Residential Fuel Transition and Fuel Interchangeability in Current Self-Aspirating Combustion Applications: Historical Development and Future Expectations
Abstract
:1. Introduction
2. Residential Fuel Consumption Historical Variation
2.1. Before the 1900s: Transition from Wood to Coal
2.2. From the 19th Century to the 1950s: Coal to Manufactured Gases
2.3. From the 1950s to the 1990s: Manufactured Gas to Natural Gas and LNG
- The gasification of alternative, lower-grade coal.
- Gas production from petroleum instead of coal.
- The introduction of natural gas to enrich manufactured gas.
2.4. Since the 1980s: Renewable Gases
- Reducing carbon emissions, thus combating climate change.
- Extending the gaseous fuel from singular fossil fuel sources to more renewable sources, such as biogas or hydrogen.
- Obtaining energy security by alleviating the influence of natural gas price fluctuation.
2.4.1. Biogas
2.4.2. Renewable Hydrogen
2.5. Summary
3. Domestic Gas Appliances vs. Electric Appliances
3.1. Lighting Market
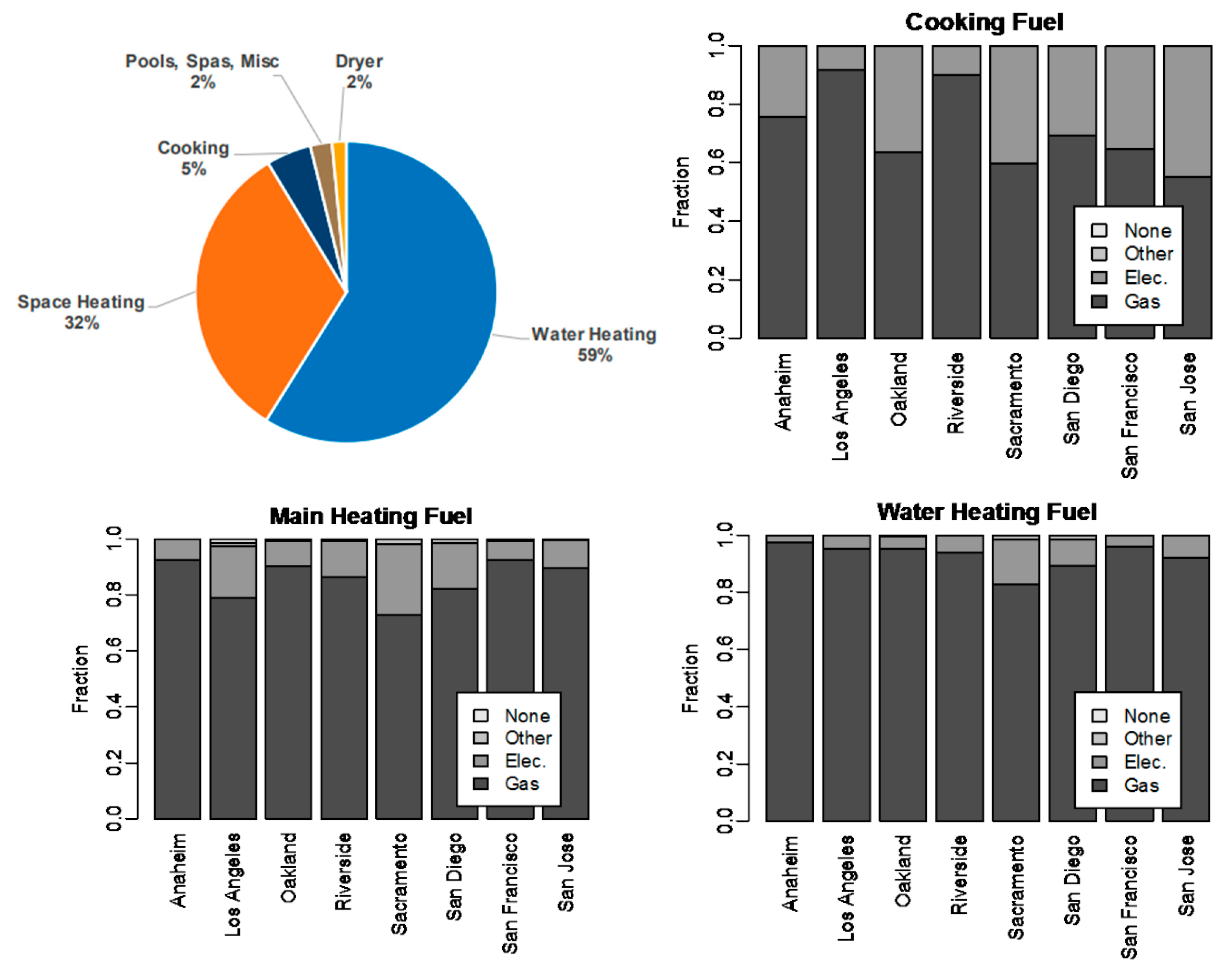
3.2. Cooking, Air/Water Heating, and Other Applications
3.3. Summary
4. Technical Considerations of Adopting Renewable Fuels in Residential Burners
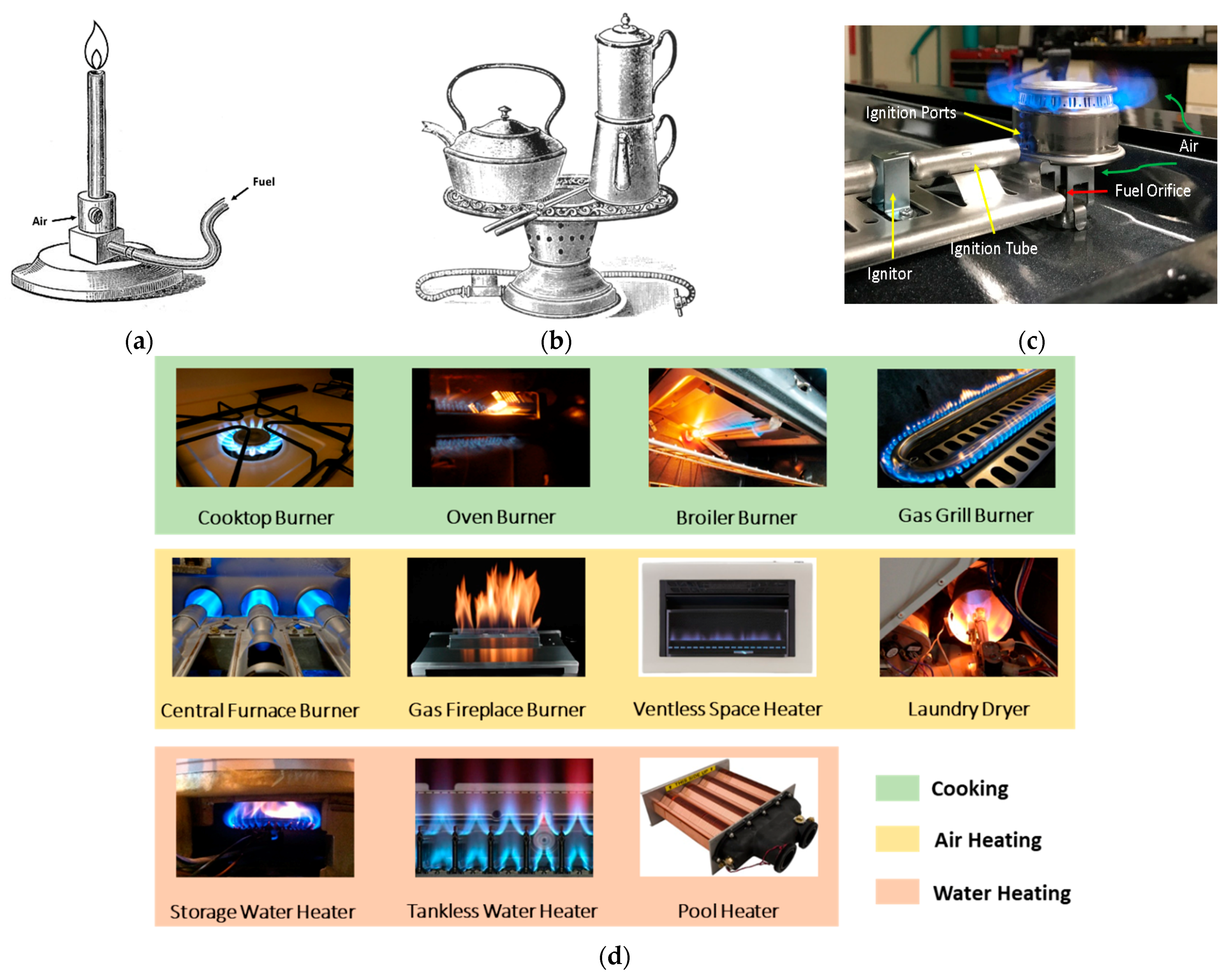
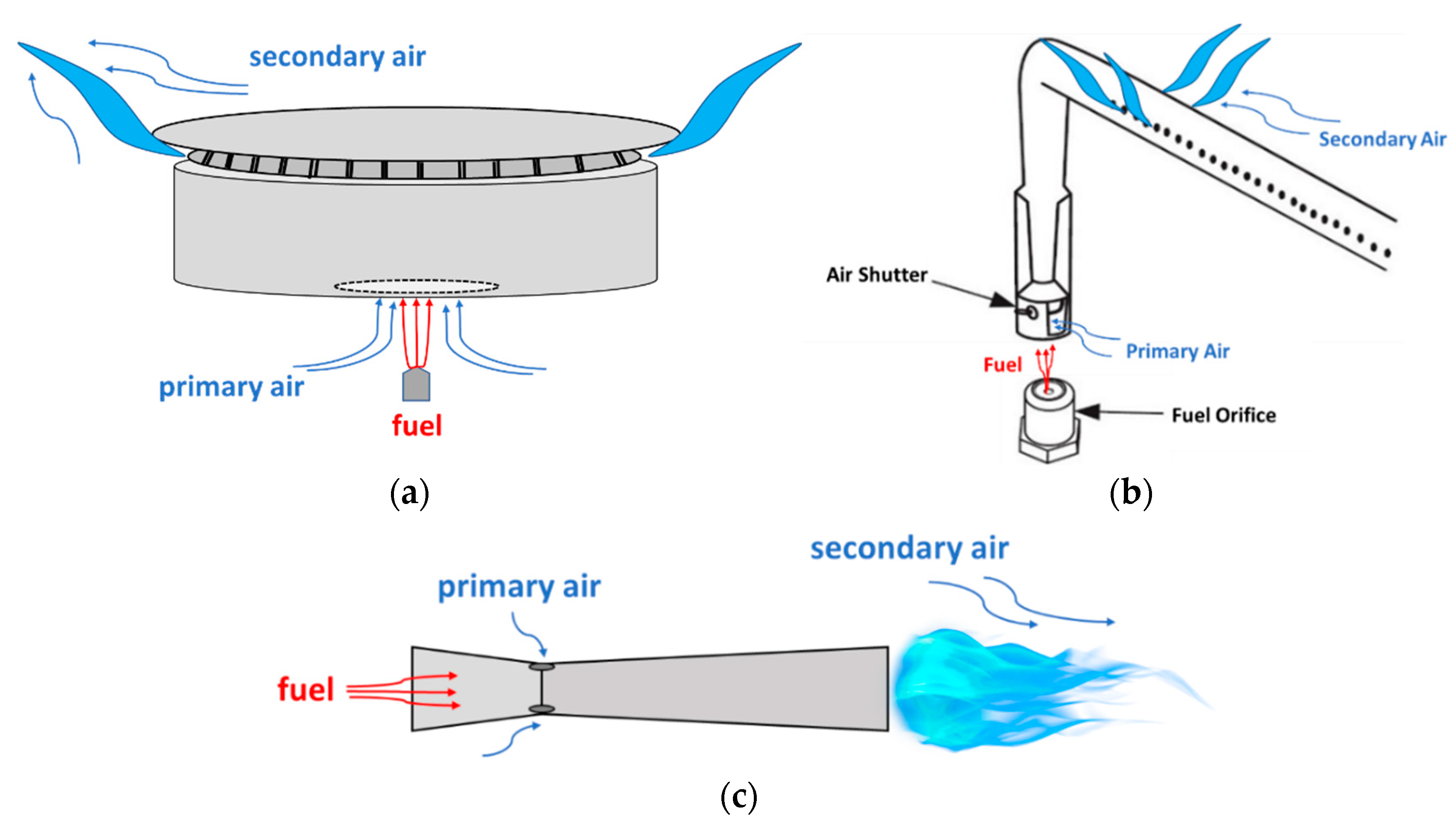
4.1. Working Principles of Residential Burners
4.2. Fuel Properties
4.3. Interchangeability Considerations
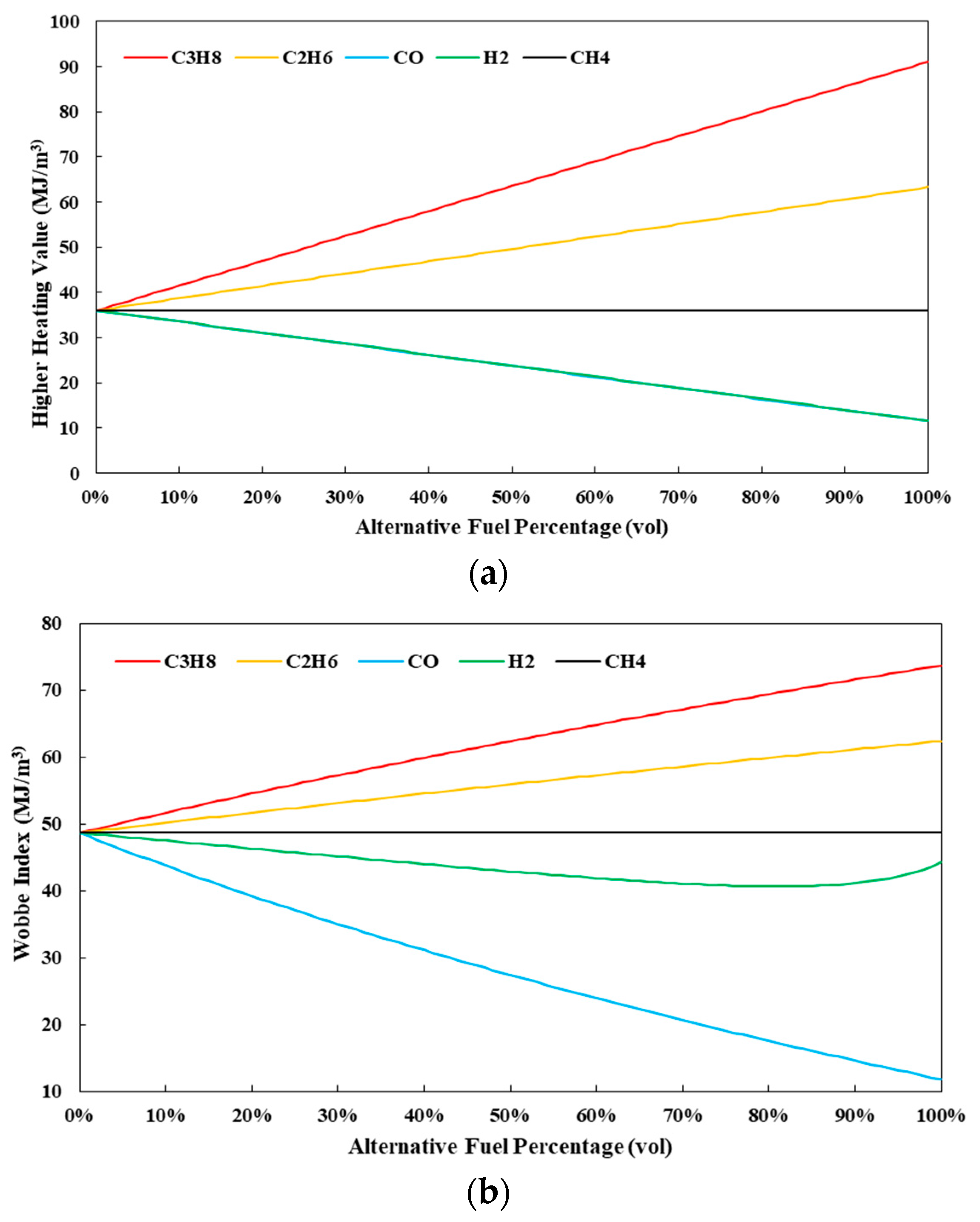
4.3.1. Heating Value and Wobbe Index
4.3.2. AGA Indices
- -
- K: lifting limit constant; a: volume of air theoretically required for complete combustion; f: primary air factor; H: a higher heating value of the fuel (unit: kJ/N·m3); Y: yellow tip coefficient.
- -
- Subscripts a and s: designating adjustment and substitute gases, respectively.
4.3.3. Weaver Indices
- -
- D: specific density of the fuel
- -
- a: volume of air theoretically required for complete combustion
- -
- S: flame speed of the fuel in the air.
- -
- Q: percentage of oxygen in the gas.
- -
- N: the number, in 100 molecules of gas, of the total carbon atoms minus one, except for methane.
- -
- R: the ratio of hydrogen to carbon atoms contained in the fuel.
4.3.4. Other Indices
5. Residential Burner Performance Evaluation
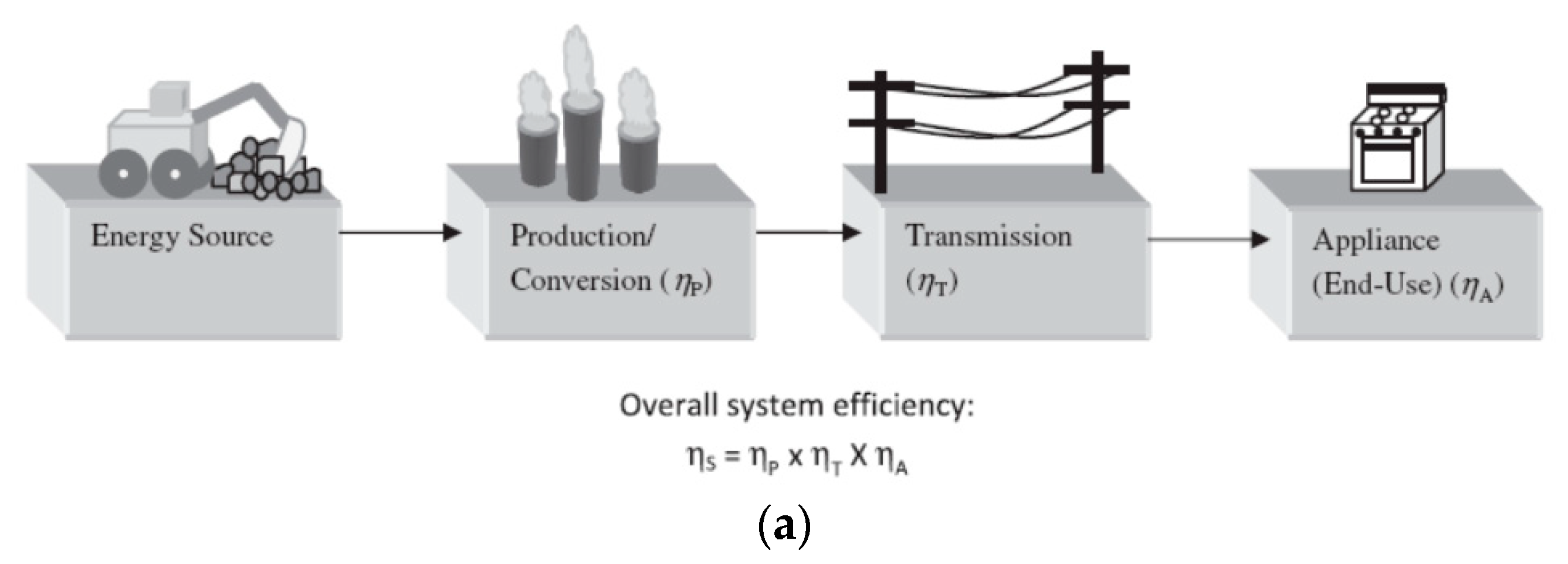
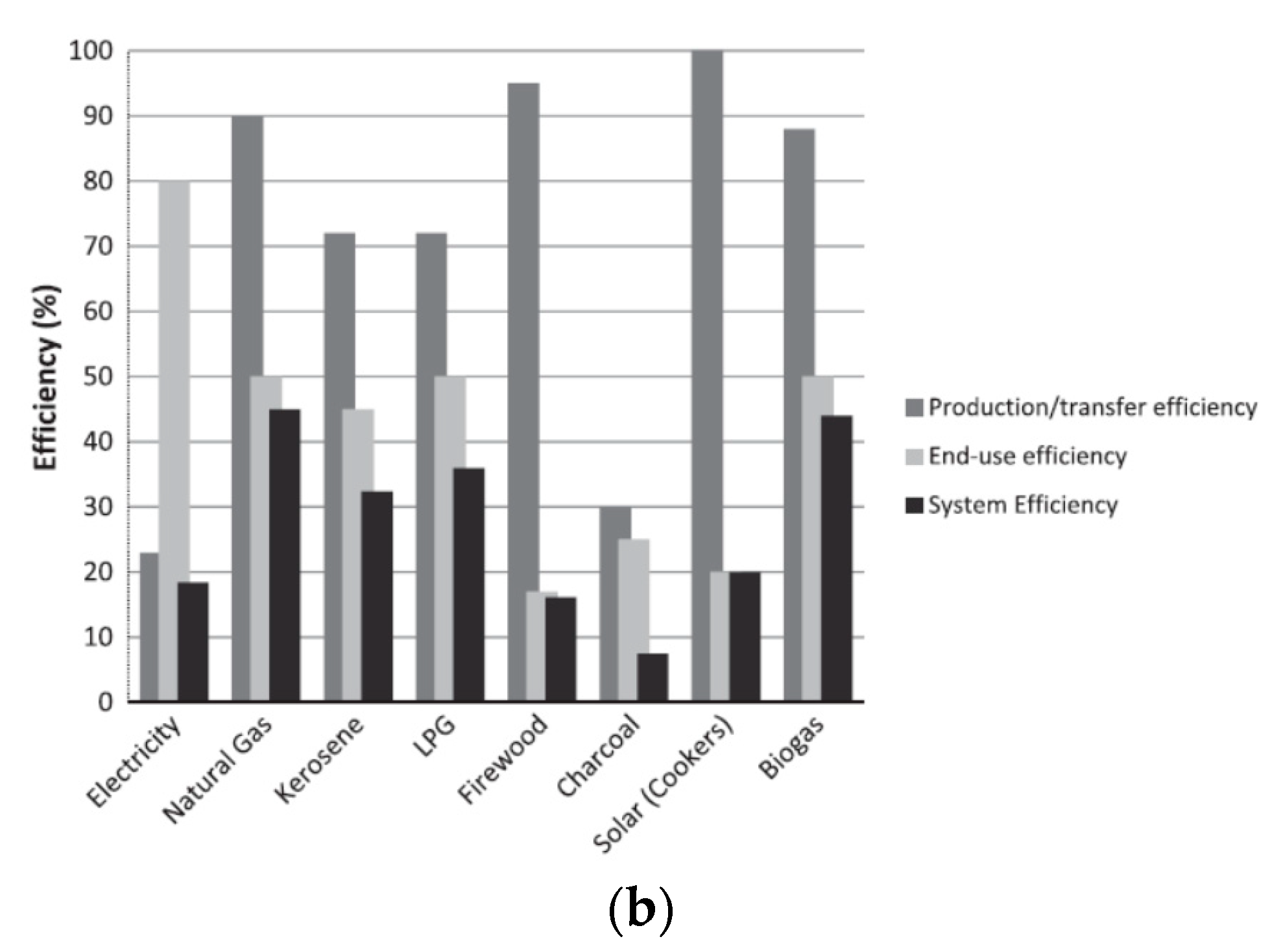
5.1. Efficiency
5.2. Emissions
5.2.1. Residential Appliance Emission Regulations
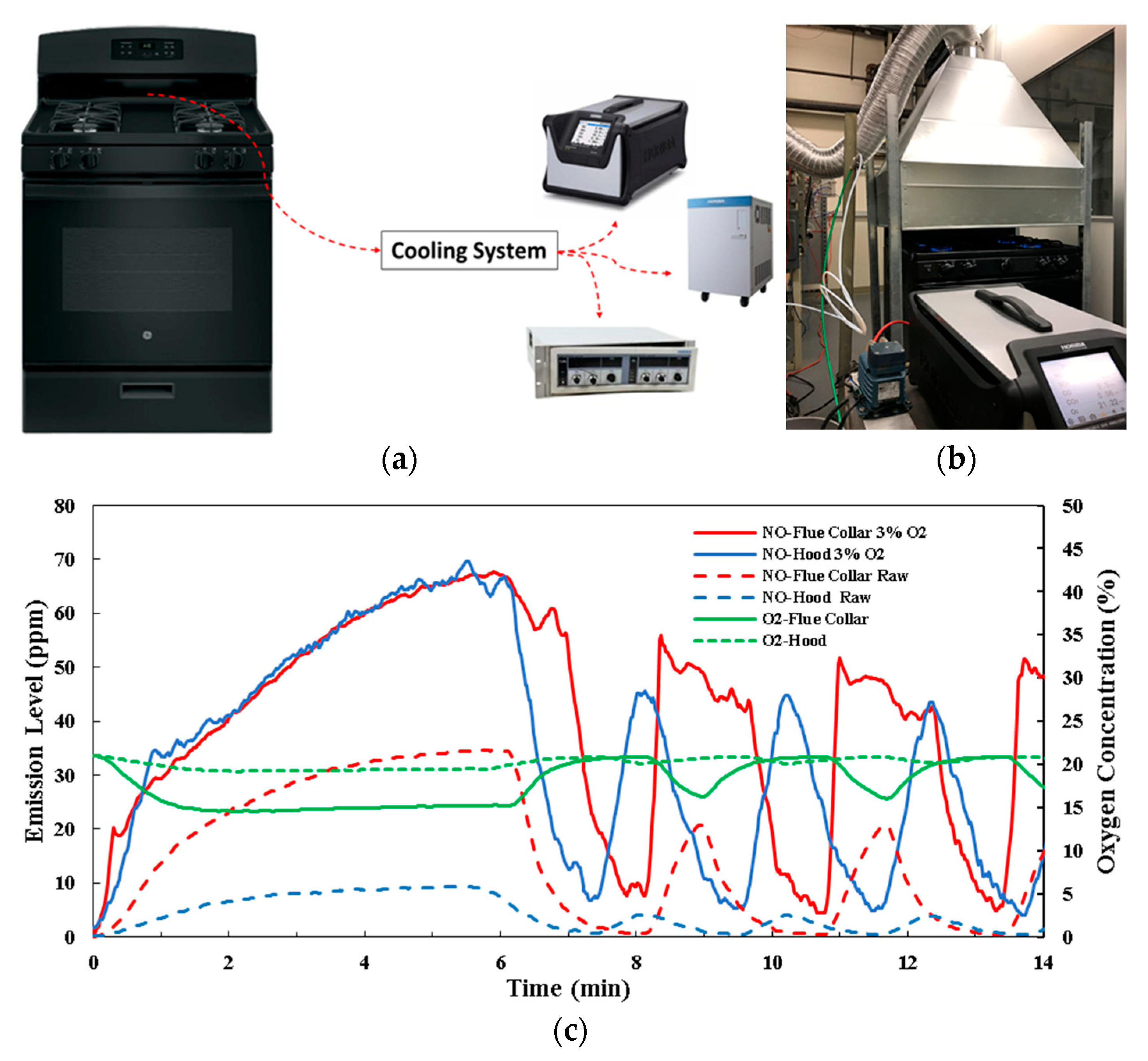
5.2.2. Emission Evaluation Methods’ Conclusion
5.3. Flame Characteristics
5.4. Ignition Performance
5.5. Other Aspects
6. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fouquet, R. The slow search for solutions: Lessons from historical energy transitions by sector and service. Energy Policy 2010, 38, 6586–6596. [Google Scholar] [CrossRef] [Green Version]
- Fouquet, R.; Pearson, P.J. Past and prospective energy transitions: Insights from history. Energy Policy 2012, 50, 1–7. [Google Scholar] [CrossRef]
- Samuelsen, S. Rich burn, quick-mix, lean burn (RQL) combustor. In Gas Turbine Handbook; 2006; Chapter 3.2.1.3, pp. 227–233. Available online: https://www.netl.doe.gov/sites/default/files/gas-turbine-handbook/3-2-1-3.pdf (accessed on 20 March 2022).
- Dunn-Rankin, D. (Ed.) Lean Combustion: Technology and Control; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Cavaliere, A.; de Joannon, M. Mild combustion. Prog. Energy Combust. Sci. 2004, 30, 329–366. [Google Scholar] [CrossRef]
- Forzatti, P. Present status and perspectives in de-NOx SCR catalysis. Appl. Catal. A Gen. 2001, 222, 221–236. [Google Scholar] [CrossRef]
- Jones, H.R. The Application of Combustion Principles to Domestic Gas Burner Design; Taylor & Francis: London, UK, 1989. [Google Scholar]
- Singer, B.C. Natural Gas Variability in California: Environmental Impacts and Device Performance: Literature Review and Evaluation for Residential Appliances: PIER Final Project Report; California Energy Commission: Sacramento, CA, USA, 2007. [Google Scholar]
- Krishnamurti, T.; Schwartz, D.; Davis, A.; Fischhoff, B.; DE Bruin, W.B.; Lave, L.; Wang, J. Preparing for smart grid technologies: A behavioral decision research approach to understanding consumer expectations about smart meters. Energy Policy 2011, 41, 790–797. [Google Scholar] [CrossRef]
- Arabul, F.K.; Arabul, A.Y.; Kumru, C.F.; Boynuegri, A.R. Providing energy management of a fuel cell–battery–wind turbine–solar panel hybrid off grid smart home system. Int. J. Hydrogen Energy 2017, 42, 26906–26913. [Google Scholar] [CrossRef]
- Wei, M.; Nelson, J.H.; Greenblatt, J.B.; Mileva, A.; Johnston, J.; Ting, M.; Yang, C.; Jones, C.; E McMahon, J.; Kammen, D.M. Deep carbon reductions in California require electrification and integration across economic sectors. Environ. Res. Lett. 2013, 8, 014038. [Google Scholar] [CrossRef]
- Fowler, R.; Elmhirst, O.; Richards, J. Electrification in the United Kingdom: A Case Study Based on Future Energy Scenarios. IEEE Power Energy Mag. 2018, 16, 48–57. [Google Scholar] [CrossRef]
- Weiland, P. Biogas production: Current state and perspectives. Appl. Microbiol. Biotechnol. 2009, 85, 849–860. [Google Scholar] [CrossRef]
- Urban, W. Biomethane injection into natural gas networks. In The Biogas Handbook; Woodhead Publishing: Wolfgang, Germany, 2013; pp. 378–403. [Google Scholar]
- Turner, J.; Sverdrup, G.; Mann, M.K.; Maness, P.C.; Kroposki, B.; Ghirardi, M.; Evans, R.J.; Blake, D. Renewable hydrogen production. Int. J. Energy Res. 2008, 32, 379–407. [Google Scholar] [CrossRef] [Green Version]
