Effect of Temperature Gradient on Chloride Ion Diffusion in Nuclear Reactor Containment Building Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Concrete and Test Samples
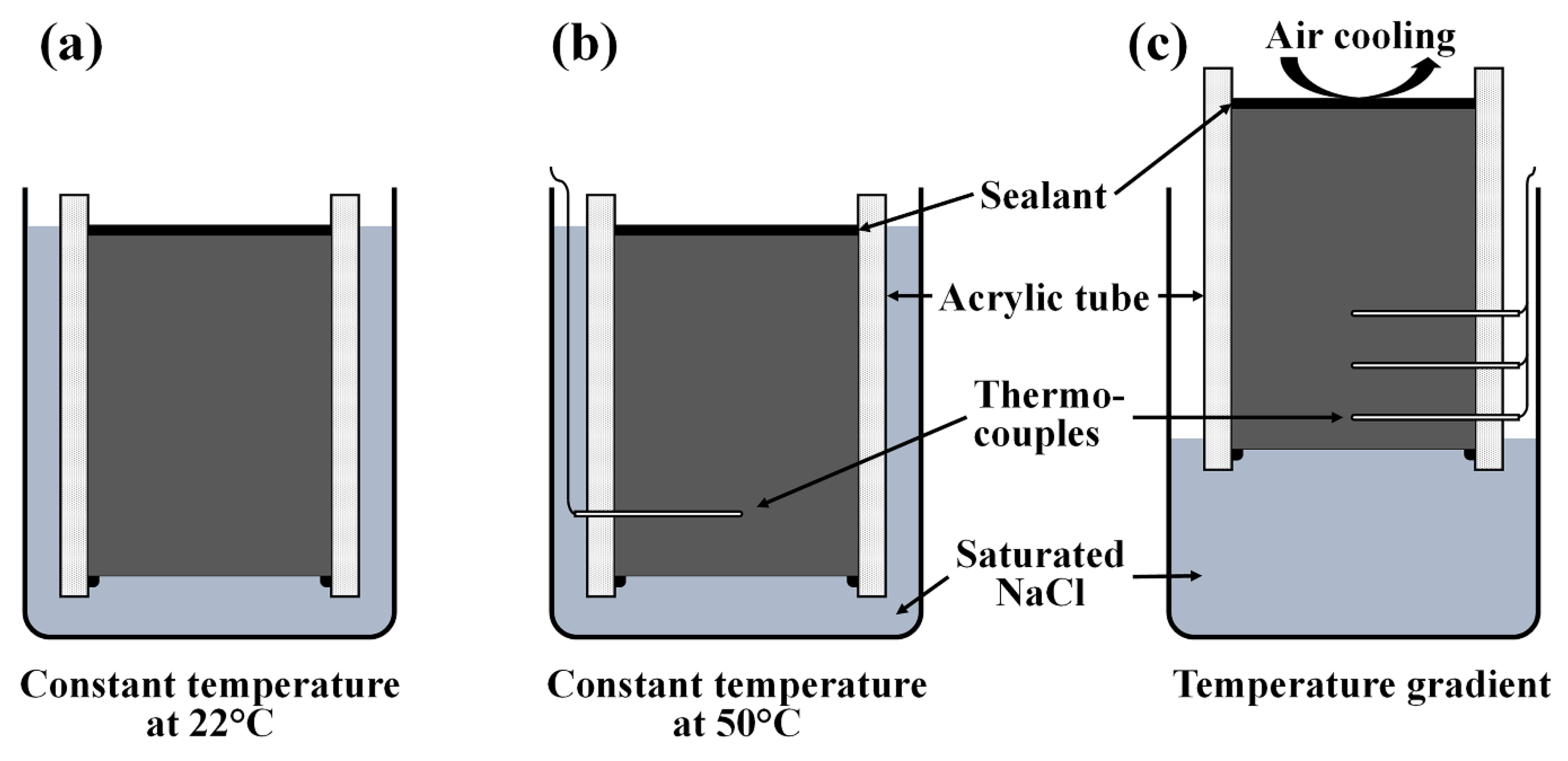
2.2. Chloride Diffusion Tests
2.3. Chloride Ion Analysis
3. Results and Discussion
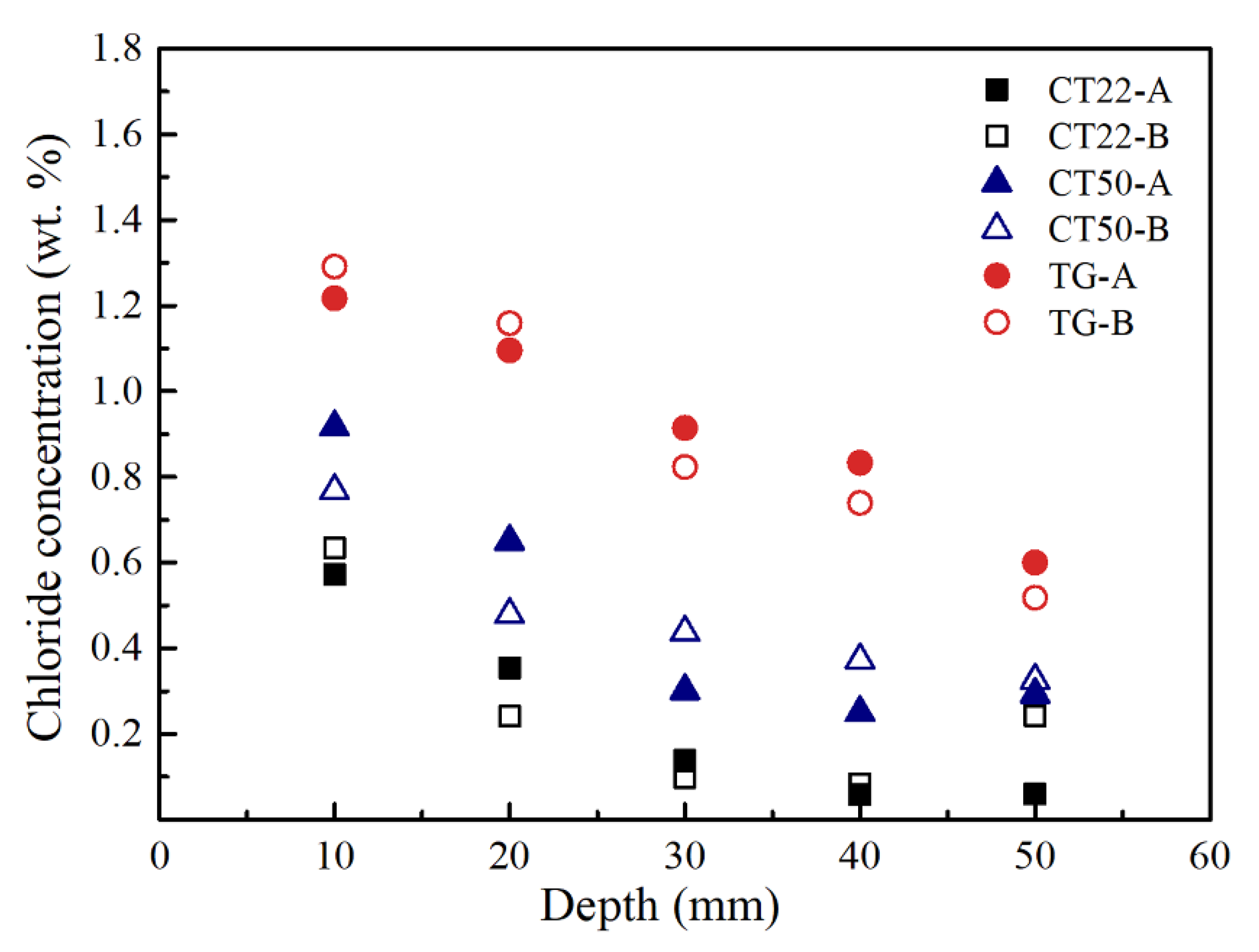
3.1. Chloride Concentrations in Concrete
3.2. Determination of Chloride Diffusion Coefficients in Constant Temperatures
- (i)
- Cs is equal to the chloride concentration in the concrete surface of which pores are filled with the NaCl solution;
- (ii)
- Cs does not change with time;
- (iii)
- From Assumption (i), Cs is a single value for all the temperature conditions.
