The Role of the Accumulated Surface Charge on Nanoparticles in Improving the Breakdown Strength of Liquid and Solid Insulation
Abstract
:1. Introduction
2. Sample Preparation and Characterization
2.1. Sample Preparation
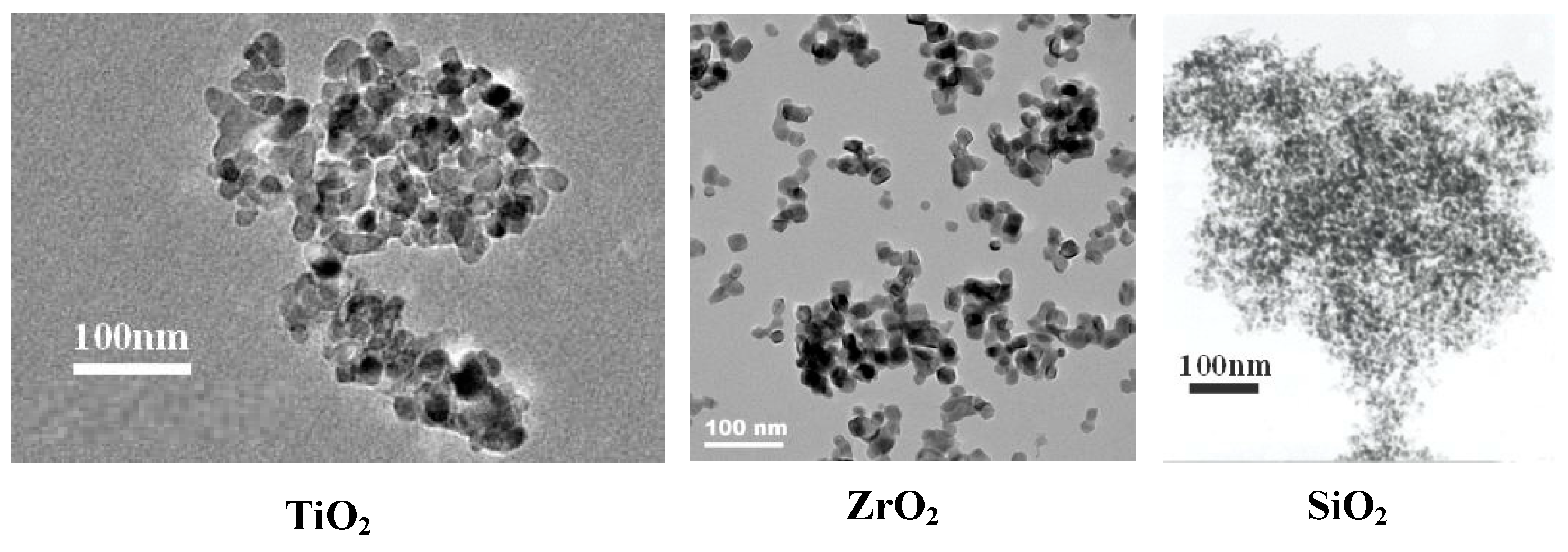
2.2. Sample Characterization
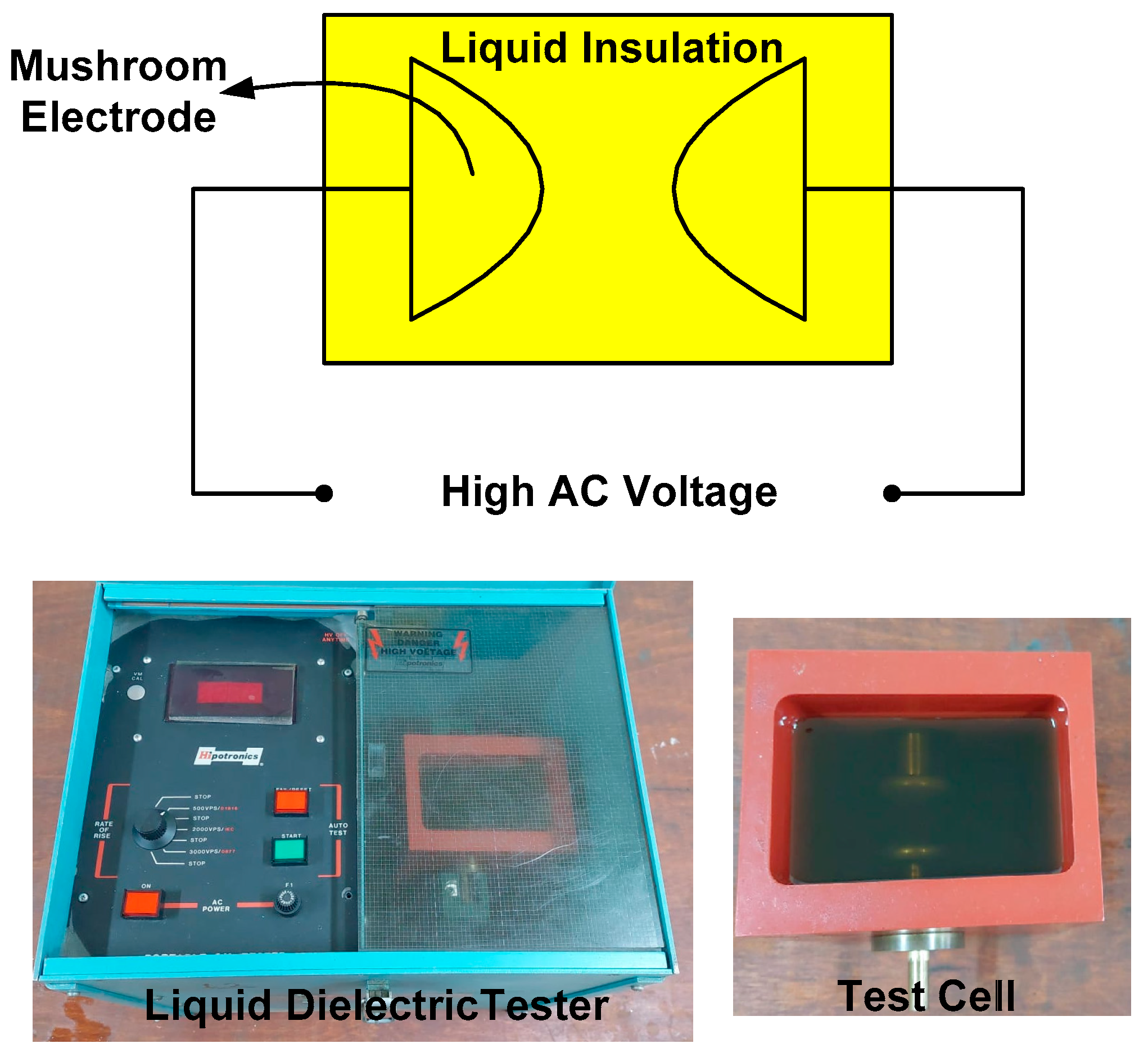
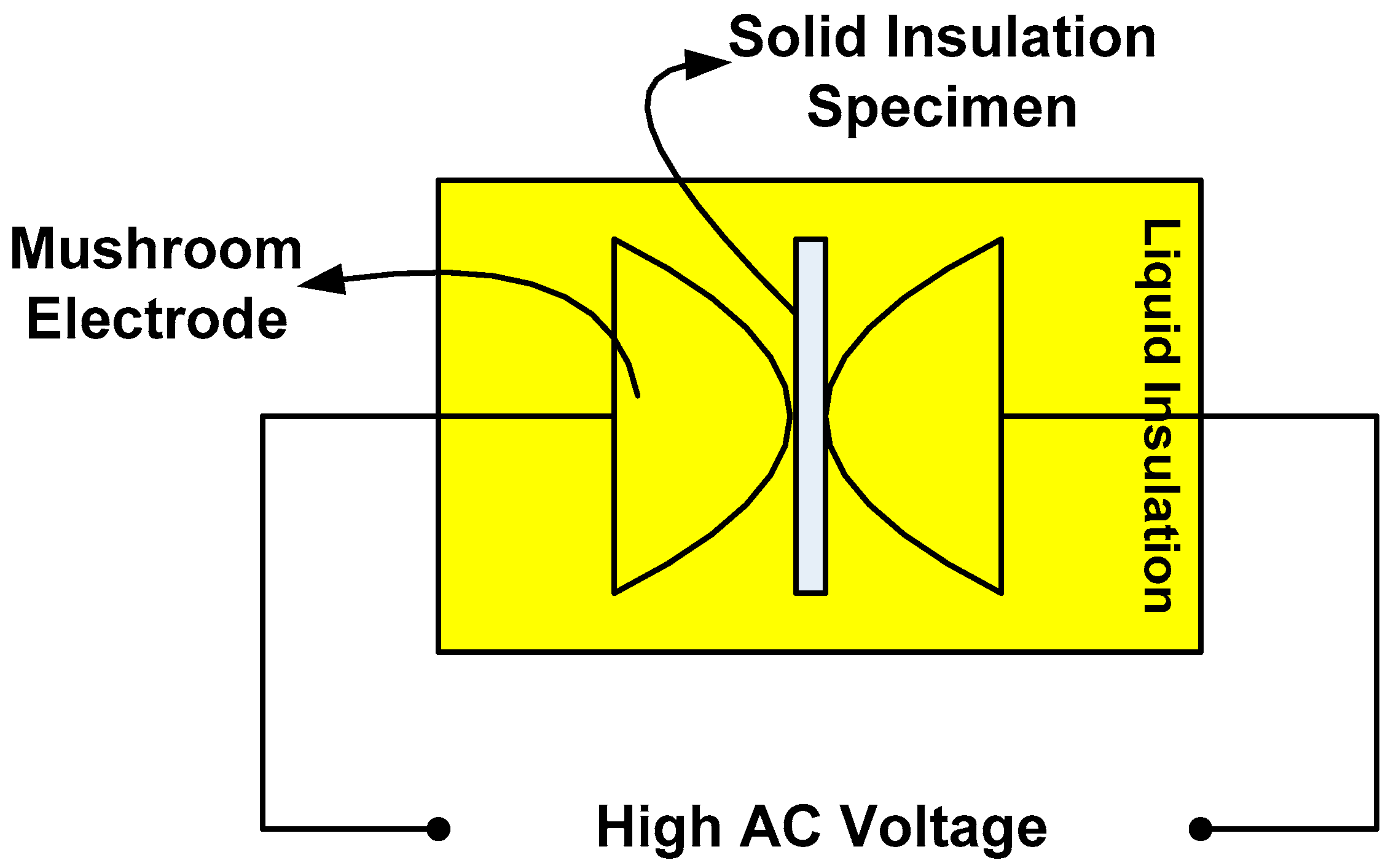
3. Breakdown Strength Measurements
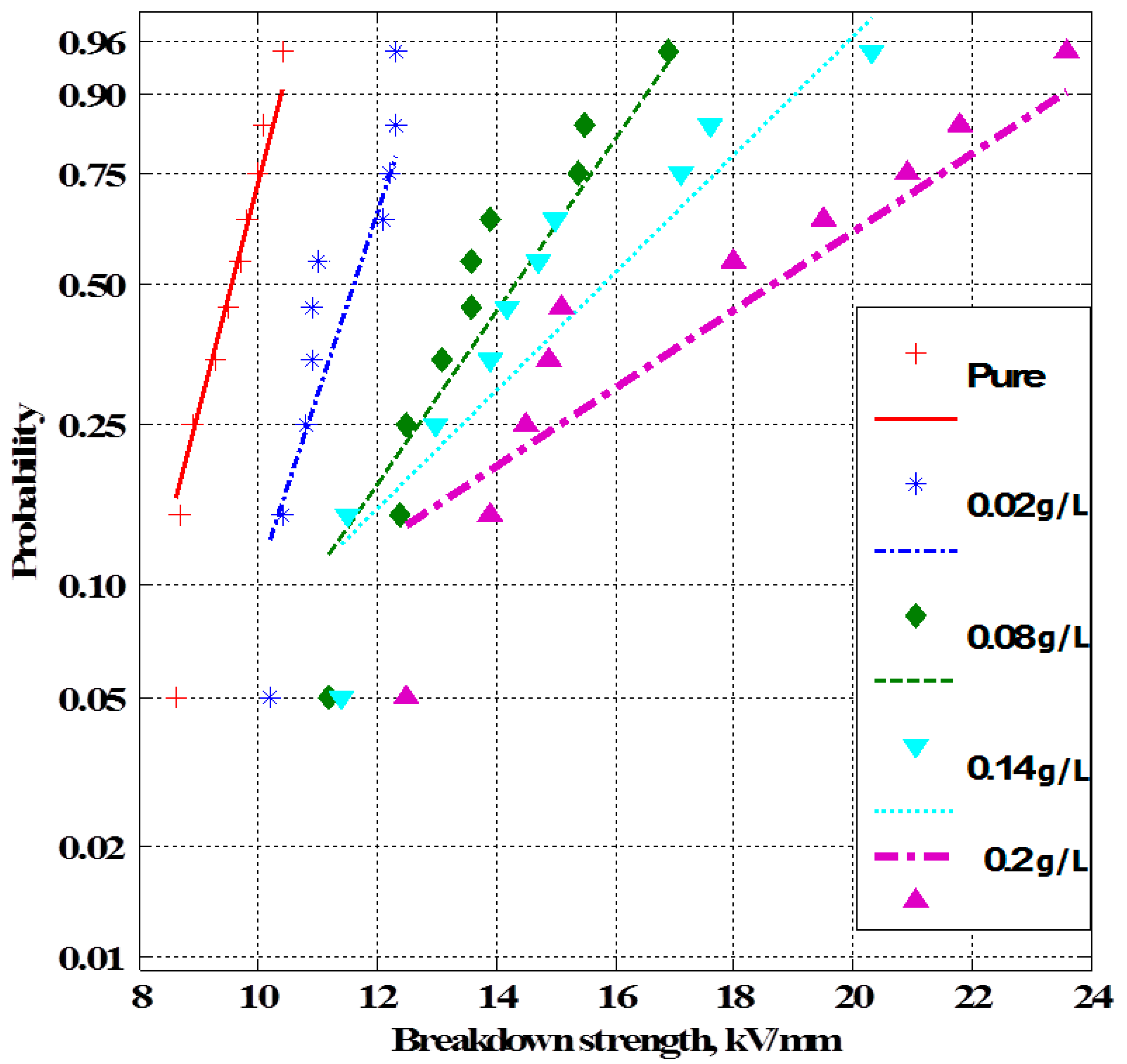
