1. Introduction
Food waste (FW) includes a variety of residual wastes generated at any point of the food production supply chain and post-consumption wastes from domestic, or commercial activity. It is an abundant biomass resource worldwide with a high carbon content and energy potential. FW can be associated either directly with agricultural wastes, such as waste from the food industry, or final consumers (household or restaurant wastes), with the latter often resulting in large amounts entering municipal waste streams.
In addition to the large quantities of food wasted, its disposal results in several inherent problems. The most common methods for the disposal of food waste include landfill and ocean dumping [
1]. Both methods of disposal result in significant environmental impact due to the emission of greenhouse gases since approximately 45% of the carbon content in food waste is released in gaseous form after landfill [
2]. A large fraction of the released carbon is in the form of methane, which presents a global warming potential (GWP) of 25 times the greenhouse effect of CO
2 in a 100-year horizon [
3]. The decomposition of organic matter after dumping and landfilling causes nutrient imbalances in soil and water bodies. In addition to environmental issues, released carbon represents a large amount of wasted energy. To counter these problems, developed countries have developed more advanced methods of disposal, the most common of which include composting and anaerobic digestion (AD) [
1]. However, these technologies have technical challenges, such as long processing times and the need for pre-treatment of feedstock to achieve optimal conversion.
In recent years, hydrothermal carbonization (HTC) has positioned itself as a potential technology for valorizing food waste because it overcomes many of the technical challenges of using FW as a feedstock in comparison to other technologies.
HTC involves the treatment of biomass submerged in water at sub-critical conditions, at temperatures ranging from 180 to 260 °C, and autogenic pressures ranging from 10 to 40 bar, resulting in a solid phase known as hydrochar and a liquid phase known as process water, which contains considerable solubilized matter [
4,
5]. At higher temperatures and pressures, e.g., from 260–350 °C, the main product is a liquid, and the process is referred to as hydrothermal liquefaction (HTL). At higher reaction severity (>375 °C and >22.1 MPa), the process favors the gasification of the feedstock-producing syngas. This process is referred to as hydrothermal gasification (HTG) [
6]. The processing conditions explored in this paper fall within the HTC range. The reaction times explored range between 20–70 min; however, carbonization reactions proceed quickly, and operating at longer reaction times is more energy intensive. The solid product has coal-like properties and is commonly known as hydrochar to differentiate it from biochar from dry-carbonization technologies, such as pyrolysis. Hydrochar exhibits enhanced stability, storage, and energetic properties compared to the original biomass [
7]. In addition, there is a considerable volume reduction from the original feedstock. In general, hydrochar exhibits a lower oxygen content, enhancing the O:C and H:C ratios due to dehydration and decarboxylation reactions, enhanced fixed carbon, and reduced volatile matter. Meanwhile, the HTC liquid fraction is generally comprised of sugars, volatile fatty acids (e.g., acetic and formic acid), N-containing compounds (e.g., pyrrole and phenolic and furanic compounds (e.g., 5-hydroxymethyl furfural), and dissolved inorganics, such as alkali metals [
8].
The HTC process offers numerous advantages that make it more suitable for treating food waste in comparison to other technologies, such as pyrolysis or gasification. Unlike the latter, HTC does not require an energy-demanding drying process, which would make it more energetically favorable [
9]. The utilization of HTC to treat high moisture biomass can reduce its carbon footprint compared to other treatments without generating an odor [
10]. HTC offers several advantages to biological treatments, such as a shorter process time, as it takes only hours instead of days. In addition, HTC could employ FW with large variability in chemical composition, which can significantly affect the performance of biological processes, such as anaerobic digestion [
9]. In addition, due to intense process conditions, HTC can eliminate pathogens and inactivate other potential organic contaminants [
10].
