Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment
Abstract
1. Introduction
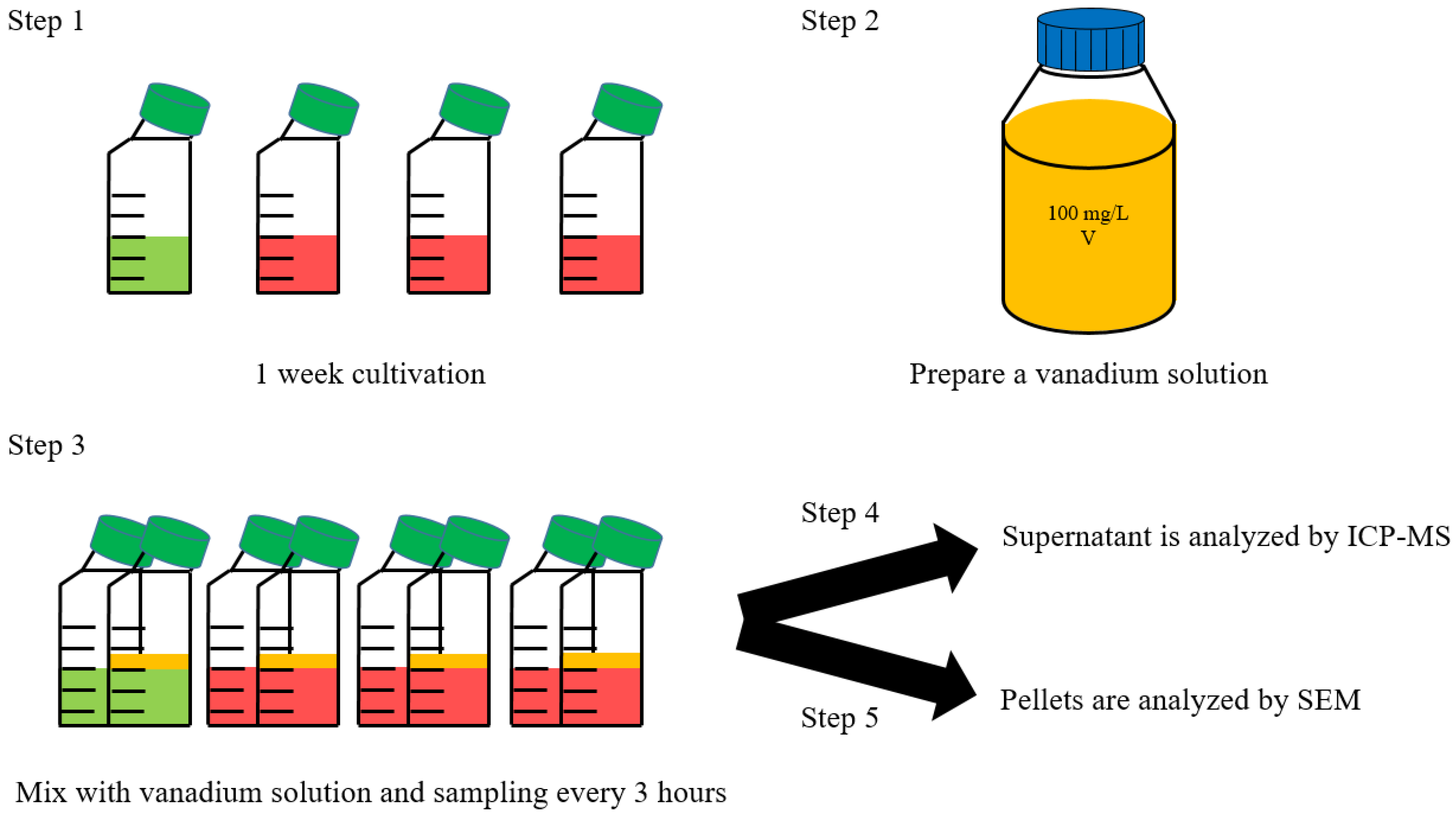
2. Materials and Methods
2.1. Strain and Maintenance Condition
2.2. Cellular Growth Measurement
2.3. Vanadium Ion Measurement Using ICP-MS
2.4. Scanning Electron Microscope (SEM)
2.5. Statistical Analysis
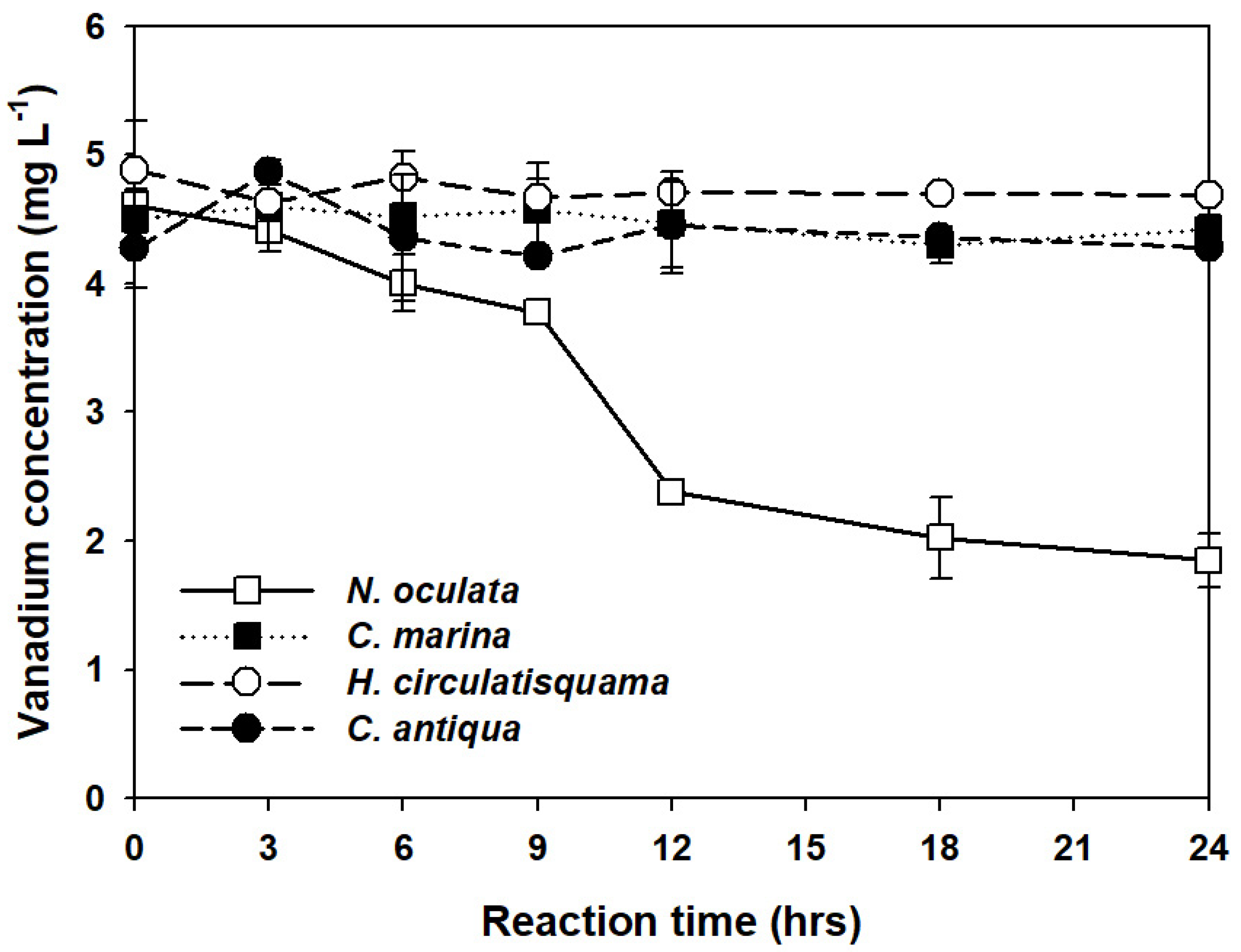
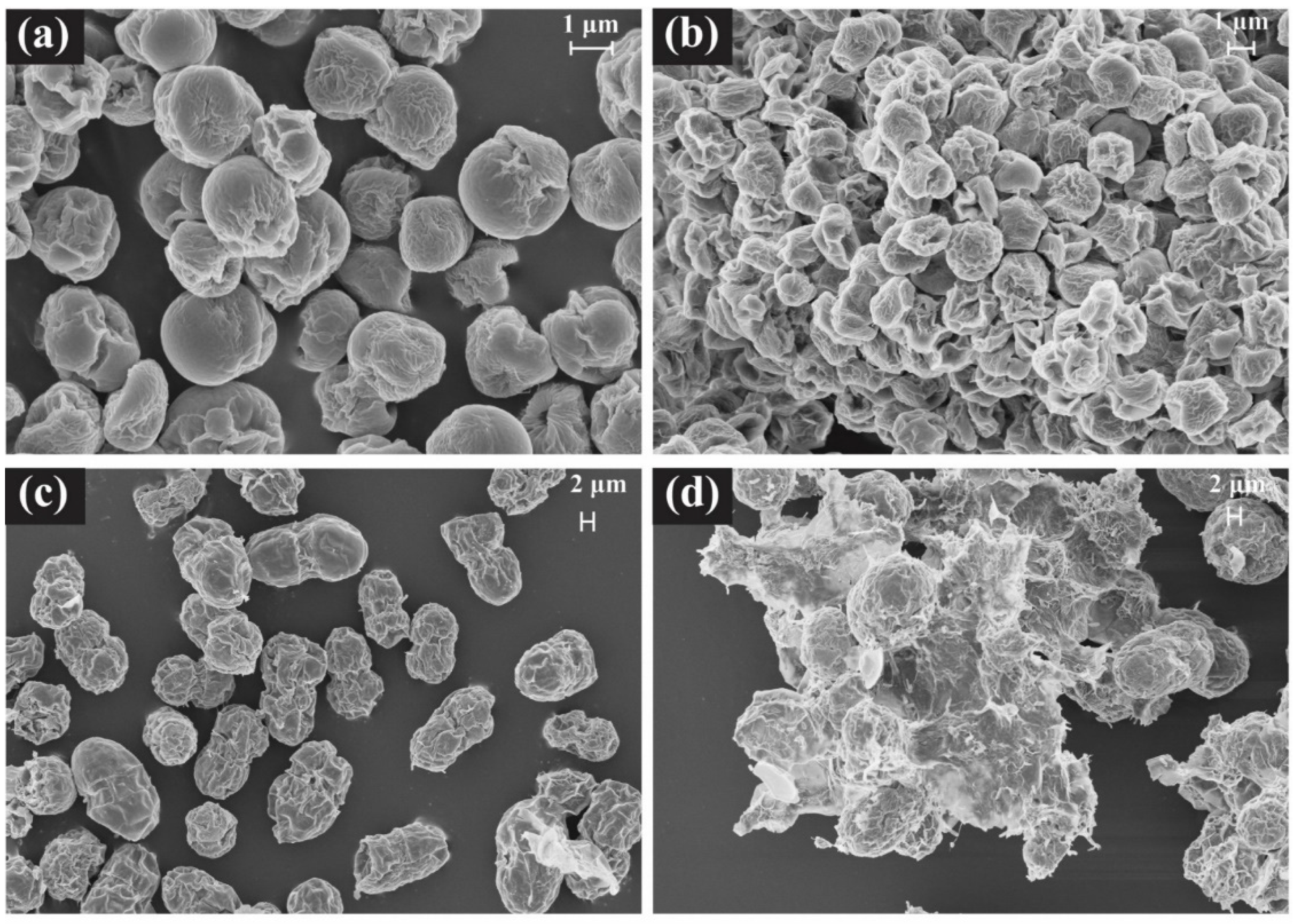
3. Results and Discussion
3.1. Cellular Growth and Physiology of Microalgae
3.2. Vanadium Ion Concentration Measurement by ICP-MS
3.3. SEM Observation of Cellular Morphology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Xiang, L.; Wang, X.; Ren, H.; Wei, L.; Chen, P. Residual effects of organochlorine pesticides (OCPs) in an e-waste recycling area compared with heavy metal pollution. Ecotoxicol. Environ. Saf. 2020, 198, 110651. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Xiang, J.; Ko, J.H. Municipal plastic recycling at two areas in China and heavy metal leachability of plastic in municipal solid waste. Environ. Pollut. 2020, 260, 114074. [Google Scholar] [CrossRef] [PubMed]
- Lou, X.-Y.; Xu, Z.; Bai, A.-P.; Resina-Gallego, M.; Ji, Z.-G. Separation and Recycling of Concentrated Heavy Metal Wastewater by Tube Membrane Distillation Integrated with Crystallization. Membranes 2020, 10, 19. [Google Scholar] [CrossRef]
- Platzer, S.; Kar, M.; Leyma, R.; Chib, S.; Roller, A.; Jirsa, F.; Krachler, R.; MacFarlane, D.R.; Kandioller, W.; Keppler, B.K. Task-specific thioglycolate ionic liquids for heavy metal extraction: Synthesis, extraction efficacies and recycling properties. J. Hazard. Mater. 2017, 324, 241–249. [Google Scholar] [CrossRef]
