Comparison of Nature and Synthetic Zeolite for Waste Battery Electrolyte Treatment in Fixed-Bed Adsorption Column
Abstract
:1. Introduction
2. Materials and Methods
2.1. Adsorption Experiment Raw Materials
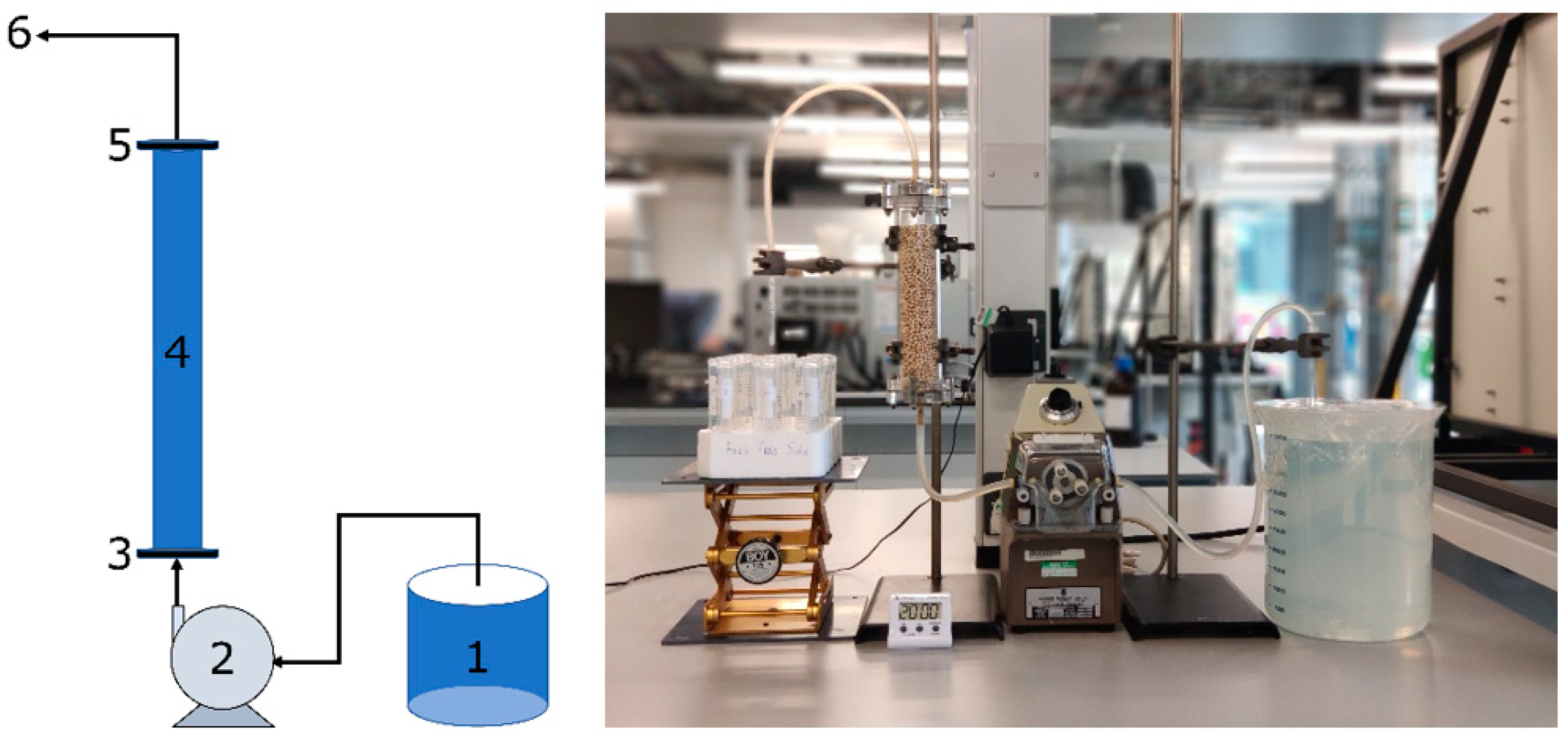
2.2. Fixed-Bed Adsorption
2.3. Characterization of the Zeolites Associated with Heavy Metal Adsorption Ions
2.4. Regeneration Experiments
3. Results
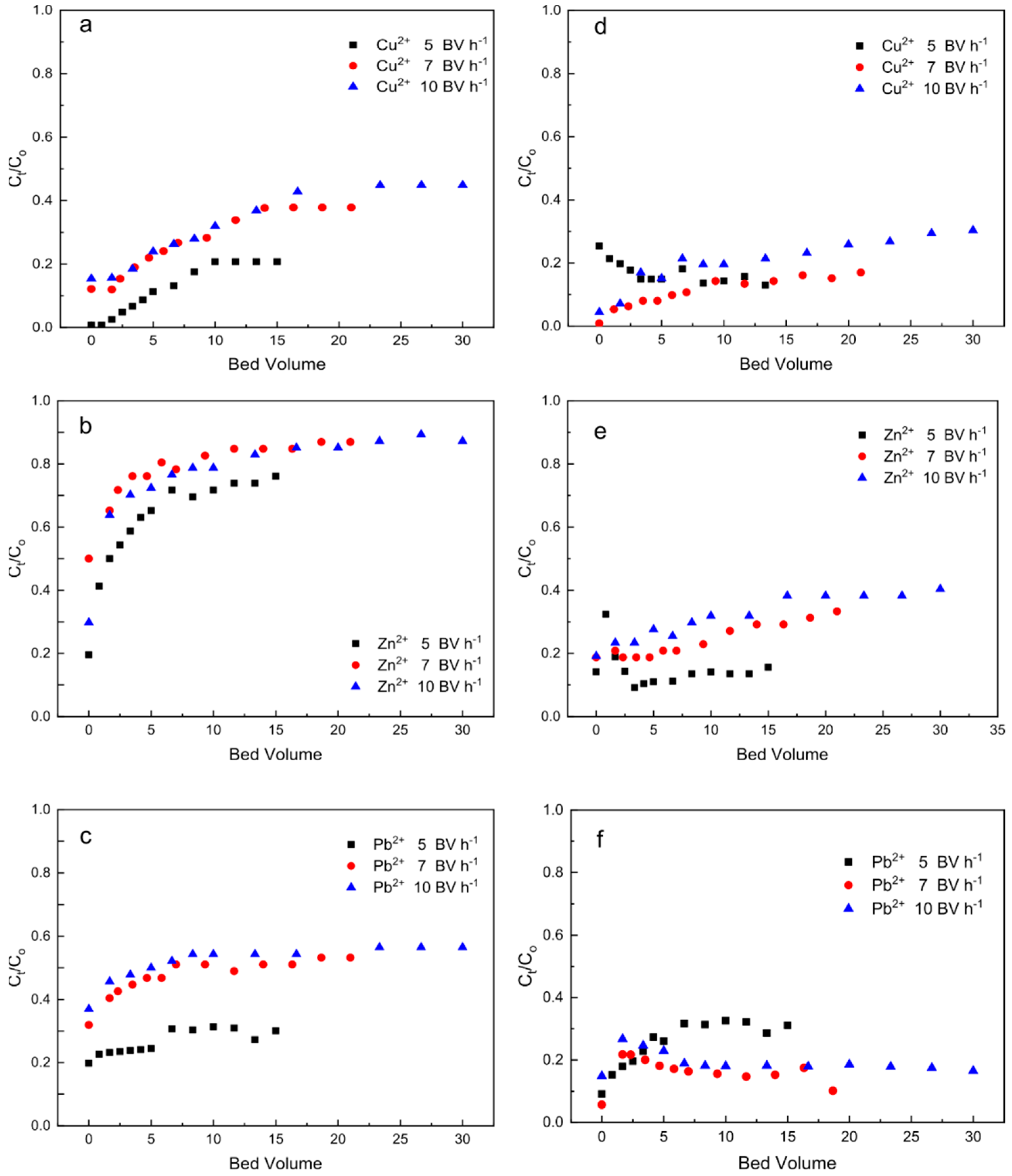
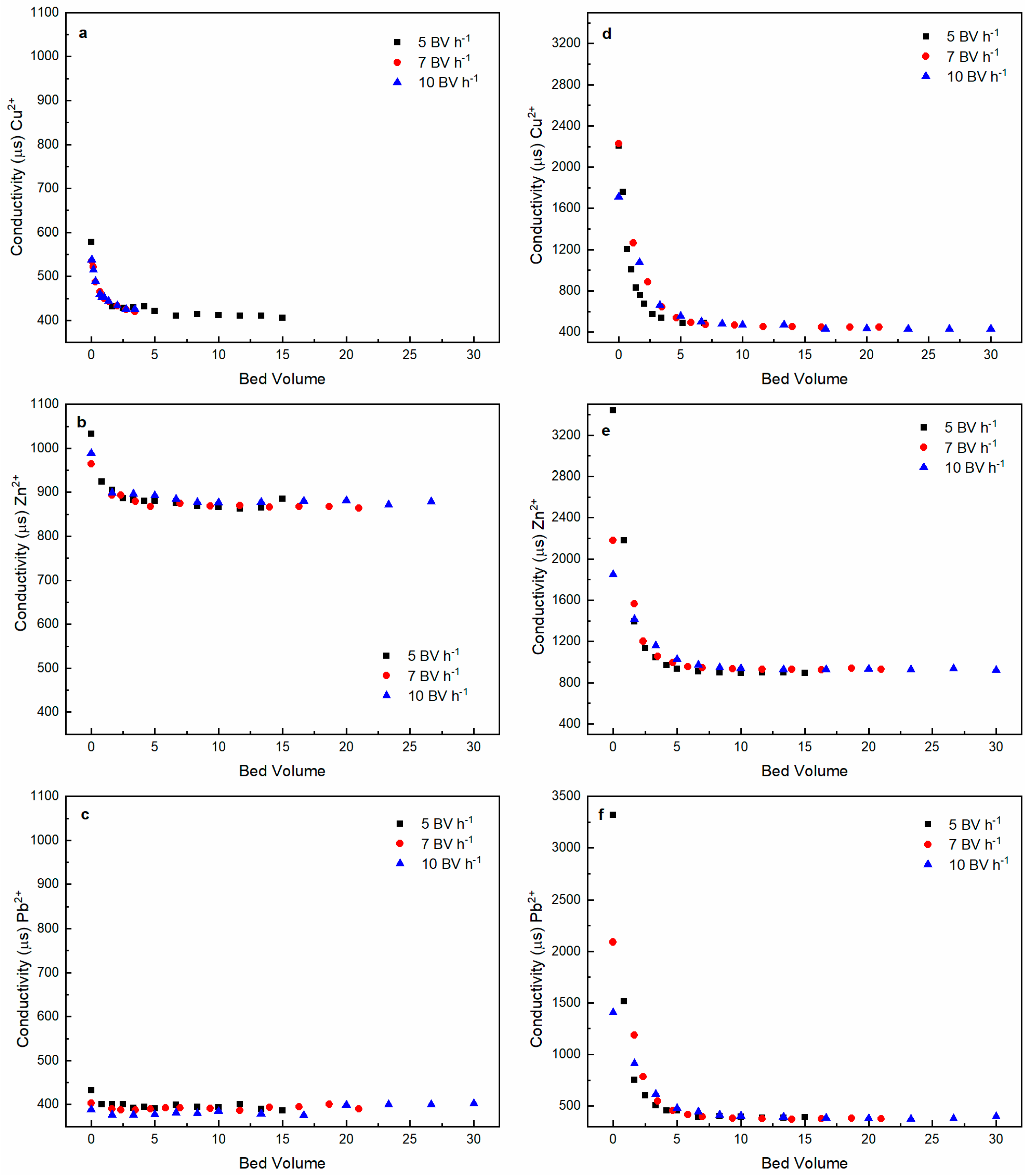
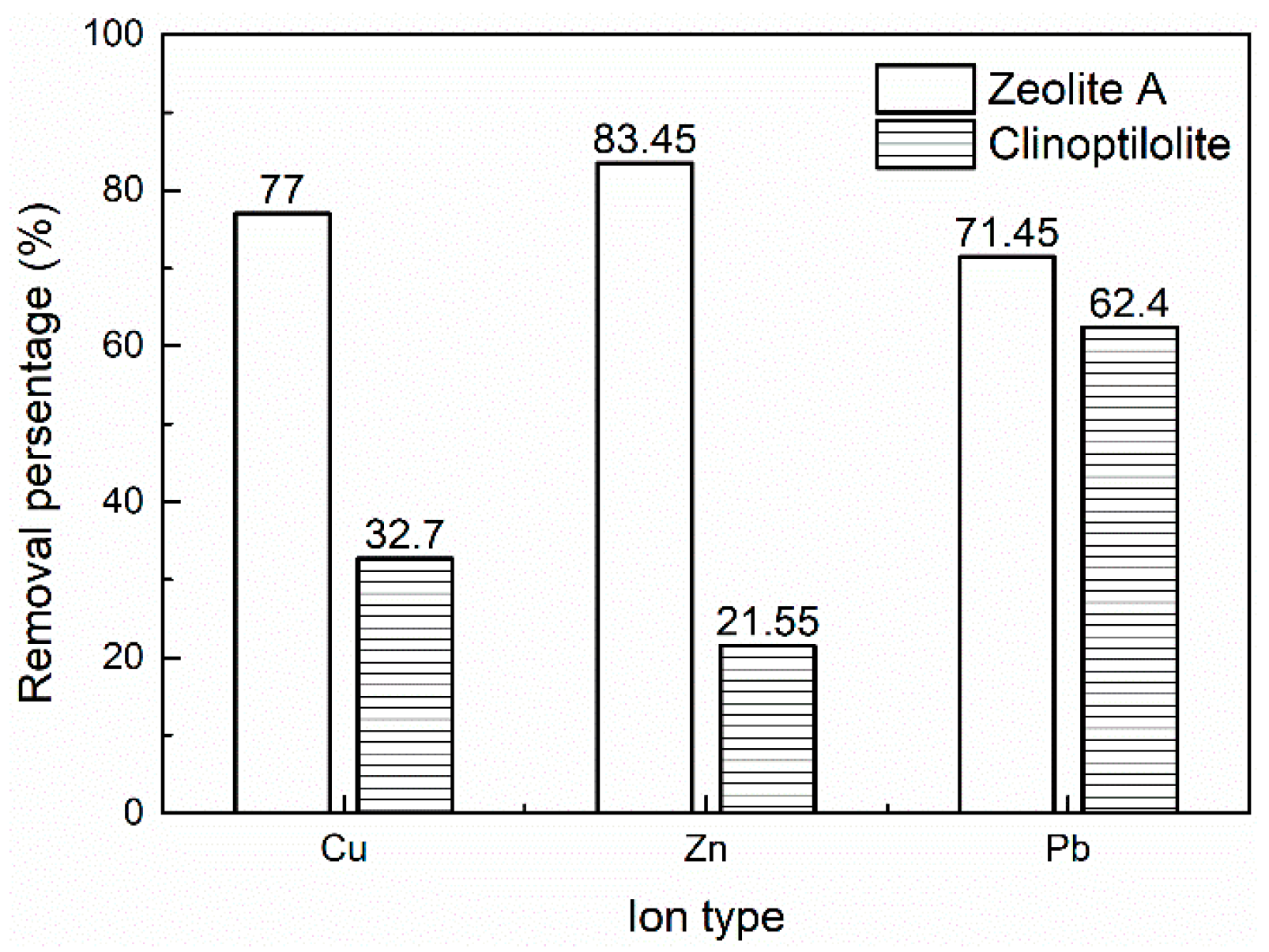
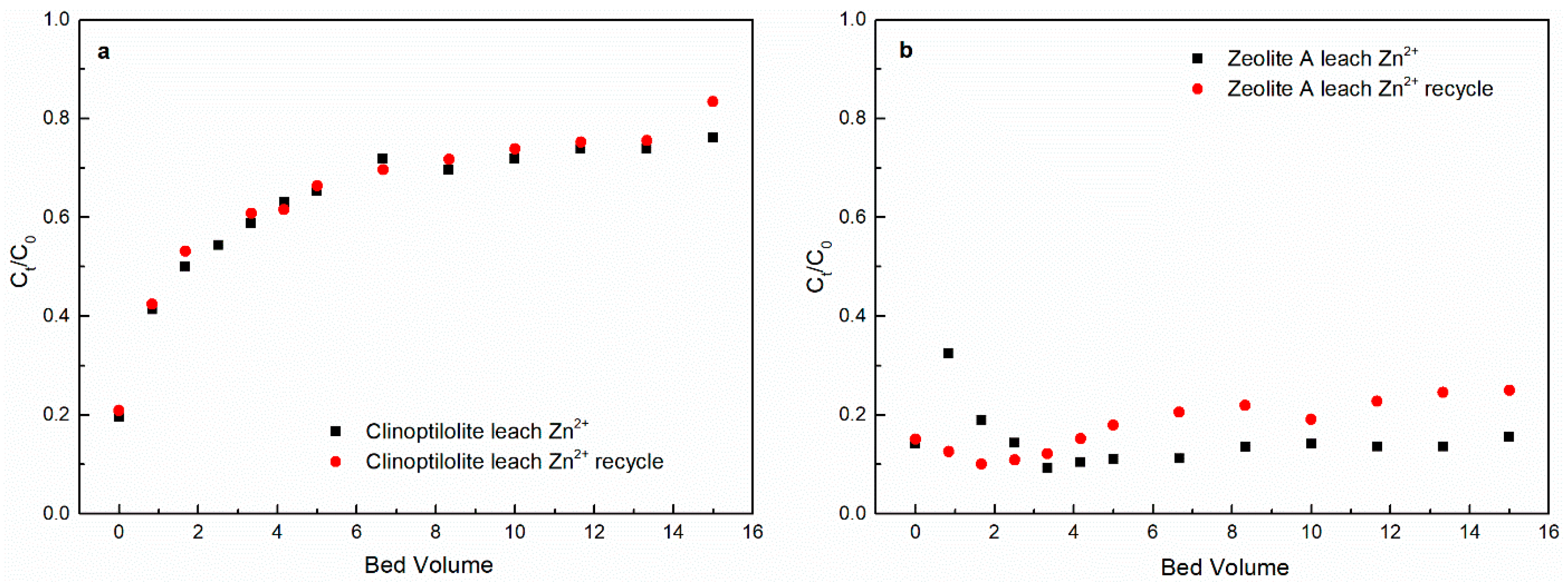
3.1. Heavy Metal Ions Leaching Experiments
3.2. Regeneration Experiments
3.3. Supportive Characterization of the Zeolites Associated with Heavy Metal Adsorption
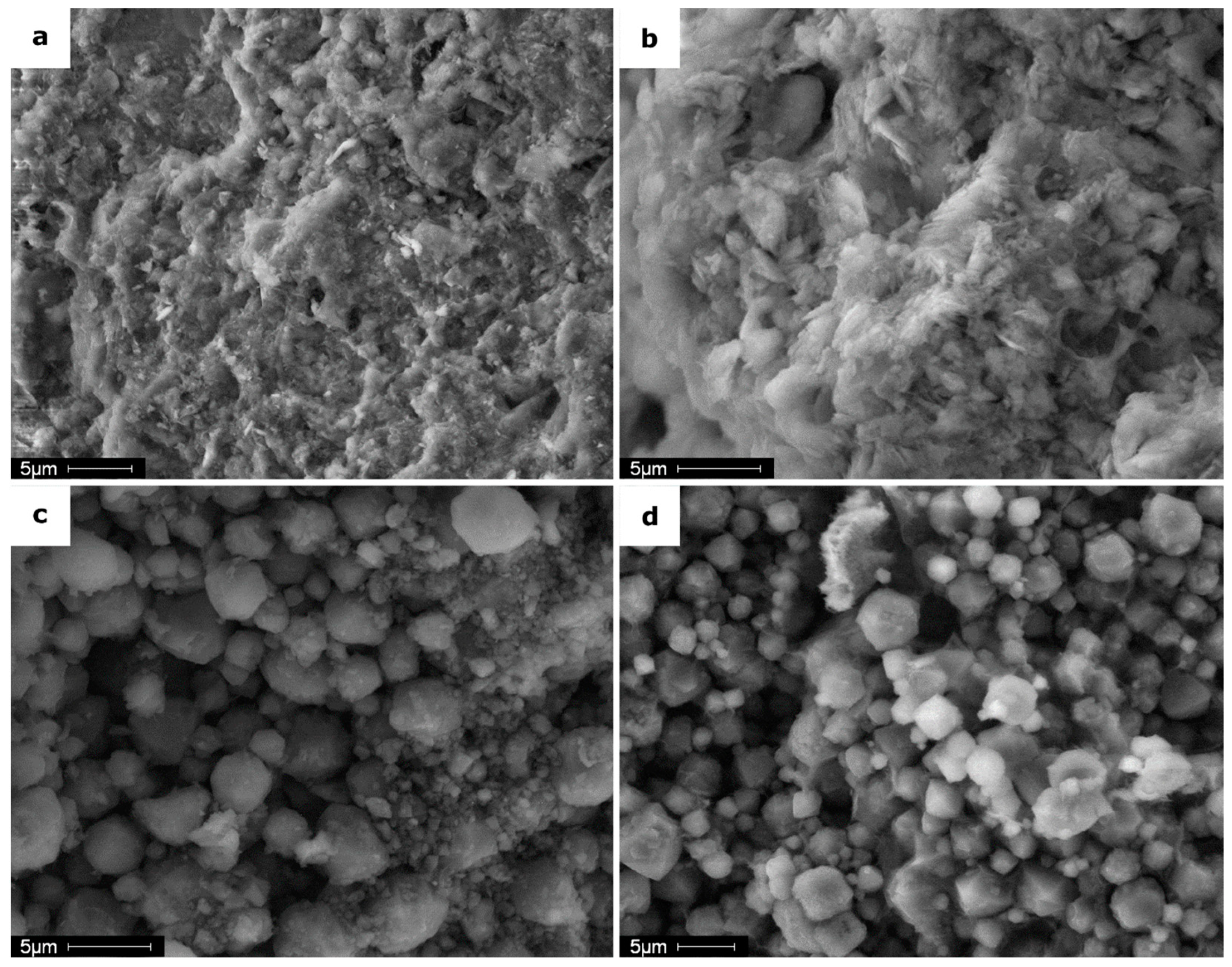
3.3.1. SEM and EDX
3.3.2. BET
3.3.3. XRD
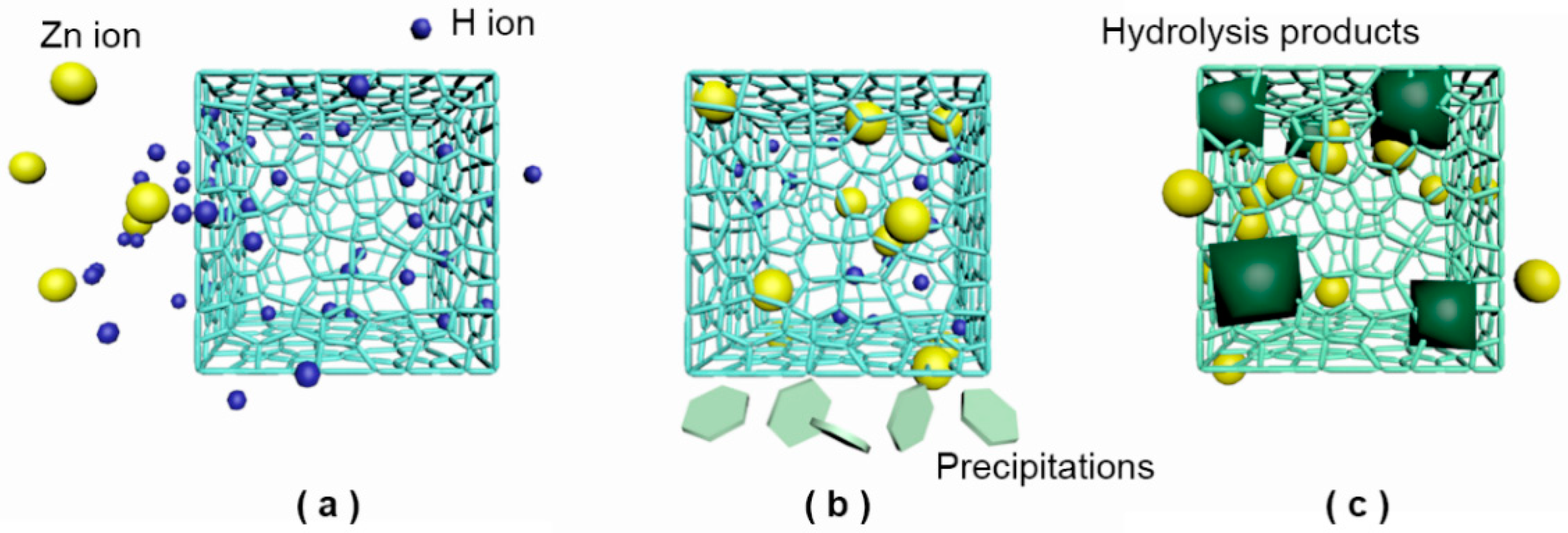
3.4. Leaching Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Juboori, O.; Sher, F.; Khalid, U.; Niazi, M.B.K.; Chen, G.Z. Electrochemical Production of Sustainable Hydrocarbon Fuels from CO2 Co-electrolysis in Eutectic Molten Melts. ACS Sustain. Chem. Eng. 2020, 8, 12877–12890. [Google Scholar] [CrossRef]
- Al-Juboori, O.; Sher, F.; Hazafa, A.; Khan, M.K.; Chen, G.Z. The effect of variable operating parameters for hydrocarbon fuel formation from CO2 by molten salts electrolysis. J. CO2 Util. 2020, 40, 101193. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Yaqoob, A.; Chen, G.Z. Electrochemical investigation of novel reference electrode Ni/Ni(OH)₂ in comparison with silver and platinum inert quasi-reference electrodes for electrolysis in eutectic molten hydroxide. Int. J. Hydrogen Energy 2019, 44, 27224–27236. [Google Scholar] [CrossRef]
