Three-Dimensional Direct Numerical Simulation of Near-Field Ozone-Enhanced Lean Premixed Syngas Turbulent Jet Flame
Abstract
:1. Introduction
2. Method and Definition
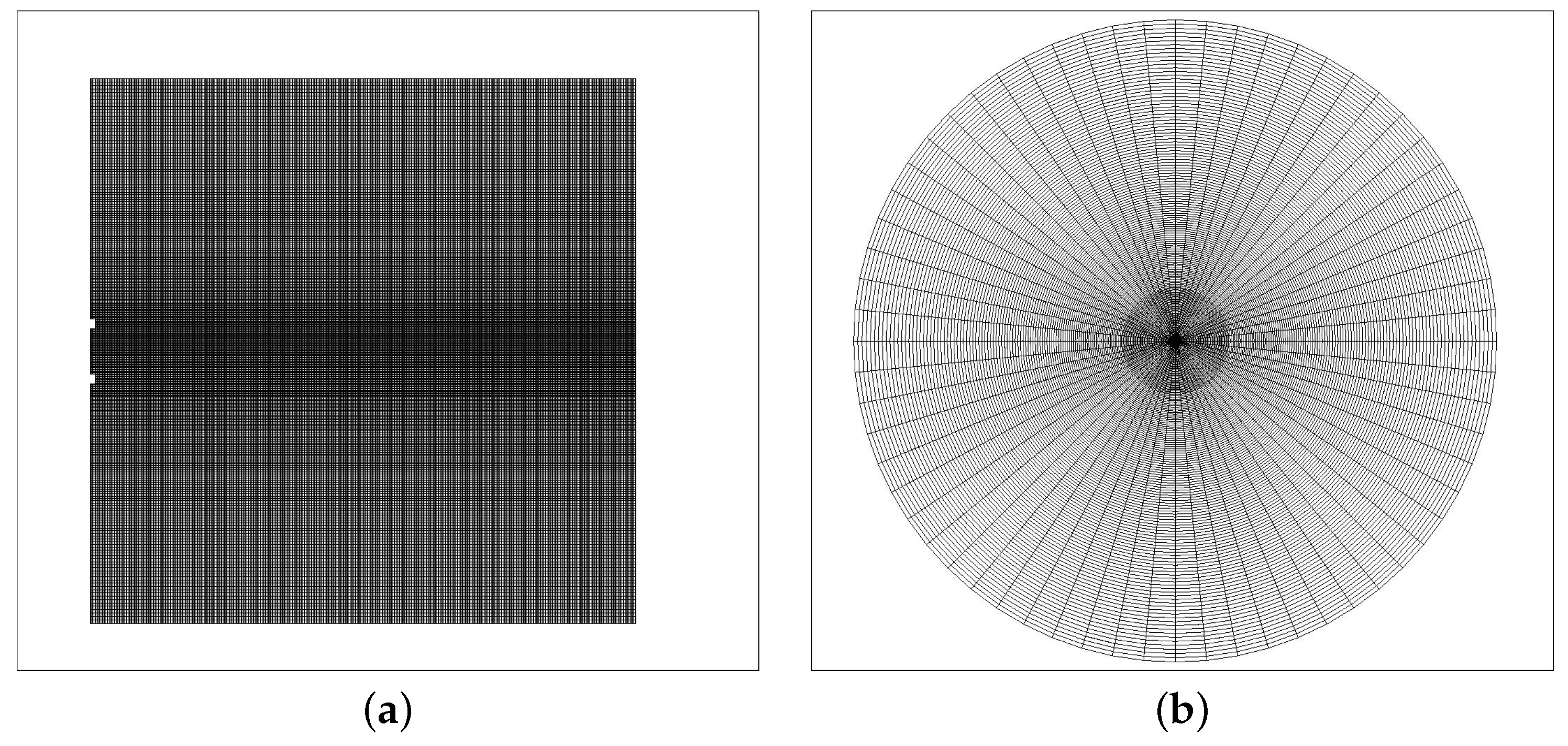
2.1. Numerical Method
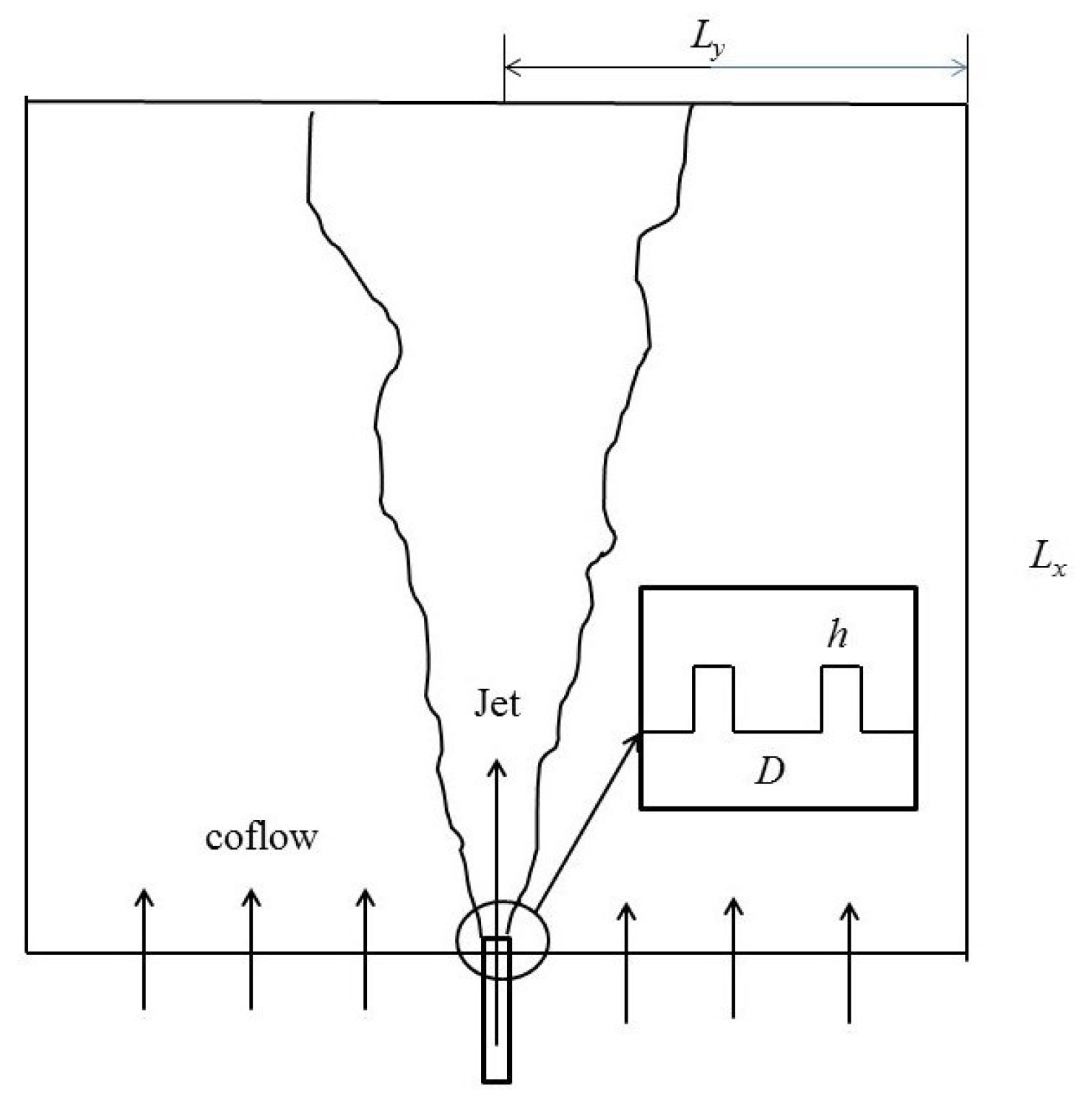
2.2. Case Definition