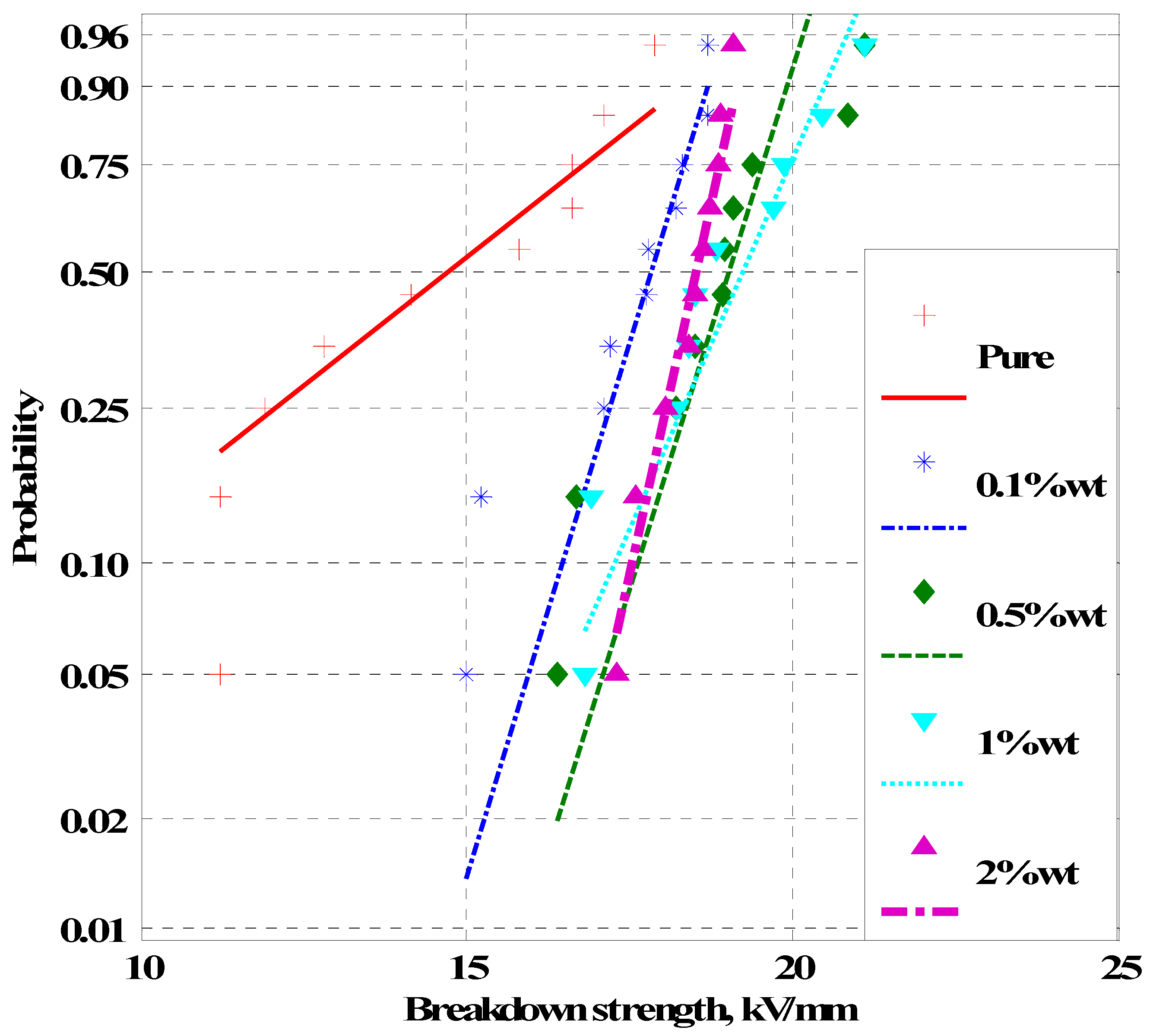
4. Experimental Results
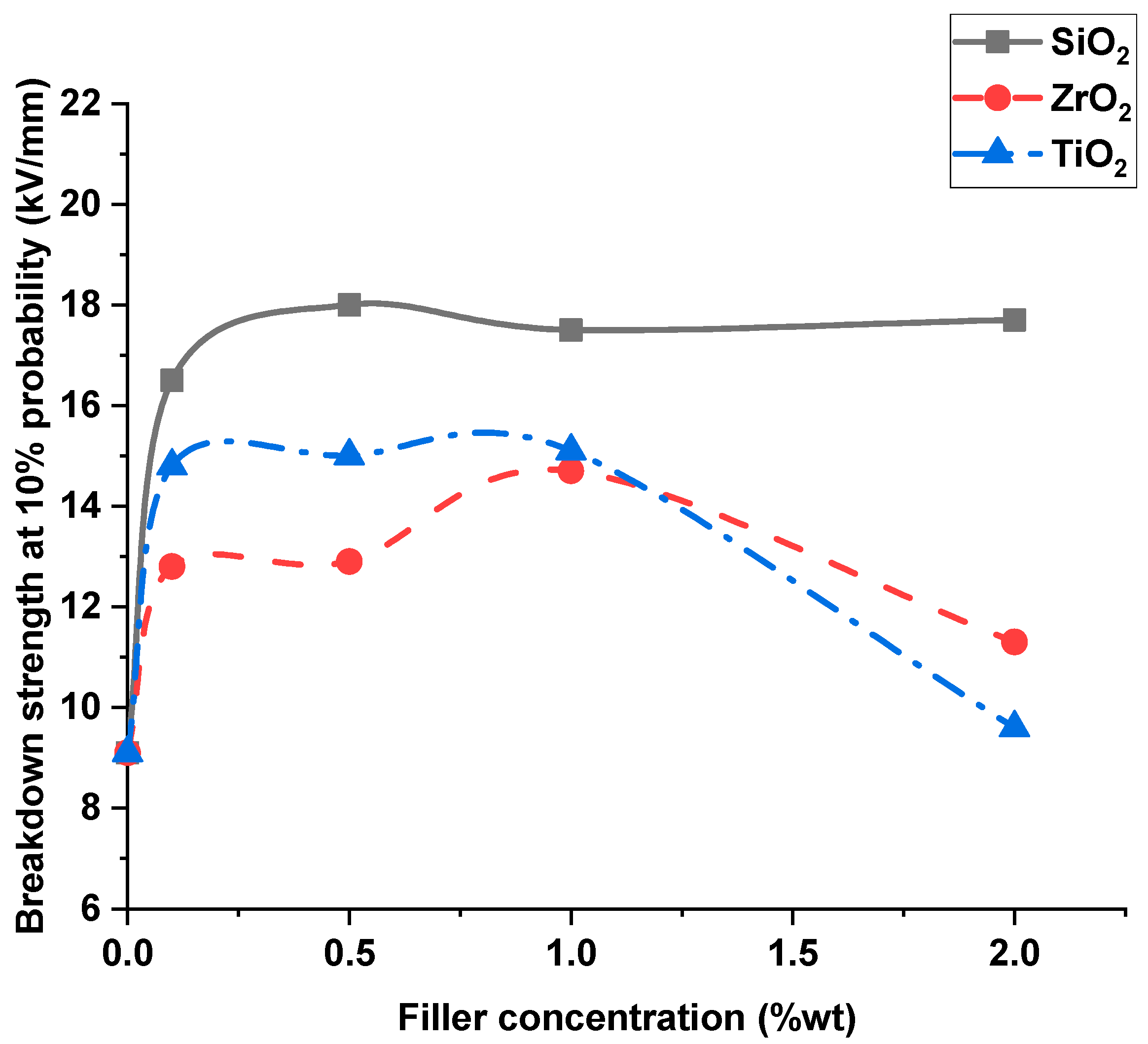
4.1. Breakdown Strength of Transformer Oil Samples
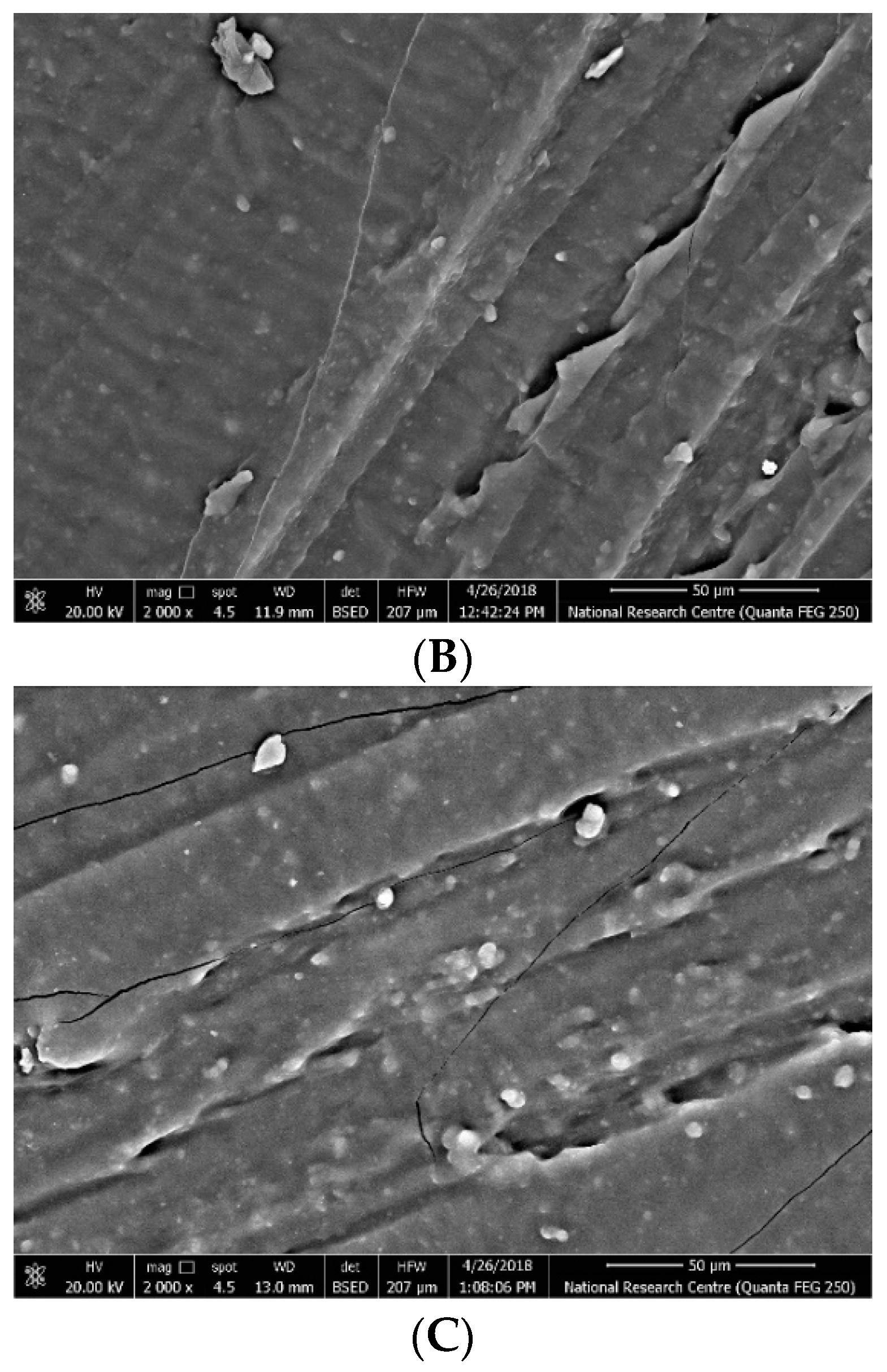
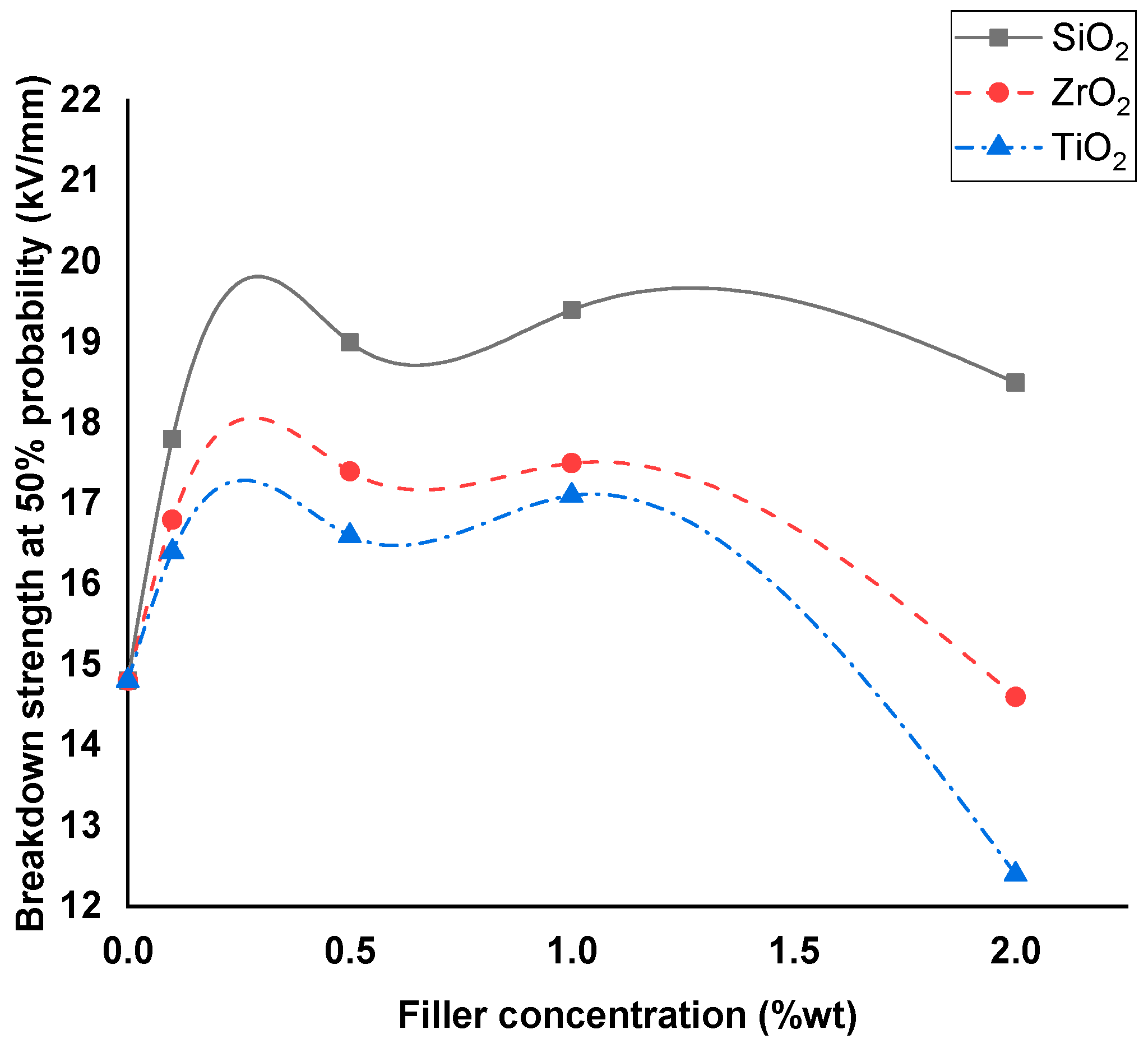