- Paulose, B.; Chhikara, S.; Coomey, J.; Jung, H.I.; Vatamaniuk, O.; Dhankher, O.P. A gamma-glutamyl cyclotransferase protects Arabidopsis plants from heavy metal toxicity by recycling glutamate to maintain glutathione homeostasis. Plant Cell 2013, 25, 4580–4595. [Google Scholar] [CrossRef] [PubMed]
- Moskalyk, R.R.; Alfantazi, A.M. Processing of vanadium: A review. Miner. Eng. 2003, 16, 793–805. [Google Scholar] [CrossRef]
- Santos Andrade, T.; Keramidas, A.; Lianos, P. Use of Chalcogenide-Semiconductor-Sensitized Titania to Directly Charge a Vanadium Redox Battery. Nanomaterials 2020, 10, 1137. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Duong, D.L.; Ha, D.M.; Singh, K.; Phan, T.L.; Choi, W.; Kim, Y.-M.; Lee, Y.H. Ferromagnetic Order at Room Temperature in Monolayer WSe2 Semiconductor via Vanadium Dopant. Adv. Sci. 2020, 7, 1903076. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, B.; Sebastian, A.; Olson, D.H.; Liu, M.; Fujisawa, K.; Pham, Y.T.H.; Jimenez, V.O.; Kalappattil, V.; Miao, L.; et al. Monolayer Vanadium-Doped Tungsten Disulfide: A Room-Temperature Dilute Magnetic Semiconductor. Adv. Sci. 2020, 7, 2001174. [Google Scholar] [CrossRef]
- Cho, H.; Atanasov, V.; Krieg, H.M.; Kerres, J.A. Novel Anion Exchange Membrane Based on Poly(Pentafluorostyrene) Substituted with Mercaptotetrazole Pendant Groups and Its Blend with Polybenzimidazole for Vanadium Redox Flow Battery Applications. Polymers 2020, 12, 915. [Google Scholar] [CrossRef]
- Jeon, H.; Baek, S.H.; Han, Y.; Go, B.; Jeong, D.; Kim, S.M. Developing technology necessary to produce domestic vanadium resources. J. Korean Soc. Miner. Energy Resour. 2021, 58, 66–74. [Google Scholar] [CrossRef]
- Wold, A.; Ward, R. Perowskite-type oxides of cobalt, chromium and vanadium with some rare earth elements. J. Am. Chem. Soc. 1954, 76, 1029–1030. [Google Scholar] [CrossRef]
