Mild Ethanol-Based Organosolv Pre-Treatment for the Selective Valorization of Polysaccharides and Lignin from Agricultural and Forestry Residues. Energies. 2022; 15(15):5654. https://doi.org/10.3390/en15155654
Chicago/Turabian StyleCarvalheiro, Florbela, Luís C. Duarte, Filipa Pires, Vanmira Van-Dúnem, Luís Sanfins, Luísa B. Roseiro, and Francisco Gírio. 2022. "Effective Mild Ethanol-Based Organosolv Pre-Treatment for the Selective Valorization of Polysaccharides and Lignin from Agricultural and Forestry Residues" Energies 15, no. 15: 5654. https://doi.org/10.3390/en15155654
APA StyleCarvalheiro, F., Duarte, L. C., Pires, F., Van-Dúnem, V., Sanfins, L., Roseiro, L. B., & Gírio, F. (2022). Effective Mild Ethanol-Based Organosolv Pre-Treatment for the Selective Valorization of Polysaccharides and Lignin from Agricultural and Forestry Residues. Energies, 15(15), 5654. https://doi.org/10.3390/en15155654







