Critical Review on Nanomaterials for Enhancing Bioconversion and Bioremediation of Agricultural Wastes and Wastewater
Abstract
:1. Introduction
2. Recycling and Valorizing Agricultural Wastes and Wastewater
2.1. Agricultural Solid Wastes and Wastewater
2.2. Bioconversion and Bioremediation of Agricultural Wastes
2.3. Nanomaterials for Enhancing Bioconversion and Bioremediation of Agricultural Wastes
3. Nanoparticles for the Enhancement of Anaerobic Digestion
3.1. Nanomaterials for Facilitating Direct Interspecies Electron Transfer (DIET) in AD
3.2. Nanomaterials for Preventing Sulfur/Ammonia Inhibition in AD
3.3. Nanoparticles as Trace Micronutrients
3.4. Nanomaterials for Immobilizing of Enzymes and Microorganisms
4. Nanoparticles for the Enhancement of Microalgae Cultivation
4.1. Metallic Nanoparticles as Micronutrients for Algal Cultivation
4.2. Nanoparticles for CO2 Supply
4.3. Nanoparticles for Improving Light Harvesting and Usage Efficiency in Algal Photosynthesis
4.4. Nanoparticles for Improvement on Algae Harvesting
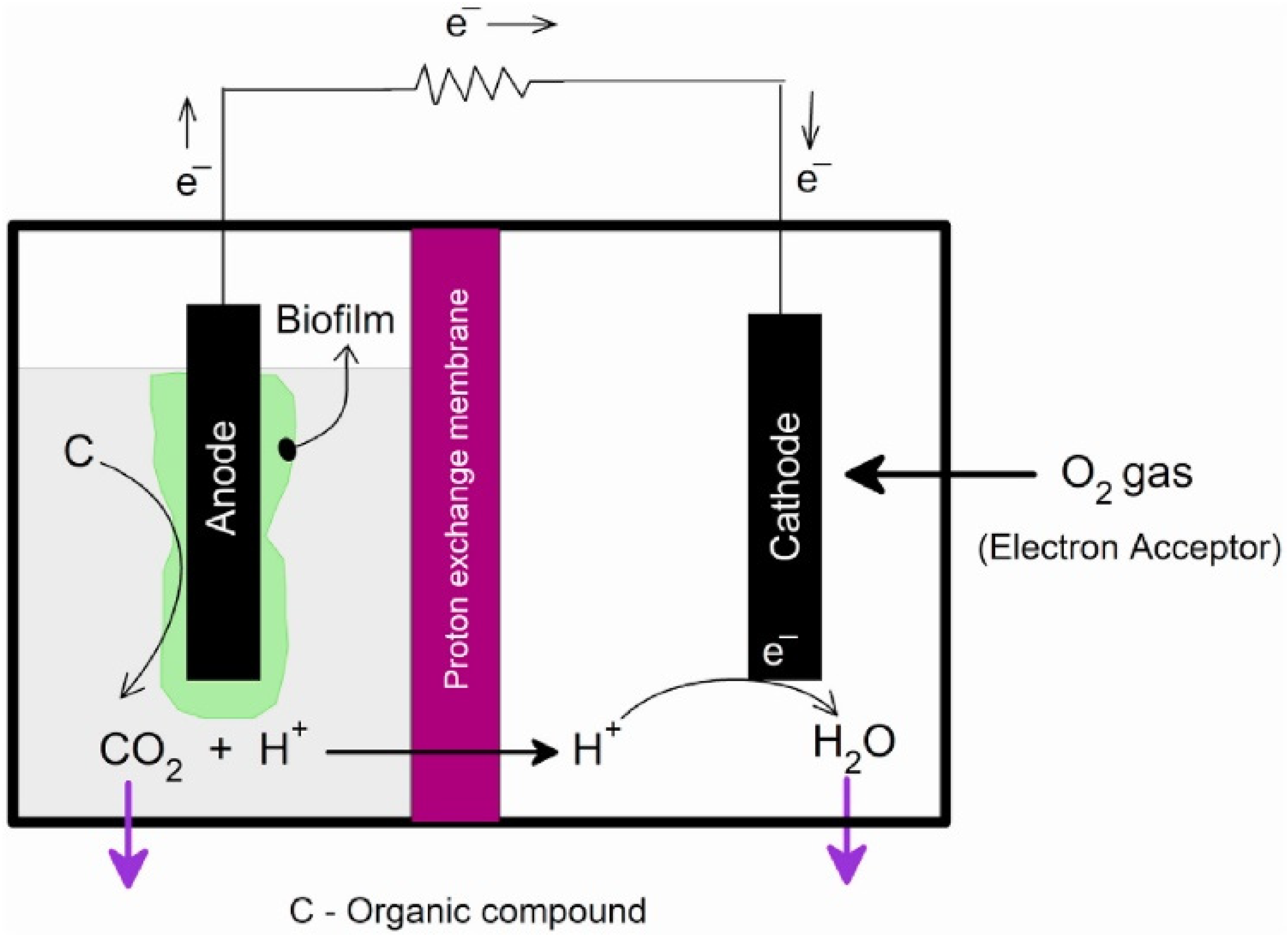
5. Nanomaterials for Microbial Fuel Cells
5.1. Nanostructured Bioelectrodes
5.2. Nanostructured Proton Exchange Membranes
6. Perspectives and Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.J.; Salehi, B.; Zhang, B. Sustainable recycling and valorization of organic solid wastes for fuels and fertilizers. In Production of Biofuels and Chemicals from Sustainable Recycling of Organic Solid Waste; Fang, Z., Smith, R.L., Jr., Xu, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Duque-Acevedo, M.; Belmonte-Urena, L.J.; Cortés-García, F.J.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Rajagopal, R.; Massé, D.I.; Singh, G. A critical review on inhibition of anaerobic digestion process by excess ammonia. Bioresour. Technol. 2013, 143, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Amini, H.; Hashemisohi, A.; Wang, L.; Shahbazi, A.; Bikdash, M.; Dukka, K.; Yuan, W. Numerical and experimental investigation of hydrodynamics and light transfer in open raceway ponds at various algal cell concentrations and medium depths. Chem. Eng. Sci. 2016, 156, 11–23. [Google Scholar] [CrossRef] [Green Version]
- Amini, H.; Wang, L.; Shahbazi, A. Effects of harvesting cell density, medium depth and environmental factors on biomass and lipid productivities of Chlorella vulgaris grown in swine wastewater. Chem. Eng. Sci. 2016, 152, 403–412. [Google Scholar] [CrossRef] [Green Version]
- Amini, H.; Wang, L.; Hashemisohi, A.; Shahbazi, A.; Bikdash, M.; Dukka, K.; Yuan, W. An integrated growth kinetics and computational fluid dynamics model for the analysis of algal productivity in open raceway ponds. Comput. Electron. Agric. 2018, 145, 363–372. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Riddicka, B.A.; Li, R.; Able, J.R.; Boakye-Boaten, N.A.; Shahbazi, A. Sustainable production of algal biomass and biofuels using swine wastewater in North Carolina, US. Sustainability 2016, 8, 477. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B. Cultivation of microalgae on agricultural wastewater for recycling energy, water, and fertilizer nutrients. In Integrated Wastewater Management and Valorization Using Algal Cultures; Elsevier: Amsterdam, The Netherlands, 2022; pp. 235–264. [Google Scholar]
- ElMekawy, A.; Srikanth, S.; Bajracharya, S.; Hegab, H.M.; Nigam, P.S.; Singh, A.; Mohan, S.V.; Pant, D. Food and agricultural wastes as substrates for bioelectrochemical system (BES): The synchronized recovery of sustainable energy and waste treatment. Food Res. Int. 2015, 73, 213–225. [Google Scholar] [CrossRef]