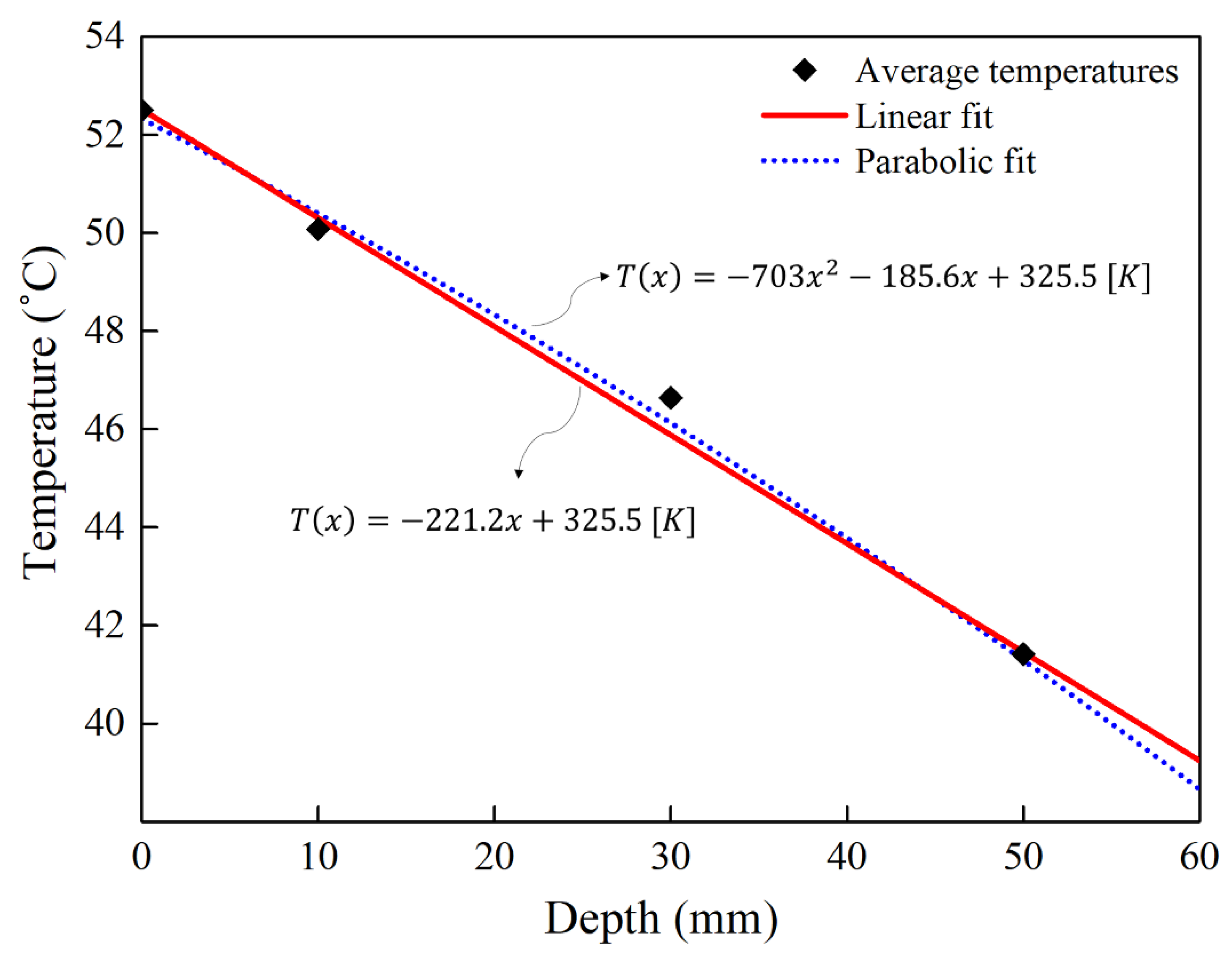
3.3. Temperature Profile in Concrete in Temperature Gradient Condition
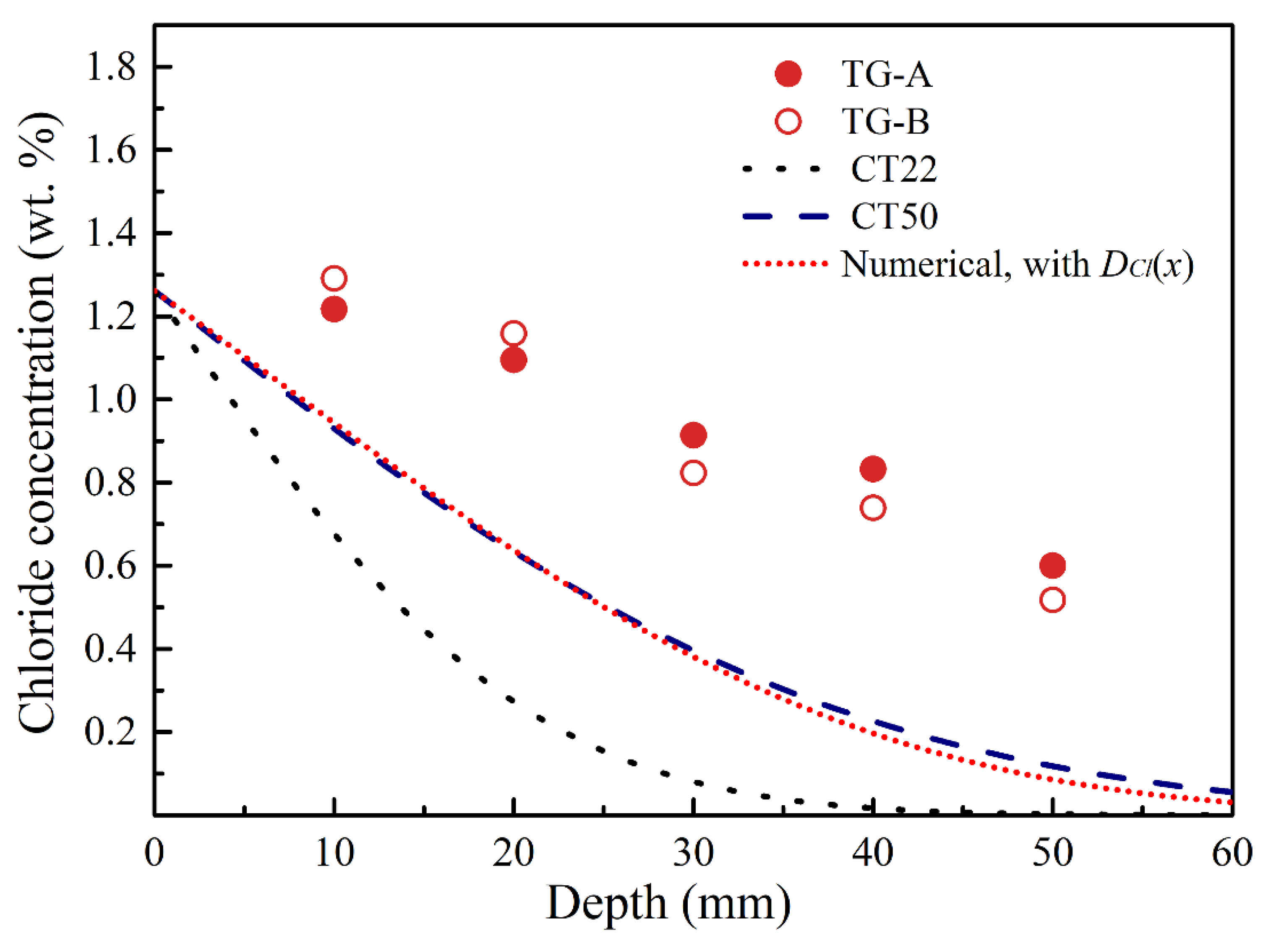
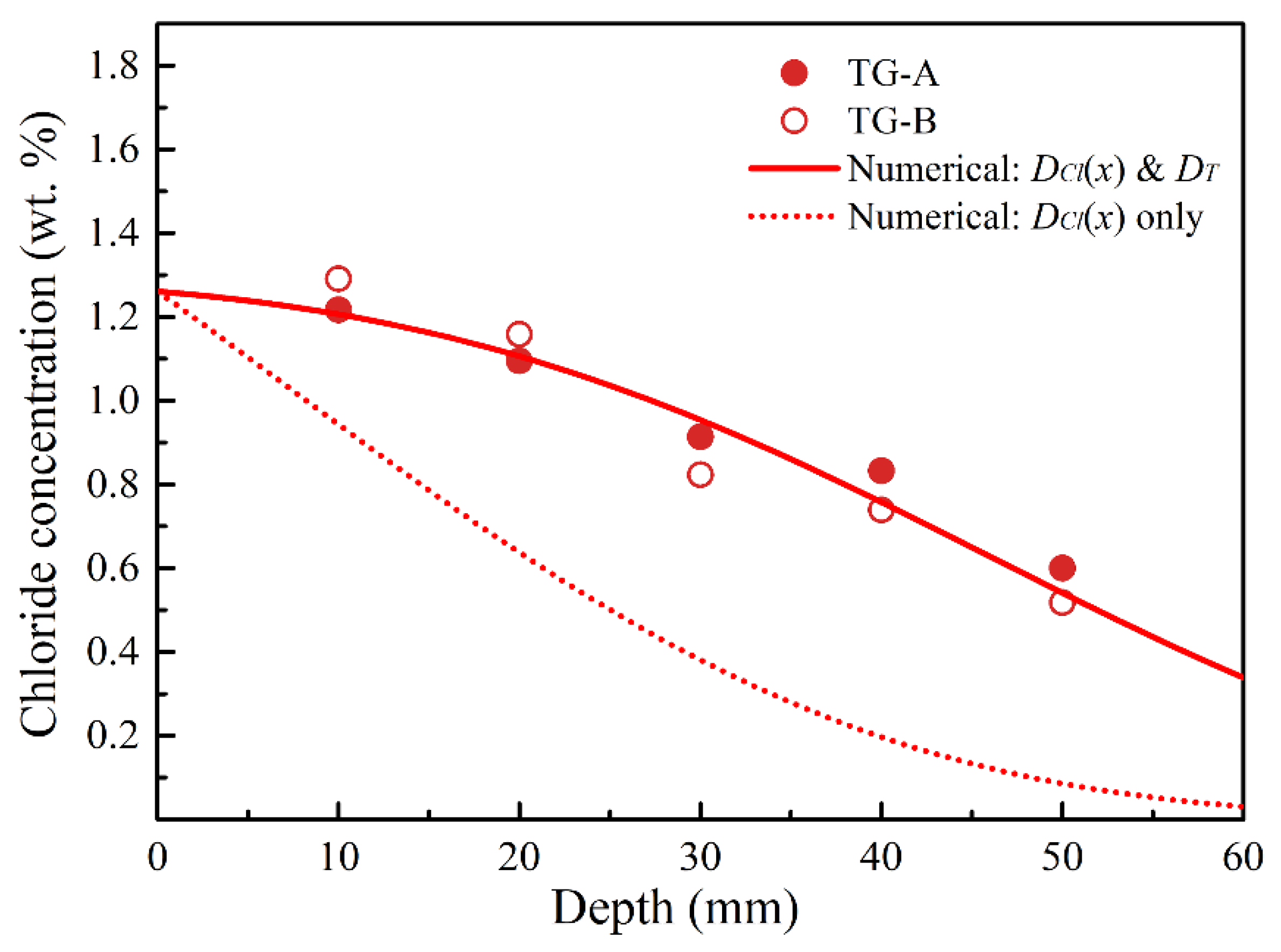
3.4. Determination of Chloride Diffusion Coefficient