4.2. Breakdown Strength of Silicone Rubber Samples
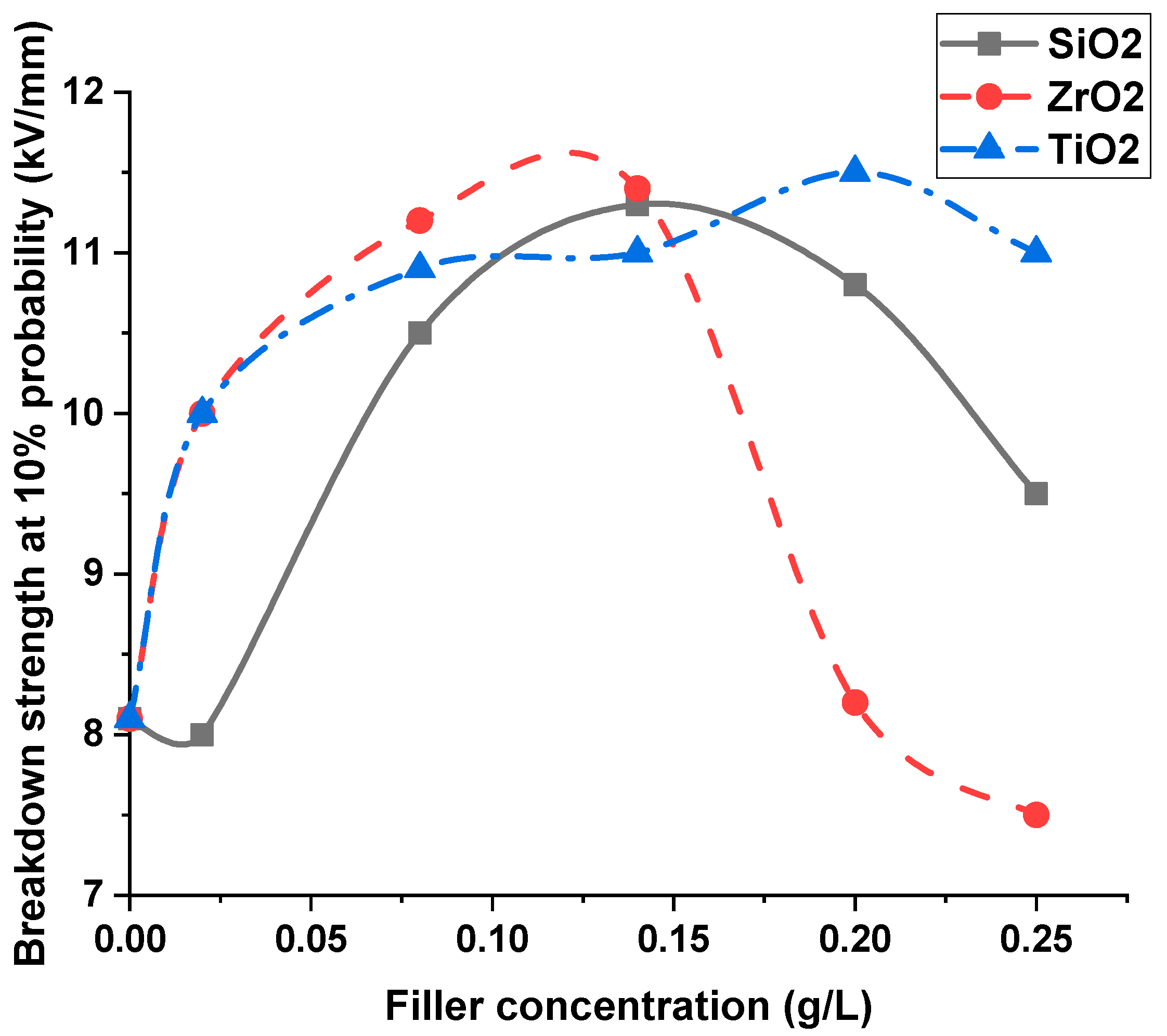
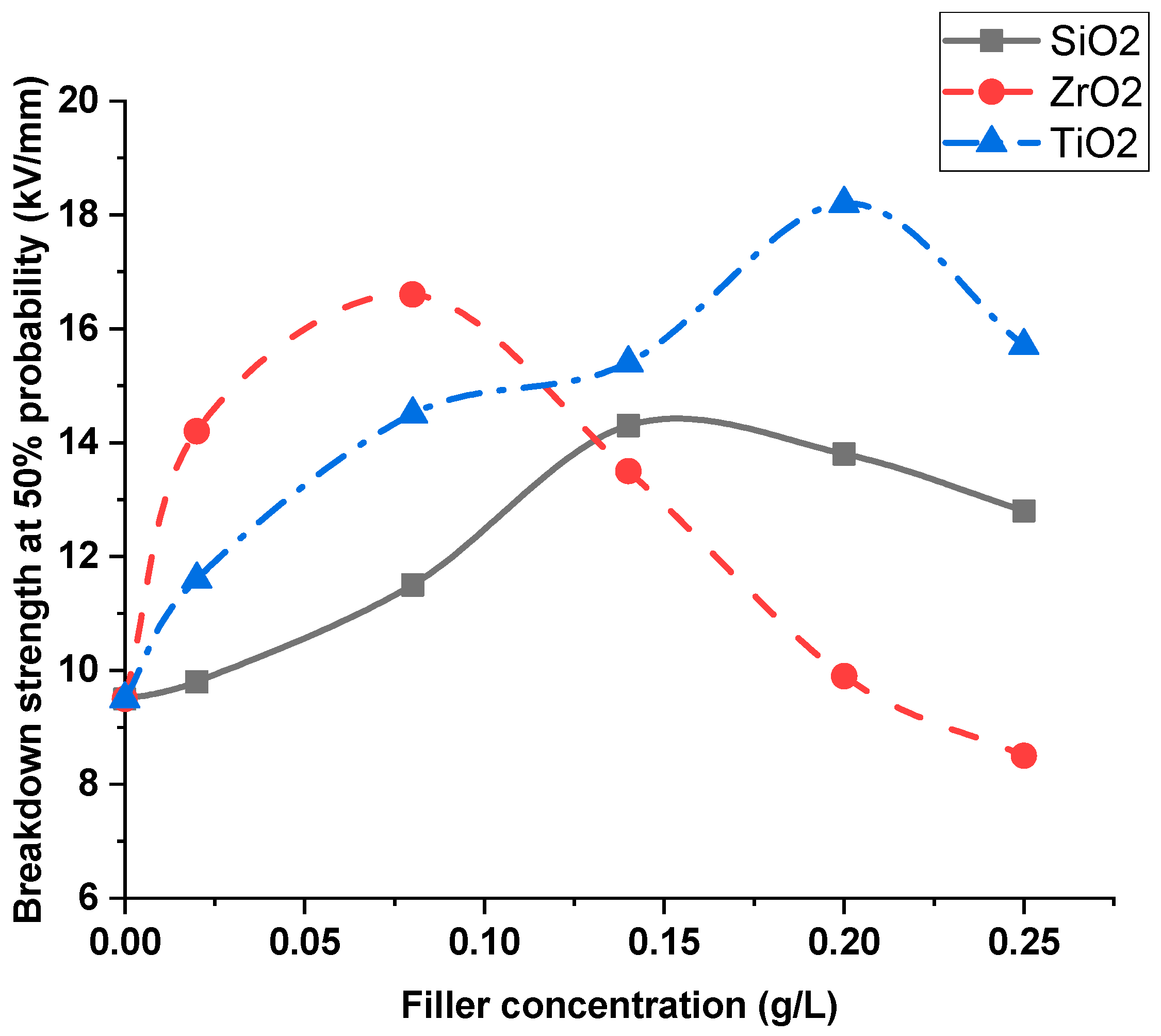
5. Discussion and Interpretations
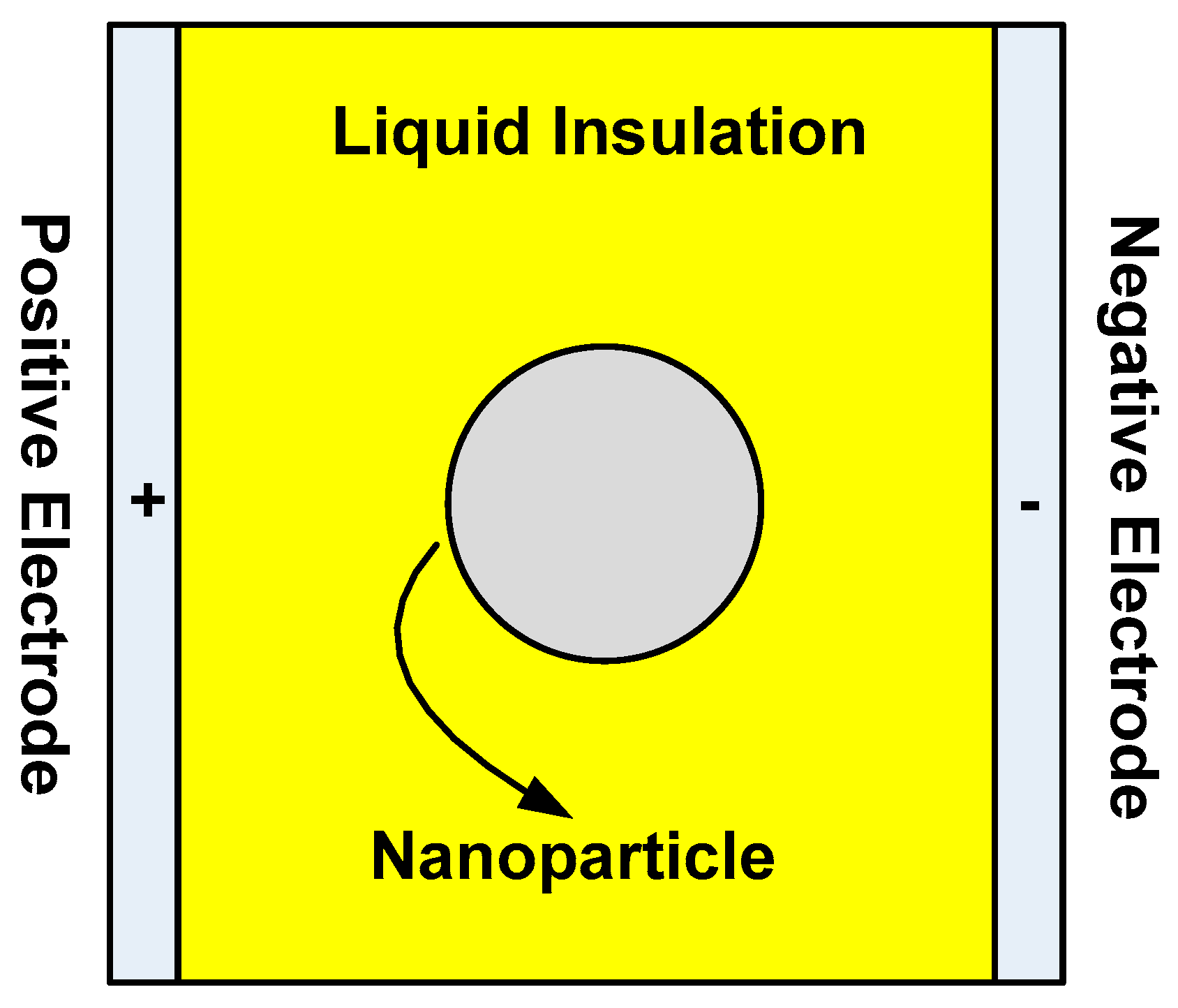