- Al-Shara, N.K.; Sher, F.; Iqbal, S.Z.; Sajid, Z.; Chen, G.Z. Electrochemical study of different membrane materials for the fabrication of stable, reproducible and reusable reference electrode. J. Energy Chem. 2020, 49, 33–41. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.-M.J.S. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Guo, Z.; Ma, Y.; Bin, D.; Wang, Y.; Xia, Y. Recent Progress of Rechargeable Batteries Using Mild Aqueous Electrolytes. Small Methods 2019, 3, 1800272. [Google Scholar] [CrossRef] [Green Version]
- Leong, K.W.; Wang, Y.; Ni, M.; Pan, W.; Luo, S.; Leung, D.Y.C. Rechargeable Zn-air batteries: Recent trends and future perspectives. Renew. Sustain. Energy Rev. 2022, 154, 111771. [Google Scholar] [CrossRef]
- Heidari-Chaleshtori, M.; Nezamzadeh-Ejhieh, A. Clinoptilolite nano-particles modified with aspartic acid for removal of Cu(II) from aqueous solutions: Isotherms and kinetic aspects. New J. Chem. 2015, 39, 9396–9406. [Google Scholar] [CrossRef]
- Enya, O.; Lin, C.; Qin, J. Heavy metal contamination status in soil-plant system in the Upper Mersey Estuarine Floodplain, Northwest England. Mar. Pollut. Bull. 2019, 146, 292–304. [Google Scholar] [CrossRef]
- Tamiji, T.; Nezamzadeh-Ejhieh, A. A comprehensive study on the kinetic aspects and experimental design for the voltammetric response of a Sn(IV)-clinoptilolite carbon paste electrode towards Hg(II). J. Electroanal. Chem. 2018, 829, 95–105. [Google Scholar] [CrossRef]
- Nosuhi, M.; Nezamzadeh-Ejhieh, A. Voltammetric determination of trace amounts of permanganate at a zeolite modified carbon paste electrode. New J. Chem. 2017, 41, 15508–15516. [Google Scholar] [CrossRef]
- Luo, X.; Yuan, J.; Liu, Y.; Liu, C.; Zhu, X.; Dai, X.; Ma, Z.; Wang, F. Improved Solid-Phase Synthesis of Phosphorylated Cellulose Microsphere Adsorbents for Highly Effective Pb2+ Removal from Water: Batch and Fixed-Bed Column Performance and Adsorption Mechanism. ACS Sustain. Chem. Eng. 2017, 5, 5108–5117. [Google Scholar] [CrossRef]
