Possibility of Advanced Modified-Silica-Based Porous Materials Utilisation in Water Adsorption Processes—A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
- Metal Organic Silica: AFSPd-Cu in the form and colour similar to the fine sand, AFSPd-Cu (NP) (MOS with metal nanoparticles (NP)) material was similar to the previous one, but the colour of the sample was grey, AFSMo-Cu in the form of small blue crystals,
- High-porous silica material MPSilica: which was a very fine white powder.
- Narrow porous silica gel of particle size 2–7 mm.
2.2. Methods
3. Results and Discussion
3.1. Structural Analysis and Morphology
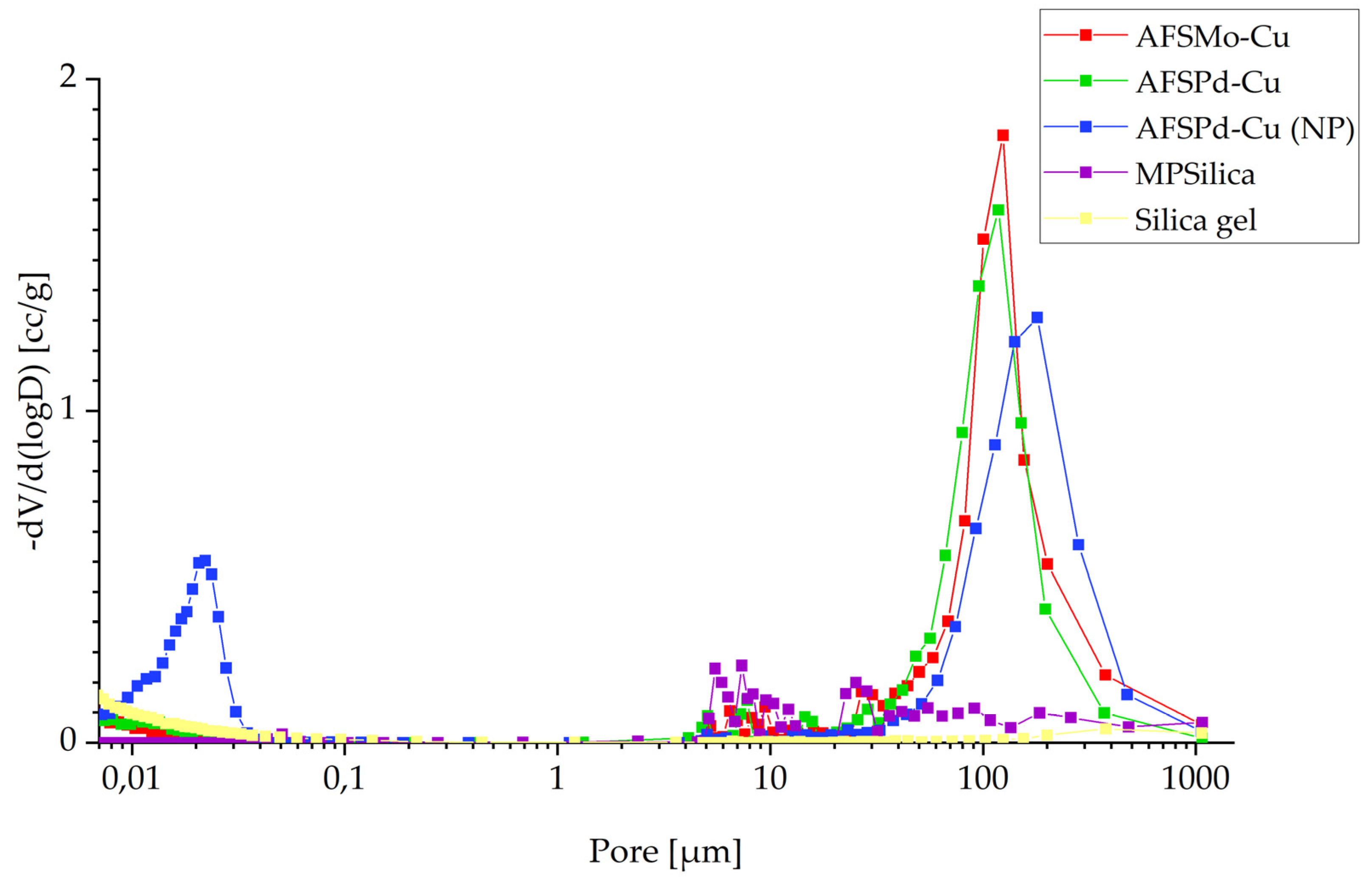
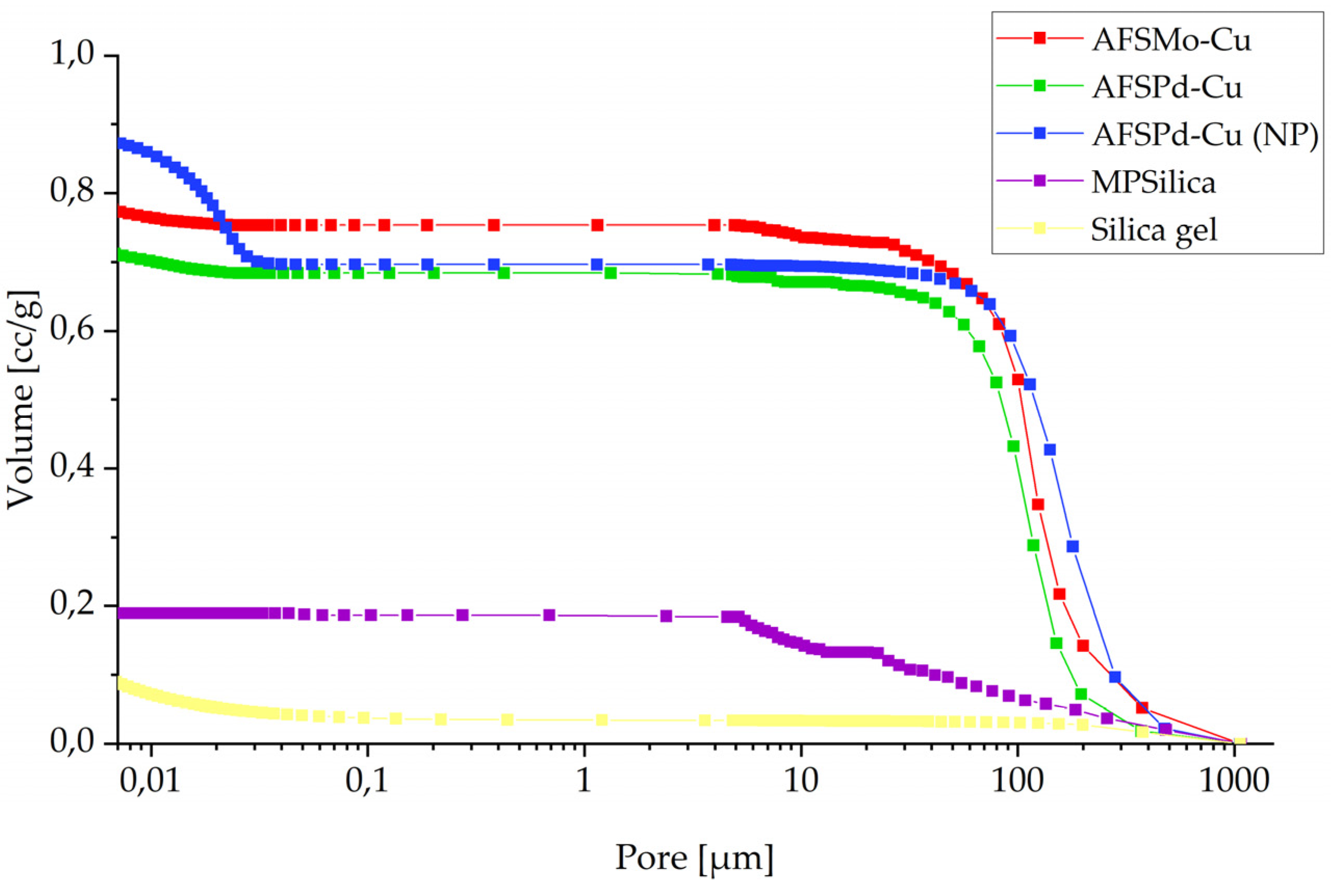
3.2. Porosity
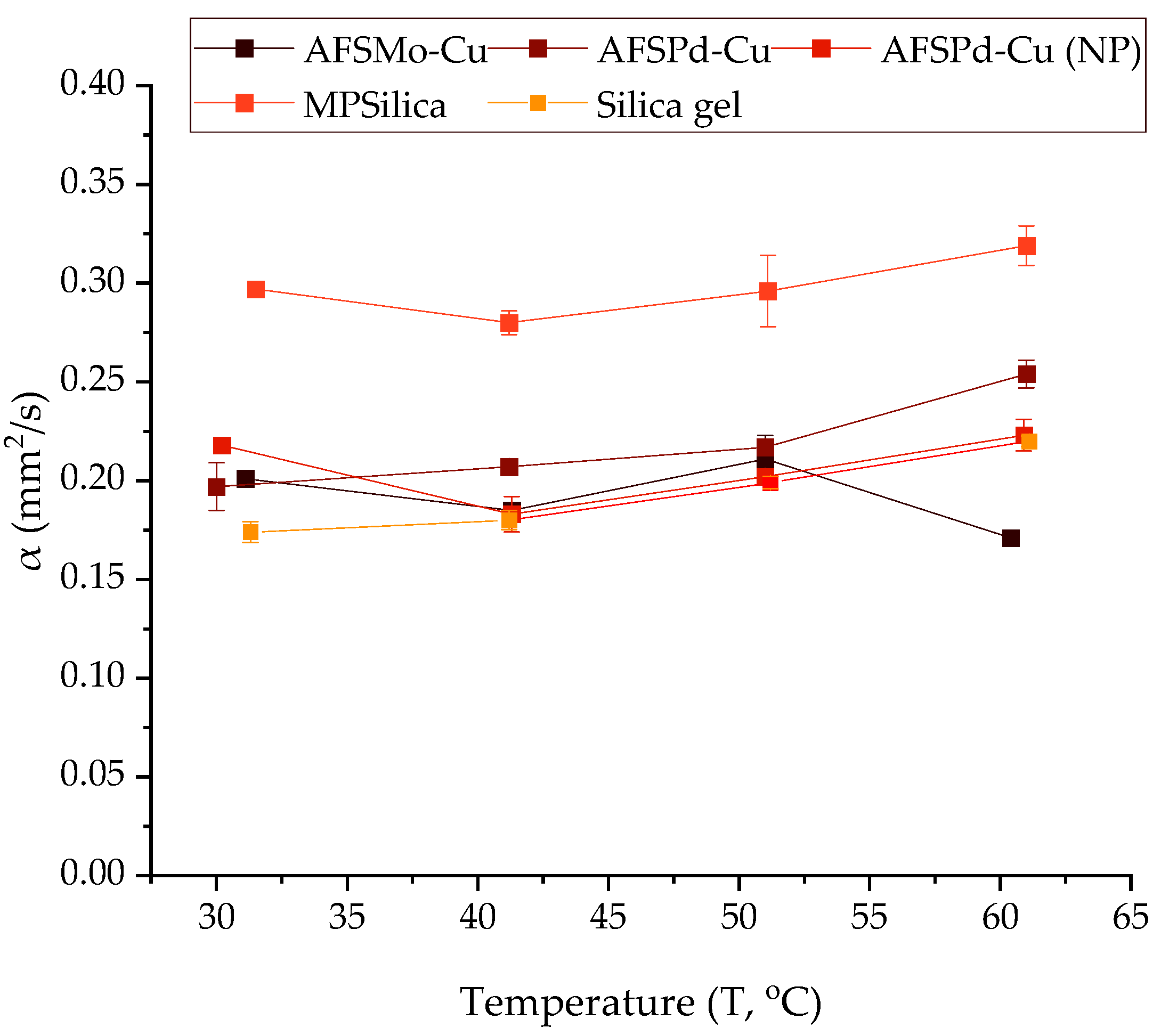
3.3. Thermal Diffusivity Coefficient
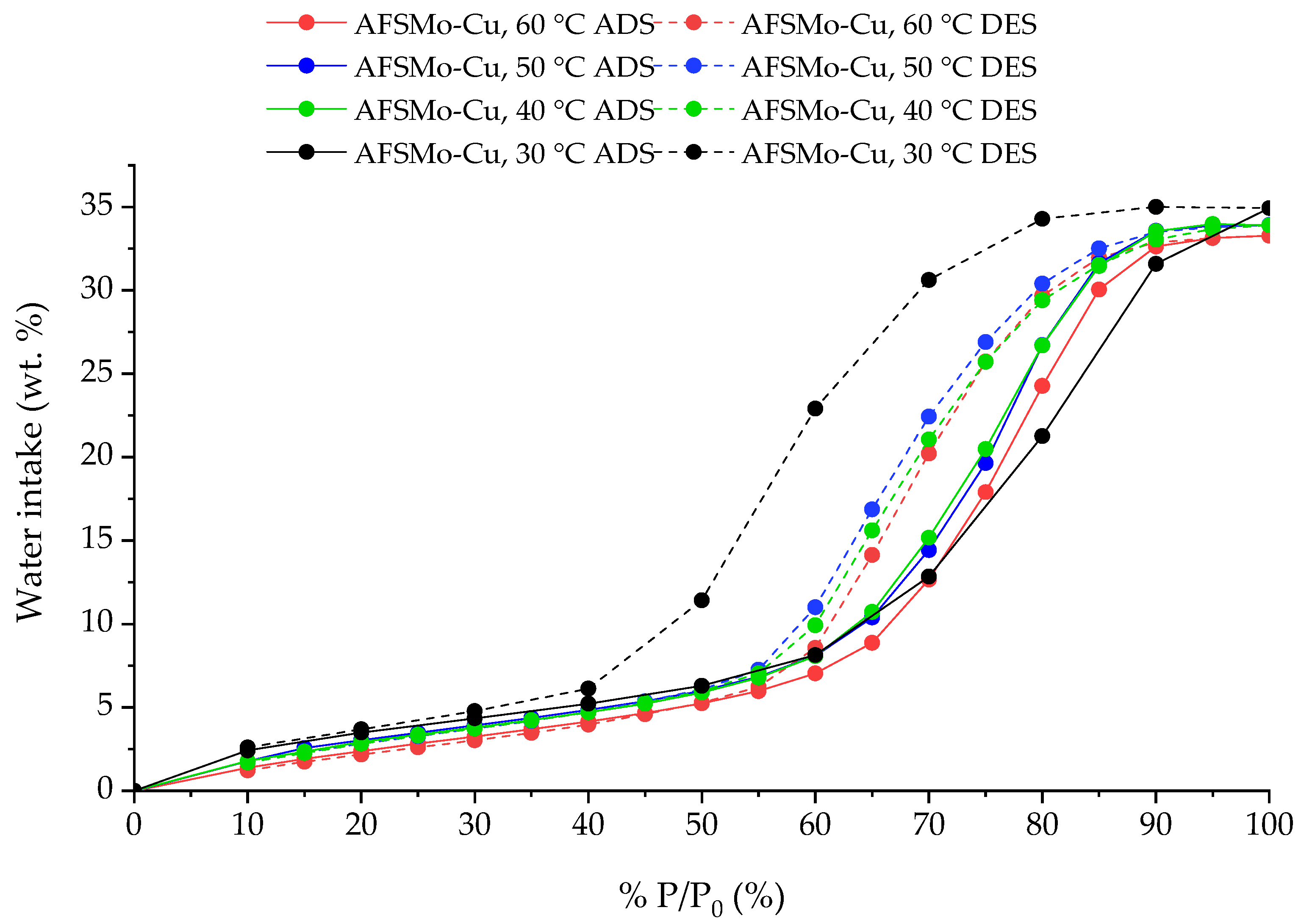
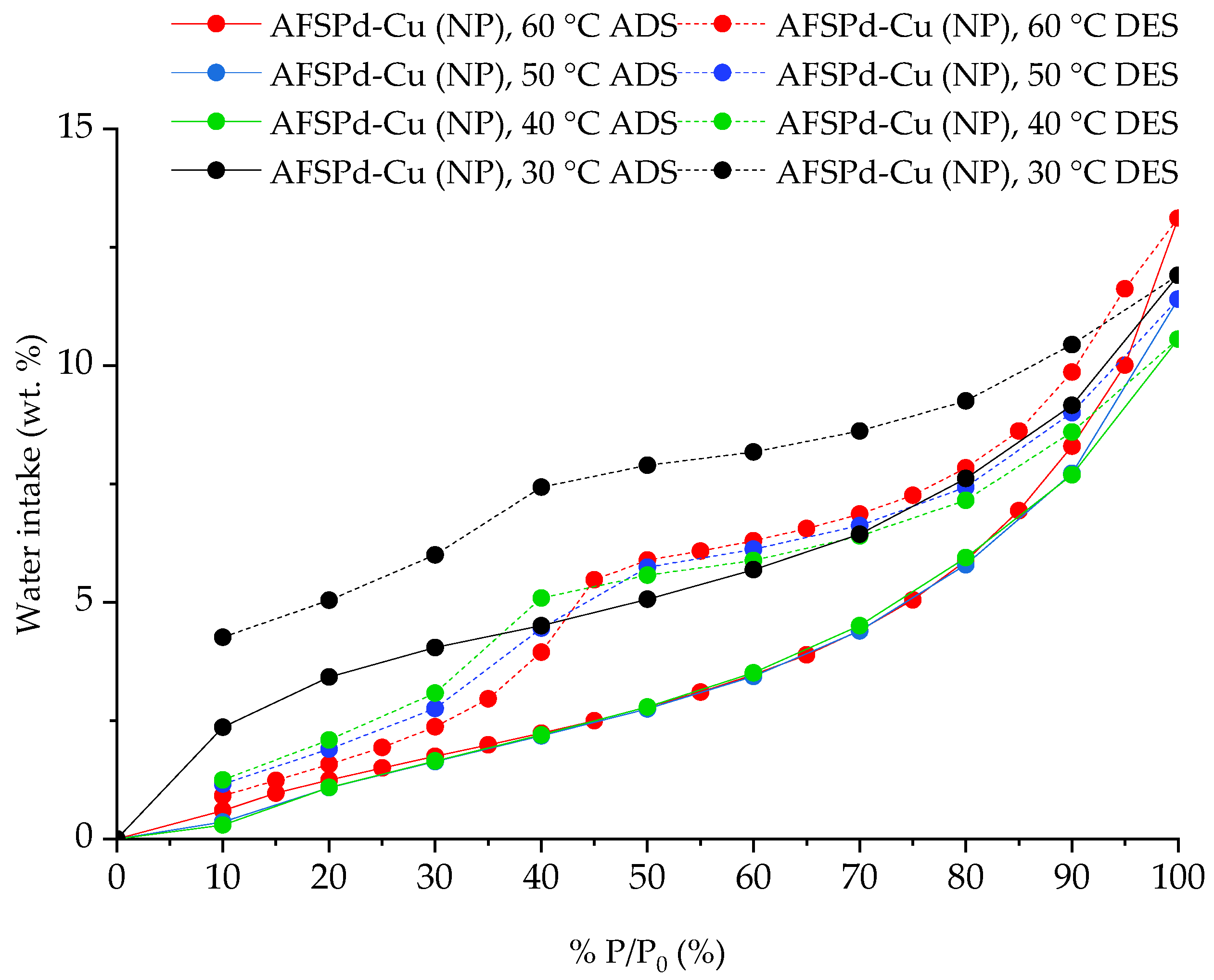
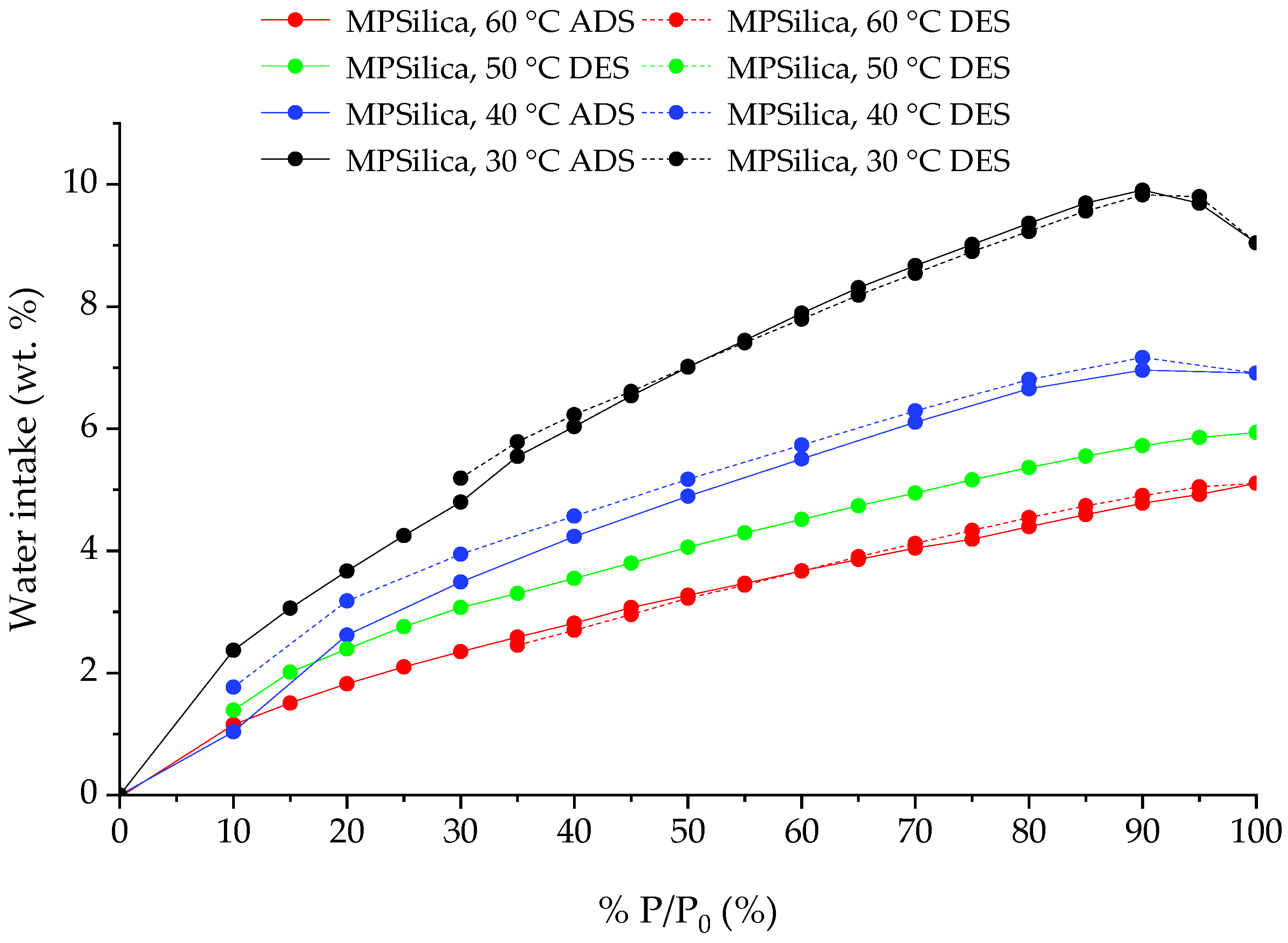
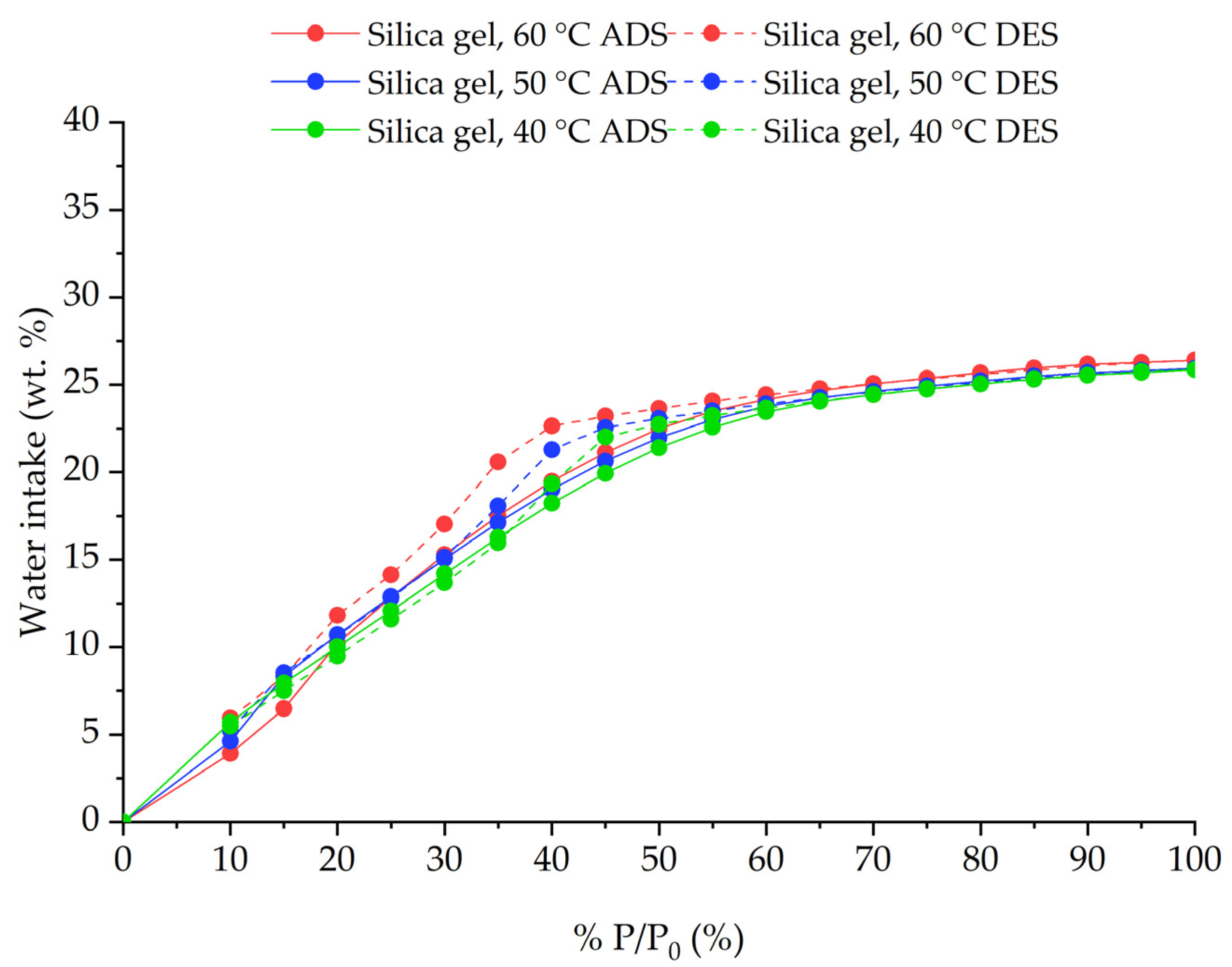
3.4. Sorption Characteristics
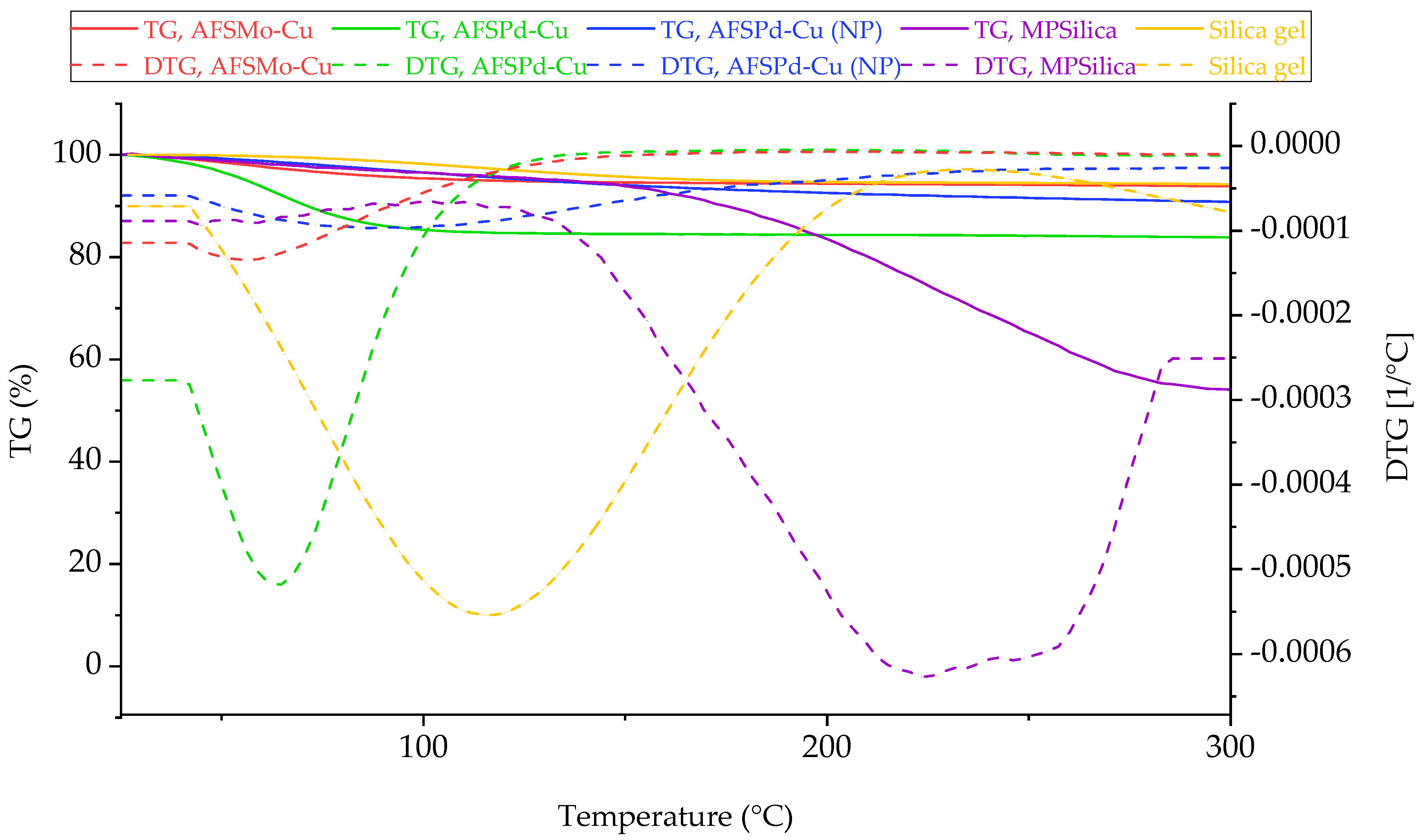
3.5. Simultaneous Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sztekler, K.; Kalawa, W.; Mlonka-Medrala, A.; Nowak, W.; Mika, L.; Krzywanski, J.; Grabowska, K.; Sosnowski, M.; Debniak, M. The effect of adhesive additives on silica gel water sorption properties. Entropy 2020, 22, 327. [Google Scholar] [CrossRef] [Green Version]
- Sztekler, K.; Kalawa, W.; Mika, Ł.; Mlonka-Mędrala, A.; Sowa, M.; Nowak, W. Effect of additives on the sorption kinetics of a silica gel bed in adsorption chiller. Energies 2021, 14, 83. [Google Scholar] [CrossRef]