- Baroch, E.F. Updated by Staff. Vanadium and vanadium alloys. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, NY, USA, 2000; pp. 1–18. [Google Scholar]
- Peng, H. A literature review on leaching and recovery of vanadium. J. Environ. Chem. Eng. 2020, 7, 103313. [Google Scholar] [CrossRef]
- Touarssi, I.; Mourtah, I.; Chaouqi, Y.; Kamal, O.; Sefiani, N.; Lebrun, L.; Hlaïbi, M. Conceptualization and quantification of oriented membrane processes for recovering vanadium ions from acidic industrial discharges. J. Environ. Chem. Eng. 2020, 7, 103182. [Google Scholar] [CrossRef]
- Petranikova, M.; Tkaczyk, A.H.; Bartl, A.; Amato, A.; Lapkovskis, V.; Tunsu, C. Vanadium sustainability in the context of innovative recycling and sourcing development. Waste Manag. 2020, 113, 521–544. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Jiang, Q.; Gao, L.; Chen, J.; Peng, J.; Koppala, S.; Omran, M.; Chen, G. Investigations on the microwave absorption properties and thermal behavior of vanadium slag: Improvement in microwave oxidation roasting for recycling vanadium and chromium. J. Hazard. Mater. 2020, 395, 122698. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, A.K.; Li, J. An overview of the potential of eco-friendly hybrid strategy for metal recycling from WEEE. Resour. Conserv. Recycl. 2017, 126, 228–239. [Google Scholar] [CrossRef]
- Kumar, K.S.; Dahms, H.-U.; Won, E.-J.; Lee, J.-S.; Shin, K.-H. Microalgae–A promising tool for heavy metal remediation. Ecotoxicol. Environ. Saf. 2015, 113, 329–352. [Google Scholar] [CrossRef]
- Van Hille, R.P.; Boshoff, G.A.; Rose, P.D.; Duncan, J.R. A continuous process for the biological treatment of heavy metal contaminated acid mine water. Resour. Conserv. Recycl. 1999, 27, 157–167. [Google Scholar] [CrossRef]