3. Results and Discussion
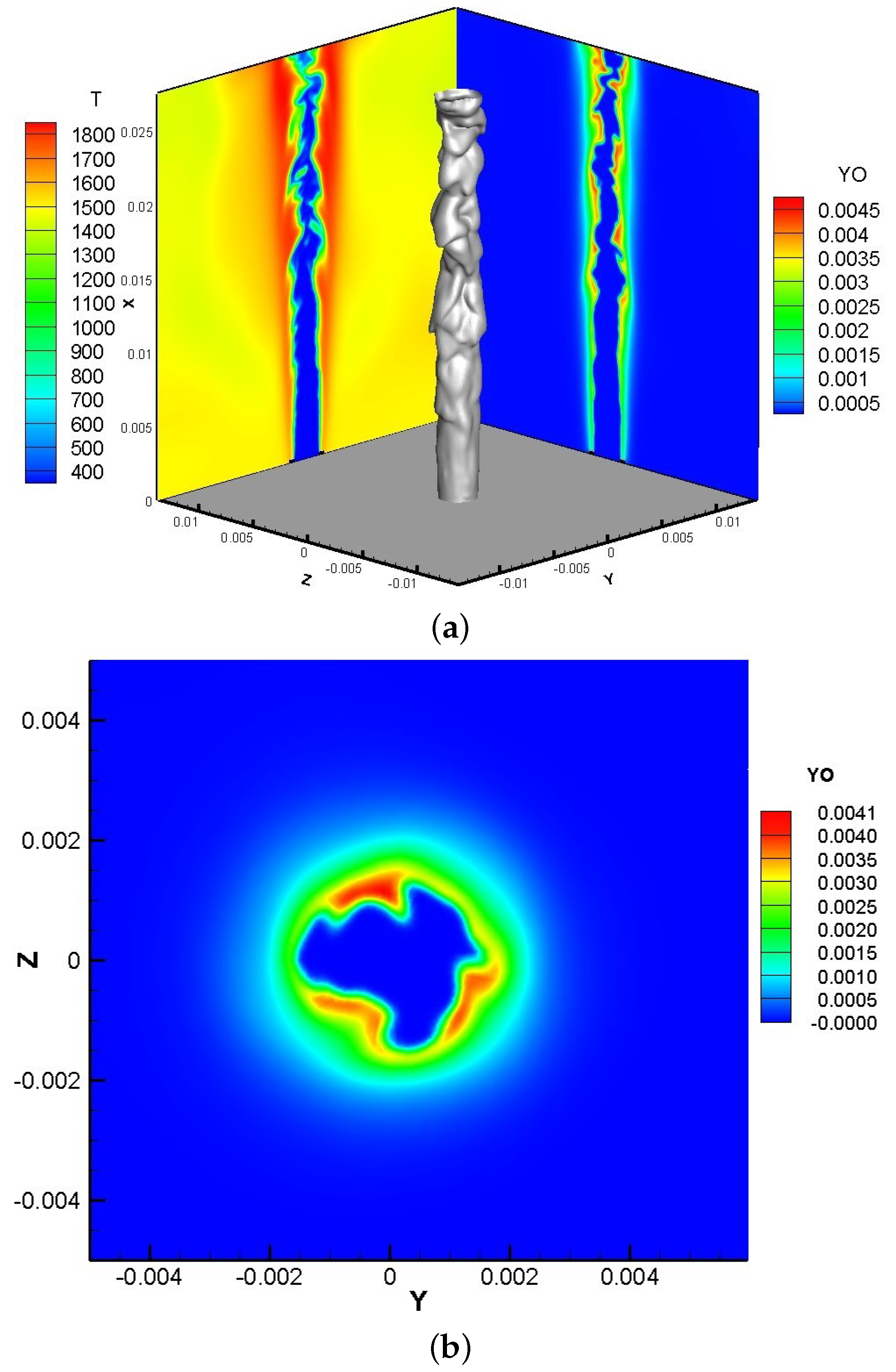
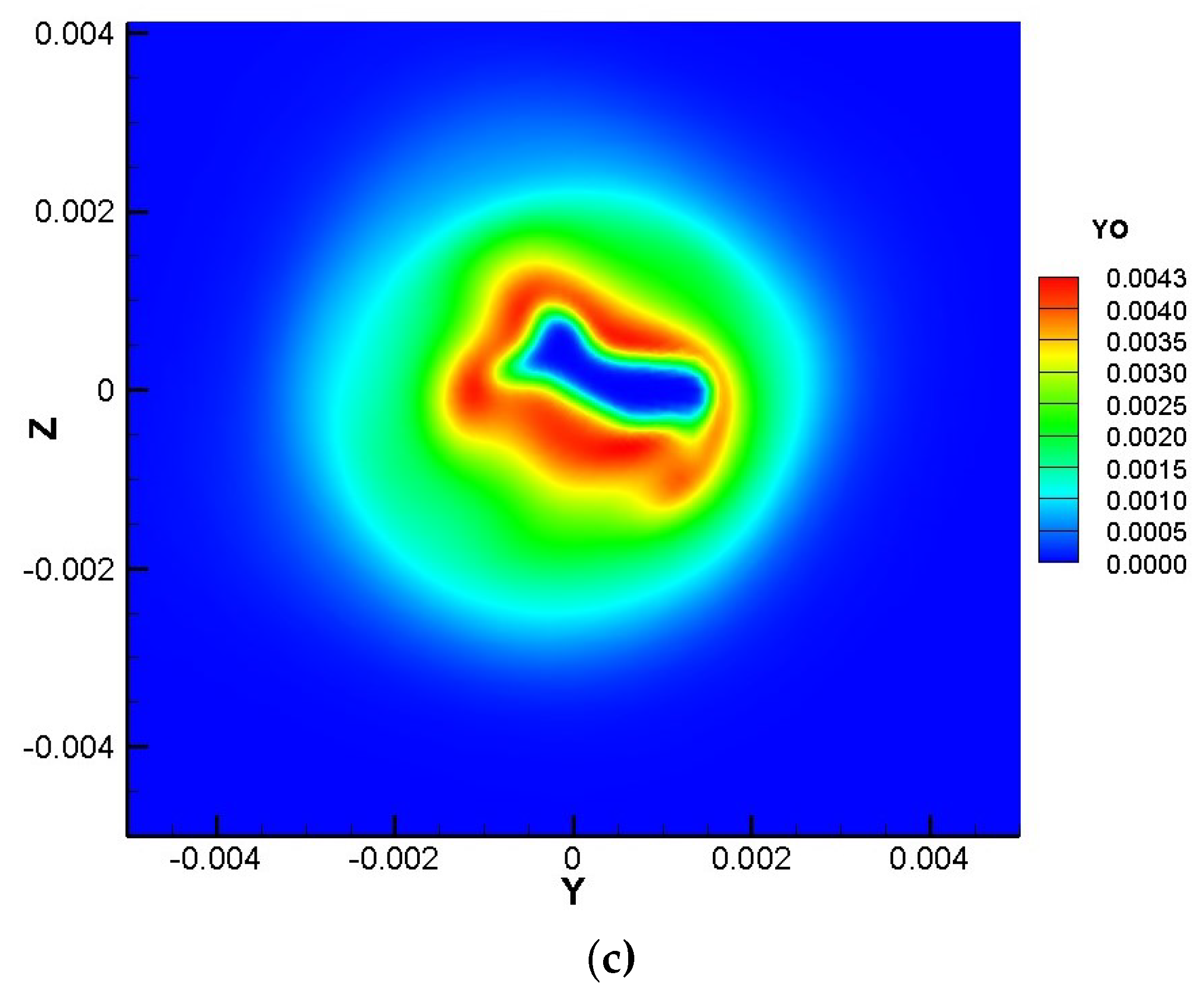
3.1. Transient Field Analysis
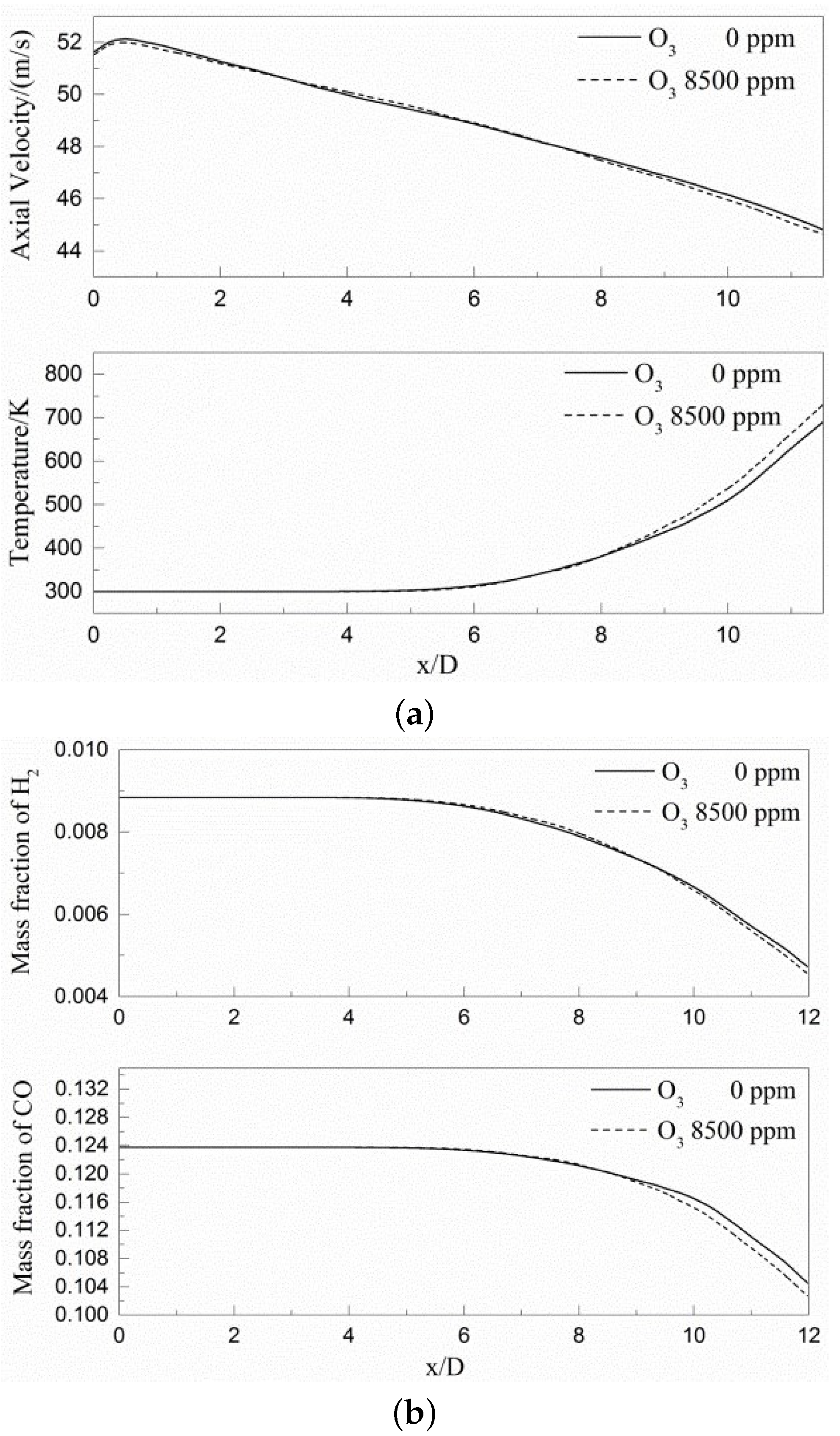
3.2. Time-Averaged Statistical Analysis
3.3. The Effect on Ignition
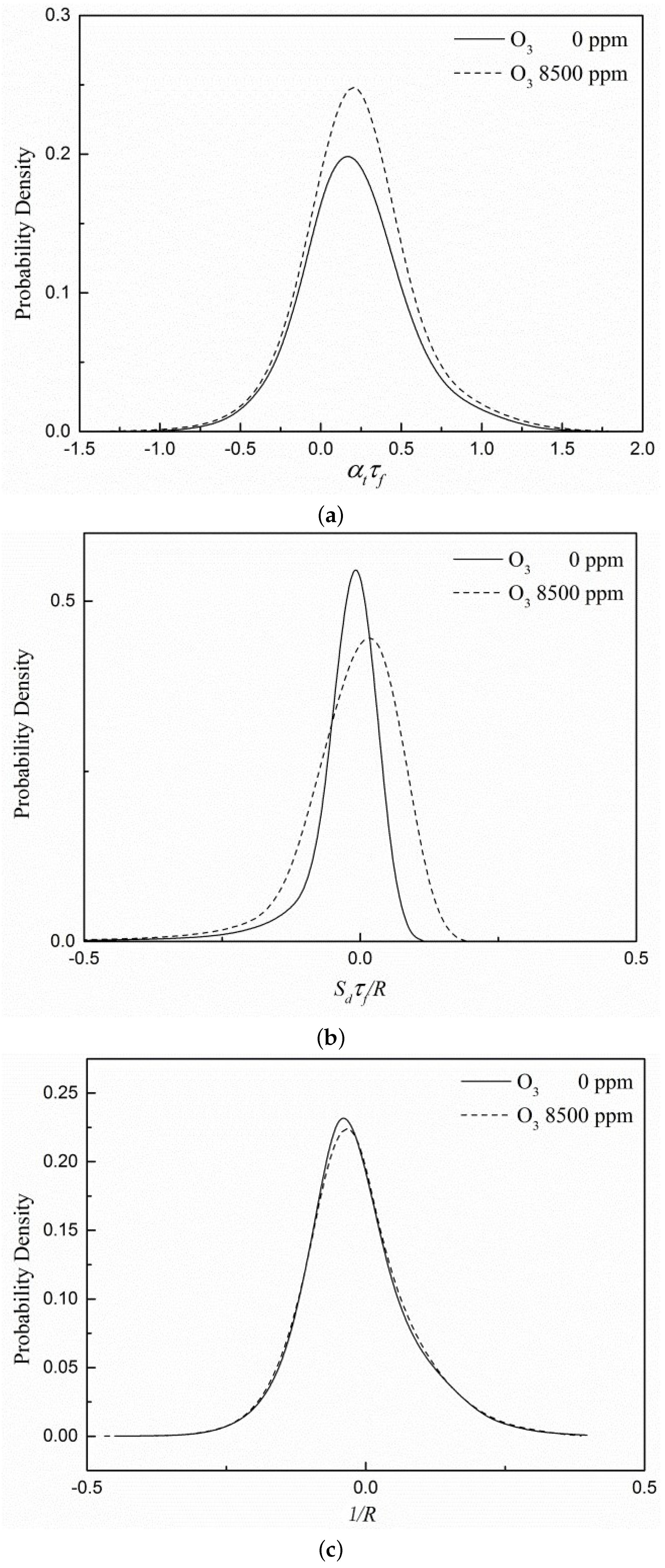
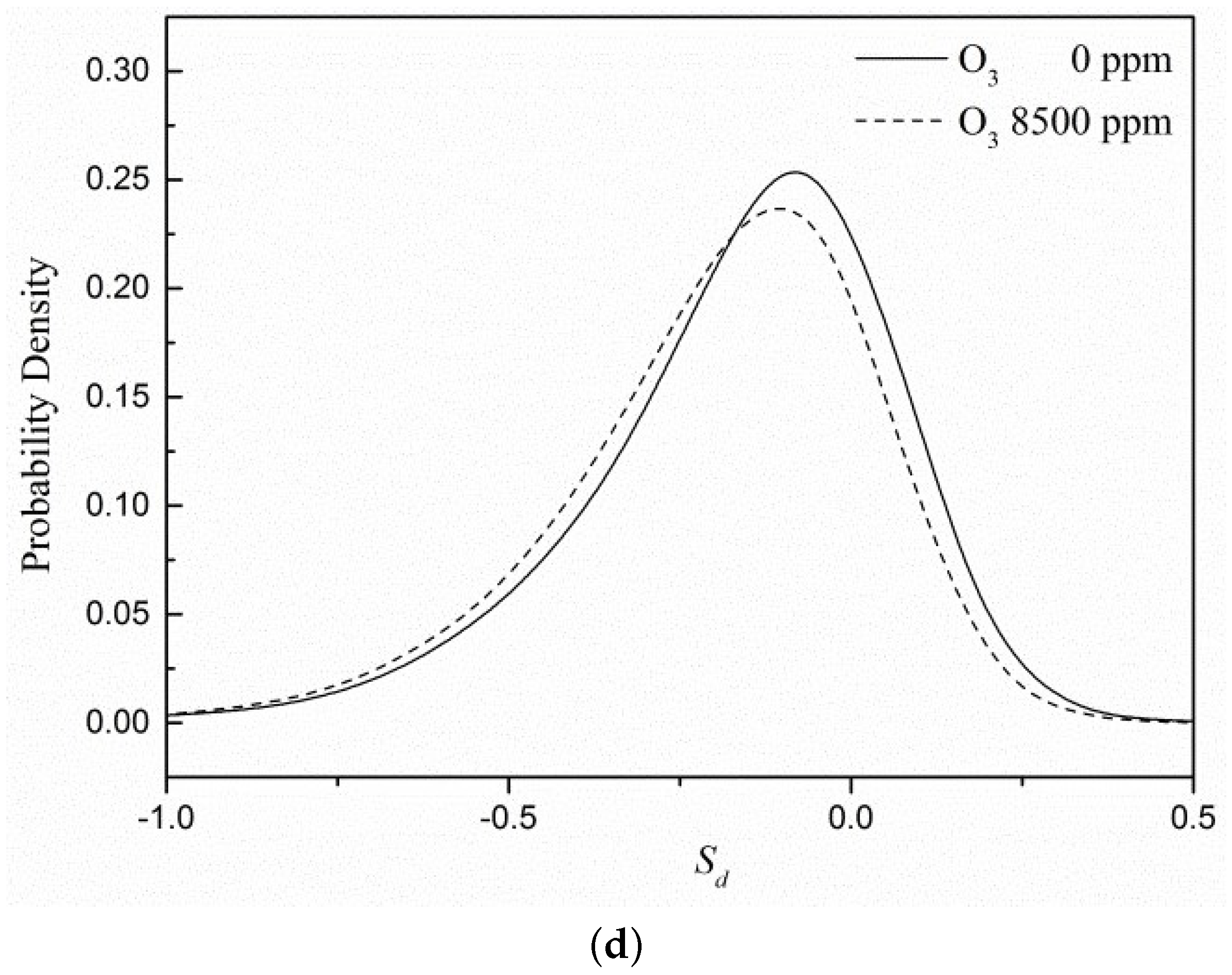
3.4. Flame Surface Statistics Analysis
4. Conclusions
- (1)
- It can be seen from the transient field and the time-averaged results that turbulence begins to significantly affect the flame surface to produce wrinkles in lean premixed gas flame after 4D, while ozone starts to affect the composition field and temperature field after 8D and accelerates the generation of intermediate products OH and O radicals and will promote the production of CO in the near field range.
- (2)
- It can be seen from the ignition process that ozone will increase the flame surface area of the lean premixed syngas flame by comparing the area of the flame surface during the ignition period, and the conclusion can be obtained that ozone can promote the ignition process and make the combustion occur earlier.
- (3)
- It can be seen from the flame surface statistics that the flame surface of the case with ozone added is more easily stretched by turbulence, and adding ozone can improve the stability of combustion. Ozone does not affect the effective radius of the flame curvature but will broaden the distribution of the curvature term because of the enhancement effect on the displacement speed of the flame surface.