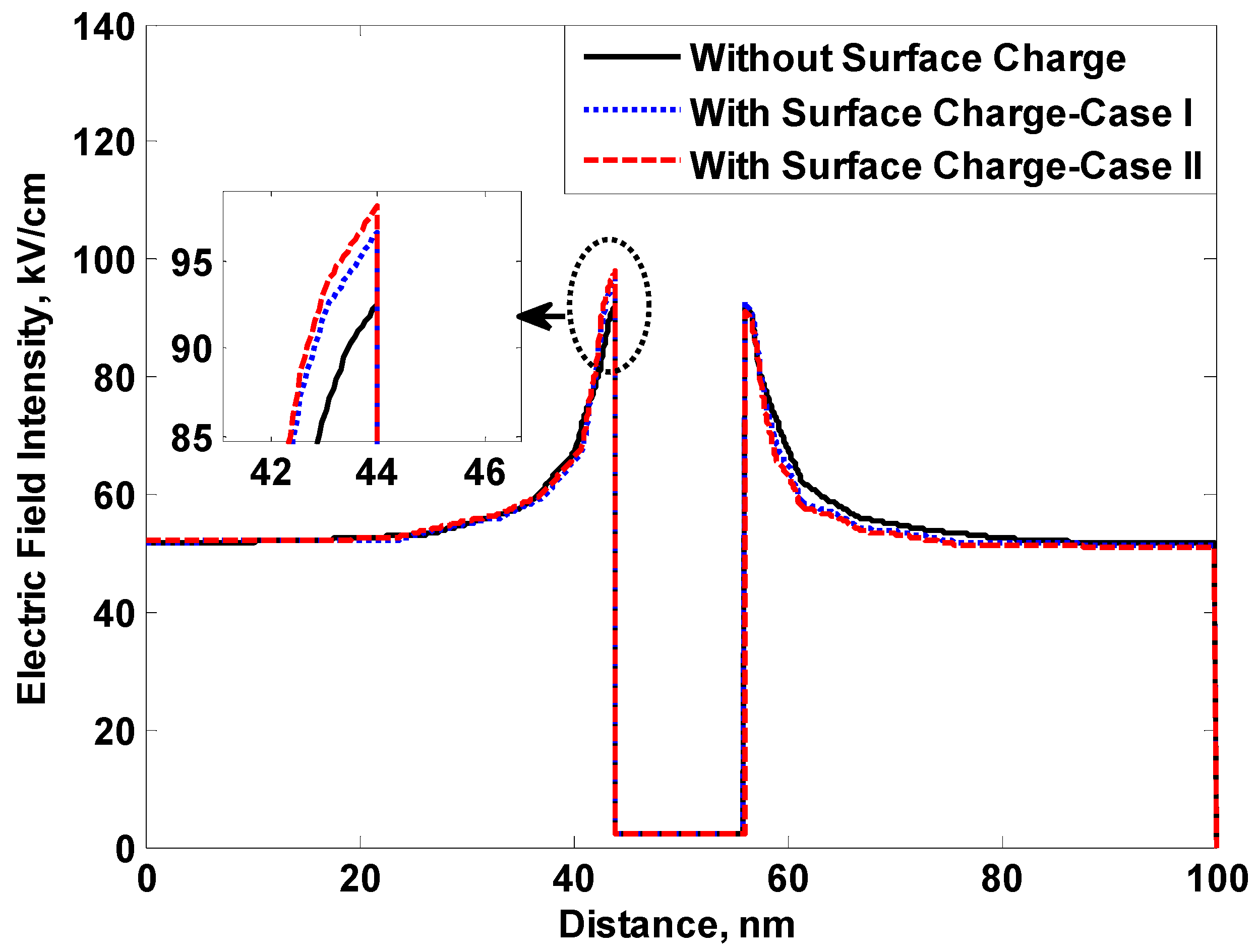
5.1. Surface Charge Formation on Nanoparticle Surface
- Charges accumulate on nanoparticle surfaces when exposed to an external electric field.
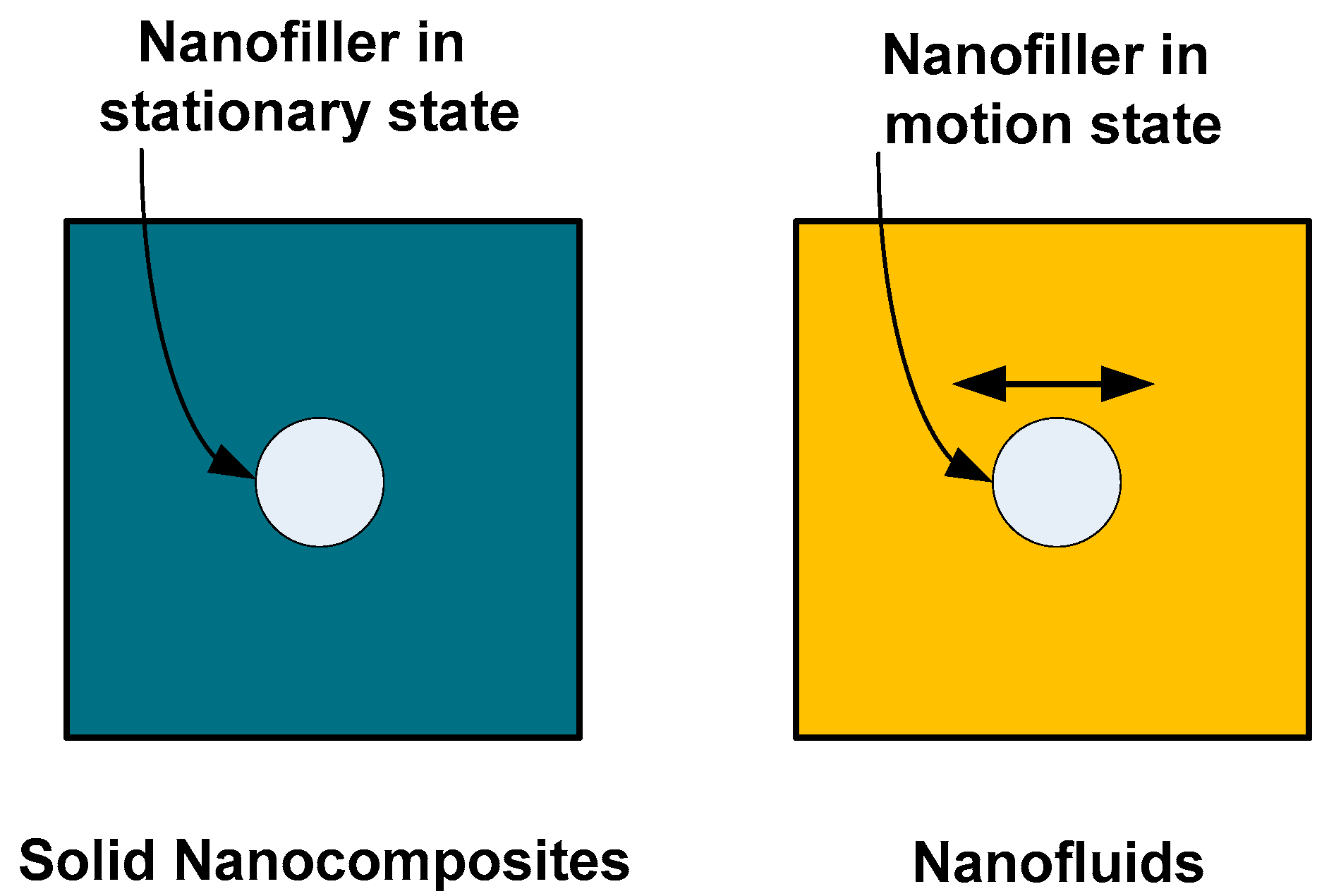
- Charged nanoparticles can move within the fluid due to the effect of an external electric field.
- However, nanoparticles cannot move within a solid dielectric due to the nature of bonding between the particle and the solid material matrix.