- Hosseini, S.E.; Wahid, M.A. Hydrogen production from renewable and sustainable energy resources: Promising green energy carrier for clean development. Renew. Sustain. Energy Rev. 2016, 57, 850–866. [Google Scholar] [CrossRef]
- O’Connor, P.; Cleveland, C. US energy transitions 1780–2010. Energies 2014, 7, 7955–7993. [Google Scholar] [CrossRef] [Green Version]
- Smil, V. Energy Transitions: History, Requirements, Prospects; ABC-CLIO: Santa Barbara, CA, USA, 2010; Available online: https://www.abc-clio.com/products/a2598c/ (accessed on 20 March 2022).
- Humphrey, W.S.; Stanislaw, J. Economic growth and energy consumption in the UK, 1700–1975. Energy Policy 1979, 7, 29–42. [Google Scholar] [CrossRef]
- Edomah, N.; Foulds, C.; Jones, A. Energy Transitions in Nigeria: The Evolution of Energy Infrastructure Provision (1800–2015). Energies 2016, 9, 484. [Google Scholar] [CrossRef] [Green Version]
- Jones, C. The Carbon-Consuming Home: Residential Markets and Energy Transitions. Enterp. Soc. 2011, 12, 790–823. [Google Scholar] [CrossRef] [PubMed]
- Nef, J.U. An Early Energy Crisis and its Consequences. Sci. Am. 1977, 237, 140–151. [Google Scholar] [CrossRef]
- Kernot, C. The Coal Industry; Elsevier: Cambridge, UK, 2000. [Google Scholar]
- Lewis, W.D.; Binder, F.M. Coal Age Empire: Pennsylvania Coal and Its Utilization to 1860. Technol. Cult. 1976, 17, 140. [Google Scholar] [CrossRef]
- Bowers, B.; Anastas, P. Lengthening the Day: A History of Lighting Technology; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Rose, M.H. Cities of Light and Heat: Domesticating Gas and Electricity in Urban America; Penn State Press: University Park, TX, USA, 1995. [Google Scholar]
- Gibbs, L.; Anderson, B.; Barnes, K.; Engeler, G.; Freel, J.; Horn, J.; Ingham, M.; Kohler, D.; Lesnini, D.; MacArthur, R.; et al. Motor Gasolines Technical Review; Chevron Products Company: San Ramon, CA, USA, 2009. [Google Scholar]
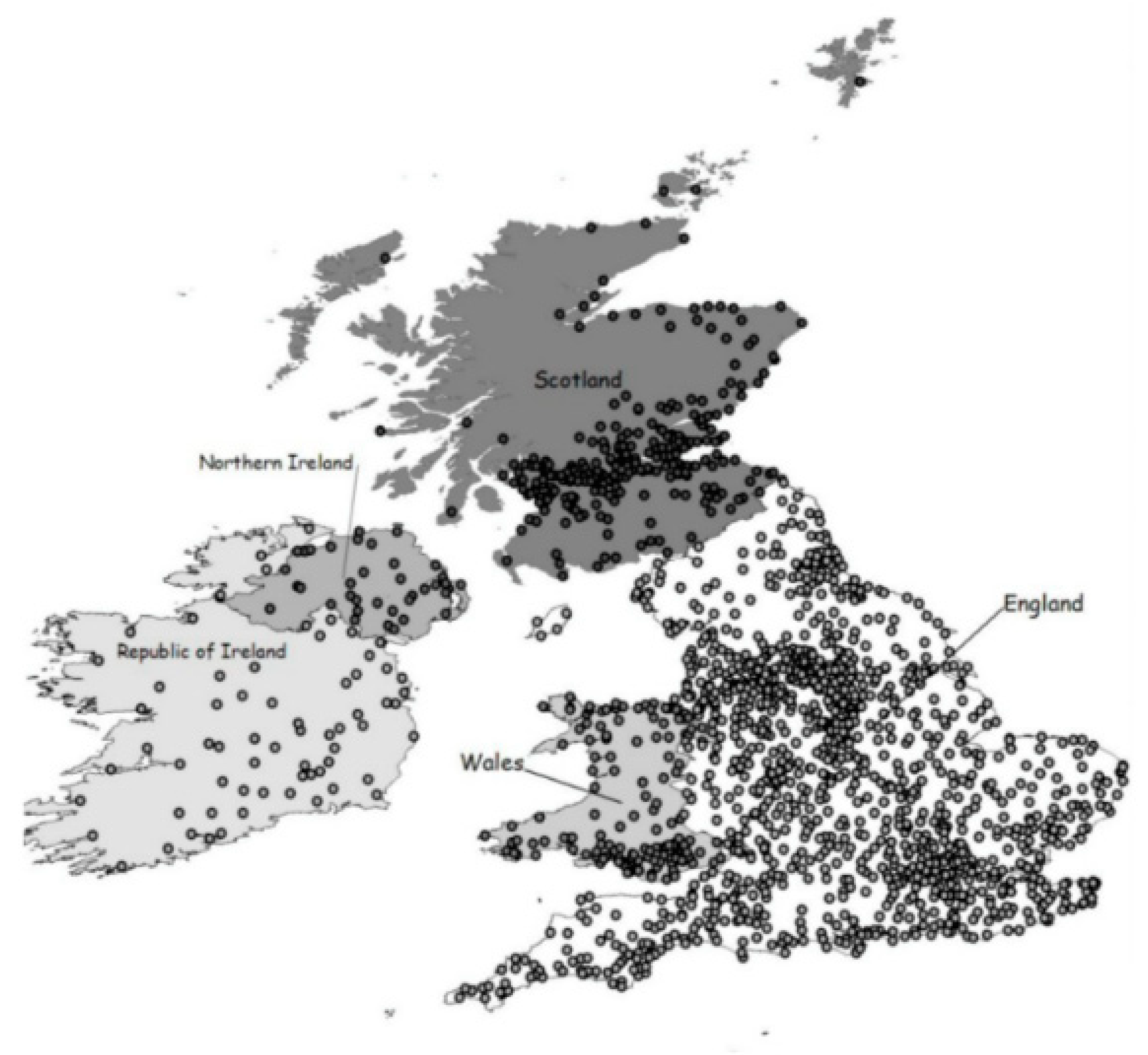
- Outhwaite, R.B.; Houston, R.A. The Population History of Britain and Ireland, 1500–1750. Econ. Hist. Rev. 1993, 46, 821. [Google Scholar] [CrossRef]
- Fouquet, R. Heat, Power and Light: Revolutions in Energy Services; Edward Elgar Publishing: Cheltenham, UK, 2008. [Google Scholar]
- Wilson, C. England’s Apprenticeship, 1603–1763; Longman Group: London, UK, 1967. [Google Scholar]
- Evelyn, J. Fumifugium; Rota: Exeter, UK, 1772. [Google Scholar]
- Brimblecombe, P. The Big Smoke (Routledge Revivals): A History of Air Pollution in London since Medieval Times; Routledge: London, UK, 2012. [Google Scholar]
- Price, W.H. The English Patents of Monopoly; Harvard University Press: Cambridge, MA, USA, 1920. [Google Scholar]
- Peter, S.R. The Subterranean Forest; The White Horse Press: Cambridge, UK, 2001. [Google Scholar]
- Hatcher, J. The History of the British Coal Industry; Clarendon Press: Oxford, USA, 1993. [Google Scholar]
- Nef, J.U. The Rise of the British Coal Industry; Routledge: London, UK, 2013. [Google Scholar]
- Brimblecombe, P. Attitudes and Responses towards Air Pollution in Medieval England. J. Air Pollut. Control Assoc. 1976, 26, 941–945. [Google Scholar] [CrossRef]
- Jenner, M. The politics of London air John Evelyn’s Fumifugium and the Restoration. Hist. J. 1995, 38, 535–551. [Google Scholar] [CrossRef] [Green Version]
- O’Connor Peter, A. Aspects of Energy Transitions: History and Determinants. Ph.D. Thesis, Boston University, Boston, MA, USA, 2014. [Google Scholar]
- Höök, M.; Aleklett, K. Historical trends in American coal production and a possible future outlook. Int. J. Coal Geol. 2009, 78, 201–216. [Google Scholar] [CrossRef]
- Taylor, G.R. The Transportation Revolution, 1815–1860; Routledge: London, UK, 2015. [Google Scholar]
- Powell, H.B. Philadelphia’s First Fuel Crisis: Jacob Cist and the Developing Market for Pennsylvania Anthracite; Pennsylvania State University Press: State College, PA, USA, 1978. [Google Scholar]
- Taylor, R.C. Statistics of Coal; JW Moore: Archbald, PA, USA, 1848. [Google Scholar]
- Brewer, P.J. From Fireplace to Cookstove: Technology and the Domestic Ideal in America. J. Early Repub. 2001, 21, 566. [Google Scholar] [CrossRef]
- Adams, S.P. Warming the poor and growing consumers: Fuel philanthropy in the early republic’s urban north. J. Am. Hist. 2008, 95, 69–94. [Google Scholar] [CrossRef]
- Auzanneau, M. Oil, Power, and War: A Dark History; Chelsea Green Publishing: White River Junction, VT, USA, 2018. [Google Scholar]
- Van Vactor, S.A. Historical Perspective on Energy Transitions (May 14, 2018); USAEE Working Paper; SSRN: Online, 2018. [Google Scholar]
- Williamson, H.F.; Daum, A.R. The American Petroleum Industry: 1859–1899. The Age of Illumination; Northwestern University Press: Evanston, IL, USA, 1959. [Google Scholar]
- Gordon, R.J. The Rise and Fall of American Growth: The US Standard of Living since the Civil War; Princeton University Press: Princeton, NJ, USA, 2017. [Google Scholar]
- Peebles, M.W. Evolution of the Gas Industry; Macmillan International Higher Education: London, UK, 1980. [Google Scholar]
- Clendinning, A. Gas Cooker. Vic. Rev. 2008, 34, 56–62. [Google Scholar] [CrossRef]
- Jensen, W.B. The Origin of the Bunsen Burner. J. Chem. Educ. 2005, 82, 518. [Google Scholar] [CrossRef]
- Baumgartner, E. Carl Auer von Welsbach: A pioneer in the industrial application of rare earths. In Episodes from the History of the Rare Earth Elements; Springer: Dordrecht, The Netherlands, 1996; pp. 113–129. [Google Scholar]