In recent years, the use of HTC for the treatment of food waste has gained momentum, and experimental and literature reports are becoming more numerous each year. The works cover different end uses for hydrochar, including soil conditioner or fertilizer [
11,
12], adsorbents of metals [
13], and N and P recovery [
14,
15]. Nonetheless, the proposed main end use of hydrochar from food waste is solid fuel, either for combustion or gasification [
2,
16,
17,
18,
19,
20,
21,
22,
23]. These experimental works, along with feasibility and process evaluation, have made advances in the development of technology for food waste valorization or treatment [
9,
24,
25,
26,
27].
Finding the optimal conditions is a crucial step for assessing the viability of HTC as a commercial technology and achieving its implementation as a biomass pre-treatment in larger processes. However, the optimization of this process is hindered by the complexity of the HTC reaction environment and the heterogeneity of food waste feedstock, which complicates the generation of a general reaction model for the HTC process and the prediction of HTC products, limiting the extent of the simulation works [
28]. Thus, statistical and empirical models are powerful tools for developing the optimization of the HTC process.
It has been noted that most studies focus on expanding feedstock options and often evaluate one variable at a time [
29]. This suggests that the evaluation of how multiple variables and their interactions affect the HTC process represents a significant gap in HTC research. Filling this gap is an important step in continuing the maturation process of technology. Studies and multi-response analysis could help develop opportunities for HTC technology applications.
Previous studies have used response surface methods to understand the effect of multiple variables on HTC parameters, such as temperature, reaction time, solid load, and pH [
30,
31,
32]. However, studies on optimization are limited. Graphical optimization has been reported for hydrochar yields [
33,
34]. Nonetheless, this optimization approach is limited to one response at a time. More recently, the multiple-response optimization of HTC is beginning to appear. Stutzenstein et al. [
35] optimized the solid yield and the difference of O/C in hydrochar using a desirability function for anaerobic digestion digestate. In the same manner, El Ouadrhiri et al. [
36] reported the optimization of date stones HTC for solid yield and carbon retention. Therefore, combining end-utilization responses in multiple-response optimization could provide better insight into the HTC process and overcome the trade-offs of the reaction.
This work aims to evaluate the effect of HTC process conditions on the composition and utilization capabilities of hydrochar from food waste. To this day, there is a lack of studies evaluating the effects of the multiple variables of HTC on food waste. Thus, a Design of Experiment (DoE) approach has been used to investigate the influence of three process variables on the product composition and properties of the solid product, hydrochar, from the HTC of food waste. Parametric analysis is carried out with a design of experiments of central composite rotatable design (CCRD) and response surface methodology (RSM). The desirability function was employed to perform a multi-response evaluation. To advance the utilization of HTC on food waste valorization, it is necessary to understand the effect of process variables on product distribution and their trade-offs.
2. Materials and Methods
2.1. Food Waste Collection
Food waste was collected from a hall of residence canteen at the University of Leeds. The food waste had the accumulation of three meals for two days, comprised of significant amounts of eggs, sausages, cooked vegetables, cooked potatoes, bread, and fresh fruit, as observed during the initial visual inspection. The sample was blended to a more easily handled size with a Nutribullet
® blender and mixed to homogenization. The homogenized sample was stored in 1.5 kg bags and frozen at −20 °C to preserve its initial composition. For characterization analysis, the food waste sample was freeze-dried and analyzed by proximate, ultimate analysis, and biochemical analysis; the measurements were performed by duplicate on the homogenized sample. A summary of the food waste characterization is shown in
Table 1. Neutral detergent fiber (NDF), acid detergent fiber (ADF), and lignin were determined using the Gerhardt Fibrecap system (C. Gerhardt GmbH & Co. KG, Königswinter, Germany) complying with the van Soest methods [
37,
38]. Where NDF is the sample’s remaining fraction after treatment with a neutral detergent solution, NDF includes hemicellulose, cellulose, and lignin fractions. The ADF fraction is obtained after acid detergent digestion and consists of cellulose and lignin fractions, while the hemicellulose fraction is removed by filtration. Finally, lignin is determined after treating the ADF fraction with a sulfuric acid solution, where cellulose is removed. Total oil was determined using the Soxtec method [
39]. The total protein content was determined using a nitrogen-to-protein using the DUMAS method, with a conversion factor of 5.13.