- Shirzadi, H.; Nezamzadeh-Ejhieh, A. An efficient modified zeolite for simultaneous removal of Pb(II) and Hg(II) from aqueous solution. J. Mol. Liq. 2017, 230, 221–229. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Haddad, S.A.; Lemanowicz, J. Benefits of Corn-Cob Biochar to the Microbial and Enzymatic Activity of Soybean Plants Grown in Soils Contaminated with Heavy Metals. Energies 2021, 14, 5763. [Google Scholar] [CrossRef]
- Ku, Y.; Jung, I.-L. Photocatalytic reduction of Cr(VI) in aqueous solutions by UV irradiation with the presence of titanium dioxide. Water Res. 2001, 35, 135–142. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Sillanpää, M. Chapter 1—Electrocoagulation in the treatment of industrial waters and wastewaters. In Advanced Water Treatment; Sillanpää, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–78. [Google Scholar]
- Kurniawan, T.A.; Chan, G.Y.S.; Lo, W.-H.; Babel, S. Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem. Eng. J. 2006, 118, 83–98. [Google Scholar] [CrossRef]
- Pessoa, M.E.; de Sousa, K.S.; Clericuzi, G.Z.; Ferreira, A.L.; Soares, M.C.; Neto, J.C. Adsorption of Reactive Dye onto Uçá Crab Shell (Ucides cordatus): Scale-Up and Comparative Studies. Energies 2021, 14, 5876. [Google Scholar] [CrossRef]
- Yang, C.; Wang, L.; Yu, Y.; Wu, P.; Wang, F.; Liu, S.; Luo, X. Highly efficient removal of amoxicillin from water by Mg-Al layered double hydroxide/cellulose nanocomposite beads synthesized through in-situ coprecipitation method. Int. J. Biol. Macromol. 2020, 149, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Burakov, A.E.; Galunin, E.V.; Burakova, I.V.; Kucherova, A.E.; Agarwal, S.; Tkachev, A.G.; Gupta, V.K. Adsorption of heavy metals on conventional and nanostructured materials for wastewater treatment purposes: A review. Ecotoxicol. Environ. Saf. 2018, 148, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, J.-R.; Wang, K.; Han, T.; Tong, M.; Li, L.; Xie, Y.; Yang, Q.; Liu, D.; Zhong, C. An in situ self-assembly template strategy for the preparation of hierarchical-pore metal-organic frameworks. Nat. Commun. 2015, 6, 8847. [Google Scholar] [CrossRef] [Green Version]