- Webber, W.H. Town Gas and Its Uses for the Production of Light, Heat, and Motive Power; A. Constable & Company, Limited: London, UK, 1907. [Google Scholar]
- Hatheway, A.W.; Doyle, B.C. Technical History of the Town Gas Plants of the British Isles. In Proceedings of the International Association of Engineering Geologists Conference; 2006; Volume 564. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.472.7729&rep=rep1&type=pdf (accessed on 20 March 2022).
- Newsome, D.S. The water-gas shift reaction. Catal. Rev. Sci. Eng. 1980, 21, 275–318. [Google Scholar] [CrossRef]
- Murphy, B.L.; Sparacio, T.; Shields, W.J. Manufactured gas plants—Processes, historical development, and key issues in insur-ance coverage disputes. Environ. Forensics 2005, 6, 161–173. [Google Scholar] [CrossRef]
- Hamper, M.J. Manufactured Gas History and Processes. Environ. Forensics 2006, 7, 55–64. [Google Scholar] [CrossRef]
- Ray, H.S. Energy in Minerals and Metallurgical Industries; Allied Publishers: New Delhi, India, 2005. [Google Scholar]
- Fry, J.F. Town gas explosions in dwellings. Fire Saf. Sci. 1970, 813, 1. [Google Scholar]
- Ernst, A.; Zibrak, J.D. Carbon monoxide poisoning. N. Engl. J. Med. 1998, 339, 1603–1608. [Google Scholar] [CrossRef] [Green Version]
- Chalke, H.D.; Dewhurst, J.R.; Ward, C.W. Loss of Sense of Smell in Old People. A Possible Contributory Factor in Accidental Poisoning from Town Gas. Public Health 1958, 72, 223–230. [Google Scholar] [CrossRef]
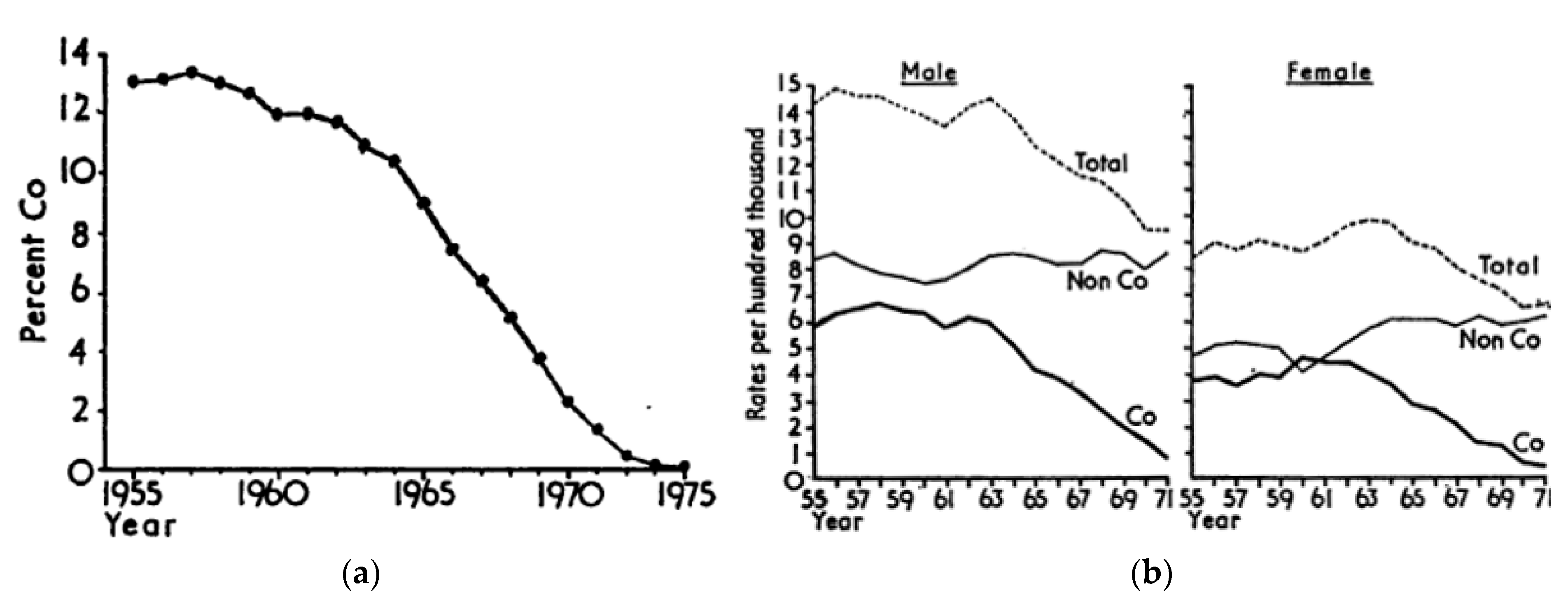
- Clarke, R.V.; Mayhew, P. The British Gas Suicide Story and Its Criminological Implications. Crime Justice 1988, 10, 79–116. [Google Scholar] [CrossRef]
- Kreitman, N. The coal gas story. United Kingdom suicide rates, 1960–1971. J. Epidemiol. Community Health 1976, 30, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arapostathis, S.; Carlsson-Hyslop, A.; Pearson, P.J.G.; Thornton, J.; Gradillas, M.; Laczay, S.; Wallis, S. Governing transitions: Cases and insights from two periods in the history of the UK gas industry. Energy Policy 2013, 52, 25–44. [Google Scholar] [CrossRef]
- Williams, M. A History of the British Gas Industry; Oxford University Press: Oxford, UK, 1981. [Google Scholar]
- Tiratsoo, E.N. Natural Gas; Scientific Press: Beaconsfield, UK, 1972. [Google Scholar]
- Elliott, C. The History of Natural Gas Conversion in Great Britain; Cambridge Information and Research Services: Cambridge, UK, 1980. [Google Scholar]
- Falkus, M.E. Always under Pressure: A History of North Thames Gas since 1949; Palgrave Macmillan: London, UK, 1988. [Google Scholar]
- Falkus, M. The Canvey Experiment and the Beginnings of Conversion. In Always under Pressure; Palgrave Macmillan: London, UK, 1988; pp. 89–122. [Google Scholar]
- Morton, F. Report of the Inquiry into the Safety of Natural Gas as a Fuel; HM Stationery Office: London, UK, 1970. [Google Scholar]
- Lash, G.G.; Lash, E.P. Early History of the Natural Gas Industry, Fredonia, New York. In Proceedings of the AAPG Annual Conventional and Exhibition, Houston, TX, USA, 6–9 April 2014. [Google Scholar]
- Tarr, J.A.; Clay, K.; Clay, J.A.T. Boom and Bust in Pittsburgh Natural Gas History: Development, Policy, and Environmental Effects, 1878–1920. Pa. Mag. Hist. Biogr. 2015, 139, 323. [Google Scholar] [CrossRef]
- Troxel, C.E. Long-distance natural gas pipe lines. J. Land Public Util. Econ. 1936, 12, 344–354. [Google Scholar] [CrossRef]
- Liu, H. Pipeline Engineering; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Rao, A. Sustainable Energy Conversion for Electricity and Coproducts: Principles, Technologies, and Equipment; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Southern California Gas Company. Rule No. 30: Transportation of Customer-Owned Gas; Southern California Gas Company: Los Angeles, CA, USA, 2019. [Google Scholar]
- American Gas Association. Report #4A Natural Gas Contract Measurement and Quality Clauses; American Gas Association: Washington, DC, USA, 2009. [Google Scholar]
- Berry, W.M.; Brumbaugh, I.V.; Moulton, G.F.; Shawn, G.B. Design of Atmospheric Gas Burners; US Government Printing Office: Washington, DC, USA, 1921. [Google Scholar]
- Brumbaugh, I.V.; Jones, G.W. Carbon Monoxide in the Products of Combustion from Natural Gas Burners; US Government Printing Office: Washington, DC, USA, 1922. [Google Scholar]
- Eiserman, J.; Weaver, E.; Smith, F. A method for determining the most favorable design of gas burners. Bur. Stand. J. Res. 1932, 8, 669. [Google Scholar] [CrossRef]
- Weaver, E. Formulas and graphs for representing the interchangeability of fuel gases. J. Res. Natl. Inst. Stand. Technol. 1951, 46, 213. [Google Scholar] [CrossRef]
- Protocol, K. United Nations Framework Convention on Climate Change; Kyoto Protocol: Kyoto, Japan, 1997. [Google Scholar]
- DNV. Hydrogen and Other Routes to Decarbonization of the Gas Network; FCH2 Conference: Birmingham, UK, 2018. [Google Scholar]
- Hobson, P.N. Production of biogas from agricultural wastes. In Advances in Agricultural Microbiology; Butterworth Scientific: London, UK, 1982; pp. 523–550. [Google Scholar]
- Osman, G.A.; El-Tinay, A.H.; Mohamed, E.F. Biogas production from agricultural wastes. J. Food Technol. 2006, 4, 37–39. [Google Scholar]
- Mackuľak, T.; Prousek, J.; Švorc, Ľ.; Drtil, M. Increase of biogas production from pretreated hay and leaves using wood-rotting fungi. Chem. Pap. 2012, 66, 649–653. [Google Scholar] [CrossRef]
- Gardner, N.; Probert, S. Forecasting landfill-gas yields. Appl. Energy 1993, 44, 131–163. [Google Scholar] [CrossRef]