- Elshobary, M.E.; Zabed, H.M.; Yun, J.; Zhang, G.; Qi, X. Recent insights into microalgae-assisted microbial fuel cells for generating sustainable bioelectricity. Int. J. Hydrogen Energy 2021, 46, 3135–3159. [Google Scholar] [CrossRef]
- He, Z.; Pagliari, P.H.; Waldrip, H.M. Applied and Environmental Chemistry of Animal Manure: A Review. Pedosphere 2016, 26, 779–816. [Google Scholar] [CrossRef]
- Lemke, R.L.; VandenBygaart, A.J.; Campbell, C.A.; Lafond, G.P.; Grant, B. Crop residue removal and fertilizer N: Effects on soil organic carbon in a long-term crop rotation experiment on a Udic Boroll. Agric. Ecosyst. Environ. 2010, 135, 42–51. [Google Scholar] [CrossRef]
- Guo, W.; Lei, Z.; Wang, J.; Wei, D. Special issue on challenges in biological wastewater treatment and resource recovery. Bioresour. Technol. Rep. 2019, 7, 100243. [Google Scholar] [CrossRef]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 2018, 621, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Donner, M.; Verniquet, A.; Broeze, J.; Kayser, K.; De Vries, H. Critical success and risk factors for circular business models valorising agricultural waste and by-products. Resour. Conserv. Recycl. 2021, 165, 105236. [Google Scholar] [CrossRef]
- Ryckebosch, E.; Drouillon, M.; Vervaeren, H. Techniques for transformation of biogas to biomethane. Biomass Bioenergy 2011, 35, 1633–1645. [Google Scholar] [CrossRef]
- Almomani, F.; Bhosale, R.R. Enhancing the production of biogas through anaerobic co-digestion of agricultural waste and chemical pre-treatments. Chemosphere 2020, 255, 126805. [Google Scholar] [CrossRef] [PubMed]
- Chiew, Y.L.; Spångberg, J.; Baky, A.; Hansson, P.-A.; Jönsson, H. Environmental impact of recycling digested food waste as a fertilizer in agriculture—A case study. Resour. Conserv. Recycl. 2015, 95, 1–14. [Google Scholar] [CrossRef]
- Orner, K.D.; Camacho-Céspedes, F.; Cunningham, J.A.; Mihelcic, J.R. Assessment of nutrient fluxes and recovery for a small-scale agricultural waste management system. J. Environ. Manag. 2020, 267, 110626. [Google Scholar] [CrossRef]
- Jiang, E.; Cheng, S.; Tu, R.; He, Z.; Jia, Z.; Long, X.; Wu, Y.; Sun, Y.; Xu, X. High yield self-nitrogen-oxygen doped hydrochar derived from microalgae carbonization in bio-oil: Properties and potential applications. Bioresour. Technol. 2020, 314, 123735. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Hasan, R.; Shahbazi, A. Characterization of a native algae species chlamydomonas debaryana: Strain selection, bioremediation ability, and lipid characterization. BioResources 2014, 9, 6130–6140. [Google Scholar] [CrossRef]
- Cui, B.; Chen, Z.; Guo, D.; Liu, Y. Investigations on the pyrolysis of microalgal-bacterial granular sludge: Products, kinetics, and potential mechanisms. Bioresour. Technol. 2022, 349, 126328. [Google Scholar] [CrossRef]
- Sousa, C.; de Winter, L.; Janssen, M.; Vermuë, M.H.; Wijffels, R.H. Growth of the microalgae Neochloris oleoabundans at high partial oxygen pressures and sub-saturating light intensity. Bioresour. Technol. 2012, 104, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Iasimone, F.; Panico, A.; De Felice, V.; Fantasma, F.; Iorizzi, M.; Pirozzi, F. Effect of light intensity and nutrients supply on microalgae cultivated in urban wastewater: Biomass production, lipids accumulation and settleability characteristics. J. Environ. Manag. 2018, 223, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Rosli, S.S.; Amalina Kadir, W.N.; Wong, C.Y.; Han, F.Y.; Lim, J.W.; Lam, M.K.; Yusup, S.; Kiatkittipong, W.; Kiatkittipong, K.; Usman, A. Insight review of attached microalgae growth focusing on support material packed in photobioreactor for sustainable biodiesel production and wastewater bioremediation. Renew. Sustain. Energy Rev. 2020, 134, 110306. [Google Scholar] [CrossRef]