- Haw, J.F. Zeolite acid strength and reaction mechanisms in catalysis. Phys. Chem. Chem. Phys. 2002, 4, 5431–5441. [Google Scholar] [CrossRef]
- Perić, J.; Trgo, M.; Vukojević Medvidović, N. Removal of zinc, copper and lead by natural zeolite—A comparison of adsorption isotherms. Water Res. 2004, 38, 1893–1899. [Google Scholar] [CrossRef]
- Vaca Mier, M.; López Callejas, R.; Gehr, R.; Jiménez Cisneros, B.E.; Alvarez, P.J.J. Heavy metal removal with mexican clinoptilolite: Multi-component ionic exchange. Water Res. 2001, 35, 373–378. [Google Scholar] [CrossRef]
- Alidusty, F.; Nezamzadeh-Ejhieh, A. Considerable decrease in overvoltage of electro-catalytic oxidation of methanol by modification of carbon paste electrode with Cobalt(II)-clinoptilolite nanoparticles. Int. J. Hydrogen Energy 2016, 41, 6288–6299. [Google Scholar] [CrossRef]
- Nosuhi, M.; Nezamzadeh-Ejhieh, A. High catalytic activity of Fe(II)-clinoptilolite nanoparticales for indirect voltammetric determination of dichromate: Experimental design by response surface methodology (RSM). Electrochim. Acta 2017, 223, 47–62. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Zanin, E.; Scapinello, J.; de Oliveira, M.; Rambo, C.L.; Franscescon, F.; Freitas, L.; de Mello, J.M.M.; Fiori, M.A.; Oliveira, J.V.; Dal Magro, J. Adsorption of heavy metals from wastewater graphic industry using clinoptilolite zeolite as adsorbent. Process Saf. Environ. Prot. 2017, 105, 194–200. [Google Scholar] [CrossRef]