- Bove, R.; Lunghi, P. Electric power generation from landfill gas using traditional and innovative technologies. Energy Convers. Manag. 2006, 47, 1391–1401. [Google Scholar] [CrossRef]
- Kamalan, H.; Sabour, M.; Shariatmadari, N. A review on available landfill gas models. J. Environ. Sci. Technol. 2011, 4, 79–92. [Google Scholar] [CrossRef]
- Midilli, A.; Dogru, M.; Howarth, C.R.; Ling, M.J.; Ayhan, T. Combustible gas production from sewage sludge with a downdraft gasifier. Energy Convers. Manag. 2001, 42, 157–172. [Google Scholar] [CrossRef]
- Elango, D.; Pulikesi, M.; Baskaralingam, P.; Ramamurthi, V.; Sivanesan, S. Production of biogas from municipal solid waste with domestic sewage. J. Hazard. Mater. 2007, 141, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Acelas, N.Y.; López, D.P.; Brilman, D.W.; Kersten, S.R.; Kootstra, A.M. Supercritical water gasification of sewage sludge: Gas production and phosphorus recovery. Bioresour. Technol. 2014, 174, 167–175. [Google Scholar] [CrossRef]
- Deublein, D.; Steinhauser, A. Biogas from Waste and Renewable Resources: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Wellinger, A.; Murphy, J.D.; Baxter, D. (Eds.) The Biogas Handbook: Science, Production and Applications; Woodhead Publishing: Sawston, UK, 2013. [Google Scholar]
- Scarlat, N.; Dallemand, J.-F.; Fahl, F. Biogas: Developments and perspectives in Europe. Renew. Energy 2018, 129, 457–472. [Google Scholar] [CrossRef]
- Gamba, S.; Pellegrini, L.A. Biogas upgrading: Analysis and comparison between water and chemical scrubbings. Chem. Eng. 2013, 32, 1273–1278. [Google Scholar]
- Morin, P.; Marcos, B.; Moresoli, C.; Laflamme, C.B. Economic and environmental assessment on the energetic valorization of organic material for a municipality in Quebec, Canada. Appl. Energy 2010, 87, 275–283. [Google Scholar] [CrossRef]
- Fujino, J.; Morita, A.; Matsuoka, Y.; Sawayama, S. Vision for utilization of livestock residue as bioenergy resource in Japan. Biomass-Bioenergy 2005, 29, 367–374. [Google Scholar] [CrossRef]
- Persson, M.; Jönsson, O.; Wellinger, A. Biogas upgrading to vehicle fuel standards and grid injection. In IEA Bioenergy Task; IEA: Paris, France, 2006; Volume 37, pp. 1–34. [Google Scholar]
- Available online: www.iea-biogas.net (accessed on 20 March 2022).
- Kim, S.H.; Kumar, G.; Chen, W.H.; Khanal, S.K. Renewable hydrogen production from biomass and wastes (ReBioH2-2020). Bioresour. Technol. 2021, 331, 125024. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, R.; Sahu, S.; James, O.O.; Maity, S. A review on development of industrial processes and emerging techniques for production of hydrogen from renewable and sustainable sources. Renew. Sustain. Energy Rev. 2013, 23, 443–462. [Google Scholar] [CrossRef]
- Haldane, J.B.; Daedalus, O. Science and the Future. A Paper Read to the Heretics, Cambridge, on February 4th. 1923; Kegan Paul: Whitelackington, UK, 1924. [Google Scholar]
- Das, D.; Veziroǧlu, T.N. Hydrogen production by biological processes: A survey of literature. Int. J. Hydrogen Energy 2001, 26, 13–28. [Google Scholar] [CrossRef]
- Muradov, N.Z.; Veziroğlu, T.N. “Green” path from fossil-based to hydrogen economy: An overview of carbon-neutral technologies. Int. J. Hydrogen Energy 2008, 33, 6804–6839. [Google Scholar] [CrossRef]
- Sorgulu, F.; Dincer, I. Cost evaluation of two potential nuclear power plants for hydrogen production. Int. J. Hydrogen Energy 2018, 43, 10522–10529. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef] [Green Version]
- Quarton, C.J.; Samsatli, S. Power-to-gas for injection into the gas grid: What can we learn from real-life projects, economic assessments and systems modelling? Renew. Sustain. Energy Rev. 2018, 98, 302–316. [Google Scholar] [CrossRef]
- Pangborn, J.; Scott, M.; Sharer, J. Technical prospects for commercial and residential distribution and utilization of hydrogen. Int. J. Hydrogen Energy 1977, 2, 431–445. [Google Scholar] [CrossRef]
- De Vries, H.; Florisson, O.; Tiekstra, G.C. Safe Operation of Natural Gas Appliances Fueled with Hydrogen/Natural Gas Mixtures (Progress Obtained in the Naturalhy-Project). In Proceedings of the International Conference on Hydrogen Safety, San Sebastian, Spain, 11–13 September 2007. [Google Scholar]
- Van Essen, V.M.; De Vries, H.; Levinsky, H.B. Possibilities for admixing gasification gases: Combustion aspects in domestic natural gas appliances in The Netherlands. In Proceedings of the International Gas Union Research Conference, Seoul, Korea, 19–21 October 2011. [Google Scholar]
- De Vries, H.; Mokhov, A.V.; Levinsky, H.B. The impact of natural gas/hydrogen mixtures on the performance of end-use equipment: Interchangeability analysis for domestic appliances. Appl. Energy 2017, 208, 1007–1019. [Google Scholar] [CrossRef]
- De Vries, H.; Levinsky, H.B. Flashback, burning velocities and hydrogen admixture: Domestic appliance approval, gas regulation and appliance development. Appl. Energy 2019, 259, 114116. [Google Scholar] [CrossRef]
- Jones, D.R.; Al-Masry, W.A.; Dunnill, C.W. Hydrogen-enriched natural gas as a domestic fuel: An analysis based on flash-back and blow-off limits for domestic natural gas appliances within the UK. Sustain. Energy Fuels 2018, 2, 710–723. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Choudhury, S.; McDonell, V. Influence of Renewable Gas Addition to Natural Gas on the Combustion Performance of Cooktop Burners. In Proceedings of the ASME 2018 International Mechanical Engineering Congress and Exposition, Pittsburgh, PA, USA, 9–15 November 2018. [Google Scholar]
