Getting Value from Pulp and Paper Industry Wastes: On the Way to Sustainability and Circular Economy
Abstract
1. Introduction
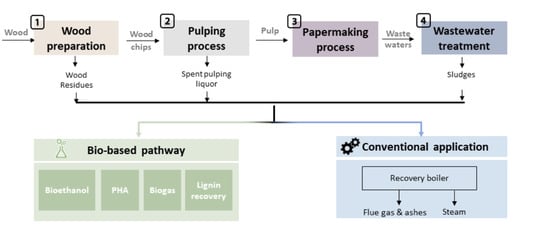
2. Wastes Resulting from Each Step of Pulp and Paper Processing: Composition and Potential for Valorization
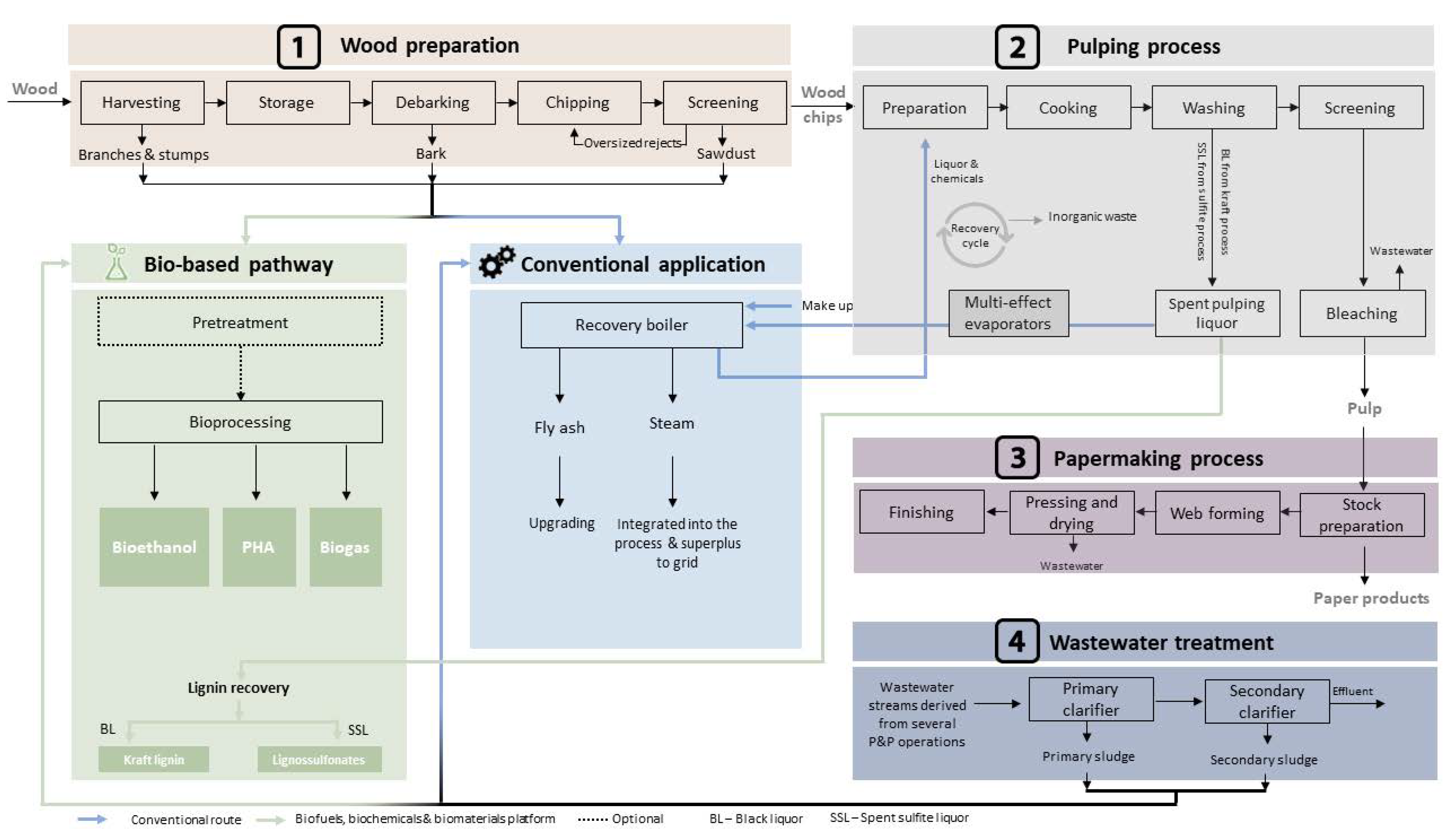
2.1. Wood Preparation
2.1.1. Harvesting
2.1.2. Debarking
2.1.3. Chipping and Screening
2.2. Pulping Processes
2.2.1. Sulfite Process
2.2.2. Kraft Process
2.3. Papermaking Process
2.4. Wastewater Treatment
3. Deconstructing Residues for Further Valorization through Bioprocessing
3.1. Residues from Wood Preparation
3.1.1. Lignocellulosic Biomass Pretreatments
3.1.2. Hydrolysis of Polysaccharides
3.2. Residues from Pulping Processes
3.3. Residues from Wastewater Treatment
4. Getting Value from Wastes: Production of Bioethanol, PHA and Biogas
4.1. Bioethanol
4.1.1. Bioethanol Production from Sawdust
4.1.2. Bioethanol Production from Bark
4.1.3. Bioethanol Production from SSL
4.1.4. Bioethanol Production from Pulp and Paper Mill Sludge (PPMS)
| Substrate | Pretreatment | Configuration | Enzymatic Consortium | Hydrolysis Efficiency (%) | Microorganism | [Ethanol]max (g L−1) | Prodethanol (g L−1 h−1) | Yethanol (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Eucalyptus sawdust | Autohydrolysis + soda pulping | PS-SSF | Cellic Ctec2 | N.A. | S. cerevisiae PE-2 | 58 ± 3 | 1.2 ± 0.3 | 85 ± 1 | [48] |
| E. globulus bark | Kraft | SHF | Cellic Ctec2 | N.R. | Ethanol Red® | 50.8 ± 0.5 | 2.48 ± 0.02 | 81.0 ± 0.6 | [132] |
| E. globulus bark | Hydrothermal | PS-SSF | Cellic Ctec2 | N.A. | Ethanol Red® | 38.03 ± 0.33 | 0.52 | 73.14 | [37] |
| Spruce bark | HWE+SE | SSF | Celluclast 1.5 L, Novozym 188 and Pectinex Ultra SP-L | N.A. | S. cerevisiae VTT-B-08014 | 21.0 | N.R. | 66.4 | [145] |
| Spruce wood chips mixed with bark | SO2-catalysed steam | SSF | Cellic Ctec3 | N.A. | Ethanol Red® | 34.5 ± 0.4 | 2.5 ± 0 | 77.5 ± 1.3 | [38] |
| HSSL | Boiling + overliming with Ca(OH)2 | N.A. | N.A. | N.A. | S. stipitis NRRL-7124 | 20.20 ± 0.43 | 0.44 ± 0.02 | 82.00 ± 0.41 | [172] |
| HSSL | Ion-exchange resins | N.A. | N.A. | N.A. | S. stipitis NRRL-7124 | 8.1 | 1.22 | 96 | [147] |
| HSSL | Biological detoxification by using P. variotii NRRL-1115 | N.A. | N.A. | N.A. | S. stipitis NRRL-7124 | 2.4 | 0.09 | 47.0 | [119] |
| HSSL | pH adjustment to 7.0 with KOH followed by aeration | N.A. | N.A. | N.A. | S. stipitis C4 isolate | 4.60 | 0.05 | 32 | [173] |
| HSSL | pH adjustment to 7.0 with KOHfollowed by aeration | N.A. | N.A. | N.A. | S. stipitis C4 isolate | 12.2 | 0.03 | 74.4 | [74] |
| Primary PPMS | N.A. | SSF | Enzymatic extract NS 22192 | N.A. | S. cerevisiae ATCC 26602 | 41.7 ± 1.2 | 0.78 ± 0.03 | 48.9 ± 1.4 | [174] |
| Primary PPMS | HCl | SHF | Cellic CTec2 | 88 | S. stipitis DSM 3651 | 10.5 | 0.20 | 76.5 | [105] |
| Primary PPMS | Spent acid | SHF | Cellic CTec2 | 72 | S. stipitis DSM 3651 | 8.5 | 0.16 | 76.5 | [105] |
| Primary PPMS | N.A. | SSF | Spezyme CP and Novozyme-188 | N.R. | S. cerevisiae ATCC-200062 | 25.5 | N.R. | 74.5 | [175] |
| Primary PPMS | N.A. | Fed-batch SSF | Spezyme CP and Novozyme-188 | N.R. | S. cerevisiae ATCC-200062 | 45.0 | N.R. | 70 | [175] |
| Primary PPMS | N.A. | SSCF | Spezyme CP and Novozyme-188 | N.R. | E. coli ATCC 55124 | 32.5 | N.R. | 95.8 | [175] |
| Primary PPMS | N.A. | Fed-batch SSCF | Spezyme CP and Novozyme-188 | N.R. | E. coli ATCC 55124 | 42.0 | N.R. | 68 | [175] |
| Primary PPMS | De-ashing | SSF | Spezyme CP and Novozyme-188 | N.R. | S. cerevisiae ATCC-200062 | 24.7 | N.R. | 72.8 | [120] |
| Primary PPMS | De-ashing | Fed-batch SSF | Spezyme CP and Novozyme-188 | N.R. | S. cerevisiae ATCC-200062 | 60.0 | N.R. | 70 | [120] |
| Primary PPMS | De-ashing | SSCF | Spezyme CP and Novozyme-188 | N.R. | E. coli ATCC 55124 | 30.3 | N.R. | 73.6 | [120] |
| Primary PPMS | De-ashing | Fed-batch SSCF | Spezyme CP and Novozyme-188 | N.R. | E. coli ATCC 55124 | 47.8 | N.R. | 70 | [120] |
4.2. Polyhydroxyalkanoates (PHA)
4.2.1. PHA Production from Residues of Wood Preparation
4.2.2. PHA from Lignin
4.2.3. PHA from Wastewaters
4.2.4. PHA from Spent Liquor
| Substrate | Pretreatment | Microorganism | PHA Composition | PHA (% w w−1) | Biomass (g L−1) | PHA (g L−1) | YPHA/S (gPHA g substrate−1) | ProdVOL (gPHA L−1 h−1) | ProdSP (gPHA gbiomass−1 h−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Wood particles | HWE + EH | MMC | PHB:PHV (85:15%) | N.R. | N.R. | 2.3 | N.R. | N.R. | N.R. | [171] |
| Waste wood | Hydrothermal + EH | MMC | PHB:PHV (94:6%) | 50 | 6.30 | 3.15 | 0.711 | 0.237 | 0.029 | [180] |
| Kraft lignin | N.A. | C. basilensis B-8 | P(S3HB):P(R3HB):PHB (98:1:0.4%) | 19 | 3.87 | 0.74 | 0.15 | 0.015 | 0.004 | [182] |
| Kraft lignin | N.A. | Pandoraea sp. ISTKB | PHB:PHV | 21 | 0.08 | 0.02 | N.R. | N.R | N.R. | [183] |
| Wood mill effluent | N.A. | MMC | PHB:PHV (46:54%) | 29 | 3.93 | 1.14 | 0.232 | N.R. | N.R. | [187] |
| N.A. | MMC | PHB:PHV (81:19%) | 25 | 7.88 | 1.97 | 0.572 | 0.303 | 0.038 | [186] | |
| Paper mill effluent | N.A. | MMC | N.R. | 48 | 2.63 | 1.26 | 0.113 | 0.152 | 0.058 | [188] |
| PHB:PHV:PHMV (6:47:47%) | 42 | 2.63 | 1.10 | 0.103 | 0.244 | 0.093 | [188] | |||
| Kraft mill effluent | N.A. | MMC | N.R. | N.R. | N.R. | 0.08 | 0.143 | 0.001 | N.R. | [189] |
| N.A. | S. chilensis S37 | PHB (100%) | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | [190] | |
| N.A. | Wautersia sp. PZK | Long chain length PHA | N.R. | N.R. | N.R. | N.R. | N.R. | N.R. | [190] | |
| HSSL | N.A. | MMC | PHB:PHV (68:32%) | 22 | 3.4 | 0.75 | 0.42 | 0.170 | 0.051 | [192] |
| PHB (100%) | 67 | 0.88 | 0.60 | 0.773 | 0.050 | 0.057 | [191] | |||
| N.A. | MMC | PHB:PHV (86:14%) | 77 | N.R | 0.83 | 0.801 | 0.083 | N.R. | [178] |
4.3. Biogas
4.4. Challenges and Future Perspectives for Conversion of Wastes into Bioethanol, PHA, and Biogas
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADF | Aerobic dynamic feeding |
| AN/AE | Anaerobic/aerobic system |
| AD | Anaerobic digestion |
| BL | Black liquor |
| C/N | Carbon/nitrogen ratio |
| Cdw | Cell dry weight |
| COD | Chemical oxygen demand |
| CBP | Consolidated bioprocessing |
| EH | Enzymatic hydrolysis |
| [Ethanol]max | Maximum ethanol concentration (g L−1) |
| EU | European Union |
| HB | 3-hydroxybutyrate |
| HSSL | Hardwood spent sulfite liquor |
| HV | 3-hydroxyvalerate |
| HWE | Hot water extraction |
| LCB | Lignocellulosic biomass |
| MMC | Mixed microbial cultures |
| P(3HB-co-3HV)) | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PHA | Polyhydroxyalkanoates |
| Prodethanol | Volumetric productivity (gethanol L−1 h−1) |
| ProdSP | Specific productivity (gPHA gbiomass−1 h−1) |
| ProdVOL | Volumetric productivity (gPHA L−1 h−1) |
| PS-SSF | Pre-saccharification and simultaneous saccharification and fermentation |
| PPMS | Pulp and paper mill sludge |
| SHF | Separate hydrolysis and fermentation |
| SBR | Sequential batch reactor |
| SCOA | Short chain organic acids |
| SSCF | Simultaneous saccharification and co-fermentation |
| SSF | Simultaneous saccharification and fermentation |
| SSSL | Softwood spent sulfite liquor |