- Chang, D.; Fukushi, K.; Ghosh, S. Stimulation of activated sludge cultures for enhanced heavy metal removal. Water Environ. Res. 1995, 67, 822–827. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Microalga-mediated bioremediation of heavy metal–contaminated surface waters. In Biomanagement of Metal-Contaminated Soils; Springer: Dordrecht, The Netherlands, 2011; pp. 365–385. [Google Scholar]
- Dirbaz, M.; Roosta, A. Adsorption, kinetic and thermodynamic studies for the biosorption of cadmium onto microalgae Parachlorella sp. J. Environ. Chem. Eng. 2018, 6, 2302–2309. [Google Scholar] [CrossRef]
- Hein, M.; Pedersen, M.F.; Sand-Jensen, K. Size-dependent nitrogen uptake in micro-and macroalgae. Mar. Ecol. Prog. Ser. 1995, 118, 247–253. [Google Scholar] [CrossRef]
- Khoshmanesh, A.; Lawson, F.; Prince, I.G. Cell surface area as a major parameter in the uptake of cadmium by unicellular green microalgae. Chem. Eng. J. 1997, 65, 13–19. [Google Scholar] [CrossRef]
- Jia, W.; Wang, B.; Wang, C.; Sun, H. Tourmaline and biochar for the remediation of acid soil polluted with heavy metals. J. Environ. Chem. Eng. 2017, 5, 2107–2114. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, C.M.; Marques, A.P.; Castro, P.M.; Xavier Malcata, F. Characterization of Desmodesmus pleiomorphus isolated from a heavy metal-contaminated site: Biosorption of zinc. Biodegradation 2009, 20, 629–641. [Google Scholar] [CrossRef]
- Schiewer, S.; Wong, M.H. Ionic strength effects in biosorption of metals by marine algae. Chemosphere 2000, 41, 271–282. [Google Scholar] [CrossRef]
- Kim, D.; Nakamura, A.; Okamoto, T.; Komatsu, N.; Oda, T.; Iida, T.; Ishimatsu, A.; Muramatsu, T. Mechanism of superoxide anion generation in the toxic red tide phytoplankton Chattonella marina: Possible involvement of NAD (P) H oxidase. Biochim. Biophys. Acta 2000, 1524, 220–227. [Google Scholar] [CrossRef]