- Zhao, Y.; McDonell, V.; Samuelsen, S. Influence of hydrogen addition to pipeline natural gas on the combustion performance of a cooktop burner. Int. J. Hydrogen Energy 2019, 44, 12239–12253. [Google Scholar] [CrossRef]
- Zhao, Y.; Leytan, K.N.S.; McDonell, V.; Samuelsen, S. Investigation of visible light emission from hydrogen-air research flames. Int. J. Hydrogen Energy 2019, 44, 22347–22354. [Google Scholar] [CrossRef]
- Zhao, Y.; McDonell, V.; Samuelsen, S. Experimental assessment of the combustion performance of an oven burner operated on pipeline natural gas mixed with hydrogen. Int. J. Hydrogen Energy 2019, 44, 26049–26062. [Google Scholar] [CrossRef]
- Zhao, Y.; Morales, D.; McDonell, V. Influence of Blending Hydrogen and Biogas Into Natural Gas on the Combustion Performance of a Tankless Water Heater. In Proceedings of the ASME 2019 International Mechanical Engineering Congress and Exposition, Salt Lake City, UT, USA, 11–14 November 2019. [Google Scholar]
- Choudhury, S.; McDonell, V.G.; Samuelsen, S. Combustion performance of low-NOx and conventional storage water heaters operated on hydrogen enriched natural gas. Int. J. Hydrogen Energy 2019, 45, 2405–2417. [Google Scholar] [CrossRef]
- Zhao, Y.; McDonell, V.; Samuelsen, S. Assessment of the combustion performance of a room furnace operating on pipeline natural gas mixed with simulated biogas or hydrogen. Int. J. Hydrogen Energy 2020, 45, 11368–11379. [Google Scholar] [CrossRef]
- Gimeno-Escobedo, E.; Cubero, A.; Ochoa, J.S.; Fueyo, N. A reduced mechanism for the prediction of methane-hydrogen flames in cooktop burners. Int. J. Hydrogen Energy 2019, 44, 27123–27140. [Google Scholar] [CrossRef]
- E.ON. Hydrogen Levels in German Gas Distribution System to be Raised to 20 Percent for the First Time; E.ON: Essen, Germany, 2019. [Google Scholar]
- Lewis, J. Short-run and long-run effects of household electrification. In Economic History Workshop; Queen’s University: Kingston, ON, Canada, 2014. [Google Scholar]
- Deason, J.; Borgeson, M. Electrification of Buildings: Potential, Challenges, and Outlook. Curr. Sustain./Renew. Energy Rep. 2019, 6, 131–139. [Google Scholar] [CrossRef]
- Brittain, J.E. The International Diffusion of Electrical Power Technology, 1870–1920. J. Econ. Hist. 1974, 34, 108–121. [Google Scholar] [CrossRef]
- Bourne, R. The beginnings of electric street lighting in the City of London. Eng. Sci. Educ. J. 1996, 5, 81–88. [Google Scholar] [CrossRef]
- Gutmann, V. More light: A short historical sketch of Carl Auer von Welsbach. J. Chem. Educ. 1970, 47, 209. [Google Scholar] [CrossRef]
- Moran, M.E. The light bulb, cystoscopy, and Thomas Alva Edison. J. Endourol. 2010, 24, 1395–1397. [Google Scholar] [CrossRef] [PubMed]
- Edison, T.A. Electric Lamp. U.S. Patent 223,898, 27 January 1880. [Google Scholar]
- Hughes, T.P. British Electrical Industry Lag: 1882–1888. Technol. Cult. 1962, 3, 27. [Google Scholar] [CrossRef]
- Shiman, D.R. Explaining the collapse of the British electrical supply industry in the 1880s: Gas versus electric lighting prices. Bus. Econ. Hist. 1993, 21, 318–327. [Google Scholar]
- Fouquet, R.; Pearson, P.J.G. Seven Centuries of Energy Services: The Price and Use of Light in the United Kingdom (1300–2000). Energy J. 2006, 27, 139–177. [Google Scholar] [CrossRef]
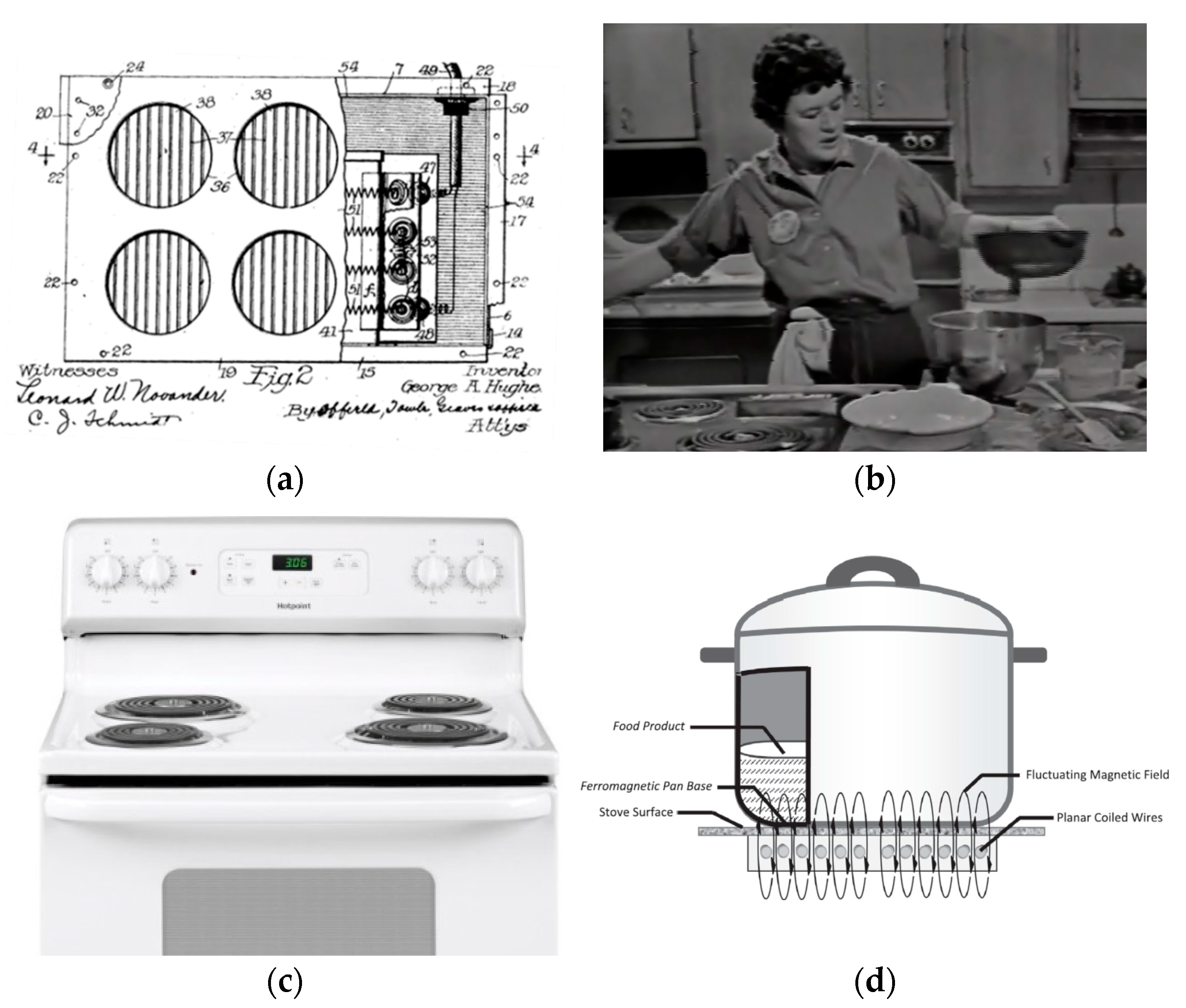
- Hughes, G.A. Electrical Stove. U.S. Patent 1,169,827, 1 February 1916. [Google Scholar]
- Bowden, S.; Offer, A. Household appliances and the use of time: The United States and Britain since the 1920s. Econ. Hist. Rev. 1994, 725–748. [Google Scholar] [CrossRef]
- Ronald, W. The Electrical Workers: A History of Labor at General Electric and Westinghouse, 1923–1960; University of Illinois Press: Champaign, IL, USA, 1987. [Google Scholar]
- Morton, D.L., Jr. Reviewing the history of electric power and electrification. Endeavour 2002, 26, 60–63. [Google Scholar] [CrossRef]
- WGBH. Picture Clipped form The French Chef by Julia Child—The Potato Show; WGBH: Boston, MA, USA, 1963. [Google Scholar]
- Hager, T.J.; Morawicki, R. Energy consumption during cooking in the residential sector of developed nations: A review. Food Policy 2013, 40, 54–63. [Google Scholar] [CrossRef]
- DOE. U.S. Department of Energy: Office of Energy Efficiency and Renewable Energy. Technical Support Document: Energy Efficiency Program for Consumer Products and Commercial and Industrial Equipment: Residential Dishwashers, Dehumidifiers, and Cooking Products, and Commercial Clothes Washers (Chapters 4–6; Appendix 6A); DOE: Washington, DC, USA, 2008. [Google Scholar]
- Breeze, P. Gas-Turbine Power Generation, Chapter 7: Combined Cycle Power Plants; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- United States Environmental Protection Agency. Combined Heat and Power Partnership. Available online: https://www.epa.gov/chp/chp-benefits (accessed on 20 March 2022).
- Han, H.J.; Jeon, Y.I.; Lim, S.H.; Kim, W.W.; Chen, K. New developments in illumination, heating and cooling technologies for energy-efficient buildings. Energy 2010, 35, 2647–2653. [Google Scholar] [CrossRef]
- EIA. EIA NG Consumption in 2018; EIA: Washington, DC, USA, 2018. Available online: https://www.eia.gov/dnav/ng/ng_cons_sum_dcu_nus_a.htm (accessed on 20 March 2022).
- California Legislative Information; Senate Bill No. 100. 2018. Available online: https://leginfo.legislature.ca.gov/faces/billTextClient.xhtml?bill_id=201720180SB100 (accessed on 20 March 2022).