| SE | Steam explosion |
| SSL | Sulfite spent liquor |
| Yethanol | Ethanol yield (%) |
| YPHA/S | PHA yield (gPHA gsubstrate−1) |
References
- Liguori, R.; Faraco, V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour. Technol. 2016, 215, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Vea, E.B.; Romeo, D.; Thomsen, M. Biowaste Valorisation in a Future Circular Bioeconomy. In Proceedings of the 25th CIRP Life Cycle Engineering (LCE) Conference, Copenhagen, Denmark, 30 April–2 May 2018; Volume 69, pp. 591–596. [Google Scholar]
- Ghisellini, P.; Cialani, C.; Ulgiati, S. A review on circular economy: The expected transition to a balanced interplay of environmental and economic systems. J. Clean. Prod. 2016, 114, 11–32. [Google Scholar] [CrossRef]
- Loiseau, E.; Saikku, L.; Antikainen, R.; Droste, N.; Hansjürgens, B.; Pitkänen, K.; Leskinen, P.; Kuikman, P.; Thomsen, M. Green economy and related concepts: An overview. J. Clean. Prod. 2016, 139, 361–371. [Google Scholar] [CrossRef]
- Reichel, A.; De Schoenmakere, M.; Gillabel, J. Circular Economy in Europe: Developing the Knowledge Base (EEA Report | No 2/2016); EEA: Copenhagen, Denmark, 2016. [Google Scholar]
- Korhonen, J.; Honkasalo, A.; Seppälä, J. Circular Economy: The Concept and its Limitations. Ecol. Econ. 2018, 143, 37–46. [Google Scholar] [CrossRef]
- European Commission. Towards a Circular Economy: A Zero Waste Programme for Europe; Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions COM(2014) 398 Final; European Commission: Brussels, Belgium, 2014. [Google Scholar]
- Lee, P.; Sims, E.; Bertham, O.; Symington, H.; Bell, N.; Lucie, P.; Sjögren, P. Towards a Circular Economy-Waste Management in the EU; EU: Brussels, Belgium, 2017. [Google Scholar]
- Borrero-López, A.M.; Valencia, C.; Franco, J.M. Lignocellulosic Materials for the Production of Biofuels, Biochemicals and Biomaterials and Applications of Lignocellulose-Based Polyurethanes: A Review. Polymers 2022, 14, 881. [Google Scholar] [CrossRef]
- Stafford, W.; De Lange, W.; Nahman, A.; Chunilall, V.; Lekha, P.; Andrew, J.; Johakimu, J.; Sithole, B.; Trotter, D. Forestry biorefineries. Renew. Energy 2020, 154, 461–475. [Google Scholar] [CrossRef]
- Branco, R.; Serafim, L.; Xavier, A. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2019, 5, 4. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Hodge, D.B. Integration of (Hemi)-Cellulosic Biofuels Technologies with Chemical Pulp Production. In Biorefineries; Qureshi, N., Hodge, D., Vertes, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 73–100. ISBN 9780444595041. [Google Scholar]
- Fernandes, A.; Cruz-Lopes, L.; Dulyanska, Y.; Domingos, I.; Ferreira, J.; Evtuguin, D.; Esteves, B. Eco Valorization of Eucalyptus globulus Bark and Branches through Liquefaction. Appl. Sci. 2022, 12, 3775. [Google Scholar] [CrossRef]
- Yamakawa, C.K.; Qin, F.; Mussatto, S.I. Advances and opportunities in biomass conversion technologies and biorefineries for the development of a bio-based economy. Biomass Bioenergy 2018, 119, 54–60. [Google Scholar] [CrossRef]
- Romaní, A.; Michelin, M.; Domingues, L.; Teixeira, J.A. Valorization of wastes from agrofood and pulp and paper industries within the biorefinery concept: Southwestern Europe scenario. In Waste Biorefinery: Potential and Perspectives; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 487–504. ISBN 9780444639936. [Google Scholar]
- Bajpai, P. Generation of Waste in Pulp and Paper Mills. In Management of Pulp and Paper Mill Waste; Bajpai, P., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 9–17. ISBN 9783319117881. [Google Scholar]
- Monte, M.C.; Fuente, E.; Blanco, A.; Negro, C. Waste management from pulp and paper production in the European Union. Waste Manag. 2009, 29, 293–308. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Pinto, P.C.O.R.; Barreiro, M.F.; Costa, C.A.E.; Mota, M.I.F.; Fernandes, I. Chemical Pulp Mills as Biorefineries. In An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries: Vanillin, Syringaldehyde, Polyphenols, and Polyurethane; Springer Nature Switzerland AG: Évora, Portugal, 2018; pp. 1–51. ISBN 9783319993126. [Google Scholar]
- Miranda, I.; Gominho, J.; Mirra, I.; Pereira, H. Fractioning and chemical characterization of barks of Betula pendula and Eucalyptus globulus. Ind. Crops Prod. 2013, 41, 299–305. [Google Scholar] [CrossRef]
- Gominho, J.; Lourenço, A.; Miranda, I.; Pereira, H. Chemical and fuel properties of stumps biomass from Eucalyptus globulus plantations. Ind. Crops Prod. 2012, 39, 12–16. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; Carneiro, A.C.; Pereira, H. Potential of Eucalyptus globulus industrial bark as a biorefinery feedstock: Chemical and fuel characterization. Ind. Crop. Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Resquin, F.; Barrichelo, L.E.G.; da Silva, F.G.; Brito, J.O.; Sansigolo, C.A. Wood quality for kraft pulping of Eucalyptus globulus origins planted in Uruguay. Sci. For. Sci. 2006, 72, 57–66. [Google Scholar]
- Gominho, J.; Costa, R.A.; Lourenço, A.; Quilhó, T.; Pereira, H. Eucalyptus globulus Stumps Bark: Chemical and Anatomical Characterization Under a Valorisation Perspective. Waste Biomass Valorization 2021, 12, 1253–1265. [Google Scholar] [CrossRef]
- Sixta, H.; Potthast, A.; Andreas, W. Krotschek Chemical Pulping Processes. In Handbook of Pulp; Sixta, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Volume 1, p. 2264. [Google Scholar]
- Banaś, J.; Utnik-Banaś, K. Using Timber as a Renewable Resource for Energy Production in Sustainable Forest Management. Energies 2022, 15, 2264. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Oliveira, C.; Costa, C.A.E.; Rodrigues, A.E. Performance of Side-Streams from Eucalyptus Processing as Sources of Polysaccharides and Lignins by Kraft Delignification. Ind. Eng. Chem. Res. 2016, 55, 516–526. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Sousa, G.D.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus globulus biomass residues from pulping industry as a source of high value triterpenic compounds. Ind. Crops Prod. 2010, 31, 65–70. [Google Scholar] [CrossRef]
- Luís, A.; Neiva, D.; Pereira, H.; Gominho, J.; Domingues, F.; Duarte, A.P. Stumps of Eucalyptus globulus as a source of antioxidant and antimicrobial polyphenols. Molecules 2014, 19, 16428–16446. [Google Scholar] [CrossRef]
- Pinto, F.; Gominho, J.; André, R.N.; Gonçalves, D.; Miranda, M.; Varela, F.; Neves, D.; Santos, J.; Lourenço, A.; Pereira, H. Improvement of gasification performance of Eucalyptus globulus stumps with torrefaction and densification pre-treatments. Fuel 2017, 206, 289–299. [Google Scholar] [CrossRef]
- Gominho, J.; Lopes, C.; Lourenço, A.; Simões, R.; Pereira, H. Eucalyptus globulus stumpwood as a raw material for pulping. BioResources 2014, 9, 4038–4049. [Google Scholar] [CrossRef][Green Version]
- Pinto, F.; André, R.; Lopes, H.; Neves, D.; Varela, F.; Santos, J.; Miranda, M. Benefits and drawbacks of energetic valorisation of Eucalyptus globulus stumps by thermochemical processes. Chem. Eng. Trans. 2015, 43, 2449–2454. [Google Scholar] [CrossRef]