- Khdary, N.H.; Ghanem, M.A.; Merajuddine, M.G.; Bin Manie, F.M. Incorporation of Cu, Fe, Ag, and Au nanoparticles in mercapto-silica (MOS) and their CO2 adsorption capacities. J. CO2 Util. 2014, 5, 17–23. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhang, H.; Liu, C.G.; Kanubaddi, K.R.; Lee, C.H.; Wang, S.B.; Cui, W.; Santos, H.A.; Lin, K.L.; Chen, A.Z. Metal species—Encapsulated mesoporous silica nanoparticles: Current advancements and latest breakthroughs. Adv. Funct. Mater. 2019, 29, 1902652. [Google Scholar] [CrossRef]
- García-Fernández, A.; Sancenón, F.; Martínez-Máñez, R. Mesoporous silica nanoparticles for pulmonary drug delivery. Adv. Drug Deliv. Rev. 2021, 177, 113953. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, W.; Wei, X.; Ding, J.; Yang, J. Experimental and numerical study on water sorption over modified mesoporous silica. Adsorption 2015, 21, 67–75. [Google Scholar] [CrossRef]
- Sierosławska, A.; Borówka, A.; Rymuszka, A.; Żukociński, G.; Sobczak, K. Mesoporous silica nanoparticles containing copper or silver synthesized with a new metal source: Determination of their structure parameters and cytotoxic and irritating effects. Toxicol. Appl. Pharmacol. 2021, 429, 115685. [Google Scholar] [CrossRef]
- Sachan, D.; Ramesh, A.; Das, G. Green synthesis of silica nanoparticles from leaf biomass and its application to remove heavy metals from synthetic wastewater: A comparative analysis. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100467. [Google Scholar] [CrossRef]