- Mahone, A.; Subin, Z.; Orans, R.; Miller, M.; Regan, L.; Calviou, M.; Saenz, M.; Bacalao, N. On the path to decarbonization: Electrification and renewables in California and the Northeast United States. IEEE Power Energy Mag. 2018, 16, 58–68. Available online: https://ieeexplore.ieee.org/abstract/document/8386887 (accessed on 20 March 2022). [CrossRef]
- Raghavan, S.V.; Wei, M.; Kammen, D.M. Scenarios to decarbonize residential water heating in California. Energy Policy 2017, 109, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, S.; Mac Kinnon, M.; Brouwer, J. California end-use electrification impacts on carbon neutrality and clean air. Appl. Energy 2018, 213, 435–449. [Google Scholar] [CrossRef]
- DNV GL Energy Insights USA, Inc. 2019 California Residential Appliance Saturation Study; California Energy Commission: Sacramento, CA, USA, 2020. [Google Scholar]
- Fischer, M.L.; Chan, W.; Jeong, S.; Zhu, Z.; Lawrence Berkeley National Laboratory. Natural Gas Methane Emissions from California Homes; California Energy Commission: Sacramento, CA, USA, 2018; Publication Number: CEC-500-2018-021. [Google Scholar]
- Nemec, R. California Reports Show Homeowners Prefer NatGas over Electrification. Natural Gas Intelligence. 2018. Available online: https://www.naturalgasintel.com/california-reports-show-homeowners-prefer-natgas-over-electrification/ (accessed on 20 March 2022).
- Kellner, S. 5 Reasons Top Chefs Prefer Gas Cooktops. The Daily Meal. 2014. Available online: https://www.thedailymeal.com/cook/5-reasons-top-chefs-prefer-gas-cooktops (accessed on 20 March 2022).
- Child, J.; Prud’homme, A. My Life in France; Knopf Doubleday Publishing Group: New York, NY, USA, 2006. [Google Scholar]
- Fitch, N.R. Appetite for Life: The Biography of Julia Child; Knopf Doubleday Publishing Group: New York, NY, USA, 2012. [Google Scholar]
- Reardon, J. (Ed.) As Always, Julia: The Letters of Julia Child and Avis DeVoto; Houghton Mifflin Harcourt: Boston, MA, USA, 2010. [Google Scholar]
- California Energy Commission. 2019 Building Energy Efficiency Standards. Available online: https://www.energy.ca.gov/programs-and-topics/programs/building-energy-efficiency-standards/2019-building-energy-efficiency (accessed on 20 March 2022).
- Turns, S.R. Introduction to Combustion; McGraw-Hill Companies: New York, NY, USA, 1996. [Google Scholar]
- Glassman, I.; Yetter, R.A.; Glumac, N.G. Combustion; Academic Press: Burlington, MA, USA, 2014. [Google Scholar]
- Dong, C.; Zhou, Q.; Zhao, Q.; Zhang, Y.; Xu, T.; Hui, S. Experimental study on the laminar flame speed of hydrogen/carbon mon-oxide/air mixtures. Fuel 2009, 88, 1858–1863. [Google Scholar] [CrossRef]
- Fischer, M.L.; Chan, W.; Delp, W.; Jeong, S.; Rapp, V.; Zhu, Z. An Estimate of Natural Gas Methane Emissions from California Homes. Environ. Sci. Technol. 2018, 52, 10205–10213. [Google Scholar] [CrossRef] [PubMed]
- Mejia, A.H.; Brouwer, J.; Mac Kinnon, M. Hydrogen leaks at the same rate as natural gas in typical low-pressure gas infrastructure. Int. J. Hydrogen Energy 2020, 45, 8810–8826. [Google Scholar] [CrossRef]
- Barley, C.D.; Gawlik, K. Buoyancy-driven ventilation of hydrogen from buildings: Laboratory test and model validation. Int. J. Hydrogen Energy 2009, 34, 5592–5603. [Google Scholar] [CrossRef] [Green Version]
- Hajji, Y.; Jouini, B.; Bouteraa, M.; Elcafsi, A.; Belghith, A.; Bournot, P. Numerical study of hydrogen release accidents in a residen-tial garage. Int. J. Hydrogen Energy 2015, 40, 9747–9759. [Google Scholar] [CrossRef]
- Interchangeability of Other Fuel Gases with Natural Gas. In American Gas Association Research Bulletin No. 36; American Gas Association: Washington, DC, USA, 1946.
- Interchangeability of Various Fuel Gases with Manufactured Gas. In American Gas Association Research Bulletin No. 60; American Gas Association: Washington, DC, USA, 1950.
- Briggs, T. The Combustion and Interchangeability of Natural Gas on Domestic Burners. Combustion 2014, 4, 67–87. [Google Scholar]
- Honus, S.; Kumagai, S.; Yoshioka, T. Replacing conventional fuels in USA, Europe, and UK with plastic pyrolysis gases—Part II: Multi-index interchangeability methods. Energy Convers. Manag. 2016, 126, 1128–1145. [Google Scholar] [CrossRef]
- Visser, P. The testing of cookstoves: Data of water-boiling tests as a basis to calculate fuel consumption. Energy Sustain. Dev. 2005, 9, 16–24. [Google Scholar] [CrossRef]
- Grimsby, L.; Rajabu, H.; Treiber, M. Multiple biomass fuels and improved cook stoves from Tanzania assessed with the water boiling test. Sustain. Energy Technol. Assess. 2016, 14, 63–73. [Google Scholar] [CrossRef]
- Turiel, I. Present status of residential appliance energy efficiency standards—An international review. Energy Build. 1997, 26, 5–15. [Google Scholar] [CrossRef]
- Brown, R.; Webber, C.; Koomey, J. Status and future directions of the Energy Star program. Energy 2002, 27, 505–520. [Google Scholar] [CrossRef] [Green Version]
- Ashman, P.J.; Junus, R.; Stubington, J.F.; Sergeant, G.D. The effects of load height on the emissions from a natural gas-fired domestic cooktop burner. Combust. Sci. Technol. 1994, 103, 283–298. [Google Scholar] [CrossRef]
- Oberascher, C.; Stamminger, R.; Pakula, C. Energy efficiency in daily food preparation. Int. J. Consum. Stud. 2011, 35, 201–211. [Google Scholar] [CrossRef]
- Cheng, Q.; Sun, D.-W.; Scannell, A.G. Feasibility of water cooking for pork ham processing as compared with traditional dry and wet air cooking methods. J. Food Eng. 2005, 67, 427–433. [Google Scholar] [CrossRef]
- ANSI Z21.1-2016/CSA 1.1-2016; Household Cooking Gas Appliances; ANSI: New York, NY, USA, 2016.