- Tanger, P.; Field, J.L.; Jahn, C.E.; DeFoort, M.W.; Leach, J.E. Biomass for thermochemical conversion: Targets and challenges. Front. Plant Sci. 2013, 4, 218. [Google Scholar] [CrossRef] [PubMed]
- Ressel, J.B. Wood Yard Operations. In Handbook of Pulp; Sixta, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Volume 1, pp. 69–107. ISBN 3527309993. [Google Scholar]
- Ek, M.; Gellerstedt, G.; Henriksson, G. Pulping Chemistry and Technology. In Pulp and Paper Chemistry and Technology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2009; Volume 2. [Google Scholar]
- Haile, A.; Gelebo, G.G.; Tesfaye, T.; Mengie, W.; Mebrate, M.A.; Abuhay, A.; Limeneh, D.Y. Pulp and paper mill wastes: Utilizations and prospects for high value-added biomaterials. Bioresour. Bioprocess. 2021, 8, 35. [Google Scholar] [CrossRef]
- Frankó, B.; Galbe, M.; Wallberg, O. Bioethanol production from forestry residues: A comparative techno-economic analysis. Appl. Energy 2016, 184, 727–736. [Google Scholar] [CrossRef]
- Gomes, D.G.; Michelin, M.; Romaní, A.; Domingues, L.; Teixeira, J.A. Co-production of biofuels and value-added compounds from industrial Eucalyptus globulus bark residues using hydrothermal treatment. Fuel 2021, 285, 119265. [Google Scholar] [CrossRef]
- Frankó, B.; Galbe, M.; Wallberg, O. Influence of bark on fuel ethanol production from steam-pretreated spruce. Biotechnol. Biofuels 2015, 8, 15–25. [Google Scholar] [CrossRef]
- Feng, S.; Cheng, S.; Yuan, Z.; Leitch, M.; Xu, C. Valorization of bark for chemicals and materials: A review. Renew. Sustain. Energy Rev. 2013, 26, 560–578. [Google Scholar] [CrossRef]
- Neiva, D.M.; Gominho, J.; Fernandes, L.; Lourenço, A.; Chemetova, C.; Simões, R.M.S.; Pereira, H. The Potential of Hydrothermally Pretreated Industrial Barks From E. globulus as a Feedstock for Pulp Production. J. Wood Chem. Technol. 2016, 36, 383–392. [Google Scholar] [CrossRef]
- Miranda, I.; Gominho, J.; Pereira, H. Incorporation of bark and tops in Eucalyptus globulus wood pulping. BioResources 2012, 7, 4350–4361. [Google Scholar] [CrossRef]
- Santos, S.A.O.; Villaverde, J.J.; Silva, C.M.; Neto, C.P.; Silvestre, A.J.D. Supercritical fluid extraction of phenolic compounds from Eucalyptus globulus Labill bark. J. Supercrit. Fluids 2012, 71, 71–79. [Google Scholar] [CrossRef]
- de Melo, M.M.R.; Oliveira, E.L.G.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of triterpenic acids from Eucalyptus globulus bark. J. Supercrit. Fluids 2012, 70, 137–145. [Google Scholar] [CrossRef]
- Patinha, D.J.S.; Domingues, R.M.A.; Villaverde, J.J.; Silva, A.M.S.; Silva, C.M.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Lipophilic extractives from the bark of Eucalyptus grandis x globulus, a rich source of methyl morolate: Selective extraction with supercritical CO2. Ind. Crops Prod. 2013, 43, 340–348. [Google Scholar] [CrossRef]
- Domingues, R.M.A.; Sousa, G.D.A.; Silva, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. High value triterpenic compounds from the outer barks of several Eucalyptus species cultivated in Brazil and in Portugal. Ind. Crops Prod. 2011, 33, 158–164. [Google Scholar] [CrossRef]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Mota, M.I.F.; Pinto, P.C.O.R.; Novo, C.C.; Sousa, G.D.A.; Guerreiro, O.; Guerra, Â.; Duarte, M.F.P.; Rodrigues, A.E. Eucalyptus globulus bark as a source of polyphenolic compounds with biological activity. O Pap. 2013, 74, 57–64. [Google Scholar]
- Guigou, M.; Cabrera, M.N.; Vique, M.; Bariani, M.; Guarino, J.; Ferrari, M.D.; Lareo, C. Combined pretreatments of eucalyptus sawdust for ethanol production within a biorefinery approach. Biomass Convers. Biorefinery 2019, 9, 293–304. [Google Scholar] [CrossRef]
- Bajpai, P. Biermann’s Handbook of Pulp and Paper, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128142387. [Google Scholar]
- Cebreiros, F.; Clavijo, L.; Boix, E.; Ferrari, M.D.; Lareo, C. Integrated valorization of eucalyptus sawdust within a biorefinery approach by autohydrolysis and organosolv pretreatments. Renew. Energy 2020, 149, 115–127. [Google Scholar] [CrossRef]
- Arshadi, M.; Sellstedt, A. Production of energy from biomass. In Introduction to Chemicals from Biomass; Clark, J.H., Deswarte, F.E.I., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; pp. 143–178. ISBN 9781118714478. [Google Scholar]
- Gil, M.V.; Oulego, P.; Casal, M.D.; Pevida, C.; Pis, J.J.; Rubiera, F. Mechanical durability and combustion characteristics of pellets from biomass blends. Bioresour. Technol. 2010, 101, 8859–8867. [Google Scholar] [CrossRef]
- Li, W.; Bu, W.; Guo, W.; Jiang, Y.; Wang, Y.; Yin, X. Preparation for industrial pellet production from blends of eucalyptus sawdust and hydrolysis lignin: The optimal variable combinations of co-pelletization. Biomass Convers. Biorefinery 2019, 10, 513–521. [Google Scholar] [CrossRef]
- Dekker, R.F.H.; Karageorge, H.; Wallis, A.F.A. Pretreatment of hardwood (Eucalyptus regnans) sawdust by autohydrolysis explosion and its saccharification by trichodermal cellulases. Biocatalysis 1987, 1, 47–61. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Area, M.C. Hydrothermal Treatments Applied to Agro- and Forest- Industrial Waste to Produce High Added-Value Compounds. BioResources 2017, 12, 2058–2080. [Google Scholar]
- Xu, J.; Jiang, J.; Dai, W.; Xu, Y. Liquefaction of sawdust in hot compressed ethanol for the production of bio-oils. Process Saf. Environ. Prot. 2012, 90, 333–338. [Google Scholar] [CrossRef]
- Mafei, T.D.T.; Neto, F.S.P.P.; Peixoto, G.; de Baptista Neto, Á.; Monti, R.; Masarin, F. Extraction and Characterization of Hemicellulose from Eucalyptus By-product: Assessment of Enzymatic Hydrolysis to Produce Xylooligosaccharides. Appl. Biochem. Biotechnol. 2020, 190, 197–217. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.G.; Lima, G.F.; Rodrigues, R.C.L.B.; Cesarino, I.; Leão, A.L.; Rosa, D.S. A New Approach for Conversion of Eucalyptus Lignocellulosic Biomass into Cellulose Nanostructures: A Method that Can Be Applied in Industry. J. Nat. Fibers 2019, 18, 1501–1511. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Felissia, F.E.; Area, M.C.; Ehman, N.V.; Tarrés, Q.; Mutjé, P. Nanofibrillated cellulose (CNF) from eucalyptus sawdust as a dry strength agent of unrefined eucalyptus handsheets. Carbohydr. Polym. 2016, 139, 99–105. [Google Scholar] [CrossRef]
- Couto, G.M.; Dessimoni, A.L.A.; Bianchi, M.L.; Perígolo, D.M.; Trugilho, P.F. Use of sawdust Eucalyptus sp. in the preparation of activated carbons. Ciência e Agrotecnologia 2012, 36, 69–77. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, P.; Huang, Y.; Tong, Z.; Li, Z. Preparation and Characterization of Activated Carbon from Eucalyptus Sawdust I. Activated by NaOH. J. Inorg. Organomet. Polym. Mater. 2013, 23, 1201–1209. [Google Scholar] [CrossRef]
- Chemani, B.; Chemani, H. Effect of Adding Sawdust on Mechanical- Physical Properties of Ceramic Bricks to Obtain Lightweight Building Material. Int. J. Mech. Mechatron. Eng. 2012, 6, 2521–2525. [Google Scholar]