- Yuan, H.; Zhang, X.; Jiang, Z.; Wang, X.; Wang, Y.; Cao, L.; Zhang, X. Effect of light spectra on microalgal biofilm: Cell growth, photosynthetic property, and main organic composition. Renew. Energy 2020, 157, 83–89. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Yan, T.; Jiang, Z.; Zhang, X.; Zuo, Y.Y. Quantitatively Predicting Bacterial Adhesion Using Surface Free Energy Determined with a Spectrophotometric Method. Environ. Sci. Technol. 2015, 49, 6164–6171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Zheng, Y.; Li, J.; Liao, Q.; Fu, Q.; Xia, A.; Fu, J.; Sun, Y. Enhancing microalgae biofilm formation and growth by fabricating microgrooves onto the substrate surface. Bioresour. Technol. 2018, 261, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Greenman, J.; Gajda, I.; Ieropoulos, I. Microbial fuel cells (MFC) and microalgae; photo microbial fuel cell (PMFC) as complete recycling machines. Sustain. Energy Fuels 2019, 3, 2546–2560. [Google Scholar] [CrossRef]
- Mateo, S.; Rodrigo, M.; Fonseca, L.P.; Cañizares, P.; Fernandez-Morales, F.J. Oxygen availability effect on the performance of air-breathing cathode microbial fuel cell. Biotechnol. Progr. 2015, 31, 900–907. [Google Scholar] [CrossRef]
- Neethu, B.; Bhowmick, G.D.; Ghangrekar, M.M. Enhancement of bioelectricity generation and algal productivity in microbial carbon-capture cell using low cost coconut shell as membrane separator. Biochem. Eng. J. 2018, 133, 205–213. [Google Scholar] [CrossRef]
- Kim, J.R.; Zuo, Y.; Regan, J.M.; Logan, B.E. Analysis of ammonia loss mechanisms in microbial fuel cells treating animal wastewater. Biotechnol. Bioeng. 2008, 99, 1120–1127. [Google Scholar] [CrossRef]
- Zhao, Q.; Wang, S.; Lv, Z.; Zupanic, A.; Guo, S.; Zhao, Q.; Jiang, L.; Yu, Y. Using nanomaterials to increase the efficiency of chemical production in microbial cell factories: A comprehensive review. Biotechnol. Adv. 2022, 59, 107982. [Google Scholar] [CrossRef]
- Baniamerian, H.; Isfahani, P.G.; Tsapekos, P.; Alvarado-Morales, M.; Shahrokhi, M.; Vossoughi, M.; Angelidaki, I. Application of nano-structured materials in anaerobic digestion: Current status and perspectives. Chemosphere 2019, 229, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Faisal, S.; Salama, E.-S.; Malik, K.; Lee, S.-h.; Li, X. Anaerobic digestion of cabbage and cauliflower biowaste: Impact of iron oxide nanoparticles (IONPs) on biomethane and microbial communities alteration. Bioresour. Technol. Rep. 2020, 12, 100567. [Google Scholar] [CrossRef]
- White, S.A.; Strosnider, W.H.J.; Chase, M.E.M.; Schlautman, M.A. Removal and reuse of phosphorus from plant nursery irrigation return water with reclaimed iron oxides. Ecol. Eng. 2021, 160, 106153. [Google Scholar] [CrossRef]
- Stolyar, S.V.; Krasitskaya, V.V.; Frank, L.A.; Yaroslavtsev, R.N.; Chekanova, L.A.; Gerasimova, Y.V.; Volochaev, M.N.; Bairmani, M.S.; Velikanov, D.A. Polysaccharide-coated iron oxide nanoparticles: Synthesis, properties, surface modification. Mater. Lett. 2021, 284, 128920. [Google Scholar] [CrossRef]
- Mir, R.A.; Singla, S.; Pandey, O.P. Hetero carbon structures derived from waste plastics as an efficient electrocatalyst for water splitting and high-performance capacitors. Phys. E Low-Dimens. Syst. Nanostructures 2020, 124, 114284. [Google Scholar] [CrossRef]