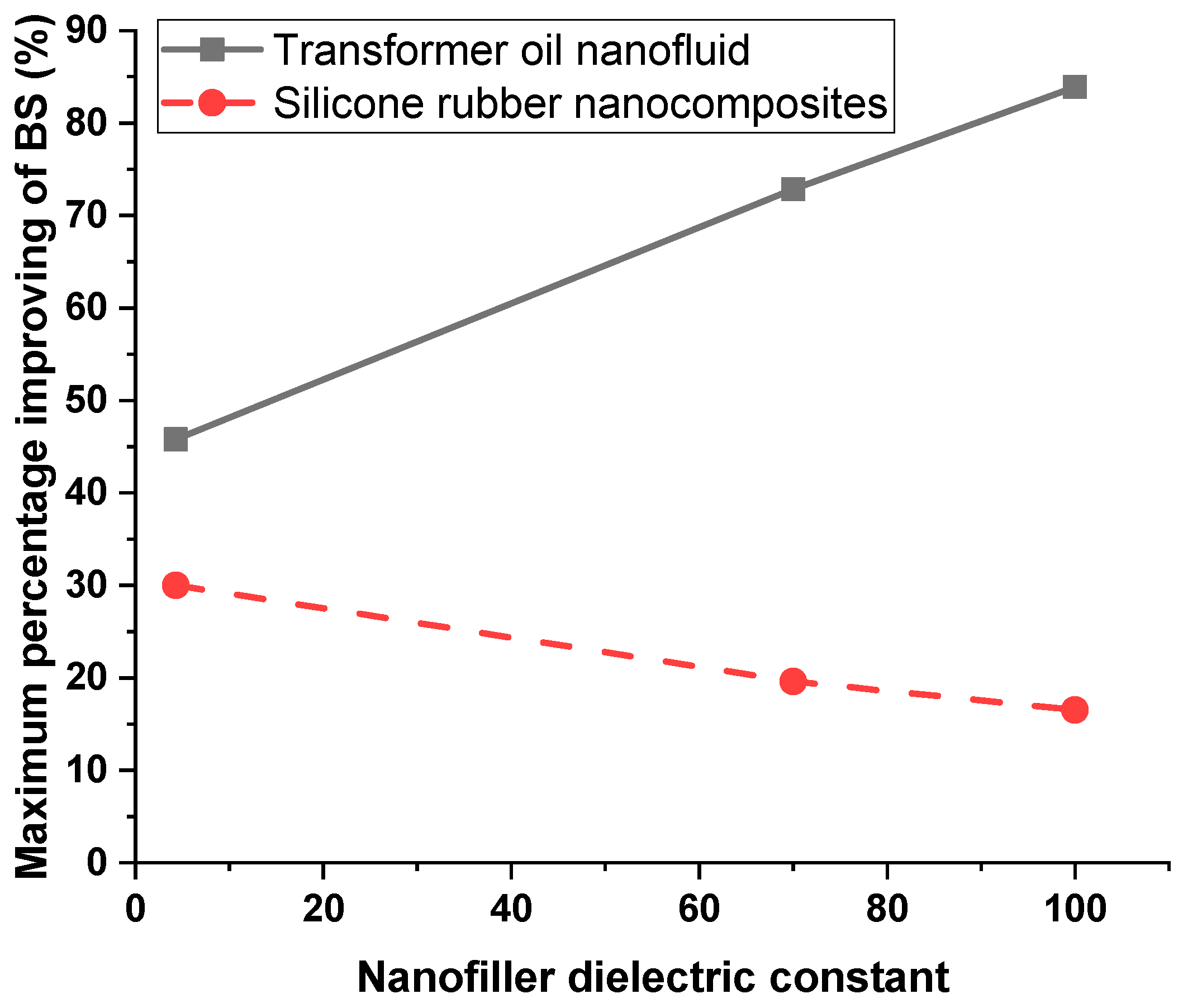
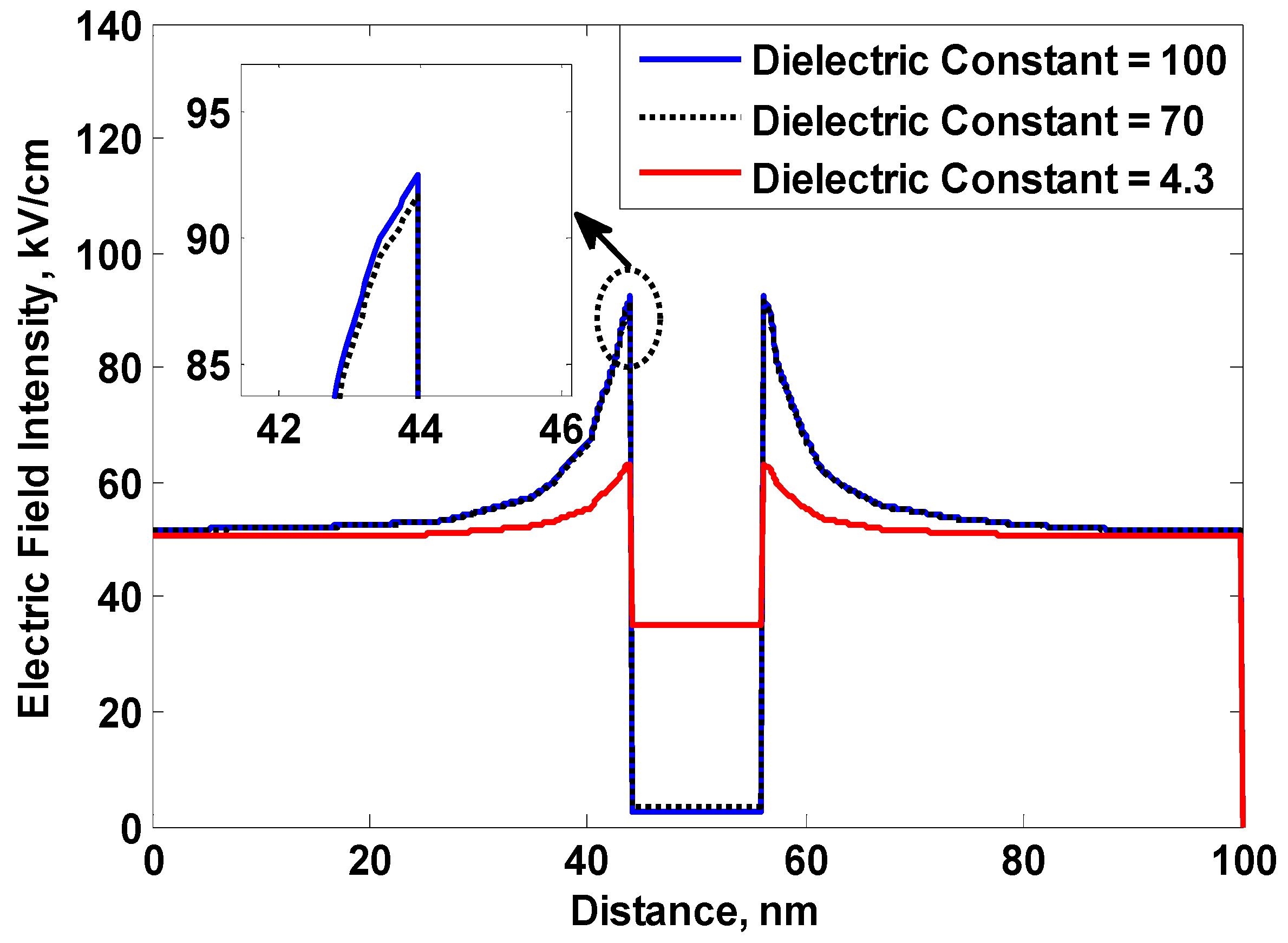
5.2. The Role of the Nanofiller Dielectric Constant in Improving Breakdown Strength of Liquid and Solid Dielectrics
6. Conclusions
- As the nanofiller concentration increases, the average breakdown strength increases above pure dielectric up to a maximum value and then decreases at higher concentrations for both nanofluids and silicone rubber nanocomposites.
- The percentage increase in breakdown strength increases with the increase in the dielectric constant of nanofillers with liquid dielectrics; however, it decreases with the increase in the nanofillers dielectric constant with solid dielectrics.
- The increase in the nanofiller dielectric constant results in an increase in the accumulated charges on a nanoparticle surface.