- Capela, M.N.; Cesconeto, F.R.; Pinto, P.C.; Tarelho, L.A.C.; Seabra, M.P.; Labrincha, J.A. Biomass Fly Ash Self-Hardened Adsorbent Monoliths for Methylene Blue Removal from Aqueous Solutions. Appl. Sci. 2022, 12, 5134. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Vlysidis, A.; Pleissner, D.; Kopsahelis, N.; Garcia, I.L.; Kookos, I.K.; Papanikolaou, S.; Kwan, T.H.; Lin, C.S.K. Valorization of industrial waste and by-product streams via fermentation for the production of chemicals and biopolymers. Chem. Soc. Rev. 2014, 43, 2587–2627. [Google Scholar] [CrossRef] [PubMed]
- Evtuguin, D.V. Sulphite Pulping. In Lignocellulosic Fibers and Wood Handbook; Belgacem, N., Pizzi, A., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 225–244. ISBN 9781118773727. [Google Scholar]
- Heinemann, S.; Blechschmidt, J. Handbook of Paper and Board, 2nd ed.; Holik, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; ISBN 9780470528105. [Google Scholar]
- Humpert, D.; Ebrahimi, M.; Czermak, P. Membrane Technology for the Recovery of Lignin: A Review. Membranes 2016, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Evtuguin, D.V.; Magina, S.; Amado, F.M.L.; Prates, A. Chemical composition of spent liquors from acidic magnesium-based sulphite pulping of Eucalyptus globulus. J. Wood Chem. Technol. 2009, 29, 322–336. [Google Scholar] [CrossRef]
- Pereira, S.R.; Portugal-Nunes, D.J.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Advances in ethanol production from hardwood spent sulphite liquors. Process Biochem. 2013, 48, 272–282. [Google Scholar] [CrossRef]
- Lawford, H.G.; Rousseau, J.D. Production of ethanol from pulp mill hardwood and softwood spent sulfite liquors by genetically engineered E. coli. Appl. Biochem. Biotechnol. 1993, 39/40, 667–685. [Google Scholar] [CrossRef]
- Humpert, D.; Ebrahimi, M.; Stroh, A.; Czermak, P. Recovery of Lignosulfonates from Spent Sulfite Liquor Using Ceramic Hollow-Fiber Membranes. Membranes 2019, 9, 45. [Google Scholar] [CrossRef]
- Lora, J. Industrial Commercial Lignins: Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Oxford, UK, 2008; pp. 225–241. ISBN 9780080453163. [Google Scholar]
- Fernandes, D.L.A.; Pereira, S.R.; Serafim, L.S.; Evtuguin, D.V.; Xavier, A.M.R.B. Second Generation Bioethanol from Lignocellulosics: Processing of Hardwood Sulphite Spent Liquor. In Bioethanol; Lima, M.A.P., Natalense, A.P.P., Eds.; InTech: Rijeka, Croatia, 2012; pp. 123–152. [Google Scholar]
- Henriques, T.M.; Pereira, S.R.; Serafim, L.S.; Xavier, A.M.R.B. Two-stage aeration fermentation strategy to improve bioethanol production by Scheffersomyces stipitis. Fermentation 2018, 4, 97. [Google Scholar] [CrossRef]
- Ladakis, D.; Michailidi, K.; Vlysidis, A.; Koutinas, A.; Kookos, I.K. Valorization of spent sulphite liquor for succinic acid production via continuous fermentation system. Biochem. Eng. J. 2018, 137, 262–272. [Google Scholar] [CrossRef]
- Queirós, D.; Sousa, R.; Pereira, S.; Serafim, L.S. Valorization of a pulp industry by-product through the production of short-chain organic acids. Fermentation 2017, 3, 20. [Google Scholar] [CrossRef]
- Rueda, C.; Calvo, P.A.; Moncalián, G.; Ruiz, G.; Coz, A. Biorefinery options to valorize the spent liquor from sulfite pulping. J. Chem. Technol. Biotechnol. 2015, 90, 2218–2226. [Google Scholar] [CrossRef]
- Magina, S.; Rudnitskaya, A.; Soreto, S.; Costa, L.C.; Barros-Timmons, A.; Evtuguin, D.V. Lignosulfonate-Based Conducting Flexible Polymeric Membranes for Liquid Sensing Applications. Materials 2021, 14, 5331. [Google Scholar] [CrossRef] [PubMed]
- Magina, S.; Gama, N.; Carvalho, L.; Barros-Timmons, A.; Evtuguin, D.V. Lignosulfonate-Based Polyurethane Adhesives. Materials 2021, 14, 7072. [Google Scholar] [CrossRef] [PubMed]
- Magina, S.; Barros-Timmons, A.; Evtuguin, D.V. Synthesis of Lignosulfonate-Based Dispersants for Application in Concrete Formulations. Materials 2021, 14, 7388. [Google Scholar] [CrossRef]
- Pinto, P.I.F.; Magina, S.; Budjav, E.; Pinto, P.C.R.; Liebner, F.; Evtuguin, D. Cationization of Eucalyptus Kraft LignoBoost Lignin: Preparation, Properties, and Potential Applications. Ind. Eng. Chem. Res. 2022, 61, 3503–3515. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, E. Wood Chemistry: Fundamentals and Applications, 2nd ed.; Academic Press, Inc.: San Diego, CA, USA, 1993; ISBN 0-12-647481-8. [Google Scholar]
- Becker, J.; Wittmann, C. A field of dreams: Lignin valorization into chemicals, materials, fuels, and health-care products. Biotechnol. Adv. 2019, 37, 107360. [Google Scholar] [CrossRef]
- Pettersson, K.; Mahmoudkhani, M.; Schenck, A. Opportunities for Biorefineries in the Pulping Industry. In Systems Perspectives on Biorefineries; Sandén, B., Pettersson, K., Eds.; Chalmers University of Technology: Göteborg, Sweden, 2014; pp. 68–80. [Google Scholar]
- Hubbe, M.A.; Alén, R.; Paleologou, M.; Kannangara, M.; Kihlman, J. Lignin recovery from spent alkaline pulping liquors using acidification, membrane separation, and related processing steps: A review. BioResources 2019, 14, 2300–2351. [Google Scholar] [CrossRef]
- Bajpai, P. Overview of Pulp and Papermaking Processes. In Environmentally Friendly Production of Pulp and Paper; Bajpai, P., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 8–45. [Google Scholar]
- Cabrera, M.N. Pulp Mill Wastewater: Characteristics and Treatment. In Biological Wastewater Treatment and Resource Recovery; Farooq, R., Ahmad, Z., Eds.; InTechOpen: Rijeka, Croatia, 2017; pp. 119–139. [Google Scholar]
- Wallberg, O.; Jönsson, A.S.; Wimmerstedt, R. Fractionation and concentration of kraft black liquor lignin with ultrafiltration. Desalination 2003, 154, 187–199. [Google Scholar] [CrossRef]
- Kouisni, L.; Gagné, A.; Maki, K.; Holt-Hindle, P.; Paleologou, M. The LignoForce System for the recovery of lignin from black liquor: Feedstock options, odour profile and product characterization. ACS Sustain. Chem. Eng. 2016, 4, 5152–5159. [Google Scholar] [CrossRef]
- AL-Kaabi, Z.; Pradhan, R.; Thevathasan, N.; Arku, P.; Gordon, A.; Dutta, A. Beneficiation of renewable industrial wastes from paper and pulp processing. AIMS Energy 2018, 6, 880–907. [Google Scholar] [CrossRef]
- Rodrigues, A.E.; Pinto, P.C.O.R.; Barreiro, M.F.; da Costa, C.A.E.; da Mota, M.I.F.; Fernandes, I. An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries; Springer International Publishing: Cham, Switzerland, 2018; ISBN 978-3-319-99312-6. [Google Scholar]
- Manorma; Ferreira, I.; Alves, P.; Gil, M.H.; Gando-Ferreira, L.M. Lignin separation from black liquor by mixed matrix polysulfone nanofiltration membrane filled with multiwalled carbon nanotubes. Sep. Purif. Technol. 2021, 260, 118231. [Google Scholar] [CrossRef]
- Liu, L.Y.; Chen, S.; Ji, L.; Jang, S.K.; Renneckar, S. One-pot route to convert technical lignin into versatile lignin esters for tailored bioplastics and sustainable materials. Green Chem. 2021, 23, 4567–4579. [Google Scholar] [CrossRef]
- Demuner, I.F.; Colodette, J.L.; Demuner, A.J.; Jardim, C.M. Biorefinery Review: Wide-Reaching Products Through Kraft Lignin | Demuner | BioResources. BioResources 2019, 14, 7543–7581. [Google Scholar] [CrossRef]
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefining 2018, 12, 756–787. [Google Scholar] [CrossRef] [PubMed]