- Khdary, N.H.; Ghanem, M.A.; Abdesalam, M.E.; Al-Garadah, M.M. Sequestration of CO2 using Cu nanoparticles supported on spherical and rod-shape mesoporous silica. J. Saudi Chem. Soc. 2018, 22, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Salazar Hoyos, L.A.; Faroldi, B.M.; Cornaglia, L.M. A coke-resistant catalyst for the dry reforming of methane based on Ni nanoparticles confined within rice husk-derived mesoporous materials. Catal. Commun. 2020, 135, 105898. [Google Scholar] [CrossRef]
- Garbarino, G.; Cavattoni, T.; Riani, P.; Busca, G. Support effects in metal catalysis: A study of the behavior of unsupported and silica-supported cobalt catalysts in the hydrogenation of CO2 at atmospheric pressure. Catal. Today 2020, 345, 213–219. [Google Scholar] [CrossRef]
- Di Natale, F.; Gargiulo, V.; Alfè, M. Adsorption of heavy metals on silica-supported hydrophilic carbonaceous nanoparticles (SHNPs). J. Hazard. Mater. 2020, 393, 122374. [Google Scholar] [CrossRef] [PubMed]
- El Kurdi, R.; Chebl, M.; Sillanpää, M.; El-Rassy, H.; Patra, D. Chitosan oligosaccharide/silica nanoparticles hybrid porous gel for mercury adsorption and detection. Mater. Today Commun. 2021, 28, 102707. [Google Scholar] [CrossRef]
- Venkatesvaran, H.; Kannan, A.; Mayavan, A.; Dhanabal, A.; Gandhi, S. Metal nanoparticle ornated mesoporous silica: A potential nano-interface for uric acid detection. Microporous Mesoporous Mater. 2021, 324, 111313. [Google Scholar] [CrossRef]