in Temperature Gradient Condition
3.5. Determination of Thermo-Diffusion Coefficient
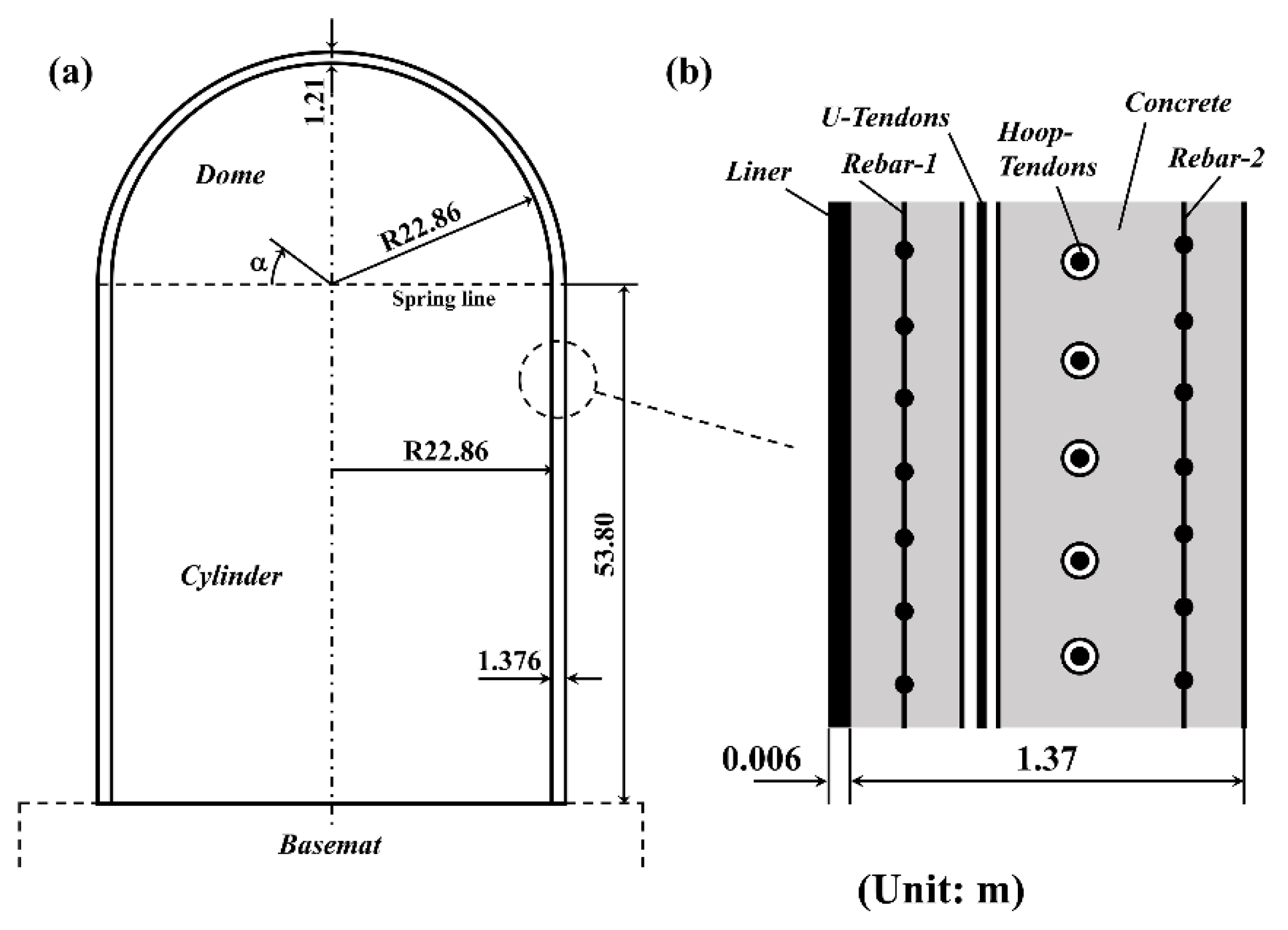
3.6. Estimation of Cl Diffusion in RCBs
4. Conclusions
- Using isothermal diffusion test results, the mass diffusion coefficients (DCl) of chloride ions were determined by applying Fick’s law of diffusion. In the curve fitting, the surface chloride concentration was estimated by the concrete sample porosity, which was judged to be a reasonable value.