- Dessbesell, L.; Paleologou, M.; Leitch, M.; Pulkki, R.; Xu, C. (Charles) Global lignin supply overview and kraft lignin potential as an alternative for petroleum-based polymers. Renew. Sustain. Energy Rev. 2020, 123, 109768. [Google Scholar] [CrossRef]
- Pinto, J.A.; Fernandes, I.P.; Pinto, V.D.; Gomes, E.; Oliveira, C.F.; Pinto, P.C.R.; Mesquita, L.M.R.; Piloto, P.A.G.; Rodrigues, A.E.; Barreiro, M.F. Valorization of lignin side-streams into polyols and rigid polyurethane foams—A contribution to the pulp and paper industry biorefinery. Energies 2021, 14, 3825. [Google Scholar] [CrossRef]
- Moretti, C.; Corona, B.; Hoefnagels, R.; van Veen, M.; Vural-Gürsel, I.; Strating, T.; Gosselink, R.; Junginger, M. Kraft lignin as a bio-based ingredient for Dutch asphalts: An attributional LCA. Sci. Total Environ. 2022, 806, 150316. [Google Scholar] [CrossRef]
- Patel, K.; Patel, N.; Vaghamshi, N.; Shah, K.; Murty, S. Trends and strategies in the effluent treatment of pulp and paper industries: A review highlighting reactor options. Curr. Res. Microb. Sci. 2021, 2, 100077. [Google Scholar] [CrossRef]
- Ashrafi, O.; Yerushalmi, L.; Haghighat, F. Wastewater treatment in the pulp-and-paper industry: A review of treatment processes and the associated greenhouse gas emission. J. Environ. Manag. 2015, 158, 146–157. [Google Scholar] [CrossRef]
- Mandeep; Gupta, G.K.; Shukla, P. Insights into the resources generation from pulp and paper industry wastes: Challenges, perspectives and innovations. Bioresour. Technol. 2020, 297, 122496. [Google Scholar] [CrossRef]
- Faubert, P.; Barnabé, S.; Bouchard, S.; Côté, R.; Villeneuve, C. Pulp and paper mill sludge management practices: What are the challenges to assess the impacts on greenhouse gas emissions? Resour. Conserv. Recycl. 2016, 108, 107–133. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Rocha, J.M.S.; Menezes, F.F.; Carvalho, M.G.V.S. Batch and fed-batch simultaneous saccharification and fermentation of primary sludge from pulp and paper mills. Environ. Technol. 2017, 38, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Simão, L.; Hotza, D.; Raupp-Pereira, F.; Labrincha, J.A.; Montedo, O.R.K. Wastes from pulp and paper mills—A review of generation and recycling alternatives. Ceramica 2018, 64, 443–453. [Google Scholar] [CrossRef]
- Mendes, C.V.T.; Rocha, J.M.S.; Carvalho, M.G.V.S. Valorization of residual streams from pulp and paper mills: Pretreatment and bioconversion of primary sludge to bioethanol. Ind. Eng. Chem. Res. 2014, 53, 19398–19404. [Google Scholar] [CrossRef]
- Gottumukkala, L.D.; Haigh, K.; Collard, F.X.; van Rensburg, E.; Görgens, J. Opportunities and prospects of biorefinery-based valorisation of pulp and paper sludge. Bioresour. Technol. 2016, 215, 37–49. [Google Scholar] [CrossRef]
- Puhakka, J.A.; Alavakeri, M.; Shieh, W.K. Anaerobic treatment of kraft pulp-mill waste activated-sludge: Gas production and solids reduction. Bioresour. Technol. 1992, 39, 61–68. [Google Scholar] [CrossRef]
- Peng, L.; Chen, Y. Conversion of paper sludge to ethanol by separate hydrolysis and fermentation (SHF) using Saccharomyces cerevisiae. Biomass Bioenergy 2011, 35, 1600–1606. [Google Scholar] [CrossRef]
- Mahmood, T.; Elliott, A. A review of secondary sludge reduction technologies for the pulp and paper industry. Water Res. 2006, 40, 2093–2112. [Google Scholar] [CrossRef]
- Söderholm, P.; Bergquist, A.K.; Söderholm, K. Environmental Regulation in the Pulp and Paper Industry: Impacts and Challenges. Curr. For. Reports 2019, 5, 185–198. [Google Scholar] [CrossRef]
- Cherian, C.; Siddiqua, S. Pulp and paper mill fly ash: A review. Sustain. 2019, 11, 4394. [Google Scholar] [CrossRef]
- Erhardt, W.; Prüeß, A. Organic Contaminants in Sewage Sludge for Agricultural Use; European Commission & Joint Research Centre Institute for Environment and Sustainability Soil and Waste Unit: Perugia, Italy, 2001. [Google Scholar]
- Bhutto, A.W.; Qureshi, K.; Harijan, K.; Abro, R.; Abbas, T.; Bazmi, A.A.; Karim, S.; Yu, G. Insight into progress in pre-treatment of lignocellulosic biomass. Energy 2017, 122, 724–745. [Google Scholar] [CrossRef]
- Canilha, L.; Rodrigues, R.C.L.B.; Antunes, F.A.F.; Chandel, A.K.; Milessi, T.S.D.S.; Felipe, M.D.G.A.; da Silva, S.S. Bioconversion of Hemicellulose from Sugarcane Biomass Into Sustainable Products. In Sustainable Degradation of Lignocellulosic Biomass-Techniques, Applications and Commercialization; Chandel, A.K., da Silva, S.S., Eds.; InTech: Rijeka, Croatia, 2013; pp. 15–45. [Google Scholar]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Sohail, I.; Shahid, M.; Tariq, M.; Tabassum, R. Lignocellulosic Biomass: A Sustainable Bioenergy Source for Future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of Second Generation Bioethanol Production from Residual Biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Gominho, J.; Costa, R.; Lourenço, A.; Neiva, D.M.; Pereira, H. The effect of different pre-treatments to improve delignification of eucalypt stumps in a biorefinery context. Bioresour. Technol. Rep. 2019, 6, 89–95. [Google Scholar] [CrossRef]
- Pereira, S.R.; Ivanuša, Š.; Evtuguin, D.V.; Serafim, L.S.; Xavier, A.M.R.B. Biological treatment of eucalypt spent sulphite liquors: A way to boost the production of second generation bioethanol. Bioresour. Technol. 2012, 103, 131–135. [Google Scholar] [CrossRef]
- Kang, L.; Wang, W.; Pallapolu, V.R.; Lee, Y.Y. Enhanced ethanol production from de-ashed paper sludge by simultaneous saccharification and fermentation and simultaneous saccharification and Co-Fermentation. BioResources 2011, 6, 3791–3808. [Google Scholar] [CrossRef]
- Pino, M.S.; Rodríguez-Jasso, R.M.; Michelin, M.; Flores-Gallegos, A.C.; Morales-Rodriguez, R.; Teixeira, J.A.; Ruiz, H.A. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem. Eng. J. 2018, 347, 119–136. [Google Scholar] [CrossRef]
- Chen, H.; Fu, X. Industrial technologies for bioethanol production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2016, 57, 468–478. [Google Scholar] [CrossRef]
- Seidl, P.R.; Goulart, A.K. Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr. Opin. Green Sustain. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Ahorsu, R.; Medina, F.; Constantí, M. Significance and Challenges of Biomass as a Suitable Feedstock for Bioenergy and Biochemical Production: A Review. Energies 2018, 11, 3366. [Google Scholar] [CrossRef]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Pan, L.; He, M.; Wu, B.; Wang, Y.; Hu, G.; Ma, K. Simultaneous concentration and detoxification of lignocellulosic hydrolysates by novel membrane filtration system for bioethanol production. J. Clean. Prod. 2019, 227, 1185–1194. [Google Scholar] [CrossRef]