- Kocasoy, G.; Şahin, V. Heavy metal removal from industrial wastewater by clinoptilolite. J. Environ. Sci. Health Part A 2007, 42, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Çoruh, S. The removal of zinc ions by natural and conditioned clinoptilolites. Desalination 2008, 225, 41–57. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Banan, Z. A comparison between the efficiency of CdS nanoparticles/zeolite A and CdO/zeolite A as catalysts in photodecolorization of crystal violet. Desalination 2011, 279, 146–151. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Mirzaeyan, E. Hexadecylpyridinium surfactant modified zeolite A as an active component of a polymeric membrane sulfite selective electrode. Mater. Sci. Eng. C 2013, 33, 4751–4758. [Google Scholar] [CrossRef]
- Fareed, B.; Sher, F.; Sehar, S.; Rasheed, T.; Zafar, F.; Ameen, M.; Lima, E.C. Tailor made Functional Zeolite as Sustainable Potential Candidates for Catalytic Cracking of Heavy Hydrocarbons. Catal. Lett. 2021, 1–13. [Google Scholar] [CrossRef]
- Turan, M.; Mart, U.; Yüksel, B.; Çelik, M.S. Lead removal in fixed-bed columns by zeolite and sepiolite. Chemosphere 2005, 60, 1487–1492. [Google Scholar] [CrossRef]
- Netzer, A.; Hughes, D.E. Adsorption of copper, lead and cobalt by activated carbon. Water Res. 1984, 18, 927–933. [Google Scholar] [CrossRef]
- Arulanantham, A.; Balasubramanian, N.; Ramakrishna, T.V. Coconut shell carbon for treatment of cadmium and lead containing wastewater. Met. Finish. 1989, 87, 51–55. [Google Scholar]
- Stylianou, M.A.; Hadjiconstantinou, M.P.; Inglezakis, V.J.; Moustakas, K.G.; Loizidou, M.D. Use of natural clinoptilolite for the removal of lead, copper and zinc in fixed bed column. J. Hazard. Mater. 2007, 143, 575–581. [Google Scholar] [CrossRef]
- Demirel, S.; Uyanik, I. Simultaneous fluoride and nitrate removal from drinking water using mixotrophic denitrification processes in a fixed bed column reactor. Desalination Water Treat. 2019, 164, 56–61. [Google Scholar] [CrossRef]