- Decker, T.; Baumgardner, M.; Prapas, J.; Bradley, T. A mixed computational and experimental approach to improved biogas burner flame port design. Energy Sustain. Dev. 2018, 44, 37–46. [Google Scholar] [CrossRef]
- Natarajan, R.; Karthikeyan, N.; Agarwaal, A.; Sathiyanarayanan, K. Use of vegetable oil as fuel to improve the efficiency of cooking stove. Renew. Energy 2008, 33, 2423–2427. [Google Scholar] [CrossRef]
- Karunanithy, C.; Shafer, K. Heat transfer characteristics and cooking efficiency of different sauce pans on various cooktops. Appl. Therm. Eng. 2016, 93, 1202–1215. [Google Scholar] [CrossRef]
- Anozie, A.; Bakare, A.; Sonibare, J.; Oyebisi, T. Evaluation of cooking energy cost, efficiency, impact on air pollution and policy in Nigeria. Energy 2007, 32, 1283–1290. [Google Scholar] [CrossRef]
- Probert, D.; Newborough, M. Designs, thermal performances and other factors concerning cooking equipment and associated facilities. Appl. Energy 1985, 21, 81–222. [Google Scholar] [CrossRef]
- Cernela, J.; Heyd, B.; Broyart, B. Evaluation of heating performances and associated variability of domestic cooking appliances (oven-baking and pan-frying). Appl. Therm. Eng. 2014, 62, 758–765. [Google Scholar] [CrossRef]
- ANSI Z21.10.1-2017/CSA 4.1-2017; Gas Water Heaters, Volume I, Storage Water Heaters with Input Ratings of 75,000 btu per Hour or Less; ANSI: New York, NY, USA, 2017.
- ANSI Z21.10.3-2017/CSA 4.3-2017; Gas-Fired Water Heaters, Volume III, Storage Water Heaters with Input Ratings above 75,000 btu per Hour, Circulating and Instantaneous; ANSI: New York, NY, USA, 2017.
- San Diego County Air Pollution Control District. Rule 69.5.1 Natural Gas-Fired Water Heaters; San Diego County Air Pollution Control District: San Diego, CA, USA, 2015. [Google Scholar]
- South Coast Air Quality Management District. Rule 1121—Control of Nitrogen Oxides from Residentialtype, Natural Gas-Fired Water Heaters; South Coast Air Quality Management District: Diamond Bar, CA, USA, 2004. [Google Scholar]
- California Legislative Information. Health and Safety Code–HSC-Division 13. Housing-Part 3. Miscellaneous-Chapter 12: Heating Appliances and Installations. Available online: https://law.justia.com/codes/california/2013/code-hsc/division-13/part-3/chapter-12/ (accessed on 20 March 2022).
- ANSI Z21.86-2016/CSA 2.32-2016; Vented Gas-Fired Space Heating Appliances; ANSI: New York, NY, USA, 2016.
- San Joaquin Valley Air Pollution Control District. Rule 4905: Natural Gas-Fired, Fan-Type Central Furnaces; San Joaquin Valley Air Pollution Control District: Fresno, CA, USA, 2020. [Google Scholar]
- South Coast Air Quality Management District. Rule 1111: Reduction of NOX Emissions from Natural Gas-Fired, Fan-Type Central Furnaces; South Coast Air Quality Management District: Diamond Bar, CA, USA, 2018. [Google Scholar]
- South Coast Air Quality Management District. Rule 1153: Commercial Bakery Ovens; South Coast Air Quality Management District: Diamond Bar, CA, USA, 1995. [Google Scholar]
- South Coast Air Quality Management District. Rule 1138: Control of Emissions from Restaurant Operations; South Coast Air Quality Management District: Diamond Bar, CA, USA, 1997. [Google Scholar]
- South Coast Air Quality Management District. Rule 1131: Food Product Manufacturing and Processing; South Coast Air Quality Management District: Diamond Bar, CA, USA, 2003. [Google Scholar]
- South Coast Air Quality Management District. Rule 1153.1: Emissions of Oxides of Nitrogen from Commercial Food Ovens; South Coast Air Quality Management District: Diamond Bar, CA, USA, 2014. [Google Scholar]
- San Joaquin Valley Air Pollution Control District. Rule 4692: Commercial Charboiling; San Joaquin Valley Air Pollution Control District: Fresno, CA, USA, 2018. [Google Scholar]
- Schwela, D. Cooking smoke: A silent killer. People Planet 1997, 6, 24. [Google Scholar] [PubMed]
- Wang, L.; Xiang, Z.; Stevanovic, S.; Ristovski, Z.; Salimi, F.; Gao, J.; Wang, H.; Li, L. Role of Chinese cooking emissions on ambient air quality and human health. Sci. Total Environ. 2017, 589, 173–181. [Google Scholar] [CrossRef]
- Singer, B.C.; Apte, M.G.; Black, D.R.; Hotchi, T.; Lucas, D.; Lunden, M.M.; Mirer, A.G.; Spears, M.; Sullivan, D.P. Natural Gas Variability in California: Environmental Impacts and Device Performance Experimental Evaluation of Pollutant Emissions from Residential Appliances; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2009. [Google Scholar]
- Moschandreas, D.J.; Relwani, S.M.; Billick, I.H.; Macriss, R.A. Emission rates from range-top burners—Assessment of measurement methods. Atmos. Environ. 1987, 21, 285–289. [Google Scholar] [CrossRef]
- Therkelsen, P.; Cheng, R.; Sholes, D.; California Energy Commission. Research and Development of Natural Draft Ultra-Low Emissions Burners for Gas Appliances; Lawrence Berkeley National Lab. (LBNL): Berkeley, CA, USA, 2015; Publication number: CEC-500-2016-054. [Google Scholar]
- Hou, S.-S.; Lee, C.-Y.; Lin, T.-H. Efficiency and emissions of a new domestic gas burner with a swirling flame. Energy Convers. Manag. 2007, 48, 1401–1410. [Google Scholar] [CrossRef]
- Jahnke, J.A. Continuous Emission Monitoring; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Blake, D.R.; Rowland, F.S. Continuing Worldwide Increase in Tropospheric Methane, 1978 to 1987. Science 1988, 239, 1129–1131. [Google Scholar] [CrossRef]
- Zhao, Y.; McDonell, V.; Samuelsen, S. Corrigendum to “Influence of hydrogen addition to pipeline natural gas on the combustion performance of a cooktop burner”[Int J Hydrogen Energy 44 (2019) 12239–12253] & An introduction to combustion: Concepts and applications [McGraw-Hill (2012)]. Int. J. Hydrogen Energy 2021, 46, 10586–10588. [Google Scholar] [CrossRef]
- Zhao, Y. Impact of Increased Renewable Gases in Natural Gas on Combustion Performance of Self-Aspirating Flames. Ph.D. Dissertation, University of California, Irvine, CA, USA, 2020. [Google Scholar]
- Glanville, P.; Fridlyand, A.; Sutherland, B.; Liszka, M.; Zhao, Y.; Bingham, L.; Jorgensen, K. Impact of Hydrogen/Natural Gas Blends on Partially Premixed Combustion Equipment: NOx Emission and Operational Performance. Energies 2022, 15, 1706. [Google Scholar] [CrossRef]
- Zhao, Y.; Hickey, B.; Srivastava, S.; Smirnov, V.; McDonell, V. Decarbonized combustion performance of a radiant mesh burner operating on pipeline natural gas mixed with hydrogen. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]









| Commodity | 1451–1500 | 1531–1540 | 1551–1560 | 1583–1592 | 1603–1612 | 1613–1622 | 1623–1632 | 1633–1642 |
|---|---|---|---|---|---|---|---|---|
| General | 100 | 105 | 132 | 198 | 251 | 257 | 282 | 291 |
| Fuelwood | 100 | 94 | 163 | 277 | 366 | 457 | 677 | 780 |
| Coal | 100 | 89 | 147 | 186 | 295 | 371 | 442 | 321 |
| Year | Period | Tons | Notes |
|---|---|---|---|
| 1580 | 12 March–18 September | 10,785 | |
| 1585–1586 | Michaelmas–Michaelmas | 23,867 | |
| 1591–1592 | Michaelmas–Michaelmas | 34,757 | |
| 1605–1606 | Christmas–Christmas | 73,984 | |
| 1614–1615 | 91,599 | One week missing | |
| 1637–1638 | 142,579 | Two weeks missing; a year of bad trade | |
| 1667–1668 | Midsummer–Midsummer | 264,212 | |
| 1680–1681 | Michaelmas–Michaelmas | 361,189 |
| Contents | Producer Gas | Water Gas | Carbureted Water Gas | Coke Oven Gas | Blast Furnace Gas | Oil Gas | Natural Gas |
|---|---|---|---|---|---|---|---|
| H2 | 15% | 49.7% | 40.5% | 57.0% | 3.7% | 50.0% | 0.0% |
| CO | 24.7% | 39.8% | 34% | 5.9% | 26.3% | 10.2% | 0.0% |
| CH4 | 2.3% | 1.3% | 8.9% | 29.7% | 0.0% | 27.6% | 94.5% |
| C2H6 | 0.0% | 0.1% | 1.1% | 0.0% | - | 0.5% | |
| CO2 | 4.8% | 3.4% | - | 1.5% | 12.9% | 2.6% | 0.2% |
| N2 | 52.2% | 5.5% | - | 0.7% | 57.1% | 5.1% | 4.0% |
| O2 | 0.2% | 0.2 | - | 0.0% | 0.0% | 0.2% | 0.3% |
| Lower Heating Value (MJ/m3) | 5.8 | 11.4 | 20.1 | 21.5 | 3.9 | 19.7 | 35.7 |
| Year (April to March) | Total Customers (000s) | Annual Number Converted to NG (000s) | Cumulative Number Converted to NG (000s) |
|---|---|---|---|
| 1967/68 | 13,210 | 51 | 51 |
| 1968/69 | 13,265 | 418 | 469 |
| 1969/70 | 13,347 | 1093 | 1562 |
| 1970/71 | 13,372 | 2029 | 3591 |
| 1971/72 | 13,390 | 2407 | 5998 |
| 1972/73 | 13,506 | 2100 | 8098 |
| 1973/74 | 13,559 | 2108 | 10,206 |
| 1974/75 | 13,682 | 1674 | 11,880 |
| 1975/76 | 13,925 | 1131 | 13,011 |
| 1976/77 | 14,200 | 329 | 13,340 |
| 1977/78 | 14,516 | 98 | 13,438 |
| Year | Total Customers (Millions) | Total Consumption (Trillion Btu) | Average Consumption (Million Btu/Customer) |
|---|---|---|---|
| 1945 | 18.6 | 775 | 41.6 |
| 1950 | 22.1 | 1384 | 62.6 |
| 1955 | 26.3 | 2239 | 85.2 |
| 1960 | 30.4 | 3188 | 104.8 |
| 1965 | 34.3 | 3999 | 116.5 |
| 1970 | 38.1 | 4924 | 129.2 |
| 1975 | 40.9 | 4991 | 121.9 |
| 1977 | 41.7 | 4946 | 118.7 |
| Component | Volume (%) | |||
|---|---|---|---|---|
| Mean | Standard Deviation | Minimum | Maximum | |
| CH4 | 93.0 | 5.5 | 73 | 99 |
| C2H6 | 3.0 | 2.6 | 0 | 13 |
| C3H8 | 1.0 | 1.4 | 0 | 8 |
| C4H10 | 0.5 | 1.0 | 0 | 7 |
| C5H12 | 0.1 | 0.3 | 0 | 3 |
| C6H14 | 0.1 | 0.1 | 0 | 1 |
| N2 | 1.5 | 2.9 | 0 | 17 |
| CO2 | 0.5 | 0.5 | 0 | 2 |
| Gas Company | Heating Value (Btu/scf) | Water Content | Various Inerts | Hydrogen Sulfide (H2S) | |||
|---|---|---|---|---|---|---|---|
| Min | Max | Lbs/MMscf | CO2 | O2 | Total Inerts | (Grain/100 scf) | |
| SoCalGas | 970 | 1150 | 7 | 3% | 0.2% | 4% | 0.25 |
| Dominion Transmission | 967 | 1100 | 7 | 3% | 0.2% | 5% | 0.25 |
| Equitrans LP | 970 | - | 7 | 3% | 0.2% | 4% | 0.3 |
| Florida Gas Transmission Co. | 1000 | 1110 | 7 | 1% | 0.25% | 3% | 0.25 |
| Colorado Intrastate Gas Co. | 968 | 1235 | 7 | 3% | 0.001% | - | 0.25 |
| Questar Pipeline Co. | 950 | 1150 | 5 | 2% | 0.1% | 3% | 0.25 |
| Gas Transmission Northwest Co. | 995 | - | 4 | 2% | 0.4% | - | 0.25 |
| Species | Units | Biogas | ||
|---|---|---|---|---|
| Sewage Gas | Agricultural Gas | Landfill Gas | ||
| CH4 | % | 66–75 | 45–75 | 45–55 |
| CO2 | % | 20–35 | 25–55 | 20–30 |
| CO | % | <0.2 | <0.2 | <0.2 |
| N2 | % | 3.4 | 0.01–5 | 10–25 |
| O2 | % | 0.5 | 0.01–2 | 1–5 |
| H2 | % | trace | 0.5 | 0.0 |
| H2S | mg/N·m3 | <8000 | 10–30 | <8000 |
| NH3 | mg/N·m3 | Trace | 0.01–2.5 | trace |
| Siloxanes | mg/N·m3 | <0.1–5.0 | trace | <0.1–5.0 |
| Organization | Starting Time | Appliances/Burners | Description | References |
|---|---|---|---|---|