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Techno-economic assessment of carboxylic acids, furfural, and pellet production in a pine sawdust biorefinery. Biofuels Bioprod. Biorefining 2018, 12, 997–1012. [Google Scholar] [CrossRef]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Bensah, E.C.; Mensah, M.Y. Emerging physico-chemical methods for biomass pretreatment. In Fuel Ethanol Production from Sugarcane; Basso, T.P., Basso, L.C., Eds.; InTechOpen: London, UK, 2018; pp. 1–22. [Google Scholar]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Neiva, D.M.; Gominho, J.; Pereira, H. Modeling and optimization of Eucalyptus globulus bark and wood delignification using response surface methodology. BioResources 2014, 9, 2907–2921. [Google Scholar] [CrossRef]
- Amândio, M.S.T.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Cellulosic Bioethanol from Industrial Eucalyptus globulus Bark Residues Using Kraft Pulping as a Pretreatment. Energies 2021, 14, 2185. [Google Scholar] [CrossRef]
- Asada, C.; Sasaki, C.; Nakamura, Y. High Concentration Ethanol Production from Mixed Softwood Sawdust Waste. Waste and Biomass Valorization 2019, 10, 433–439. [Google Scholar] [CrossRef]
- da Silva Morais, A.P.; Sansígolo, C.A.; de Oliveira Neto, M. Effects of autohydrolysis of Eucalyptus urograndis and Eucalyptus grandis on influence of chemical components and crystallinity index. Bioresour. Technol. 2016, 214, 623–628. [Google Scholar] [CrossRef]
- Amini, N.; Haritos, V.S.; Tanksale, A. Microwave assisted pretreatment of eucalyptus sawdust enhances enzymatic saccharification and maximizes fermentable sugar yield. Renew. Energy 2018, 127, 653–660. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Tulashie, S.K.; Akpari, E.E.A.; Appiah, G.; Adongo, A.; Andoh, E.K. Acid hydrolysis of sawdust waste into bioethanol. Biomass Convers. Biorefinery 2021, 1–14. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Kumar, A.; Naraian, R. Differential expression of the microbial β-1, 4-xylanase, and β-1, 4-endoglucanase genes. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Gupta, V.K., Jogaiah, S., Eds.; Elsevier B.V.: Amsterdam, The Netherlands, 2019; pp. 95–111. ISBN 9780444635037. [Google Scholar]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Gomes, A.C.; Moysés, D.N.; Santa Anna, L.M.M.; Castro, A.M. Fed-batch strategies for saccharification of pilot-scale mild-acid and alkali pretreated sugarcane bagasse: Effects of solid loading and surfactant addition. Ind. Crops Prod. 2018, 119, 283–289. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, W.; Paice, M.G.; Saddler, J.N. High consistency enzymatic hydrolysis of hardwood substrates. Bioresour. Technol. 2009, 100, 5890–5897. [Google Scholar] [CrossRef]
- Chen, H.Z.; Liu, Z.H. Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng. Life Sci. 2017, 17, 489–499. [Google Scholar] [CrossRef]
- He, L.; Han, Q.; Jameel, H.; Chang, H.M.; Phillips, R.; Wang, Z. Comparison of One-Stage Batch and Fed-Batch Enzymatic Hydrolysis of Pretreated Hardwood for the Production of Biosugar. Appl. Biochem. Biotechnol. 2018, 184, 1441–1452. [Google Scholar] [CrossRef]
- Kemppainen, K.; Inkinen, J.; Uusitalo, J.; Nakari-Setälä, T.; Siika-aho, M. Hot water extraction and steam explosion as pretreatments for ethanol production from spruce bark. Bioresour. Technol. 2012, 117, 131–139. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Roberto, I.C. Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresour. Technol. 2004, 93, 1–10. [Google Scholar] [CrossRef]
- Xavier, A.M.R.B.; Correia, M.F.; Pereira, S.R.; Evtuguin, D.V. Second-generation bioethanol from eucalypt sulphite spent liquor. Bioresour. Technol. 2010, 101, 2755–2761. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, D.L.A.; Silva, C.M.; Xavier, A.M.R.B.; Evtuguin, D.V. Fractionation of sulphite spent liquor for biochemical processing using ion exchange resins. J. Biotechnol. 2012, 162, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed]
- Canilha, L.; Chandel, A.K.; dos Santos Milessi, T.S.; Antunes, F.A.F.; da Costa Freitas, W.L.; das Graças Almeida Felipe, M.; da Silva, S.S. Bioconversion of Sugarcane Biomass into Ethanol: An Overview about Composition, Pretreatment Methods, Detoxification of Hydrolysates, Enzymatic Saccharification, and Ethanol Fermentation. J. Biomed. Biotechnol. 2012, 2012, 989572. [Google Scholar] [CrossRef]
- Kumar, M.; Morya, R.; Gupta, A.; Thakur, I.S. Anaerobic biovalorization of pulp and paper mill waste. In Biovalorisation of Wastes to Renewable Chemicals and Biofuels; Rathinam, N.K., Sani, R.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 41–61. ISBN 9780128179512. [Google Scholar]
- Veluchamy, C.; Kalamdhad, A.S. Influence of pretreatment techniques on anaerobic digestion of pulp and paper mill sludge: A review. Bioresour. Technol. 2017, 245, 1206–1219. [Google Scholar] [CrossRef]
- Rorke, D.C.S.; Lekha, P.; Kana, G.E.B.; Sithole, B.B. Surfactant-assisted green liquor dregs pretreatment to enhance the digestibility of paper mill sludge. Int. J. Hydrogen Energy 2021, 46, 21359–21371. [Google Scholar] [CrossRef]
- Singh, S.; Sithole, B.; Lekha, P.; Permaul, K.; Govinden, R. Pretreatment and enzymatic saccharification of sludge from a prehydrolysis kraft and kraft pulping mill. J. Wood Chem. Technol. 2020, 41, 10–24. [Google Scholar] [CrossRef]
- Dey, P.; Rangarajan, V.; Nayak, J.; Das, D.B.; Wood, S.B. An improved enzymatic pre-hydrolysis strategy for efficient bioconversion of industrial pulp and paper sludge waste to bioethanol using a semi-simultaneous saccharification and fermentation process. Fuel 2021, 294, 120581. [Google Scholar] [CrossRef]
- Arthur, W.; Diedericks, D.; Coetzee, G.; Van Rensburg, E.; Görgens, J.F. Kinetic modelling of cellulase recycling in paper sludge to ethanol fermentation. J. Environ. Chem. Eng. 2021, 9, 105981. [Google Scholar] [CrossRef]
- Zamani, A. Introduction to Lignocellulose-based Products. In Lignocellulose-Based Bioproducts (Biofuel and Biorefinery Technologies); Karimi, K., Ed.; Springer International Publishing Switzerland: Cham, Switzerland, 2015; Volume 1, pp. 1–36. ISBN 978-3-319-14032-2. [Google Scholar]
- Chandel, A.K.; Albarelli, J.Q.; Santos, D.T.; Chundawat, S.P.; Puri, M.; Meireles, M.A.A. Comparative analysis of key technologies for cellulosic ethanol production from Brazilian sugarcane bagasse at a commercial scale. Biofuels, Bioprod. Biorefining 2019, 13, 994–1014. [Google Scholar] [CrossRef]
- Azhar, S.H.M.; Abdulla, R.; Jambo, S.A.; Marbawi, H.; Gansau, J.A.; Faik, A.A.M.; Rodrigues, K.F. Yeasts in sustainable bioethanol production: A review. Biochem. Biophys. Rep. 2017, 10, 52–61. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Chandel, A.K.; Terán-Hilares, R.; Ingle, A.P.; Rai, M.; dos Santos Milessi, T.S.; da Silva, S.S.; dos Santos, J.C. Overcoming challenges in lignocellulosic biomass pretreatment for second-generation (2G) sugar production: Emerging role of nano, biotechnological and promising approaches. 3 Biotech 2019, 9, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Sievers, D.A.; Stickel, J.J.; Grundl, N.J.; Tao, L. Technical Performance and Economic Evaluation of Evaporative and Membrane-Based Concentration for Biomass-Derived Sugars. Ind. Eng. Chem. Res. 2017, 56, 11584–11592. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef] [PubMed]
- EurObserv’ER Biofuels Barometer 2020. Available online: https://www.eurobserv-er.org/biofuels-barometer-2020/ (accessed on 4 April 2022).