- Liu, H.; Peng, S.; Shu, L.; Chen, T.; Bao, T.; Frost, R.L. Magnetic zeolite NaA: Synthesis, characterization based on metakaolin and its application for the removal of Cu2+, Pb2+. Chemosphere 2013, 91, 1539–1546. [Google Scholar] [CrossRef]
- Wieland, E.; Stumm, W. Dissolution kinetics of kaolinite in acidic aqueous solutions at 25 °C. Geochim. Cosmochim. Acta 1992, 56, 3339–3355. [Google Scholar] [CrossRef]
- Ames, L.L., Jr. Cation sieve properties of the open zeolites chabazite, mordenite, erionite and clinoptilolite. Am. Mineral. 1961, 46, 1120–1131. [Google Scholar]
- Neveu, A.; Gaspard, M.; Blanchard, G.; Martin, G. La diffusion intraparticulaire dans la clinoptilolite application aux ions Na+ et NH4+. Water Res. 1985, 19, 611–618. [Google Scholar] [CrossRef]
- Kurtoğlu, A.E.; Atun, G. Determination of kinetics and equilibrium of Pb/Na exchange on clinoptilolite. Sep. Purif. Technol. 2006, 50, 62–70. [Google Scholar] [CrossRef]
- Inglezakis, V.J.; Stylianou, M.A.; Gkantzou, D.; Loizidou, M.D. Removal of Pb(II) from aqueous solutions by using clinoptilolite and bentonite as adsorbents. Desalination 2007, 210, 248–256. [Google Scholar] [CrossRef]
- Mehrali-Afjani, M.; Nezamzadeh-Ejhieh, A. Efficient solid amino acid-clinoptilolite nanoparticles adsorbent for Mn(II) removal: A comprehensive study on designing the experiments, thermodynamic and kinetic aspects. Solid State Sci. 2020, 101, 106124. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Afshari, E. Modification of a PVC-membrane electrode by surfactant modified clinoptilolite zeolite towards potentiometric determination of sulfide. Microporous Mesoporous Mater. 2012, 153, 267–274. [Google Scholar] [CrossRef]