- At 50 °C, the value of the mass diffusion coefficient (DCl) was 3.7 times higher than at 20 °C with the activation energy of 34.6 kJ/mol.
- In the temperature gradient condition (TG) varying from 52.5 °C to 22 °C, much higher chloride concentration values were measured than in the constant 50 °C condition.
- For the TG condition, Fick’s law was rewritten in a binary form including both the mass diffusion and thermo-diffusion terms. By the curve fitting of the TG results, the thermo-diffusion coefficient (DT) was determined to be 6.201 × 10−11 m2/s·K.
- The values of the mass diffusion and thermo-diffusion coefficients were applied to the RCBs in APR1400 nuclear power plants. With the temperature gradient and daily variation in atmosphere temperature being considered, the time to corrosion initiation was reduced by 30~40% compared with the prediction without considering the temperature gradient.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alhanaee, S.; Yi, Y.; Schiffer, A. Ultimate pressure capacity of nuclear reactor containment buildings under unaged and aged conditions. Nucl. Eng. Des. 2018, 335, 128–139. [Google Scholar] [CrossRef]
- Moreno, M.; Morris, W.; Alvarez, M.G.; Duffó, G.S. Corrosion of reinforcing steel in simulated concrete pore solutions. Corros. Sci. 2004, 46, 2681–2699. [Google Scholar] [CrossRef]
- Zhang, J.; Lounis, Z. Sensitivity analysis of simplified diffusion-based corrosion initiation model of concrete structures exposed to chlorides. Cem. Concr. Res. 2006, 36, 1312–1323. [Google Scholar] [CrossRef] [Green Version]
- Aveldaño, R.R.; Ortega, N.F. Characterization of concrete cracking due to corrosion of reinforcements in different environments. Constr. Build. Mater. 2011, 25, 630–637. [Google Scholar] [CrossRef]
- Angst, U.M.; Geiker, M.R.; Alonso, M.C.; Polder, R.; Isgor, O.B.; Elsener, B.; Wong, H.; Michel, A.; Hornbostel, K.; Gehlen, C.; et al. The effect of the steel–concrete interface on chloride-induced corrosion initiation in concrete: A critical review by RILEM TC 262-SCI. Mater. Struct. 2019, 52, 88. [Google Scholar] [CrossRef]
- Qasem, M.; Yi, Y.; Al Hanaei, S.; Cho, P. Electrochemical behavior of reinforcing steel for nuclear reactor containment buildings. IOSR J. Mech. Civil Eng. 2016, 13, 80–90. [Google Scholar]
- Jung, S.-H.; Ryu, H.-S.; Karthick, S.; Kwon, S.-J. Time and Crack Effect on Chloride Diffusion for Concrete with Fly Ash. Int. J. Concr. Struct. Mater. 2018, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Al Saadi, S.; Yi, Y. Dry storage of spent nuclear fuel in UAE—Economic aspect. Ann. Nucl. Energy 2015, 75, 527–535. [Google Scholar] [CrossRef]
- Tian, Y.; Jin, N.; Jin, X. Coupling effect of temperature and relative humidity diffusion in concrete under ambient conditions. Constr. Build. Mater. 2018, 159, 673–689. [Google Scholar] [CrossRef]
- Stanish, K.; Thomas, M. The use of bulk diffusion tests to establish time-dependent concrete chloride diffusion coefficients. Cem. Concr. Res. 2003, 33, 55–62. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, M. Pore-scale modeling of chloride ion diffusion in cement microstructures. Cem. Concr. Compos. 2018, 85, 92–104. [Google Scholar] [CrossRef]
- Xi, Y.; Willam, K.; Frangopol, D.M. Multiscale Modeling of Interactive Diffusion Processes in Concrete. J. Eng. Mech. 2000, 126, 258–265. [Google Scholar] [CrossRef]
- Titi, H.H.; Tabatabai, H. Effect of coarse aggregate type on chloride ion penetration in concrete. Constr. Build. Mater. 2018, 162, 871–880. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, L.; Wang, Y.; Liu, C.; Li, Q. Effects of coarse aggregates on chloride diffusion coefficients of concrete and interfacial transition zone under experimental drying-wetting cycles. Constr. Build. Mater. 2018, 185, 230–245. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Xi, Y. The effect of w/c ratio and aggregate volume fraction on chloride penetration in non-saturated concrete. Constr. Build. Mater. 2018, 191, 260–269. [Google Scholar] [CrossRef]