Author Contributions
Funding
Conflicts of Interest
References
- Halter, F.; Higelin, P.; Dagaut, P. Experimental and Detailed Kinetic Modeling Study of the Effect of Ozone on the Combustion of Methane. Energy Fuels 2011, 25, 2909–2916. [Google Scholar] [CrossRef]
- Masurier, J.B.; Foucher, F.; Dayma, G.; Daguat, P. Investigation of iso-octane combustion in a homogeneous charge compression ignition engine seeded by ozone, nitric oxide and nitrogen dioxide. Proc. Combust. Inst. 2015, 35, 3125–3132. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, M.M.; Zhang, Z.Z.; Shang, R.X.; Zhang, D.K. Ozone effect on the flammability limit and near-limit combustion of syngas/air flames with N2, CO2, and H2O dilutions. Fuel 2016, 186, 414–421. [Google Scholar] [CrossRef]
- Mehdi, G.; Bonuso, S.; De Giorgi, M.G. Effects of Nanosecond Repetitively Pulsed Discharges Timing for Aeroengines Ignition at Low Temperature Conditions by Needle-Ring Plasma Actuator. Energies 2021, 14, 5814. [Google Scholar] [CrossRef]
- Ombrello, T.; Won, S.H.; Ju, Y.G.; Williams, S. Flame propagation enhancement by plasma excitation of oxygen. Part I: Effects of O3. Combust. Flame 2010, 157, 1906–1915. [Google Scholar] [CrossRef]
- Wang, Z.H.; Yang, L.; Li, B.; Li, Z.S.; Sun, Z.W.; Alden, M.; Cen, K.F.; Konnov, A.A. Investigation of combustion enhancement by ozone additive in CH4/air flames using direct laminar burning velocity measurements and kinetic simulations. Combust. Flame 2012, 159, 120–129. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z.; Li, L.; Wan, K.; Cen, K. Reaction Mechanism Reduction for Ozone-Enhanced CH4/Air Combustion by a Combination of Directed Relation Graph with Error Propagation, Sensitivity Analysis and Quasi-Steady State Assumption. Energies 2018, 11, 1470. [Google Scholar] [CrossRef] [Green Version]
- Ji, S.B.; Lan, X.; Lian, J.; Xu, H.M.; Wang, Y.Q.; Cheng, Y.; Liu, Y.Q. Influence of Ozone on Ignition and Combustion Performance of a Lean Methane/Air Mixture. Energy Fuels 2017, 31, 14191–14200. [Google Scholar] [CrossRef]
- Wu, B.; Hastings, M.; Sun, W.T.; Ombrello, T.; Carter, C. Dynamics of laminar ethylene lifted flame with ozone addition. Proc. Combust. Inst. 2020, 38, 6773–6780. [Google Scholar] [CrossRef]
- Keil, F.B.; Amzehnhoff, M.; Ahmed, U.; Chakraborty, N.; Klein, M. Comparison of Flame Propagation Statistics Extracted from Direct Numerical Simulation Based on Simple and Detailed Chemistry—Part 1: Fundamental Flame Turbulence Interaction. Energies 2021, 14, 5548. [Google Scholar] [CrossRef]
- Keil, F.B.; Amzehnhoff, M.; Ahmed, U.; Chakraborty, N.; Klein, M. Comparison of Flame Propagation Statistics Based on Direct Numerical Simulation of Simple and Detailed Chemistry. Part 2: Influence of Choice of Reaction Progress Variable. Energies 2021, 14, 5695. [Google Scholar]
- Wang, Z.H.; Fan, J.R.; Zhou, J.H.; Cen, K.F. Direct numerical simulation of hydrogen turbulent lifted jet flame in a vitiated coflow. Chin. Sci. Bull. 2007, 52, 2147–2156. [Google Scholar] [CrossRef]
- Hawkes, E.R.; Sankaran, R.; Sutherland, J.C.; Chen, J.H. Scalar mixing in direct numerical simulations of temporally evolving plane jet flames with skeletal CO/H2 kinetics. Proc. Combust. Insititute 2007, 31, 1633–1640. [Google Scholar] [CrossRef]
- Wang, Z.H.; Lv, Y.; He, P.; Zhou, J.H.; Cen, K.F. Fully explicit implementation of direct numerical simulation for a transient near-field methane/air diffusion jet flame. Comput. Fluids 2010, 39, 1381–1389. [Google Scholar] [CrossRef]
- Wang, Z.H.; He, P.; Lv, Y.; Zhou, J.H.; Fan, J.R.; Cen, K.F. Direct Numerical Simulation of Subsonic Round Turbulent Jet. Flow Turbul. Combust 2010, 84, 669–686. [Google Scholar] [CrossRef]
- Chi, C.; Janlga, G.; Abdelsamle, A.; Zahringer, K.; Turanyi, T.; Thevenin, D. DNS Study of the Optimal Chemical Markers for Heat Release in Syngas Flames. Flow Trubulence Combust. 2017, 98, 1117–1132. [Google Scholar] [CrossRef]
- Roy, R.N.; Muto, M.; Kurose, R. Direct numerical simulation of ignition of syngas (H2/CO) mixtures with temperature and composition stratifications relevant to HCCI conditions. Int. J. Hydrog. Energy 2017, 42, 26152–26161. [Google Scholar] [CrossRef]
- Bhide, K.G.; Sreedhara, S. A DNS study on turbulence-chemistry interaction in lean premixed syngas flames. Int. J. Hydrog. Energy 2020, 45, 23615–23623. [Google Scholar] [CrossRef]