- Luo, X.; Lei, X.; Cai, N.; Xie, X.; Xue, Y.; Yu, F. Removal of Heavy Metal Ions from Water by Magnetic Cellulose-Based Beads with Embedded Chemically Modified Magnetite Nanoparticles and Activated Carbon. ACS Sustain. Chem. Eng. 2016, 4, 3960–3969. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Moeinirad, S. Heterogeneous photocatalytic degradation of furfural using NiS-clinoptilolite zeolite. Desalination 2011, 273, 248–257. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Shirvani, K. CdS loaded an Iranian clinoptilolite as a heterogeneous catalyst in photodegradation of p-aminophenol. J. Chem. 2013, 2013, 541736. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: Effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 2017, 321, 629–638. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Keane, M.A. The removal of copper and nickel from aqueous solution using Y zeolite ion exchangers. Colloids Surf. A Physicochem. Eng. Asp. 1998, 138, 11–20. [Google Scholar] [CrossRef]
- Cabrera, C.; Gabaldón, C.; Marzal, P. Sorption characteristics of heavy metal ions by a natural zeolite. J. Chem. Technol. Biotechnol. 2005, 80, 477–481. [Google Scholar] [CrossRef]
- Tamiji, T.; Nezamzadeh-Ejhieh, A. Sensitive voltammetric determination of bromate by using ion-exchange property of a Sn(II)-clinoptilolite-modified carbon paste electrode. J. Solid State Electrochem. 2019, 23, 143–157. [Google Scholar] [CrossRef]
- Ijagbemi, C.O.; Baek, M.H.; Kim, D.S. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. J. Hazard. Mater. 2009, 166, 538–546. [Google Scholar] [CrossRef] [PubMed]

| Removal Percentage (%) | ||
|---|---|---|
| Clinoptilolite leach Zn2+ at 5 BV h−1 | Original experiment | 23.91 |
| Regeneration experiment | 16.63 | |
| Zeolite A leach Zn2+ at 5 BV h−1 | Original experiment | 84.44 |
| Regeneration experiment | 74.99 |
| Element Content (%) | Si | Al | K | Na | Ca | Mg | O | Zn |
|---|---|---|---|---|---|---|---|---|
| Clean clinoptilolite | 62.59 | 15.11 | 7.01 | 0 | 3.59 | 3.38 | 8.31 | 0 |