- Thomas, D.; Fernandez, N.B.; Mullassery, M.D.; Surya, R. Iron oxide loaded biochar/polyaniline nanocomposite: Synthesis, characterization and electrochemical analysis. Inorg. Chem. Commun. 2020, 119, 108097. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Y.; Li, J.; Fu, Q.; Zhang, L. Templating synthesis of hierarchically meso/macroporous N-doped microalgae derived biocarbon as oxygen reduction reaction catalyst for microbial fuel cells. Int. J. Hydrogen Energy 2021, 46, 2530–2542. [Google Scholar] [CrossRef]
- Ganzoury, M.A.; Allam, N.K. Impact of nanotechnology on biogas production: A mini-review. Renew. Sustain. Energy Rev. 2015, 50, 1392–1404. [Google Scholar] [CrossRef]
- Suanon, F.; Sun, Q.; Li, M.; Cai, X.; Zhang, Y.; Yan, Y.; Yu, C.-P. Application of nanoscale zero valent iron and iron powder during sludge anaerobic digestion: Impact on methane yield and pharmaceutical and personal care products degradation. J. Hazard. Mater. 2017, 321, 47–53. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, J.; Hu, Z. Impact of nano zero valent iron (NZVI) on methanogenic activity and population dynamics in anaerobic digestion. Water Res. 2013, 47, 6790–6800. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, E.; Samer, M.; Attia, Y.; Abdel-Hadi, M.; Hassan, H.; Badr, Y. Comparison of nanoparticles effects on biogas and methane production from anaerobic digestion of cattle dung slurry. Renew. Energy 2016, 87, 592–598. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, D.; Dai, L.; Chen, Y.; Dai, X. Effects of metal nanoparticles on methane production from waste-activated sludge and microorganism community shift in anaerobic granular sludge. Sci. Rep. 2016, 6, 25857. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Fang, H. Comparison of enhancement of anaerobic digestion of waste activated sludge through adding nano-zero valent iron and zero valent iron. RSC Adv. 2018, 8, 27181–27190. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Lu, Y. Conductive Fe3O4 nanoparticles accelerate syntrophic methane production from butyrate oxidation in two different lake sediments. Front. Microbiol. 2016, 7, 1316. [Google Scholar] [CrossRef] [Green Version]
- Aulenta, F.; Majone, M.; Tandoi, V. Enhanced anaerobic bioremediation of chlorinated solvents: Environmental factors influencing microbial activity and their relevance under field conditions. J. Chem. Technol. Biotechnol. 2006, 81, 1463–1474. [Google Scholar] [CrossRef]
- Cruz Viggi, C.; Casale, S.; Chouchane, H.; Askri, R.; Fazi, S.; Cherif, A.; Zeppilli, M.; Aulenta, F. Magnetite nanoparticles enhance the bioelectrochemical treatment of municipal sewage by facilitating the syntrophic oxidation of volatile fatty acids. J. Chem. Technol. Biotechnol. 2019, 94, 3134–3146. [Google Scholar] [CrossRef] [Green Version]
- Cui, B.; Chen, Z.; Wang, F.; Zhang, Z.; Dai, Y.; Guo, D.; Liang, W.; Liu, Y. Facile Synthesis of Magnetic Biochar Derived from Burley Tobacco Stems towards Enhanced Cr (VI) Removal: Performance and Mechanism. J. Nanomater. 2022, 12, 678. [Google Scholar] [CrossRef]
- Zhang, Y.; LI, H.; Gong, L.; Dong, G.; Shen, L.; Wang, Y.; Li, Q. Nano-sized Fe2O3/Fe3O4 facilitate anaerobic transformation of hexavalent chromium in soil–water systems. J. Environ. Sci. 2017, 57, 329–337. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Ghimire, S.; Li, X.; Todd, M.S.; Shahbazi, A. Enhanced biomethane production via thermophilic anaerobic digestion of cattail amended with potassium phosphate-and magnesium-modified biochar. Clean Technol. Environ. Policy 2021, 23, 2399–2412. [Google Scholar] [CrossRef]