- Kim, D.; Sato, Y.; Oda, T.; Muramatsu, T.; Matsuyama, Y.; Honjo, T. Specific toxic effect of dinoflagellate Heterocapsa circularisquama on the rotifer Brachionus plicatilis. Biosci. Biotechnol. Biochem. 2000, 64, 2719–2722. [Google Scholar] [CrossRef]
- Samarakoon, K.W.; Kwon, O.-N.; Ko, J.-Y.; Lee, J.-H.; Kang, M.-C.; Kim, D.; Lee, J.B.; Lee, J.-S.; Jeon, Y.-J. Purification and identification of novel angiotensin-I converting enzyme (ACE) inhibitory peptides from cultured marine microalgae (Nannochloropsis oculata) protein hydrolysate. J. Appl. Phycol. 2013, 25, 1595–1606. [Google Scholar] [CrossRef]
- Kim, D.; Watanabe, M.; Nakayasu, Y.; Kohata, K. Production of superoxide anion and hydrogen peroxide associated with cell growth of Chattonella antiqua. Aquat. Microb. Ecol. 2004, 35, 57–64. [Google Scholar] [CrossRef]
- Kim, D.; Choi, K.-S.; Hong, H.-K.; Jiang, Z.; Zou, Y.; Choi, K.-S.; Yamasaki, Y.; Matsuyama, Y.; Yamaguchi, K.; Oda, T. Comparative study on the toxic effects of red tide flagellates Heterocapsa circularisquama and Chattonella marina on the short-necked clam (Ruditapes philippinarum). Biosci. Biotechnol. Biochem. 2011, 75, 2052–2055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, D.; Oda, T. Production of Nitric Oxide by Marine Unicellular Red Tide Phytoplankton, Chattonella marina. In Nitric Oxide in Plants: Metabolism and Role in Stress Physiology; Springer: Cham, Switzerland, 2014; pp. 75–84. [Google Scholar]
- Cha, S.-H.; Kim, M.-J.; Yang, H.-Y.; Jin, C.-B.; Jeon, Y.-J.; Oda, T.; Kim, D.-K. ACE, α-glucosidase and cancer cell growth inhibitory activities of extracts and fractions from marine microalgae, Nannochloropsis oculata. Korean J. Fish. Aquat. Sci. 2010, 43, 437–444. [Google Scholar]