| Zn2+-contaminated clinoptilolite | 61.69 | 12.26 | 5.31 | 0 | 5.21 | 2.65 | 11.55 | 1.33 |
| Clean zeolite A | 46.97 | 25.57 | 3.88 | 5.43 | 2.12 | 3.7 | 12.33 | 0 |
| Zn2+-contaminated zeolite A | 45.53 | 24.77 | 3.27 | 6.17 | 3.05 | 3.49 | 11.69 | 2.03 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| BET surface area (m2 g−1) | 30.99 | 44.17 | 40.69 | 301.48 |
| Micropore Volume (×10−2 cm3 g−1 STP) | 0.17 | 0.31 | 1.07 | 1.40 |
| Micropore Area (m2 g−1) | 2.74 | 5.23 | 18.68 | 244.28 |
| External Surface Area (m2 g−1) | 28.26 | 38.95 | 22.01 | 57.20 |
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| Major Phase | HEU (Heulandite) | CHA (Chabazite) | Sodium Aluminum Silicate | Sodium Zinc Aluminum Silicate |
| a (Å) | 17.536 | 8.845 | 24.9506 | 12.152 |
| b (Å) | 17.277 | 16.607 | 24.9506 | 12.152 |
| c (Å) | 7.409 | 9.746 | 24.9506 | 12.152 |
| α | 90° | 90° | 90° | 90° |
| β | 116.62° | 123.19° | 90° | 90° |
| γ | 90° | 90° | 90° | 90° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Wang, Y.; Alfutimie, A. Comparison of Nature and Synthetic Zeolite for Waste Battery Electrolyte Treatment in Fixed-Bed Adsorption Column. Energies 2022, 15, 347. https://doi.org/10.3390/en15010347
Yang C, Wang Y, Alfutimie A. Comparison of Nature and Synthetic Zeolite for Waste Battery Electrolyte Treatment in Fixed-Bed Adsorption Column. Energies. 2022; 15(1):347. https://doi.org/10.3390/en15010347
Chicago/Turabian StyleYang, Cong, Yifei Wang, and Abdullatif Alfutimie. 2022. "Comparison of Nature and Synthetic Zeolite for Waste Battery Electrolyte Treatment in Fixed-Bed Adsorption Column" Energies 15, no. 1: 347. https://doi.org/10.3390/en15010347
APA StyleYang, C., Wang, Y., & Alfutimie, A. (2022). Comparison of Nature and Synthetic Zeolite for Waste Battery Electrolyte Treatment in Fixed-Bed Adsorption Column. Energies, 15(1), 347. https://doi.org/10.3390/en15010347