- Tsapekos, P.; Alvarado-Morales, M.; Tong, J.; Angelidaki, I. Nickel spiking to improve the methane yield of sewage sludge. Bioresour. Technol. 2018, 270, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.; Muhammad, N.; Bhuyar, P.; Krishnan, S.; Abd Razak, A.S.; Zularisam, A.; Nasrullah, M. A review on the impact of conductive nanoparticles (CNPs) in anaerobic digestion: Applications and limitations. Environ. Technol. Innov. 2021, 23, 101526. [Google Scholar] [CrossRef]
- Choi, O.; Clevenger, T.E.; Deng, B.; Surampalli, R.Y.; Ross Jr, L.; Hu, Z. Role of sulfide and ligand strength in controlling nanosilver toxicity. Water Res. 2009, 43, 1879–1886. [Google Scholar] [CrossRef]
- Zhu, X.; Blanco, E.; Bhatti, M.; Borrion, A. Impact of metallic nanoparticles on anaerobic digestion: A systematic review. Sci. Total Environ. 2021, 757, 143747. [Google Scholar] [CrossRef]
- Oktavitri, N.I.; Nakashita, S.; Hibino, T.; Van Tran, T.; Jeong, I.; Oh, T.-G.; Kim, K. Enhancing pollutant removal and electricity generation in Sediment Microbial Fuel Cell with nano zero-valent iron. Environ. Technol. Innov. 2021, 24, 101968. [Google Scholar] [CrossRef]
- Giese, E.C.; Silva, D.D.; Costa, A.F.; Almeida, S.G.; Dussán, K.J. Immobilized microbial nanoparticles for biosorption. Crit. Rev. Biotechnol. 2020, 40, 653–666. [Google Scholar] [CrossRef]
- Arabacı, N.; Karaytuğ, T.; Demirbas, A.; Ocsoy, I.; Kati, A. Nanomaterials for enzyme immobilization. Green Synth. Nanomater. Bioenergy Appl. 2020, 165–190. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Woodard, T.; Nevin, K.; Lovley, D. Enhancing syntrophic metabolism in up-flow anaerobic sludge blanket reactors with conductive carbon materials. Bioresour. Technol. 2015, 191, 140–145. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.-Y.; Raman, A.A.A.; Ibrahim, S. Microalgae lipid and biomass for biofuel production: A comprehensive review on lipid enhancement strategies and their effects on fatty acid composition. Renew. Sustain. Energy Rev. 2018, 97, 200–232. [Google Scholar] [CrossRef]
- Vargas-Estrada, L.; Torres-Arellano, S.; Longoria, A.; Arias, D.M.; Okoye, P.U.; Sebastian, P. Role of nanoparticles on microalgal cultivation: A review. Fuel 2020, 280, 118598. [Google Scholar] [CrossRef]
- Hossain, S.Z. Biochemical conversion of microalgae biomass into biofuel. Chem. Eng. Technol. 2019, 42, 2594–2607. [Google Scholar] [CrossRef]
- Gledhill, M.; Buck, K.N. The organic complexation of iron in the marine environment: A review. Front. Microbiol. 2012, 3, 69. [Google Scholar] [CrossRef] [Green Version]
- Pádrová, K.; Lukavský, J.; Nedbalová, L.; Čejková, A.; Cajthaml, T.; Sigler, K.; Vítová, M.; Řezanka, T. Trace concentrations of iron nanoparticles cause overproduction of biomass and lipids during cultivation of cyanobacteria and microalgae. J. Appl. Phycol. 2015, 27, 1443–1451. [Google Scholar] [CrossRef]