- Kim, H.S.; Park, W.; Lee, B.; Seon, G.; Suh, W.I.; Moon, M.; Chang, Y.K. Optimization of heterotrophic cultivation of Chlorella sp. HS2 using screening, statistical assessment, and validation. Sci. Rep. 2019, 9, 19383. [Google Scholar] [CrossRef] [PubMed]
- Šebestová, A.; Baron, D.; Pechancová, R.; Pluháček, T.; Petr, J. Determination of oxaliplatin enantiomers at attomolar levels by capillary electrophoresis connected with inductively coupled plasma mass spectrometry. Talanta 2019, 205, 120151. [Google Scholar] [CrossRef] [PubMed]
- Salim, S.; Kosterink, N.; Wacka, N.T.; Vermuë, M.; Wijffels, R. Mechanism behind autoflocculation of unicellular green microalgae Ettlia texensis. J. Biotechnol. 2014, 174, 34–38. [Google Scholar] [CrossRef]
- Lee, H.; Nam, K.; Yang, J.-W.; Han, J.-I.; Chang, Y.K. Synergistic interaction between metal ions in the sea salts and the extracellular polymeric substances for efficient microalgal harvesting. Algal Res. 2016, 14, 79–82. [Google Scholar] [CrossRef]
- Huang, H.; Winchester, K.J.; Suvorova, A.; Lawn, B.R.; Liu, Y.; Hu, X.Z.; Dell, J.M.; Faraone, L. Effect of deposition conditions on mechanical properties of low-temperature PECVD silicon nitride films. Mater. Sci. 2006, 435, 453–459. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Brauer, G. Vanadium, Niobium, Tantalum. In Handbook of Preparative Inorganic Chemistry V2, 2nd ed.; Academic Press: Cambridge, MA, USA, 2012; p. 1252. [Google Scholar]
- Emission Permitted Standards, Article 34, Section 1, Chapter 3, Ministry of Environment Decree No. 553, Republic of KOREA. Available online: http://www.law.go.kr/lsInfoP.do?lsiSeq=153570&efYd=20140430#AJAX (accessed on 16 June 2022).