2.2. Hydrothermal Carbonization
HTC reactions were carried out in a 600 mL high-pressure reactor (Parr Instrument Company, Moline, IL, USA). The biomass was loaded fresh into a glass liner, and the moisture content was adjusted to the desired solid load values for the different runs. A glass liner containing the sample submerged in water was placed into the HTC reactor vessel and turned on. The reactor was counted with a heating jacket surrounding the reactor vessel that heated the sample until it reached a set temperature; only then was the reaction time started. The temperature was regulated by a proportional integral derivative (PID) controller at a heating rate of approximately 8 °C/min. After the reaction time ended, the heater was turned off, and the reactor was taken out of the furnace and left to cool down at room temperature until the temperature fell below 40 °C. The reactor was opened once it reached a manageable temperature, and the hydrochar was separated from the process water using 150 mm filter paper (Whatman, Maidstone, UK) and vacuum filtration for 1 min. The liquid filtered called ‘process water’ was frozen at −20 °C for further analysis. The solid fraction ‘hydrochar’ was oven-dried at 60°C overnight and manually ground in a mortar and stored in a zip-lock sealed bag.
2.3. Characterization Analysis
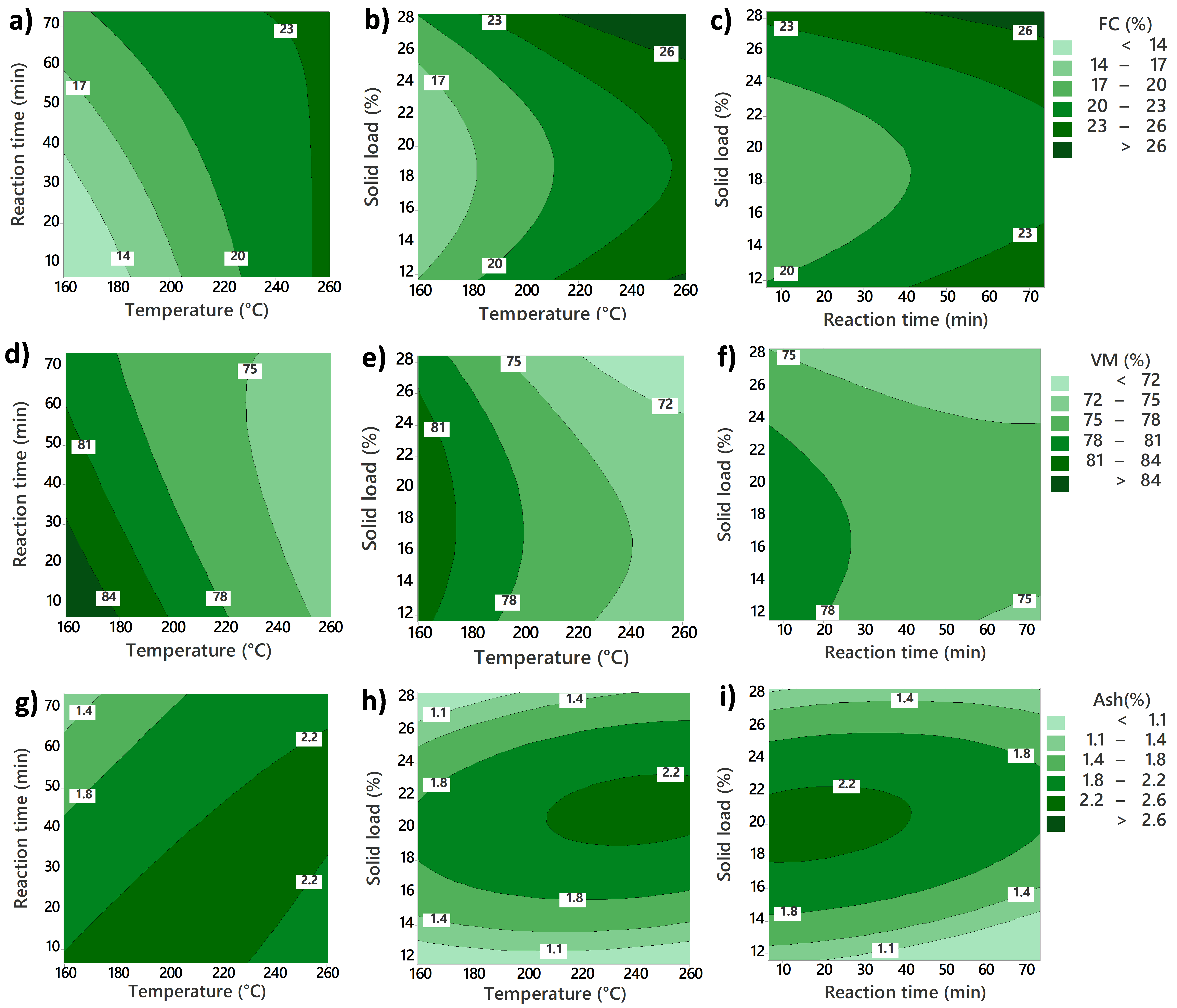
Proximate analysis was carried out with a thermogravimetric analyzer (TGA) (Mettler Toledo TGA/DSC 1). For this method, 10 mg of homogenized sample was loaded into an Alumina 70 µL ceramic crucible and then placed in the TGA equipment. The temperature was ramped from 25 to 900 °C at a heating rate of 25 °C/min, initially under a constant flow of nitrogen (50 mL/min), and finally switched to airflow. The equipment was heated from 25 to 105 °C, at this temperature, it was held for 10 min to assure the removal of moisture. This section was used to calculate moisture content. Heating was resumed until the system reached a temperature of 900 °C, and was held for 10 min to allow the release of volatile matter. After that, the nitrogen flow was switched to air, and the system was held once more at 900 °C for another 15 min. The change in the gaseous phase ensured the total combustion of the fixed carbon