- Kadar, E.; Rooks, P.; Lakey, C.; White, D.A. The effect of engineered iron nanoparticles on growth and metabolic status of marine microalgae cultures. Sci. Total Environ. 2012, 439, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Abd El Baky, H.H.; El-Baroty, G.S.; Bouaid, A.; Martinez, M.; Aracil, J. Enhancement of lipid accumulation in Scenedesmus obliquus by optimizing CO2 and Fe3+ levels for biodiesel production. Bioresour. Technol. 2012, 119, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Yuan, H.; Li, B.; Yang, J. Significance evaluation of the effects of environmental factors on the lipid accumulation of Chlorella minutissima UTEX 2341 under low-nutrition heterotrophic condition. Bioresour. Technol. 2014, 152, 177–184. [Google Scholar] [CrossRef]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2’, 7’-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, H.-Y.; Dai, Y.-Q.; Kong, F.; Xing, D.; Zhao, L.; Ren, N.-Q.; Ma, J.; Liu, B.-F. Enhanced microalgal growth and lipid accumulation by addition of different nanoparticles under xenon lamp illumination. Bioresour. Technol. 2020, 297, 122409. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vargas, B.P.; da Silva Vaz, B.; Cardias, B.B.; Costa, J.A.V. Advances in the synthesis and applications of nanomaterials to increase CO2 biofixation in microalgal cultivation. Clean Technol. Environ. Policy 2021, 1–16. [Google Scholar] [CrossRef]
- Lu, C.; Shi, X.; Liu, Y.; Xiao, H.; Li, J.; Chen, X. Nanomaterials for adsorption and conversion of CO2 under gentle conditions. Mater. Today 2021, 50, 385–399. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Zhao, W.; Chen, Y.; Xiao, H.; Shi, X.; Liu, Y.; Chen, X. Quaternized chitosan/PVA aerogels for reversible CO2 capture from ambient air. Ind. Eng. Chem. Res. 2018, 57, 4941–4948. [Google Scholar] [CrossRef]
- Cheng, H.; Song, H.; Toan, S.; Wang, B.; Gasem, K.A.; Fan, M.; Cheng, F. Experimental investigation of CO2 adsorption and desorption on multi-type amines loaded HZSM-5 zeolites. Chem. Eng. J. 2021, 406, 126882. [Google Scholar] [CrossRef]
- Beltzung, A.; Klaue, A.; Colombo, C.; Wu, H.; Storti, G.; Morbidelli, M. Polyacrylonitrile nanoparticle-derived hierarchical structure for CO2 capture. Energy Technol. 2018, 6, 718–727. [Google Scholar] [CrossRef]
- Li, K.; Tian, S.; Jiang, J.; Wang, J.; Chen, X.; Yan, F. Pine cone shell-based activated carbon used for CO2 adsorption. J. Mater. Chem. A 2016, 4, 5223–5234. [Google Scholar] [CrossRef]
- da Silva Vaz, B.; da Silveira Mastrantonio, D.J.; Costa, J.A.V.; de Morais, M.G. Green alga cultivation with nanofibers as physical adsorbents of carbon dioxide: Evaluation of gas biofixation and macromolecule production. Bioresour. Technol. 2019, 287, 121406. [Google Scholar] [CrossRef]
- da Silva Vaz, B.; Costa, J.A.V.; de Morais, M.G. Innovative nanofiber technology to improve carbon dioxide biofixation in microalgae cultivation. Bioresour. Technol. 2019, 273, 592–598. [Google Scholar] [CrossRef]
- da Silva Vaz, B.; Costa, J.A.V.; de Morais, M.G. CO2 biofixation by the cyanobacterium Spirulina sp. LEB 18 and the green alga Chlorella fusca LEB 111 grown using gas effluents and solid residues of thermoelectric origin. Appl. Biochem. Biotechnol. 2016, 178, 418–429. [Google Scholar] [CrossRef]
- Eroglu, E.; Eggers, P.K.; Winslade, M.; Smith, S.M.; Raston, C.L. Enhanced accumulation of microalgal pigments using metal nanoparticle solutions as light filtering devices. Green Chem. 2013, 15, 3155–3159. [Google Scholar] [CrossRef]
- Torkamani, S.; Wani, S.; Tang, Y.; Sureshkumar, R. Plasmon-enhanced microalgal growth in miniphotobioreactors. Appl. Phys. Lett. 2010, 97, 043703. [Google Scholar] [CrossRef]
- Giannelli, L.; Torzillo, G. Hydrogen production with the microalga Chlamydomonas reinhardtii grown in a compact tubular photobioreactor immersed in a scattering light nanoparticle suspension. Int. J. Hydrogen Energy 2012, 37, 16951–16961. [Google Scholar] [CrossRef]