| Gas Technology Institute, U.S. | 1977 | Atmospheric burner | Provided technical considerations of appliances operating on hydrogen-rich natural gas. | [110] |
| DNV GL Oil & Gas/University of Groningen, Netherlands | 2004 | Fundamental aspirating burner | Conducted fundamental analysis on natural gas/hydrogen mixture properties and conducted experiments on a Bunsen burner. | [111,114] |
| Swansea University, U.K.; King Saud University, Saudi Arabia | 2018 | Cooktop burner | Conducted experiments on a cooktop burner operating on over 30 vol% hydrogen. | [115] |
| University of California, Irvine, U.S. | 2018 | Cooktop burner, oven burner, room furnace, water heaters, etc. | Conducted experiments on multiple appliances and tested multiple performances (ignition, emissions, burner temperature, etc.) of various appliances operating on hydrogen/natural gas mixtures. | [116,122] |
| University of Zaragoza, BSH Home Appliances Group, Spain | 2019 | Cooktop burner | Developed simplified reaction mechanism to simulate the combustion performance of a cooktop burner. | [123] |
| E.ON/DVGW, Germany | 2019 | Various appliances | Around 400 residential appliances were tested while operating on hydrogen/natural gas mixtures. | [124] |
| Year | Event | References |
|---|---|---|
| Early 1870s | Introduction of the electric dynamo posed a competitive threat to kerosene and gas lighting. | [127] |
| 1877 | Carbon arc lamp was first used in Paris in May 1877, which triggered the interest of London. | [128] |
| Dr. Carl Auer von Welsbach of Vienna invented the incandescent gas light mantle. Welsbach established the first factory to make gas mantles in London in 1877. | [53,129] | |
| 1878–1879 | On 14 December 1878, the Holborn Viaduct electric lighting was switched on in London. Afterward, four circuits, each with four lamps, were powered by a 20-horse-power Robey steam engine that drove a Gramme alternator and exciter. However, the experimental lighting was shut down on 9 May 1879. The city engineer reported that the cost was 3.75 times that of gas. | [128] |
| 1879 | Edison invented the long-life light bulb and applied for a patent. | [130,131] |
| 1882 | Electric Lighting Act (U.K.) led to the establishment of electricity undertakings on a statutory basis, but the high cost of electricity hindered the wide application of electric lighting. | [132,133] |
| 1890s | Gas mantle started to be widely used for streetlights. | [133] |
| 1900s–1910s | By the early 1900s, the cost of electric lighting was becoming closer to that of gas. However, only the wealthy could afford to wire their houses until the 1910s. | [21] |
| After 1910s | World War I resulted in coal shortage and the coal gas quality fluctuation, which gave a chance for electric lighting popularization. After World War II, the power-generation technologies and electric grid were rapidly developed, which brought down the electric lighting price, and electric lighting found wide applications not only in street lighting but also in residential houses. | [134] |
| 100% Electrifying Residential Homes | Adopting Renewable Gases in Pipelines | |
|---|---|---|
| System Efficiency | System efficiency decreases due to the energy loss in the electricity-generation process. | The efficiency should not change significantly. |
| Carbon Reduction | Shifting the carbon emissions to power plants may increase total carbon emissions if not assisted by renewable energy sources. | Carbon emissions will drop in proportion to the amount of biomethane or renewable hydrogen adopted. |
| Combustion Pollutants | Shifts pressure to power plants to reduce emissions. | Pollutants do not change much [116]. |
| Appliance upgrade | Need to replace all the gas appliances with electric appliances, potentially together with upgrading the house’s wiring system. | Do not need appliance upgrades for biomethane adoption. Need studies on hydrogen fuel interchangeability. |
| Cost | Electric appliances are usually of a higher price, and electricity price is more expensive than gas. | Gas price might increase due to limitation of renewable gas source availability. |
| Technology Availability | Technology available. | Technology available. |
| Energy Security | Might be vulnerable when blackout occurs due to a single type of energy source. | Energy system is more diverse. |
| People’s Acceptance Level | Gas cooking appliances are usually preferred. | Appliances’ performances should be consistent or improved. |
| Fuel Properties | Unit | Propane (C3H8) | Ethane (C2H6) | Methane (CH4) | Carbon Monoxide (CO) | Hydrogen (H2) |
|---|---|---|---|---|---|---|
| Density | kg/m3 | 1.808 | 1.219 | 0.648 | 1.131 | 0.0813 |
| Viscosity | 10−5 Pa·s | 0.82 | 0.94 | 1.11 | 1.8 | 0.89 |
| Laminar Flame Speed (ϕ =1) | m/s | 0.46 | 0.45 | 0.43 | 0.17 [160] | 2.1 |
| Low Flammability | ϕ | 0.51 | 0.50 | 0.46 | 0.34 | 0.1 * |
| vol% | 2 | 3 | 5 | 12.5 | 4 | |
| High Flammability | ϕ | 2.83 | 2.72 | 1.64 | 6.76 | 7.2 * |
| vol% | 11 | 14 | 15 | 74 | 75 | |
| Ignition Energy (ϕ =1) | 10−5 J | 30.5 | 42 | 33 | - | 2 |
| Quenching Distance (ϕ =1) | mm | 2.0 | 2.3 | 2.5 | - | 0.64 |
| Adiabatic Flame Temperature (ϕ =1) | K | 2267 | 2259 | 2226 | 2400 | 2318 |
| Lower Heating Value | MJ/m3 | 83.9 | 57.9 | 32.4 | 11.5 | 9.8 |
| MJ/kg | 46.4 | 47.5 | 50.0 | 10.2 | 120.1 | |
| Higher Heating Value | MJ/m3 | 91.1 | 63.3 | 36.0 | 11.5 | 11.6 |
| MJ/kg | 50.4 | 51.9 | 55.5 | 10.2 | 142.1 | |
| Wobbe Index | MJ/m3 | 73.7 | 62.4 | 48.6 | 11.8 | 44.3 |
| Code | Flame Description |
|---|---|
| +5 | Flames lifting from ports with no flame on 25% or more of the ports. |
| +4 | Flames tend to lift from ports but become stable after short period of operation. |
| +3 | Short inner cone; flames may be noisy. |
| +2 | Inner cones distinct and pointed. |
| +1 | Inner cones and tips distinct. |
| 0 | Inner cones rounded; soft tips. |
| −1 | Inner cones visible; very soft tips. |
| −2 | Faint inner cones. |
| −3 | Inner cones broken at top; lazy wavering flames |
| −4 | Slight yellow streaming in the outer mantles or yellow fringes on tops of inner cones. Flames deposit no soot on impingement. |
| −5 | Distinct yellow in outer mantles or large volumes of luminous yellow tips on inner cones. Flames deposit soot on impingement. |
| Name | Description | Reference |
|---|---|---|
| Knoy index | Similar to Wobbe Index, which predicts heat-release rate interchangeability. | [167] |
| Dutton indices | This series of indices mainly considers the incomplete combustion behavior or near blow-off condition for higher hydrocarbons. It consists of incomplete combustion index, lift index, and soot index. | [168] |
| Delbourg indices | This series is composed of two indices: yellow tip index and soot-formation index. | [168,169,170] |
| Name | Method | Reference |
|---|---|---|
| Food cooking | Cooking a specific food (egg, etc.) and evaluating the food appearance to interpret efficiency | [174,175] |
| Water boiling | Boiling water and calculating the ratio of heat absorbed by water over the total heat-release rate | [116,117,176,177,178,179,180] |
| Object heating | Heating some materials (usually metal rod/disk) and measure the temperature change of the material to quantify the efficiency | [181,182] |
| Regulation Title | Agency | Adopted Time | Major Contents | |
|---|---|---|---|---|
| Water-heating appliances | ANSI Z21.10.1 [183] ANSI Z21.10.3 [184] | ANSI-CSA | 2017 | CO < 800 ppm (heating load ≤ 75,000 Btu/h). CO < 400 ppm (heating load > 75,000 Btu/h). |
| Rule 69.5.1 [185] | SDCAPCD | 2017 | NOX < 10 ng/J (calculated as NO2) or NOX < 15 ppm (@ 3% O2, dry). | |
| Rule 1121 [186] | SCAQMD | 2004 | NOX < 10 ng/J (calculated as NO2) or NOX < 15 ppm (@ 3% O2, dry). | |
| Space-heating appliances | HSC-1988 [187] | California Law | 1997 | No person shall sell, or offer for sale, any new or used unvented heater that is designed to be used inside any dwelling house or unit, with the exception of an electric heater or decorative gas logs for use in a vented fireplace. |
| ANSI Z21.86 [188] | ANSI-CSA | 2016 | CO: less than 200 ppm in the air free sample. | |
| Rule 4905 [189] | SJVAPCD | 2018 | NOX: 14 ng of oxides of nitrogen (calculated as NO2) per joule of useful heat delivered to the heated space. | |
| Rule 1111 [190] | SCAQMD | 2018 | NOX: 14 ng of oxides of nitrogen (calculated as NO2) per joule of useful heat delivered to the heated space. | |
| Cooking appliances | Rule 1153 [191] | SCAQMD | 1995 | VOC of commercial bakery ovens (≥2 million Btu/h) should be less than 50 pounds/day. |
| Rule 1138 [192] | SCAQMD | 1997 | Chain-driven charbroiler must be equipped with catalytic oxidizer reducing PM and VOC. | |
| Rule 1131 [193] | SCAQMD | 2003 | The VOC content of each solvent used ≤120 g per liter of material. | |
| Rule 1153.1 [194] | SCAQMD | 2014 | Commercial ovens: CO: 800 ppm (@ 3% O2). NOX: 40 ppm (@ 3% O2)-500 °F-60 ppm (@ 3% O2). | |
| ANSI Z21.1 [176] | ANSI-CSA | 2016 | CO: cooking appliances less than 800 ppm. | |
| Rule 4692 [195] | SJVUAPCD | 2018 | In lieu of SCAQMD-Rule 1138. The catalytic oxidizer shall have a control efficiency ≥ 83% for PM-10 emissions and a control efficiency ≥ 86% for VOC emissions. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; McDonell, V.; Samuelsen, S. Residential Fuel Transition and Fuel Interchangeability in Current Self-Aspirating Combustion Applications: Historical Development and Future Expectations. Energies 2022, 15, 3547. https://doi.org/10.3390/en15103547
Zhao Y, McDonell V, Samuelsen S. Residential Fuel Transition and Fuel Interchangeability in Current Self-Aspirating Combustion Applications: Historical Development and Future Expectations. Energies. 2022; 15(10):3547. https://doi.org/10.3390/en15103547
Chicago/Turabian StyleZhao, Yan, Vince McDonell, and Scott Samuelsen. 2022. "Residential Fuel Transition and Fuel Interchangeability in Current Self-Aspirating Combustion Applications: Historical Development and Future Expectations" Energies 15, no. 10: 3547. https://doi.org/10.3390/en15103547
APA StyleZhao, Y., McDonell, V., & Samuelsen, S. (2022). Residential Fuel Transition and Fuel Interchangeability in Current Self-Aspirating Combustion Applications: Historical Development and Future Expectations. Energies, 15(10), 3547. https://doi.org/10.3390/en15103547