fraction. Finally, the remaining produced comprised the inorganic fraction considered ash. The proximate composition of the samples was thus calculated based on the difference in mass loss during each heating stage. The analysis was done in duplicate for each sample.
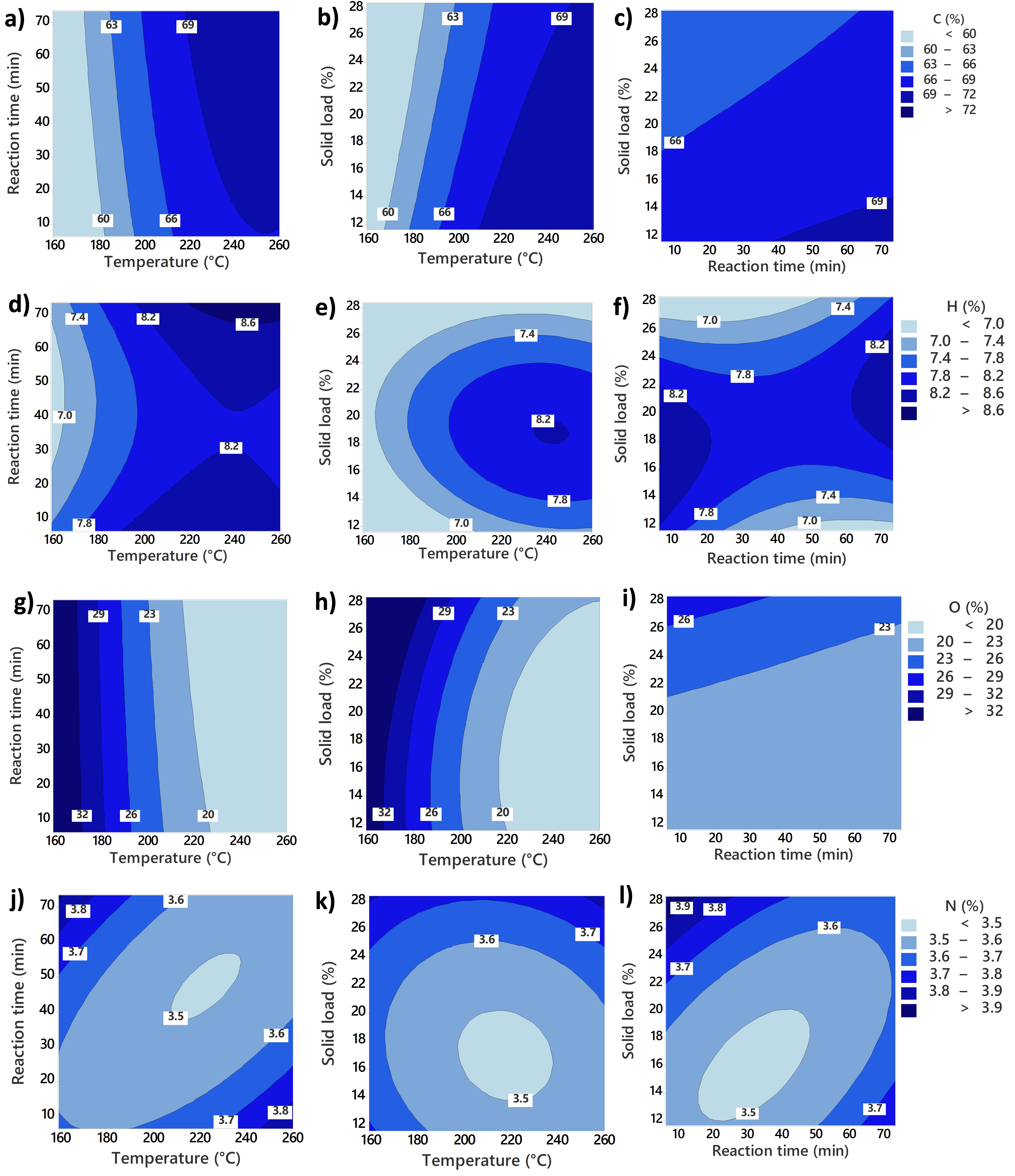
The objective of the ultimate analysis was to determine the elemental composition of the samples of study by allowing the quantification of the main elements carbon, hydrogen, oxygen, nitrogen, and sulfur (C, H, O, N, S). This technique utilized an instrument elemental analyzer Thermo Instruments Flash EA 1112 Series (Thermo Scientific, Waltham, MA, USA). In this method, 2.5 to 3.0 mg of the sample was weighed in tin foil capsules (Elemental Microanalysis D1009, Devon, UK) and crimped to remove the presence of air. The elemental composition was determined by the conversion of the different elements to their oxides, carbon to CO2, nitrogen to NOx, sulfur to SO2, and hydrogen to H2O. These compounds were measured and quantified by a gas chromatographer using a thermal conductivity detector, while oxygen was determined by difference.