- Singh, G.; Patidar, S. Microalgae harvesting techniques: A review. J. Environ. Manage. 2018, 217, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoo, G.; Lee, H.; Lim, J.; Kim, K.; Kim, C.W.; Park, M.S.; Yang, J.-W. Methods of downstream processing for the production of biodiesel from microalgae. Biotechnol. Adv. 2013, 31, 862–876. [Google Scholar] [CrossRef] [PubMed]
- Prochazkova, G.; Safarik, I.; Branyik, T. Harvesting microalgae with microwave synthesized magnetic microparticles. Bioresour. Technol. 2013, 130, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Ge, S.; Agbakpe, M.; Wu, Z.; Kuang, L.; Zhang, W.; Wang, X. Influences of surface coating, UV irradiation and magnetic field on the algae removal using magnetite nanoparticles. Environ. Sci. Technol. 2015, 49, 1190–1196. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, W.-L.; Hong, Y.; Hou, Y.-L. Magnetic nanoparticles grafted with amino-riched dendrimer as magnetic flocculant for efficient harvesting of oleaginous microalgae. Chem. Eng. J. 2016, 297, 304–314. [Google Scholar] [CrossRef]
- Hu, Y.-R.; Guo, C.; Wang, F.; Wang, S.-K.; Pan, F.; Liu, C.-Z. Improvement of microalgae harvesting by magnetic nanocomposites coated with polyethylenimine. Chem. Eng. J. 2014, 242, 341–347. [Google Scholar] [CrossRef]
- Liu, P.; Wang, T.; Yang, Z.; Hong, Y.; Xie, X.; Hou, Y. Effects of Fe3O4 nanoparticle fabrication and surface modification on Chlorella sp. harvesting efficiency. Sci. Total Environ. 2020, 704, 135286. [Google Scholar] [CrossRef]
- Priya, A.; Subha, C.; Kumar, P.S.; Suresh, R.; Rajendran, S.; Vasseghian, Y.; Soto-Moscoso, M. Advancements on sustainable microbial fuel cells and their future prospects: A review. Environ. Res. 2022, 210, 112930. [Google Scholar] [CrossRef]
- Agrahari, R.; Bayar, B.; Abubackar, H.N.; Giri, B.S.; Rene, E.R.; Rani, R. Advances in the development of electrodes material for improving reactor kinetics in Microbial Fuel Cells. Chemosphere 2021, 290, 133184. [Google Scholar] [CrossRef]
- Gadhamshetty, V.; Koratkar, N. Nano-engineered biocatalyst-electrode structures for next generation microbial fuel cells. Nano Energy 2012, 1, 3–5. [Google Scholar] [CrossRef]
- Lv, Z.; Xie, D.; Yue, X.; Feng, C.; Wei, C. Ruthenium oxide-coated carbon felt electrode: A highly active anode for microbial fuel cell applications. J. Power Sources 2012, 210, 26–31. [Google Scholar] [CrossRef]
- Alatraktchi, F.A.; Zhang, Y.; Angelidaki, I. Nanomodification of the electrodes in microbial fuel cell: Impact of nanoparticle density on electricity production and microbial community. Appl. Energy 2014, 116, 216–222. [Google Scholar] [CrossRef]
- Hou, J.; Liu, Z.; Zhang, P. A new method for fabrication of graphene/polyaniline nanocomplex modified microbial fuel cell anodes. J. Power Sources 2013, 224, 139–144. [Google Scholar] [CrossRef]
- Mashkour, M.; Rahimnejad, M.; Mashkour, M. Bacterial cellulose-polyaniline nano-biocomposite: A porous media hydrogel bioanode enhancing the performance of microbial fuel cell. J. Power Sources 2016, 325, 322–328. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Wang, R.; Yang, Y.; Liu, Y. Design and research progress of nano materials in cathode catalysts of microbial fuel cells: A review. Int. J. Hydrogen Energy 2022, 47, 18098–18108. [Google Scholar] [CrossRef]