- Petcherdchoo, A. Closed-form solutions for modeling chloride transport in unsaturated concrete under wet-dry cycles of chloride attack. Constr. Build. Mater. 2018, 176, 638–651. [Google Scholar] [CrossRef]
- Real, S.; Bogas, J.A. Chloride ingress into structural lightweight aggregate concrete in real marine environment. Mar. Struct. 2018, 61, 170–187. [Google Scholar] [CrossRef]
- Climent, M.A.; de Vera, G.; López, J.F.; Viqueira, E.; Andrade, C. A test method for measuring chloride diffusion coefficients through nonsaturated concrete Part I. The instantaneous plane source diffusion case. Cem. Concr. Res. 2002, 32, 1113–1123. [Google Scholar] [CrossRef]
- de Vera, G.; Climent, M.A.; Viqueira, E.; Antón, C.; Andrade, C. A test method for measuring chloride diffusion coefficients through partially saturated concrete. Part II: The instantaneous plane source diffusion case with chloride binding consideration. Cem. Concr. Res. 2007, 37, 714–724. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, G. A model for predicting the relative chloride diffusion coefficient in unsaturated cementitious materials. Cem. Concr. Res. 2019, 115, 133–144. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Z.; Ye, G. Dependence of unsaturated chloride diffusion on the pore structure in cementitious materials. Cem. Concr. Res. 2020, 127, 105919. [Google Scholar] [CrossRef]
- McGrath, P.F.; Hooton, R.D. Re-evaluation of the AASHTO T259 90-day salt ponding test. Cem. Concr. Res. 1999, 29, 1239–1248. [Google Scholar] [CrossRef]
- Erdoǧdu, Ş.; Kondratova, I.L.; Bremner, T.W. Determination of chloride diffusion coefficient of concrete using open-circuit potential measurements. Cem. Concr. Res. 2004, 34, 603–609. [Google Scholar] [CrossRef]
- Lizarazo-Marriaga, J.; Claisse, P. Determination of the concrete chloride diffusion coefficient based on an electrochemical test and an optimization model. Mater. Chem. Phys. 2009, 117, 536–543. [Google Scholar] [CrossRef]
- Thomas, R.J.; Ariyachandra, E.; Lezama, D.; Peethamparan, S. Comparison of chloride permeability methods for Alkali-Activated concrete. Constr. Build. Mater. 2018, 165, 104–111. [Google Scholar] [CrossRef]
- Isteita, M.; Xi, Y. The effect of temperature variation on chloride penetration in concrete. Constr. Build. Mater. 2017, 156, 73–82. [Google Scholar] [CrossRef]
- ASTM C1152/C1152M-04(2012)e1; Standard Test Method for Acid-Soluble Chloride in Mortar and Concrete. ASTM International: West Conshohocken, PA, USA, 2012.
- Climent, M.A.; Viqueira, E.; de Vera, G.; López-Atalaya, M.M. Analysis of acid-soluble chloride in cement, mortar, and concrete by potentiometric titration without filtration steps. Cem. Concr. Res. 1999, 29, 893–898. [Google Scholar] [CrossRef]
- Arya, C.; Newman, J.B. An assessment of four methods of determining the free chloride content of concrete. Mater. Struct. 1990, 23, 319–330. [Google Scholar] [CrossRef]
- Mohammed, T.U.; Hamada, H. Relationship between free chloride and total chloride contents in concrete. Cem. Concr. Res. 2003, 33, 1487–1490. [Google Scholar] [CrossRef]
- Pargar, F.; Koleva, D.A.; van Breugel, K. Determination of Chloride Content in Cementitious Materials: From Fundamental Aspects to Application of Ag/AgCl Chloride Sensors. Sensors 2017, 17, 2482. [Google Scholar] [CrossRef] [Green Version]
- Dousti, A.; Shekarchi, M. Relationship between free and total chloride profiles for optimization of service life prediction in chloride exposed RC structures. In Proceedings of the Third International Conference on Modeling, Simulation and Applied Optimization, Sharjah, United Arab Emirates, 20–22 January 2009. [Google Scholar]
- ASTM C642-13; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete. ASTM International: West Conshohocken, PA, USA, 2013.