- Chirra, S.; Siliveri, S.; Gangalla, R.; Goskula, S.; Gujjula, S.R.; Adepu, A.K.; Anumula, R.; Sivasoorian, S.S.; Wang, L.F.; Narayanan, V. Synthesis of new multivalent metal ion functionalized mesoporous silica and studies of their enhanced antimicrobial and cytotoxicity activities. J. Mater. Chem. B 2019, 7, 7235–7245. [Google Scholar] [CrossRef] [PubMed]
- Endo, A.; Inagi, Y.; Fujisaki, S.; Yamamoto, T.; Ohmori, T.; Nakaiwa, M. Synthesis of Metal-Doped Mesoporous Silica by Spray Drying and Their Adsorption Properties of Water Vapor; Elsevier: Amsterdam, The Netherlands, 2007; Volume 165, ISBN 044452178X. [Google Scholar]
- Yanagihara, H.; Yamashita, K.; Endo, A.; Daiguji, H. Adsorption-desorption and transport of water in two-dimensional hexagonal mesoporous silica. J. Phys. Chem. C 2013, 117, 21795–21802. [Google Scholar] [CrossRef]
- Oh, B.; Jabbari-hichri, A.; Bennici, S.; Auroux, A. Enhancing the heat storage density of silica—Alumina by addition. Sol. Energy Mater. Sol. Cells 2015, 140, 351–360. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kumagai, N.; Izumiya, K.; Takano, H.; Shinomiya, H.; Sasaki, Y.; Yoshida, T.; Kato, Z. The use of renewable energy in the form of methane via electrolytic hydrogen generation using carbon dioxide as the feedstock. Appl. Surf. Sci. 2016, 388, 608–615. [Google Scholar] [CrossRef]