- Wang, Z.; Mahadevan, G.D.; Wu, Y.; Zhao, F. Progress of air-breathing cathode in microbial fuel cells. J. Power Sources 2017, 356, 245–255. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Z.; Song, H. Carbon supports on preparing iron-nitrogen dual-doped carbon (Fe-N/C) electrocatalysts for microbial fuel cells: Mini-review. Chemosphere 2021, 273, 128570. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Y.-S.; Liu, Z.-M. Pyrolysis of iron phthalocyanine on activated carbon as highly efficient non-noble metal oxygen reduction catalyst in microbial fuel cells. Chem. Eng. J. 2019, 361, 416–427. [Google Scholar] [CrossRef]
- de Oliveira, M.A.C.; Mecheri, B.; D’Epifanio, A.; Placidi, E.; Arciprete, F.; Valentini, F.; Perandini, A.; Valentini, V.; Licoccia, S. Graphene oxide nanoplatforms to enhance catalytic performance of iron phthalocyanine for oxygen reduction reaction in bioelectrochemical systems. J. Power Sources 2017, 356, 381–388. [Google Scholar] [CrossRef]
- Tang, H.; Cai, S.; Xie, S.; Wang, Z.; Tong, Y.; Pan, M.; Lu, X. Metal–organic-framework-derived dual metal-and nitrogen-doped carbon as efficient and robust oxygen reduction reaction catalysts for microbial fuel cells. Adv. Sci. 2016, 3, 1500265. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Bakeri, G.; Najafpour, G.; Ghasemi, M.; Oh, S.-E. A review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Res. J. 2014, 1, 7–15. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Ghasemi, M.; Najafpour, G.; Ismail, M.; Mohammad, A.; Ghoreyshi, A.; Hassan, S.H. Synthesis, characterization and application studies of self-made Fe3O4/PES nanocomposite membranes in microbial fuel cell. Electrochim. Acta 2012, 85, 700–706. [Google Scholar] [CrossRef]
- Ghasemi, M.; Shahgaldi, S.; Ismail, M.; Yaakob, Z.; Daud, W.R.W. New generation of carbon nanocomposite proton exchange membranes in microbial fuel cell systems. Chem. Eng. J. 2012, 184, 82–89. [Google Scholar] [CrossRef]
- Pan, F.; Cheng, Q.; Jia, H.; Jiang, Z. Facile approach to polymer–inorganic nanocomposite membrane through a biomineralization-inspired process. J. Membr. Sci. 2010, 357, 171–177. [Google Scholar] [CrossRef]

| Substrate | Nanoparticles | Concentration/Average Size | Effects on AD | Refs. |
|---|---|---|---|---|
| Sewage sludge | nZVI | 0.1% sludge/160 nm |
| [42] |
| Commercial iron powder | 1.6% sludge/0.2 mm |
| ||
| Raw manure | nZVI | 20 mg/L/9 nm |
| [43] |
| Fe3O4 | 20 mg/L/7nm |
| ||
| Digested sludge | nZVI | 30 mM/55 nm |
| [44] |
| Raw manure | Co NPs | 1 mg/L |
| [45] |
| Ni NPs | 2 mg/L |
| ||
| Fe NPs | 20 mg/L |
| ||
| Fe3O4 NPs | 20 mg/L |
| ||
| Waste-activated sludge | nZVI | 10 mg/g TSS/<50 mm |
| [46] |
| Ag NPs | 100 mg/g TSS/<100 nm |
| ||
| Fe2O3 NPs | 500 mg/g TSS/< 30nm |
| ||
| MgO NPs | 500 mg/g TSS/<50 nm |
| ||
| Waste-activated sludge | nZVI | 0.6–1 g/L |
| [47] |
| 4 g/L |
| |||
| 10 g/L |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salehi, B.; Wang, L. Critical Review on Nanomaterials for Enhancing Bioconversion and Bioremediation of Agricultural Wastes and Wastewater. Energies 2022, 15, 5387. https://doi.org/10.3390/en15155387
Salehi B, Wang L. Critical Review on Nanomaterials for Enhancing Bioconversion and Bioremediation of Agricultural Wastes and Wastewater. Energies. 2022; 15(15):5387. https://doi.org/10.3390/en15155387
Chicago/Turabian StyleSalehi, Bahare, and Lijun Wang. 2022. "Critical Review on Nanomaterials for Enhancing Bioconversion and Bioremediation of Agricultural Wastes and Wastewater" Energies 15, no. 15: 5387. https://doi.org/10.3390/en15155387
APA StyleSalehi, B., & Wang, L. (2022). Critical Review on Nanomaterials for Enhancing Bioconversion and Bioremediation of Agricultural Wastes and Wastewater. Energies, 15(15), 5387. https://doi.org/10.3390/en15155387