- Amândio, M.S.T.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Bioethanol from Wastes for Mobility: Europe on the Road to Sustainability. In Clean Fuels for Mobility; Di Blasio, G., Agarwal, A.K., Belgiorno, G., Shukla, P.C., Eds.; Springer: Singapore, 2022; pp. 97–123. [Google Scholar]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Jouzani, G.S.; Taherzadeh, M.J. Advances in consolidated bioprocessing systems for bioethanol and butanol production from biomass: A comprehensive review. Biofuel Res. J. 2015, 5, 152–195. [Google Scholar] [CrossRef]
- Hasunuma, T.; Kondo, A. Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process Biochem. 2012, 47, 1287–1294. [Google Scholar] [CrossRef]
- Zhang, J.; Lynd, L.R. Ethanol production from paper sludge by simultaneous saccharification and co-fermentation using recombinant xylose-fermenting microorganisms. Biotechnol. Bioeng. 2010, 107, 235–244. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Milessi, T.S.; Candido, R.G.; Mendes, A.A.; Aguiar, A. Enzymatic catalysis as a tool in biofuels production in Brazil: Current status and perspectives. Energy Sustain. Dev. 2022, 68, 103–119. [Google Scholar] [CrossRef]
- Mbaneme, V.; Chinn, S.S.M. Consolidated bioprocessing for biofuel production: Recent advances. Energy Emiss. Control Technol. 2015, 3, 23–44. [Google Scholar] [CrossRef]
- Yin, F.; Li, D.; Ma, X.; Li, J.; Qiu, Y. Poly(3-hydroxybutyrate-3-hydroxyvalerate) production from pretreated waste lignocellulosic hydrolysates and acetate co-substrate. Bioresour. Technol. 2020, 316, 123911. [Google Scholar] [CrossRef] [PubMed]
- Nigam, J.N. Ethanol production from hardwood spent sulfite liquor using an adapted strain of Pichia stipitis. J. Ind. Microbiol. Biotechnol. 2001, 26, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.R.; Sànchez, I.; Nogué, V.; Frazão, C.J.R.; Serafim, L.S.; Gorwa-Grauslund, M.F.; Xavier, A.M.R.B. Adaptation of Scheffersomyces stipitis to hardwood spent sulfite liquor by evolutionary engineering. Biotechnol. Biofuels 2015, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Mendes, C.V.T.; Cruz, C.H.G.; Reis, D.F.N.; Carvalho, M.G.V.S.; Rocha, J.M.S. Integrated bioconversion of pulp and paper primary sludge to second generation bioethanol using Saccharomyces cerevisiae ATCC 26602. Bioresour. Technol. 2016, 220, 161–167. [Google Scholar] [CrossRef]
- Kang, L.; Wang, W.; Lee, Y.Y. Bioconversion of kraft paper mill sludges to ethanol by SSF and SSCF. Appl. Biochem. Biotechnol. 2010, 161, 53–66. [Google Scholar] [CrossRef]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The chemomechanical properties of microbial polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Koller, M.; Salerno, A.; Braunegg, G. Polyhydroxyalkanoates: Basics, Production and Applications of Microbial Biopolyesters. In Bio-Based Plastics: Materials and Applications; Kabasci, S., Ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2014; pp. 137–170. ISBN 9781118676646. [Google Scholar]
- Jiang, Y.; Marang, L.; Tamis, J.; van Loosdrecht, M.C.M.; Dijkman, H.; Kleerebezem, R. Waste to resource: Converting paper mill wastewater to bioplastic. Water Res. 2012, 46, 5517–5530. [Google Scholar] [CrossRef]
- Al-Battashi, H.S.; Annamalai, N.; Sivakumar, N.; Al-Bahry, S.; Tripathi, B.N.; Nguyen, Q.D.; Gupta, V.K. Lignocellulosic biomass (LCB): A potential alternative biorefinery feedstock for polyhydroxyalkanoates production. Rev. Environ. Sci. Biotechnol. 2019, 18, 183–205. [Google Scholar] [CrossRef]
- Li, D.; Yin, F.; Ma, X. Towards biodegradable polyhydroxyalkanoate production from wood waste: Using volatile fatty acids as conversion medium. Bioresour. Technol. 2020, 299, 122629. [Google Scholar] [CrossRef]
- Xu, Z.; Lei, P.; Zhai, R.; Wen, Z.; Jin, M. Recent advances in lignin valorization with bacterial cultures: Microorganisms, metabolic pathways, and bio-products. Biotechnol. Biofuels 2019, 12, 32. [Google Scholar] [CrossRef]
- Shi, Y.; Yan, X.; Li, Q.; Wang, X.; Liu, M.; Xie, S.; Chai, L.; Yuan, J. Directed bioconversion of Kraft lignin to polyhydroxyalkanoate by Cupriavidus basilensis B-8 without any pretreatment. Process Biochem. 2017, 52, 238–242. [Google Scholar] [CrossRef]
- Kumar, M.; Singhal, A.; Verma, P.K.; Thakur, I.S. Production and Characterization of Polyhydroxyalkanoate from Lignin Derivatives by Pandoraea sp. ISTKB. ACS Omega 2017, 2, 9156–9163. [Google Scholar] [CrossRef] [PubMed]
- Toczyłowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef]
- Mato, T.; Ben, M.; Kennes, C.; Veiga, M.C. Valuable product production from wood mill effluents. Water Sci. Technol. 2010, 62, 2294–2300. [Google Scholar] [CrossRef]
- Ben, M.; Mato, T.; Lopez, A.; Vila, M.; Kennes, C.; Veiga, M.C. Bioplastic production using wood mill effluents as feedstock. Water Sci. Technol. 2011, 63, 1196–1202. [Google Scholar] [CrossRef]
- Bengtsson, S.; Werker, A.; Welander, T. Production of polyhydroxyalkanoates by glycogen accumulating organisms treating a paper mill wastewater. Water Sci. Technol. 2008, 58, 323–330. [Google Scholar] [CrossRef]
- Pozo, G.; Villamar, A.C.; Martínez, M.; Vidal, G. Polyhydroxyalkanoates (PHA) biosynthesis from kraft mill wastewaters: Biomass origin and C:N relationship influence. Water Sci. Technol. 2011, 63, 449–455. [Google Scholar] [CrossRef]
- Tobella, L.M.; Bunster, M.; Pooley, A.; Becerra, J.; Godoy, F.; Martínez, M.A. Biosynthesis of poly-β-hydroxyalkanoates by Sphingopyxis chilensis S37 and Wautersia sp. PZK cultured in cellulose pulp mill effluents containing 2,4,6-trichlorophenol. J. Ind. Microbiol. Biotechnol. 2005, 32, 397–401. [Google Scholar] [CrossRef]
- Queirós, D.; Rossetti, S.; Serafim, L.S. PHA production by mixed cultures: A way to valorize wastes from pulp industry. Bioresour. Technol. 2014, 157, 197–205. [Google Scholar] [CrossRef]
- Pereira, J.; Queirós, D.; Lemos, P.C.; Rossetti, S.; Serafim, L.S. Enrichment of a mixed microbial culture in PHA-storing microorganisms by using fermented hardwood spent sulfite liquor. N. Biotechnol. 2020, 56, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Weissgram, M.; Gstöttner, J.; Lorantfy, B.; Tenhaken, R.; Herwig, C.; Weber, H. Generation of PHB from Spent Sulfite Liquor Using Halophilic Microorganisms. Microorganisms 2015, 3, 268–289. [Google Scholar] [CrossRef] [PubMed]
- Kamali, M.; Gameiro, T.; Costa, M.E.V.; Capela, I. Anaerobic digestion of pulp and paper mill wastes—An overview of the developments and improvement opportunities. Chem. Eng. J. 2016, 298, 162–182. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, Z. Solid-state anaerobic digestion for waste management and biogas production. In Solid State Fermentation: Research and Industrial Applications; Steudler, S., Werner, A., Cheng, J.J., Eds.; Springer Nature Switzerland AG: Cham, Switzerland, 2019; Volume 169, pp. 147–168. ISBN 0724-6145. [Google Scholar]
- Mulat, D.G.; Horn, S.J. Biogas Production from Lignin via Anaerobic Digestion. In RSC Energy and Environment Series; Beckham, G.T., Ed.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 391–412. ISBN 9781782620426. [Google Scholar]
- Kamali, M.; Khodaparast, Z. Review on recent developments on pulp and paper mill wastewater treatment. Ecotoxicol. Environ. Saf. 2015, 114, 326–342. [Google Scholar] [CrossRef]