- Kulakowska, A.; Pajdak, A.; Krzywanski, J.; Grabowska, K.; Zylka, A.; Sosnowski, M.; Wesolowska, M.; Sztekler, K.; Nowak, W. Effect of metal and carbon nanotube additives on the thermal diffusivity of a silica-gel-based adsorption bed. Energies 2020, 13, 1391. [Google Scholar] [CrossRef] [Green Version]
- Dammel, F.; Ochterbeck, J.M.; Stephan, P. Thermodynamics. In Springer Handbook of Mechanical Engineering; Grote, K., Antonsson, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 223–294. [Google Scholar]
- Wang, L.W.; Wang, R.Z.; Oliveira, R.G. A review on adsorption working pairs for refrigeration. Renew. Sustain. Energy Rev. 2009, 13, 518–534. [Google Scholar] [CrossRef]
- Furtado, A.M.B.; Liu, J.; Wang, Y.; Levan, M.D. Mesoporous silica-metal organic composite: Synthesis, characterization, and ammonia adsorption. J. Mater. Chem. 2011, 21, 6698–6706. [Google Scholar] [CrossRef]
- Palash, M.L.; Jahan, I.; Rupam, T.H.; Harish, S.; Saha, B.B. Novel technique for improving the water adsorption isotherms of metal-organic frameworks for performance enhancement of adsorption driven chillers. Inorg. Chim. Acta 2020, 501, 119313. [Google Scholar] [CrossRef]
- Yang, Z.; Cao, L.; Li, J.; Lin, J.; Wang, J. Facile synthesis of Cu-BDC/Poly(N-methylol acrylamide) HIPE monoliths via CO2-in-water emulsion stabilized by metal-organic framework. Polymer 2018, 153, 17–23. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, H.; Wang, Z.; Du, Y.; Zhong, L.; Zhang, C.; Jiang, Y.; Jia, S.; Cui, J. Three-dimensional ordered magnetic macroporous metal-organic frameworks for enzyme immobilization. J. Colloid Interface Sci. 2021, 590, 436–445. [Google Scholar] [CrossRef]