- Bird, R.B. Transport phenomena. Appl. Mech. Rev. 2002, 55, R1–R4. [Google Scholar] [CrossRef]
- Barlow, R.S.; Karpetis, A.N.; Frank, J.H.; Chen, J.Y. Scalar profiles an NO formation in laminar opposed-flow partially premixed methane/air flames. Combust. Flame 2001, 127, 2102–2118. [Google Scholar] [CrossRef]
- Najm, H.N.; Wyckoff, P.S.; Knio, O.M. A semi-implicit numerical scheme for reacting flow: I. Stiff chemistry. J. Comput. Phys. 1998, 143, 381–402. [Google Scholar] [CrossRef]
- Jiang, G.S.; Shu, C.W. Efficient Implementation of Weighted ENO Schemes. J. Comput. Phys. 1996, 126, 202–228. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.N.; Byrne, G.D.; Hindmarsh, A.C. VODE: A variable-coefficient ODE solver. SIAM J. Sci. Comput. 1989, 10, 1038–1051. [Google Scholar] [CrossRef] [Green Version]
- Davis, S.G.; Joshi, A.V.; Wang, H.; Egolfopoulos, F. An optimized kinetic model of H2/CO combustion. Proc. Combust. Insititute 2005, 30, 1283–1292. [Google Scholar] [CrossRef]
- Weng, W.; Nilsson, E.; Ehn, A.; Zhu, J.; Zhou, Y.; Wang, Z.; Li, Z.; Aldén, M.; Cen, K. Investigation of formaldehyde enhancement by ozone addition in CH4/air premixed flames. Combust. Flame 2015, 162, 1284–1293. [Google Scholar] [CrossRef]
- Pope, S.B. Turbulent Flows; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Xu, C.; Wang, Z.H.; Weng, W.B.; Wan, K.D.; Whiddon, R.; Wu, A.J. Effects of the Equivalence Ratio and Reynolds Number on Turbulence and Flame Front Interactions by Direct Numerical Simulation. Energy Fuels 2016, 30, 6727–6737. [Google Scholar] [CrossRef]
- Wan, K.D.; Xia, J.; Wang, Z.H.; Wrobel, L.C.; Cen, K.F. Online-CPD-Coupled Large-Eddy Simulation of Pulverized-Coal Pyrolysis in a Hot Turbulent Nitrogen Jet. Combust. Sci. Technol. 2017, 189, 103–131. [Google Scholar] [CrossRef]
- Wan, K.D.; Xia, J.; Vervisch, L.; Liu, Y.Z.; Wang, Z.H.; Cen, K.F. Modelling alkali metal emissions in large-eddy simulation of a preheated pulverised-coal turbulent jet flame using tabulated chemistry. Combust. Theory Model. 2018, 22, 203–236. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wan, K.D.; Li, L.; Wang, Z.H.; Cen, K.F. Verification and Validation of a Low-Mach-Number Large-Eddy Simulation Code against Manufactured Solutions and Experimental Results. Energies 2018, 11, 921. [Google Scholar] [CrossRef] [Green Version]




| Case 1 | Case 2 | |
|---|---|---|
| Inner diameter of central jet nozzle | 2.3 mm (D) | 2.3 mm (D) |
| Thickness of central jet nozzle | 0.5 mm | 0.5 mm |
| Velocity of central jet | 40.0 m/s | 40.0 m/s |
| Component in central jet (volume fraction) | 11.36% H, 11.36% CO, 77.28% Air | 11.40% H, 11.40% CO, 15.30% O, 61.25% N, 6.56% O |
| Temperature of central jet | 300 K | 300 K |
| Velocity of coflow | 0.4 m/s | 0.4 m/s |
| Temperature of coflow | 1500 K | 1500 K |
| Component in coflow (complete combust products) | 12.82% H O, 12.82% CO, 5.49% O, 68.88% N | 12.82% H O, 12.82% CO, 5.49% O, 68.88% N |
| 0.25 | 0.5 | 0.75 | 1.0 | 1.25 | 1.5 | |
|---|---|---|---|---|---|---|
| case 1 | 0.037 | 0.097 | 0.159 | 0.171 | 0.156 | 0.14 |
| case 2 | 0.041 | 0.104 | 0.162 | 0.172 | 0.158 | 0.14 |
| enhanced rate/% | 10.8 | 7.2 | 1.9 | 0.6 | 0.1 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Lin, J.; Wang, Z.; Wan, K.; Sun, S.; Zhou, Z. Three-Dimensional Direct Numerical Simulation of Near-Field Ozone-Enhanced Lean Premixed Syngas Turbulent Jet Flame. Energies 2022, 15, 3945. https://doi.org/10.3390/en15113945
Xu C, Lin J, Wang Z, Wan K, Sun S, Zhou Z. Three-Dimensional Direct Numerical Simulation of Near-Field Ozone-Enhanced Lean Premixed Syngas Turbulent Jet Flame. Energies. 2022; 15(11):3945. https://doi.org/10.3390/en15113945
Chicago/Turabian StyleXu, Cong, Junguang Lin, Zhihua Wang, Kaidi Wan, Shien Sun, and Zhijun Zhou. 2022. "Three-Dimensional Direct Numerical Simulation of Near-Field Ozone-Enhanced Lean Premixed Syngas Turbulent Jet Flame" Energies 15, no. 11: 3945. https://doi.org/10.3390/en15113945
APA StyleXu, C., Lin, J., Wang, Z., Wan, K., Sun, S., & Zhou, Z. (2022). Three-Dimensional Direct Numerical Simulation of Near-Field Ozone-Enhanced Lean Premixed Syngas Turbulent Jet Flame. Energies, 15(11), 3945. https://doi.org/10.3390/en15113945