- Platten, J.K. The Soret Effect: A Review of Recent Experimental Results. J. Appl. Mech. 2006, 73, 5–15. [Google Scholar] [CrossRef]
- Cygan, R.; Jarrell, G. Thermal Diffusion Processes in Aqueous Sodium Chloride Solutions; Sandia National Labs: Albuquerque, NM, USA, 1992. [Google Scholar]
- Caldwell, D.R.; Eide, S.A. Soret coefficient and isothermal diffusivity of aqueous solutions of five principal salt constituents of seawater, Deep Sea Research Part A. Oceanogr. Res. Pap. 1981, 28, 1605–1618. [Google Scholar] [CrossRef]
- Rahman, M.A.; Saghir, M.Z. Thermodiffusion or Soret effect: Historical review. Int. J. Heat Mass Transfer. 2014, 73, 693–705. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]




| Distilled Water | Portland Cement | Dune Sand | Coarse Aggregate * | Fine Aggregate | Total | |
|---|---|---|---|---|---|---|
| Weight (kg) | 7.284 | 12.14 | 18.21 | 18.21 | 18.21 | 74.054 |
| % w/w | 9.84 | 16.39 | 24.59 | 24.59 | 24.59 | 100.00 |
| Temperature (°C) | Sample | Depth (mm) | ||||
|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | ||
| 22 | CT22-A | 0.572 | 0.354 | 0.138 | 0.058 | 0.060 |
| CT22-B | 0.634 | 0.241 | 0.099 | 0.084 | 0.242 | |
| ΔCCl | 0.062 | 0.113 | 0.039 | 0.026 | 0.182 | |
| 50 | CT50-A | 0.916 | 0.650 | 0.301 | 0.250 | 0.292 |
| CT50-B | 0.768 | 0.478 | 0.437 | 0.372 | 0.325 | |
| ΔCCl | 0.148 | 0.172 | 0.136 | 0.122 | 0.033 | |
| Temperature gradient (TG) | TG-A | 1.217 | 1.095 | 0.914 | 0.833 | 0.600 |
| TG-B | 1.291 | 1.158 | 0.823 | 0.739 | 0.518 | |
| ΔCCl | 0.074 | 0.063 | 0.091 | 0.094 | 0.082 | |
| Sample | DCl (×10−11 m2/s) | SR |
|---|---|---|
| CT22-A | 5.37 | 0.025 |
| CT22-B | 4.73 | 0.064 |
| CT50-A | 18.1 | 0.040 |
| CT50-B | 19.2 | 0.113 |
| ΔT (°C/Wall Thickness) | Time to Corrosion Initiation (Month) | |||||
|---|---|---|---|---|---|---|
| Fixed ΔT | Daily Variation T | |||||
| Without Thermo-Diffusion (A) | With Thermo-Diffusion (B) | B/A | Without Thermo-Diffusion (C) | With Thermo-Diffusion (D) | D/C | |
| 10 | 64 | 42 | 0.66 | 84 | 59 | 0.70 |
| 20 | 42 | 25 | 0.60 | 63 | 41 | 0.65 |
| 30 | 29 | 17 | 0.59 | 55 | 29 | 0.53 |
| 40 | 20 | 12 | 0.60 | 40 | 23 | 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, B.; Cho, P.; Shittu, R.A.; Kim, T.-Y.; Rostron, P.; AlFantazi, A.; Yi, Y. Effect of Temperature Gradient on Chloride Ion Diffusion in Nuclear Reactor Containment Building Concrete. Energies 2022, 15, 5581. https://doi.org/10.3390/en15155581
An B, Cho P, Shittu RA, Kim T-Y, Rostron P, AlFantazi A, Yi Y. Effect of Temperature Gradient on Chloride Ion Diffusion in Nuclear Reactor Containment Building Concrete. Energies. 2022; 15(15):5581. https://doi.org/10.3390/en15155581
Chicago/Turabian StyleAn, Boohyun, Pyungyeon Cho, Remilekun A. Shittu, Tae-Yeon Kim, Paul Rostron, Akram AlFantazi, and Yongsun Yi. 2022. "Effect of Temperature Gradient on Chloride Ion Diffusion in Nuclear Reactor Containment Building Concrete" Energies 15, no. 15: 5581. https://doi.org/10.3390/en15155581
APA StyleAn, B., Cho, P., Shittu, R. A., Kim, T.-Y., Rostron, P., AlFantazi, A., & Yi, Y. (2022). Effect of Temperature Gradient on Chloride Ion Diffusion in Nuclear Reactor Containment Building Concrete. Energies, 15(15), 5581. https://doi.org/10.3390/en15155581