- Hinze, M.; Ranft, F.; Drummer, D.; Schwieger, W. Reduction of the heat capacity in low-temperature adsorption chillers using thermally conductive polymers as heat exchangers material. Energy Convers. Manag. 2017, 145, 378–385. [Google Scholar] [CrossRef]
- Karmakar, A.; Prabakaran, V.; Zhao, D.; Chua, K.J. A review of metal-organic frameworks (MOFs) as energy-efficient desiccants for adsorption driven heat-transformation applications. Appl. Energy 2020, 269, 115070. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal-organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef]
- Tatlier, M.; Munz, G.; Henninger, S.K. Relation of water adsorption capacities of zeolites with their structural properties. Microporous Mesoporous Mater. 2018, 264, 70–75. [Google Scholar] [CrossRef]

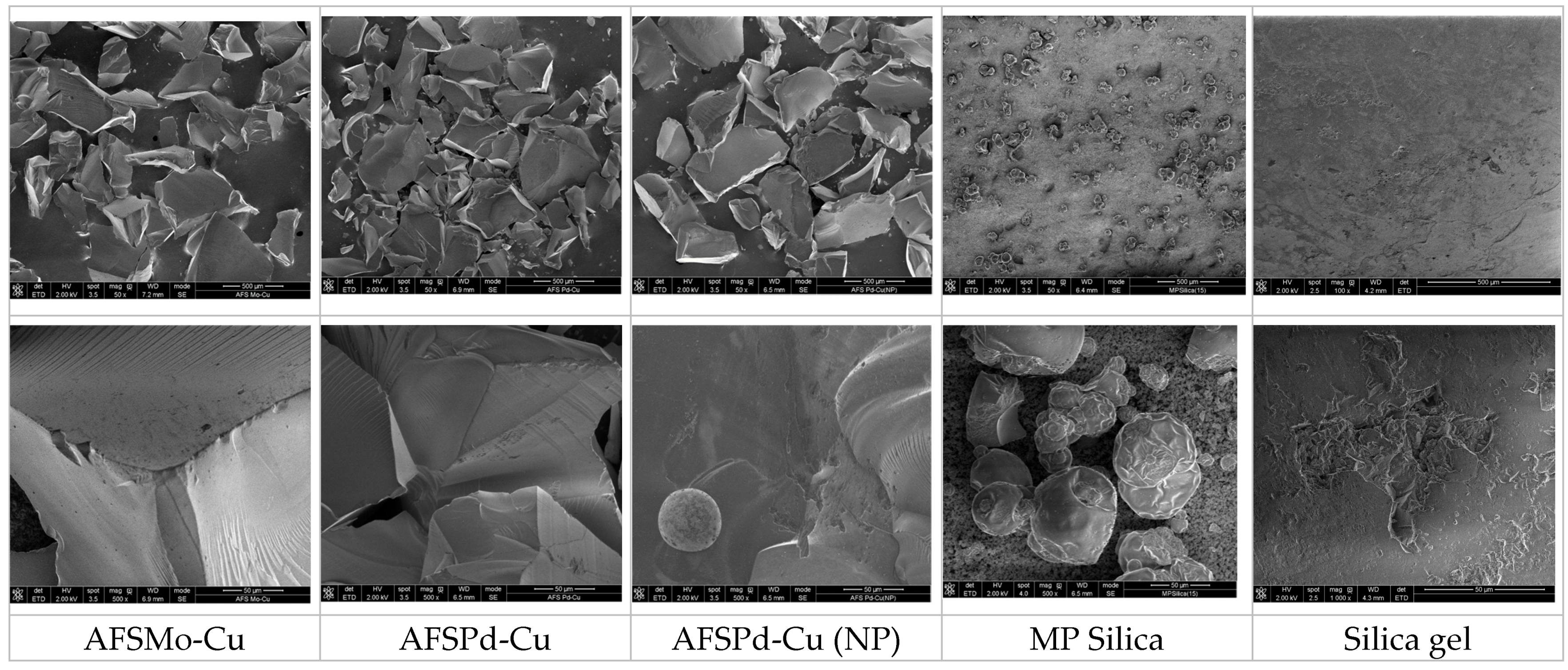

| Sample | C | O | Si | F | S | Cl | Ni | Mo | Cu | Pd | Na |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MP Silica (15) | - | 49.2 | 50.8 | - | - | - | - | - | - | - | - |
| AFS Mo-Cu | - | 54.6 | 42.9 | - | - | - | - | 1.4 | 1.2 | - | - |
| AFS Pd-Cu | - | 56.3 | 40.6 | - | - | 1.0 | - | - | 0.6 | 1.5 | - |
| AFS Pd-Cu (NP) | - | 51.7 | 42.3 | - | - | - | - | - | 0.5 | 3.5 | 2.0 |
| Silica gel | 8.6 | 49 | 42.4 | - | - | - | - | - | - | - | - |
| Sample | BET Surface Area, m2/g | Micropore Volume, m2/g | External Surface Area, m2/g | Adsorption Average Pore Diameter (4 V/A by BET), nm | Desorption Average Pore Diameter (4 V/A by BET), nm | BJH Adsorption Average Pore Diameter (4 V/A), nm | BJH Desorption Average Pore Diameter (4 V/A), nm |