2.4. Data Analysis
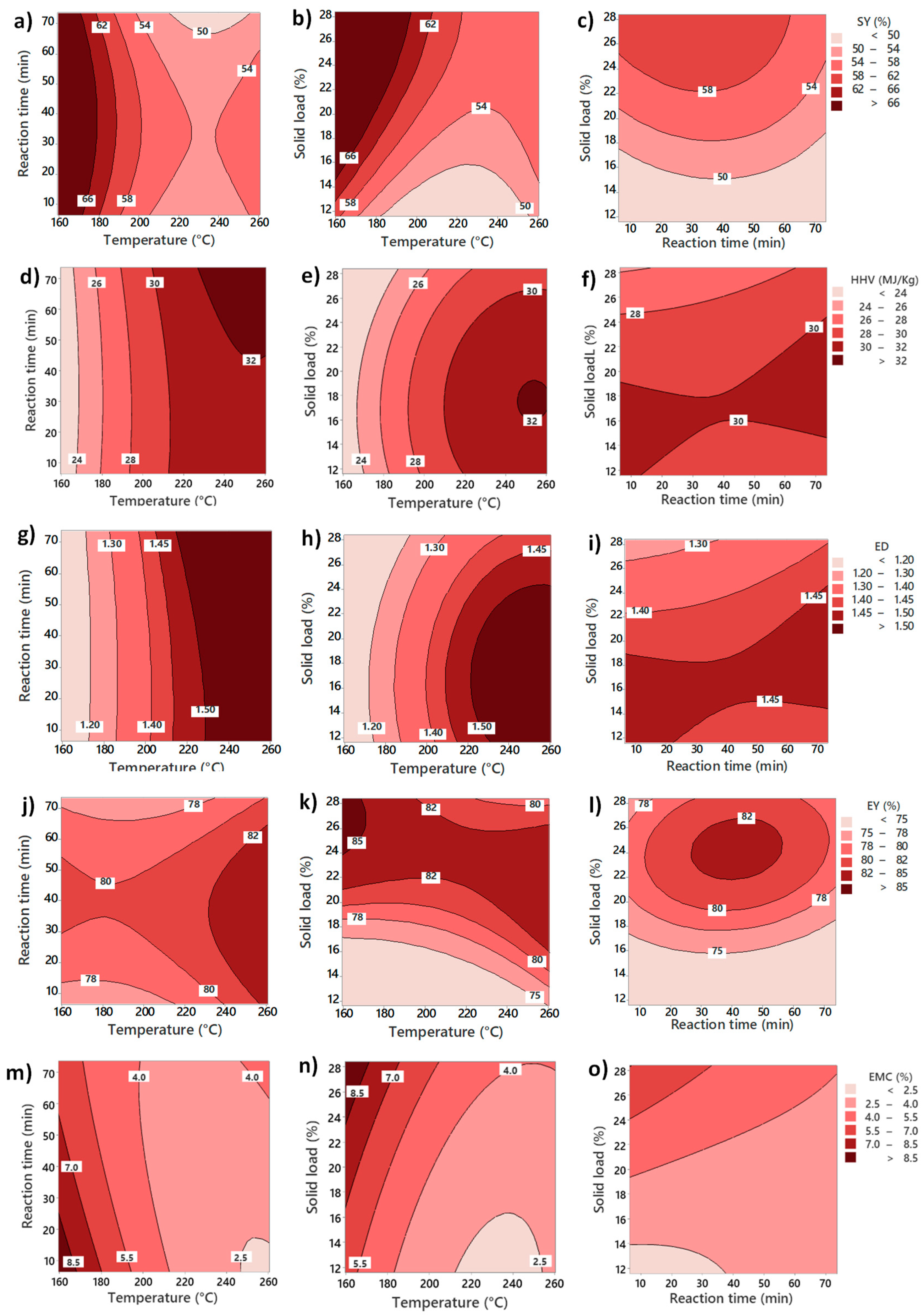
Four numeric responses were evaluated for hydrochar quality: solid yield (SY), higher heating value (HHV), energy densification (ED), and energy yield (EY). The solid yield was calculated by considering the solid fraction remaining in the hydrochar in relation to the initial solid fraction in the raw food waste. It was calculated using Equation (1), where both solid mass fractions were determined on a dry basis.
A higher heating value was calculated using the data from the proximate and ultimate analysis based on the IGT correlations [
40] in Equation (2), where C, H, A, O, and N represent the mass percentage of dry material of carbon, hydrogen, ash, oxygen, and nitrogen, respectively.
Energy densification (ED) was calculated to assess the improvement of the heating value after HTC, as in Equation (3). The energy yield (EY) to measure the content of energy remaining in hydrochar was determined using Equation (4).