- Grover, R.; Marwaha, S.S.; Kennedy, J.F. Studies on the use of an anaerobic baffled reactor for the continuous anaerobic digestion of pulp and paper mill black liquors. Process Biochem. 1999, 34, 653–657. [Google Scholar] [CrossRef]
- Buzzini, A.P.; Pires, E.C. Cellulose pulp mill effluent treatment in an upflow anaerobic sludge blanket reactor. Process Biochem. 2002, 38, 707–713. [Google Scholar] [CrossRef]
- Turkdogan, F.I.; Park, J.; Evans, E.A.; Ellis, T.G. Evaluation of pretreatment using UASB and SGBR reactors for pulp and paper plants wastewater treatment. Water. Air. Soil Pollut. 2013, 224, 1512. [Google Scholar] [CrossRef]
- Xie, K.; Lin, H.J.; Mahendran, B.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Performance and fouling characteristics of a submerged anaerobic membrane bioreactor for kraft evaporator condensate treatment. Environ. Technol. 2010, 31, 511–521. [Google Scholar] [CrossRef]
- Lin, H.J.; Xie, K.; Mahendran, B.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Sludge properties and their effects on membrane fouling in submerged anaerobic membrane bioreactors (SAnMBRs). Water Res. 2009, 43, 3827–3837. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, D.; Wu, S.; Wang, C. Alkali pretreatment enhances biogas production in the anaerobic digestion of pulp and paper sludge. J. Hazard. Mater. 2009, 170, 366–373. [Google Scholar] [CrossRef]
- Bayr, S.; Rintala, J. Thermophilic anaerobic digestion of pulp and paper mill primary sludge and co-digestion of primary and secondary sludge. Water Res. 2012, 46, 4713–4720. [Google Scholar] [CrossRef] [PubMed]
- Bayr, S.; Kaparaju, P.; Rintala, J. Screening pretreatment methods to enhance thermophilic anaerobic digestion of pulp and paper mill wastewater treatment secondary sludge. Chem. Eng. J. 2013, 223, 479–486. [Google Scholar] [CrossRef]
- Saha, M.; Eskicioglu, C.; Marin, J. Microwave, ultrasonic and chemo-mechanical pretreatments for enhancing methane potential of pulp mill wastewater treatment sludge. Bioresour. Technol. 2011, 102, 7815–7826. [Google Scholar] [CrossRef] [PubMed]
- Zwain, H.M.; Hassan, S.R.; Zaman, N.Q.; Aziz, H.A.; Dahlan, I. The start-up performance of modified anaerobic baffled reactor (MABR) for the treatment of recycled paper mill wastewater. J. Environ. Chem. Eng. 2013, 1, 61–64. [Google Scholar] [CrossRef]
- Rao, A.G.; Bapat, A.N. Anaerobic treatment of pre-hydrolysate liquor (PHL) from a rayon grade pulp mill: Pilot and full-scale experience with UASB reactors. Bioresour. Technol. 2006, 97, 2311–2320. [Google Scholar] [CrossRef]
- Yilmaz, T.; Yuceer, A.; Basibuyuk, M. A comparison of the performance of mesophilic and thermophilic anaerobic filters treating papermill wastewater. Bioresour. Technol. 2008, 99, 156–163. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Lignin degradation under anaerobic digestion: Influence of lignin modifications—A review. Biomass and Bioenergy 2019, 128, 105325. [Google Scholar] [CrossRef]
- Ahring, B.K.; Biswas, R.; Ahamed, A.; Teller, P.J.; Uellendahl, H. Making lignin accessible for anaerobic digestion by wet-explosion pretreatment. Bioresour. Technol. 2015, 175, 182–188. [Google Scholar] [CrossRef]
- Elliott, A.; Mahmood, T. Pretreatment technologies for advancing anaerobic digestion of pulp and paper biotreatment residues. Water Res. 2007, 41, 4273–4286. [Google Scholar] [CrossRef]
- Meyer, T.; Edwards, E.A. Anaerobic digestion of pulp and paper mill wastewater and sludge. Water Res. 2014, 65, 321–349. [Google Scholar] [CrossRef]
- Bajpai, P. Anaerobic Technology in Pulp and Paper Industry; Kacprzyk, J., Ed.; Springer Briefs in Applied Sciences and Technology; Springer: Singapore, 2017; ISBN 978-981-10-4129-7. [Google Scholar]
- Zhang, A.; Shen, J.; Ni, Y. Anaerobic Digestion for Use in the Pulp and Paper Industry and Other Sectors: An Introductory Mini-Review. BioResources 2015, 10, 8750–8769. [Google Scholar] [CrossRef]
- Rasi, S.; Kilpeläinen, P.; Rasa, K.; Korpinen, R.; Raitanen, J.E.; Vainio, M.; Kitunen, V.; Pulkkinen, H.; Jyske, T. Cascade processing of softwood bark with hot water extraction, pyrolysis and anaerobic digestion. Bioresour. Technol. 2019, 292, 121893. [Google Scholar] [CrossRef] [PubMed]
- Zhongjiang, W.; Yebo, L.; Jia, Z. Influence of composting pretreatment on dry anaerobic digestion of pine sawdust. Nongye Jixie Xuebao = Trans. Chin. Soc. Agric. Mach. 2014, 45, 197–200. [Google Scholar]
- Oh, J.I.; Lee, J.; Lin, K.Y.A.; Kwon, E.E.; Tsang, Y.F. Biogas production from food waste via anaerobic digestion with wood chips. Energy Environ. 2018, 29, 1365–1372. [Google Scholar] [CrossRef]
- Jantsch, T.G.; Angelidaki, I.; Schmidt, J.E.; de Hvidsten, B.E.B.; Ahring, B.K. Anaerobic biodegradation of spent sulphite liquor in a UASB reactor. Bioresour. Technol. 2002, 84, 15–20. [Google Scholar] [CrossRef]
- Schnell, A.; Hall, E.R.; Skog, S. Anaerobic and aerobic treatability of high-yield sulphite spent liquor. Water Pollut. Res. J. Canada 1992, 27, 601–620. [Google Scholar] [CrossRef]
- Yerushalmi, L.; Hodgson, J.; Jasim, K.S.; Guiot, S.R. Effects of dilution and fungal pretreatment on anaerobic biodegradability of oxygen delignification process spent liquor. Water Qual. Res. J. Canada 1999, 34, 599–613. [Google Scholar] [CrossRef]
- Raj, T.; Chandrasekhar, K.; Kumar, A.N.; Banu, J.R.; Yoon, J.J.; Bhatia, S.K.; Yang, Y.H.; Varjani, S.; Kim, S.H. Recent advances in commercial biorefineries for lignocellulosic ethanol production: Current status, challenges and future perspectives. Bioresour. Technol. 2022, 344, 126292. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Lei, F.; Jiang, J. Co-production bioethanol and xylooligosaccharides from sugarcane bagasse via autohydrolysis pretreatment. Renew. Energy 2020, 162, 2297–2305. [Google Scholar] [CrossRef]
- Álvarez, C.; Sáez, F.; González, A.; Ballesteros, I.; Oliva, J.M.; Negro, M.J. Production of xylooligosaccharides and cellulosic ethanol from steam-exploded barley straw. Holzforschung 2019, 73, 35–44. [Google Scholar] [CrossRef]
- Jiang, G.; Hill, D.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Irorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amândio, M.S.T.; Pereira, J.M.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Getting Value from Pulp and Paper Industry Wastes: On the Way to Sustainability and Circular Economy. Energies 2022, 15, 4105. https://doi.org/10.3390/en15114105
Amândio MST, Pereira JM, Rocha JMS, Serafim LS, Xavier AMRB. Getting Value from Pulp and Paper Industry Wastes: On the Way to Sustainability and Circular Economy. Energies. 2022; 15(11):4105. https://doi.org/10.3390/en15114105
Chicago/Turabian StyleAmândio, Mariana S. T., Joana M. Pereira, Jorge M. S. Rocha, Luísa S. Serafim, and Ana M. R. B. Xavier. 2022. "Getting Value from Pulp and Paper Industry Wastes: On the Way to Sustainability and Circular Economy" Energies 15, no. 11: 4105. https://doi.org/10.3390/en15114105
APA StyleAmândio, M. S. T., Pereira, J. M., Rocha, J. M. S., Serafim, L. S., & Xavier, A. M. R. B. (2022). Getting Value from Pulp and Paper Industry Wastes: On the Way to Sustainability and Circular Economy. Energies, 15(11), 4105. https://doi.org/10.3390/en15114105