|---|---|---|---|---|---|---|---|
| AFSMo-Cu | 283.71 | 21.08 | 262.63 | 5.21 | 5.21 | 4.87 | 3.95 |
| AFSPd-Cu | 636.62 | 42.11 | 594.51 | 2.50 | 2.50 | 3.29 | 2.84 |
| AFSPd-Cu (NP) | 51.86 | 4.67 | 47.19 | 21.59 | 21.14 | 23.22 | 15.12 |
| MPSilica | 2144.68 | 0 | 3956.55 | 2.64 | 2.61 | 3.02 | 2.62 |
| Silica gel | 789.18 | 204.29 | 584.89 | 2.19 | 2.19 | 3.24 | 2.75 |
| No. | Material | Process Temperature | Maximal Water Loading, % | Reference |
|---|---|---|---|---|
| 1 | AFSMo-Cu | 30 °C | 35.01 | Exp. |
| 2 | AFSMo-Cu | 40 °C | 33.90 | Exp. |
| 3 | AFSMo-Cu | 50 °C | 33.92 | Exp. |
| 4 | AFSMo-Cu | 60 °C | 33.28 | Exp. |
| 5 | AFSPd-Cu | 30 °C | 27.39 | Exp. |
| 6 | AFSPd-Cu | 40 °C | 24.66 | Exp. |
| 7 | AFSPd-Cu | 50 °C | 24.81 | Exp. |
| 8 | AFSPd-Cu | 60 °C | 25.20 | Exp. |
| 9 | AFSPd-Cu (NP) | 30 °C | 11.91 | Exp. |
| 10 | AFSPd-Cu (NP) | 40 °C | 10.56 | Exp. |
| 11 | AFSPd-Cu (NP) | 50 °C | 11.41 | Exp. |
| 12 | AFSPd-Cu (NP) | 60 °C | 13.12 | Exp. |
| 13 | MPSilica | 30 °C | 9.83 | Exp. |
| 14 | MPSilica | 40 °C | 7.16 | Exp. |
| 15 | MPSilica | 50 °C | 5.93 | Exp. |
| 16 | MPSilica | 60 °C | 5.11 | Exp. |
| 17 | Silica gel | 40 °C | 26.39 | Exp. |
| 18 | Silica gel | 50 °C | 25.94 | Exp. |
| 19 | Silica gel | 60 °C | 25.86 | Exp. |
| 20 | Silica gel | 40 °C | 30 | [27] |
| 21 | TAPSO-34 | 40 °C | 28 | [27] |
| 22 | Silica gel | 25 °C | 34.35 | [2] |