- Ellendersen, L.S.N.; Milinsk, M.C.; Feroldi, M.; Zadinelo, I.V.; Santos, L.D.; Muniz, G.I.B.; Gasparrini, L.J.; Alves, H.J. Biopolymer foam for remediation of aquatic environments contaminated with particulates and heavy metals. J. Environ. Chem. Eng. 2018, 6, 6131–6138. [Google Scholar] [CrossRef]
- Cui, Z.; Zhang, X.; Yang, H.; Sun, L. Bioremediation of heavy metal pollution utilizing composite microbial agent of Mucor circinelloides, Actinomucor sp. and Mortierella sp. J. Environ. Chem. Eng. 2017, 5, 3616–3621. [Google Scholar] [CrossRef]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Kinetic modeling of azo dye adsorption on non-living cells of Nannochloropsis oceanica. J. Environ. Chem. Eng. 2017, 5, 4121–4127. [Google Scholar] [CrossRef]
| Cell Condition | V2O5 Solution Condition (Vanadium Ion Concentration) | |||||
|---|---|---|---|---|---|---|
| OD | Cultivation Period (day) | Temperature (℃) | pH | Stock (mg L−1) | Inoculation (mg L−1) | pH |
| 0.9–0.95 | 7 | 20 | 7.5 | 100 | 5 | 4.3 |
| N. oculata | Vanadium Concentration (mg L−1) | |
|---|---|---|
| Supernatant | 0 h | 4.61 ± 0.11 |
| 24 h | 1.85 ± 0.21 | |
| Pellet | Sonication | 2.57 ± 0.27 |
| Acid * treatment | 1.01 ± 0.07 | |
| Base ** treatment | 2.34 ± 0.08 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.S.; Kim, M.; Park, W.-K.; Yang, W.-G.; Nayak, M.; Shin, H.H.; Cho, K.; Kim, D.; Oda, T. Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment. Energies 2022, 15, 4467. https://doi.org/10.3390/en15124467
Kim HS, Kim M, Park W-K, Yang W-G, Nayak M, Shin HH, Cho K, Kim D, Oda T. Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment. Energies. 2022; 15(12):4467. https://doi.org/10.3390/en15124467
Chicago/Turabian StyleKim, Hee Su, Minsik Kim, Won-Kun Park, Won-Geun Yang, Manoranjan Nayak, Hyeon Ho Shin, Kichul Cho, Daekyung Kim, and Tatsuya Oda. 2022. "Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment" Energies 15, no. 12: 4467. https://doi.org/10.3390/en15124467
APA StyleKim, H. S., Kim, M., Park, W.-K., Yang, W.-G., Nayak, M., Shin, H. H., Cho, K., Kim, D., & Oda, T. (2022). Microalgae as an Effective Recovery Agent for Vanadium in Aquatic Environment. Energies, 15(12), 4467. https://doi.org/10.3390/en15124467