2.5. Equilibrium Moisture Content
The particle size was homogenized using a mesh of 500 and 250 microns, and approximately 1 g of samples was weighed in a crucible. The samples were then placed in a closed chamber of 35 × 50 cm, accompanied by a saturated NaCl solution to generate a relative humidity of around 75%. The principle of the experiment consisted of placing the samples inside a chamber with constant relative humidity, letting the hydrochar adsorb, and absorbing moisture. Consequently, weighing the sample to record the hydrated weight, and finally drying and weighing the sample for the mass balance, as stated in Equation (5) [
41].
This experiment was not focused on obtaining the adsorption isotherms but on assessing the hygroscopicity of the hydrochars generated in the DoE and locating the conditions zone where moisture adsorption is minimized. Instead, the EMC is represented as the weight percentage gained by moisture absorption after a period of time (48 h).
2.6. Design of Experiments
Surface response methodology (RSM) was used to evaluate the effects of different process conditions on HTC products. Design of Experiment (DoE) consists of a central composite design with rotatable points. The design evaluated the linear, quadratic, and two-way interactions of the three continuous variables: temperature, reaction time, and solid load. High- and low-level values for each variable were temperature (T) (240 and 180 °C), reaction time (RT) (60 and 20 min), and solid load (SL) (15 and 25% dry basis). The values for the central points are the middle point between the higher and lower levels of each parameter (210 °C, 40 min, and 20% solid load). The matrix of the full run set is shown in
Table 2, where 1 stands for higher level, −1 for lower level, α for higher alpha level, -α for lower alpha level, and 0 central points for a complete design composed of 8 cubic points, 6 central points, and 6 axial points (α = 1.682). The order of the runs was sorted randomly to conduct the experiments.
The values for each variable were selected based on different considerations. For temperature, 180 to 240 °C (and axial points of 160 and 260 °C) cover most of the HTC temperature reaction region. Regarding reaction time, short time values with the longest run (73 min) were screened. Considering the integration of HTC into a larger process, shorter times would make it more economically viable. In the case of solid load, the final range analyzed was from 12 to 28 wt%. This range covers the variation of solid load in real food waste.
For statistical analysis, the ANOVA was conducted using the software MiniTab