| 23 | Silica gel | 40 °C | 34.21 | [2] |
| 24 | Silica gel | 60 °C | 33.79 | [2] |
| 25 | MIL-100(Fe) | 25 °C | 90 | [28] |
| 26 | MIL-100(Al) | 25 °C | 50 | [28] |
| 27 | MOF-841 | 25 °C | 64 | [29] |
| 28 | MOF-806 | 25 °C | 26 | [29] |
| 29 | Zeolite 13X | 25 °C | 33 | [29] |
| 30 | Zeolites | - | 11–38.7 | [30] |
| 31 | SMOF | - | 42 | [24] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sztekler, K.; Mlonka-Mędrala, A.; Khdary, N.H.; Kalawa, W.; Nowak, W.; Mika, Ł. Possibility of Advanced Modified-Silica-Based Porous Materials Utilisation in Water Adsorption Processes—A Comparative Study. Energies 2022, 15, 368. https://doi.org/10.3390/en15010368
Sztekler K, Mlonka-Mędrala A, Khdary NH, Kalawa W, Nowak W, Mika Ł. Possibility of Advanced Modified-Silica-Based Porous Materials Utilisation in Water Adsorption Processes—A Comparative Study. Energies. 2022; 15(1):368. https://doi.org/10.3390/en15010368
Chicago/Turabian StyleSztekler, Karol, Agata Mlonka-Mędrala, Nezar H. Khdary, Wojciech Kalawa, Wojciech Nowak, and Łukasz Mika. 2022. "Possibility of Advanced Modified-Silica-Based Porous Materials Utilisation in Water Adsorption Processes—A Comparative Study" Energies 15, no. 1: 368. https://doi.org/10.3390/en15010368
APA StyleSztekler, K., Mlonka-Mędrala, A., Khdary, N. H., Kalawa, W., Nowak, W., & Mika, Ł. (2022). Possibility of Advanced Modified-Silica-Based Porous Materials Utilisation in Water Adsorption Processes—A Comparative Study. Energies, 15(1), 368. https://doi.org/10.3390/en15010368