- It is recommended that nanoparticles with higher dielectric constants be used to increase the breakdown strength of nanofluids. However, lower nanoparticle dielectric constants are preferred to increase the breakdown strength of solid nanocomposites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhong, Y.; Zhou, J.; Li, X.; Zhou, Y. Effect of semiconductive nanoparticles on insulating performances of transformer oil. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 770–776. [Google Scholar] [CrossRef]
- Sima, W.; Shi, J.; Yang, Q.; Huang, S.; Cao, X. Effects of conductivity and permittivity of nanoparticle on transformer oil insulation performance: Experiment and theory. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 380–390. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abd-Elhady, A.; Izzularab, M. Effect of nanoparticles on transformer oil breakdown strength: Experiment and theory. IET Sci. Meas. Technol. 2016, 10, 839–845. [Google Scholar] [CrossRef]
- Sivakumar, S.; Sasikanth, P.; Mohanram, S.; Manikandan, C.; Siddharthan, A. Property Enhancement of Transformer Oil with Suspension of TiO2 Nanoparticles. J. Nanofluids 2014, 3, 336–340. [Google Scholar] [CrossRef]
- Abd-Elhady, A.M.; Ibrahim, M.E.; Taha, T.A.; Izzularab, M.A. Dielectric and Thermal Properties of Transformer Oil Modified by Semiconductive CdS Quantum Dots. J. Electron. Mater. 2016, 45, 4755–4761. [Google Scholar] [CrossRef]
- Jang, S.P.; Choi, S.U.S. Role of Brownian motion in the enhanced thermal conductivity of nanofluids. Appl. Phys. Lett. 2004, 84, 4316–4318. [Google Scholar] [CrossRef]
- Kumar, D.H.; Patel, H.E.; Kumar, V.R.R.; Sundararajan, T.; Pradeep, T.; Das, S.K. Model for heat conduction in nanofluids. Phys. Rev. Lett. 2004, 93, 144301. [Google Scholar] [CrossRef] [Green Version]
- Ghasemi, J.; Jafarmadar, S.; Nazari, M. Effect of magnetic nanoparticles on the lightning impulse breakdown voltage of transformer oil. J. Magn. Magn. Mater. 2015, 389, 148–152. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kim, W.-Y. Experimental study on the dielectric breakdown voltage of the insulating oil mixed with magnetic nanoparticles. Phys. Procedia 2012, 32, 327–334. [Google Scholar] [CrossRef] [Green Version]
- Imai, T.; Sawa, F.; Ozaki, T.; Shimizu, T.; Kuge, S.; Kozako, M.; Tanaka, T. Effects of Epoxy/Filler Interface on Properties of Nano- or Micro-composites. IEEJ Trans. Fundam. Mater. 2006, 126, 84–91. [Google Scholar] [CrossRef] [Green Version]
- Huang, F. Interface and properties of epoxy resin modified by elastomeric nano-particles. Sci. China Ser. B 2005, 48, 148. [Google Scholar] [CrossRef]
- Imai, T.; Sawa, F.; Ozaki, T.; Nakano, T.; Shimizu, T.; Yoshimitsu, T. Preparation and Insulation Properties of Epoxy-Layered Silicate Nanocomposite. IEEJ Trans. Fundam. Mater. 2004, 124, 1065–1072. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Yamaguchi, M.; Okubo, M.; Matsumoto, T. Effect of particle size on impact properties of epoxy resin filled with angular shaped silica particles. Polymer 1991, 32, 2976–2979. [Google Scholar] [CrossRef]
- Grabowski, C.; Fillery, S.; Koerner, H.; Tchoul, M.; Drummy, L.; Beier, C.; Brutchey, R.; Durstock, M.; Vaia, R. Dielectric performance of high permitivity nanocomposites: Impact of polystyrene grafting on BaTiO3 and TiO2. Nanocomposites 2016, 2, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Luo, P.; Xu, M.; Wang, S.; Xu, Y. Structural, dynamic mechanical and dielectric properties of mesoporous silica/epoxy resin nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1685–1697. [Google Scholar] [CrossRef]
- Khan, H.; Amin, M.; Ali, M.; Iqbal, M.; Yasin, M. Effect of micro/nano-SiO2 on mechanical, thermal, and electrical properties of silicone rubber, epoxy, and EPDM composites for outdoor electrical insulations. Turk. J. Electr. Eng. Comput. Sci. 2017, 25, 1426–1435. [Google Scholar] [CrossRef]
- Preetha, P.; Thomas, M. AC breakdown characteristics of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1526–1534. [Google Scholar] [CrossRef]
- Singha, S.; Thomas, M. Dielectric properties of epoxy nanocomposites. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 12–23. [Google Scholar] [CrossRef]
- Dang, Z.; Xia, Y.; Zha, J.; Yuan, J.; Bai, J. Preparation and dielectric properties of surface modified TiO2/silicone rubber nanocomposites. Mater. Lett. 2011, 65, 3430–3432. [Google Scholar] [CrossRef]
- Park, Y.; Kwon, J.; Sim, J.; Hwang, J.; Seo, C.; Kim, J.; Lim, K. DC conduction and breakdown characteristics of Al2O3/cross-linked polyethylene nanocomposites for high voltage direct current transmission cable insulation. Jpn. J. Appl. Phys. 2014, 53, 08NL05. [Google Scholar] [CrossRef]
- Jin, H.; Andritsch, T.; Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Properties of mineral Oil based silica nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1100–1108. [Google Scholar] [CrossRef]
- Hwang, J.G.; Zahn, M.; O’Sullivan, F.M.; Pettersson, L.A.A.; Hjortstam, O.; Liu, R. Effects of nanoparticle charging on streamer development in transformer oil based nanofluids. J. Appl. Phys. 2010, 107, 014310. [Google Scholar] [CrossRef] [Green Version]
- Samy, A.M.; Ibrahim, M.E.; Abd-Elhady, A.M.; Izzularab, M.A. On Electric Field Distortion for Breakdown Mechanism of Nanofilled Transformer Oil. Int. J. Electr. Power Energy Syst. 2020, 117, 105632. [Google Scholar] [CrossRef]
- Tanaka, T. Dielectric nanocomposites with insulating properties. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 914–928. [Google Scholar] [CrossRef]
- Ding, S.; Yu, S.; Zhu, X.; Xie, S.; Sun, R.; Liao, W.; Wong, C. Enhanced breakdown strength of polymer composites by low filler loading and its mechanisms. Appl. Phys. Lett. 2017, 111, 153902. [Google Scholar] [CrossRef]
- Kavitha, D.; Sindhu, T.; Nambiar, T. Impact of permittivity and concentration of filler nanoparticles on dielectric properties of polymer nanocomposites. IET Sci. Meas. Technol. 2017, 11, 179–185. [Google Scholar] [CrossRef]
- Habashy, M.M.; Abd-Elhady, A.M.; Elsad, R.A.; Izzularab, M.A. Performance of PVC/SiO2 Nanocomposites under Thermal Ageing. Appl. Nanosci. J. 2021, 11, 2143–2151. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elhady, A.M.; Ibrahim, M.E.; Taha, T.; Izzularab, M.A. Effect of Temperature on AC Breakdown Voltage of Nanofilled Transformer Oil. IET Sci. Meas. Technol. 2018, 12, 138–144. [Google Scholar] [CrossRef]
- US Research Nanomaterials, Inc. Available online: https://www.us-nano.com/inc/sdetail/47163 (accessed on 30 June 2022).




| Nanofiller | Maximum BS | Maximum Percentage Increase | ||
|---|---|---|---|---|
| Transformer Oil | Silicone Rubber | Transformer Oil | Silicone Rubber | |
| SiO2 | 13.87 kV/mm | 18.85 kV/mm | 46% | 30% |
| ZrO2 | 16.42 kV/mm | 17.34 kV/mm | 72.8% | 19.6% |
| TiO2 | 17.48 kV/mm | 16.89 kV/mm | 84% | 16.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.E.; Tag Eldin, E.; Elzoghby, S.F.; Izzularab, M.A.; Abd-Elhady, A.M. The Role of the Accumulated Surface Charge on Nanoparticles in Improving the Breakdown Strength of Liquid and Solid Insulation. Energies 2022, 15, 4860. https://doi.org/10.3390/en15134860
Ibrahim ME, Tag Eldin E, Elzoghby SF, Izzularab MA, Abd-Elhady AM. The Role of the Accumulated Surface Charge on Nanoparticles in Improving the Breakdown Strength of Liquid and Solid Insulation. Energies. 2022; 15(13):4860. https://doi.org/10.3390/en15134860
Chicago/Turabian StyleIbrahim, Mohamed E., Elsayed Tag Eldin, Safaa F. Elzoghby, Mohamed A. Izzularab, and Amr M. Abd-Elhady. 2022. "The Role of the Accumulated Surface Charge on Nanoparticles in Improving the Breakdown Strength of Liquid and Solid Insulation" Energies 15, no. 13: 4860. https://doi.org/10.3390/en15134860
APA StyleIbrahim, M. E., Tag Eldin, E., Elzoghby, S. F., Izzularab, M. A., & Abd-Elhady, A. M. (2022). The Role of the Accumulated Surface Charge on Nanoparticles in Improving the Breakdown Strength of Liquid and Solid Insulation. Energies, 15(13), 4860. https://doi.org/10.3390/en15134860