®, using a full quadratic regression model (Equation (6)). A significance test was performed for the model and each individual factor using a confidence interval of α = 0.95.
where y
i is the response of interest,
is the constant coefficient,
is the coded value for each parameter,
is the linear effect coefficient,
is the interaction coefficient and
is the quadratic coefficient. The validation of the regression models and the significant test of each factor were carried out by a Fischer F-test that considers the variance ratio distribution.
4. Conclusions
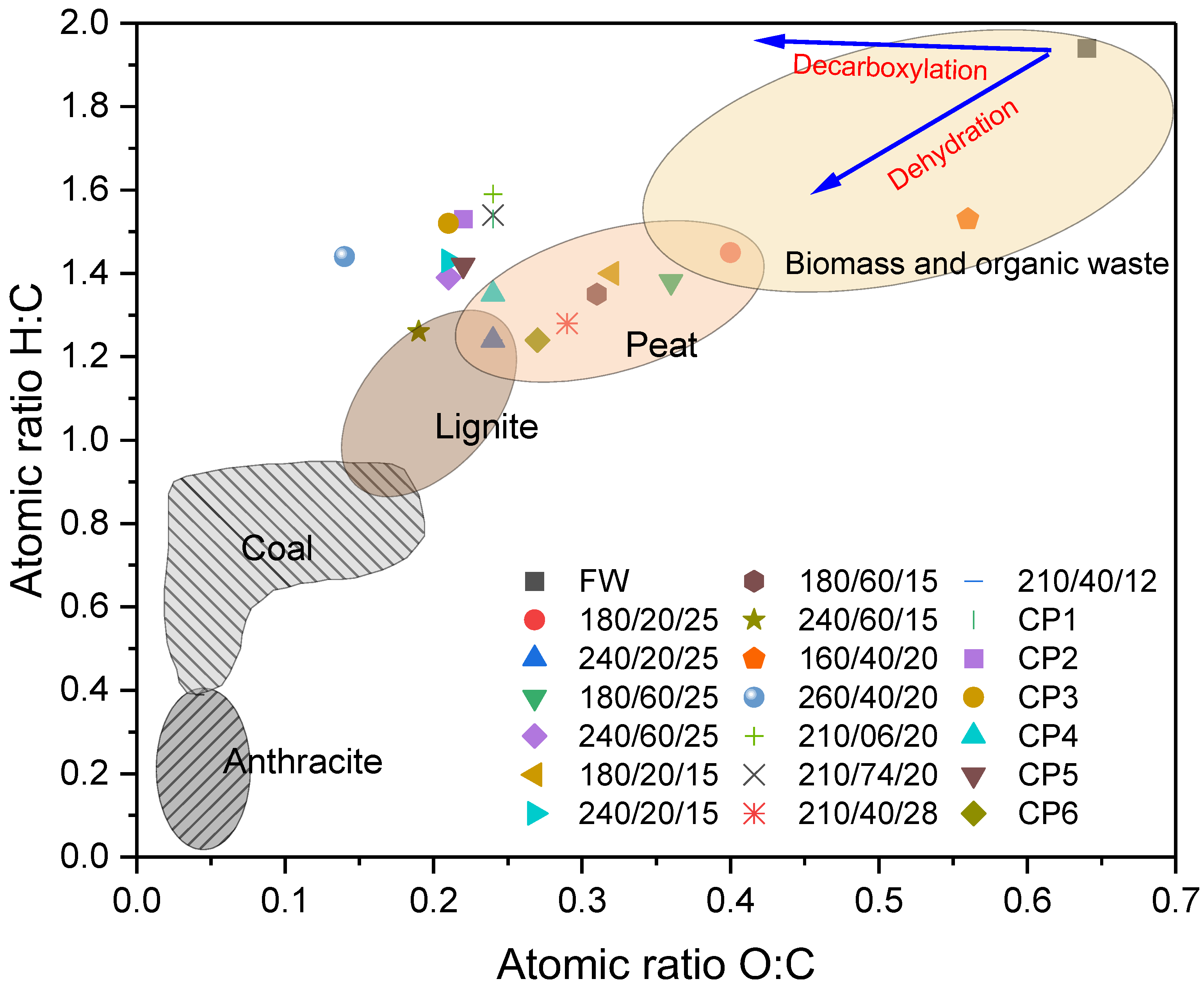
The application of a DoE provided insight into how the HTC process conditions influence the composition and energetic properties of the hydrochar from food waste. This information is valuable for the further development of HTC technology as a treatment and valorization of food waste streams. Hydrothermal carbonization has successfully improved the solid fuel characteristics of food waste. Temperature and a solid load were significant factors affecting the composition of the hydrochar within the condition range used in this work. This suggests that the reaction mechanisms, such as hydrolysis, polymerization, condensation, decarboxylation, and dehydration, were mainly governed by temperature, whereas within the reaction time range of 6–74 min, time played no important role. Decarboxylation reactions were dominant against dehydration. This was visible in the Van Krevelen plot, where the O:C ratio was successfully reduced to coal levels, while H:C remained higher. This behavior was linked to the high volatile matter content and possibly related to the considerable lipid content. It was also evident that solid load played a crucial role, likely facilitating the reaction. This is worth noting, as most modeling and mechanisms studies on HTC are conducted, or assumed to occur, in low solid load (<10%), while practical studies for treating wet wastes have considered minimizing the addition of water to increase environmental viability. This discrepancy requires further addressing, and solid load must be considered a crucial factor when optimizing HTC processes. Reaction time was insignificant for all analyzed responses. However, it could be a major factor when assessing the energy efficiency of the process and should be taken into consideration. Even though the responses evaluated in this work provided insight into the HTC process, it could be useful to encompass a wider set of variables to challenge the perspective of the process and to take advantage of the optimization versatility.