Three-Dimensional Computation Fluid Dynamics Simulation of CO Methanation Reactor with Immersed Tubes
Abstract
:1. Introduction
2. Mathematical Models
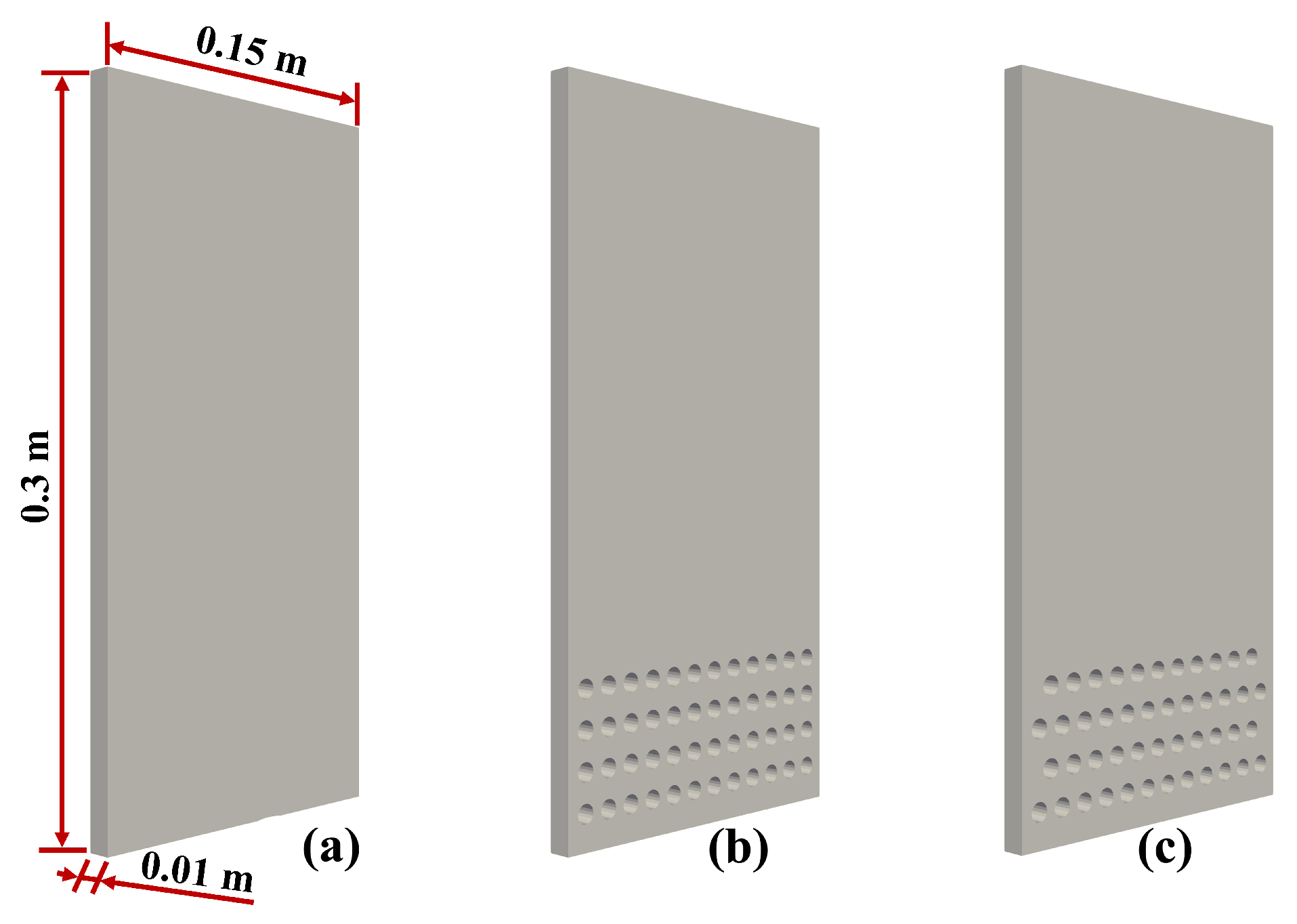
3. Setup of Simulation
4. Results and Discussion
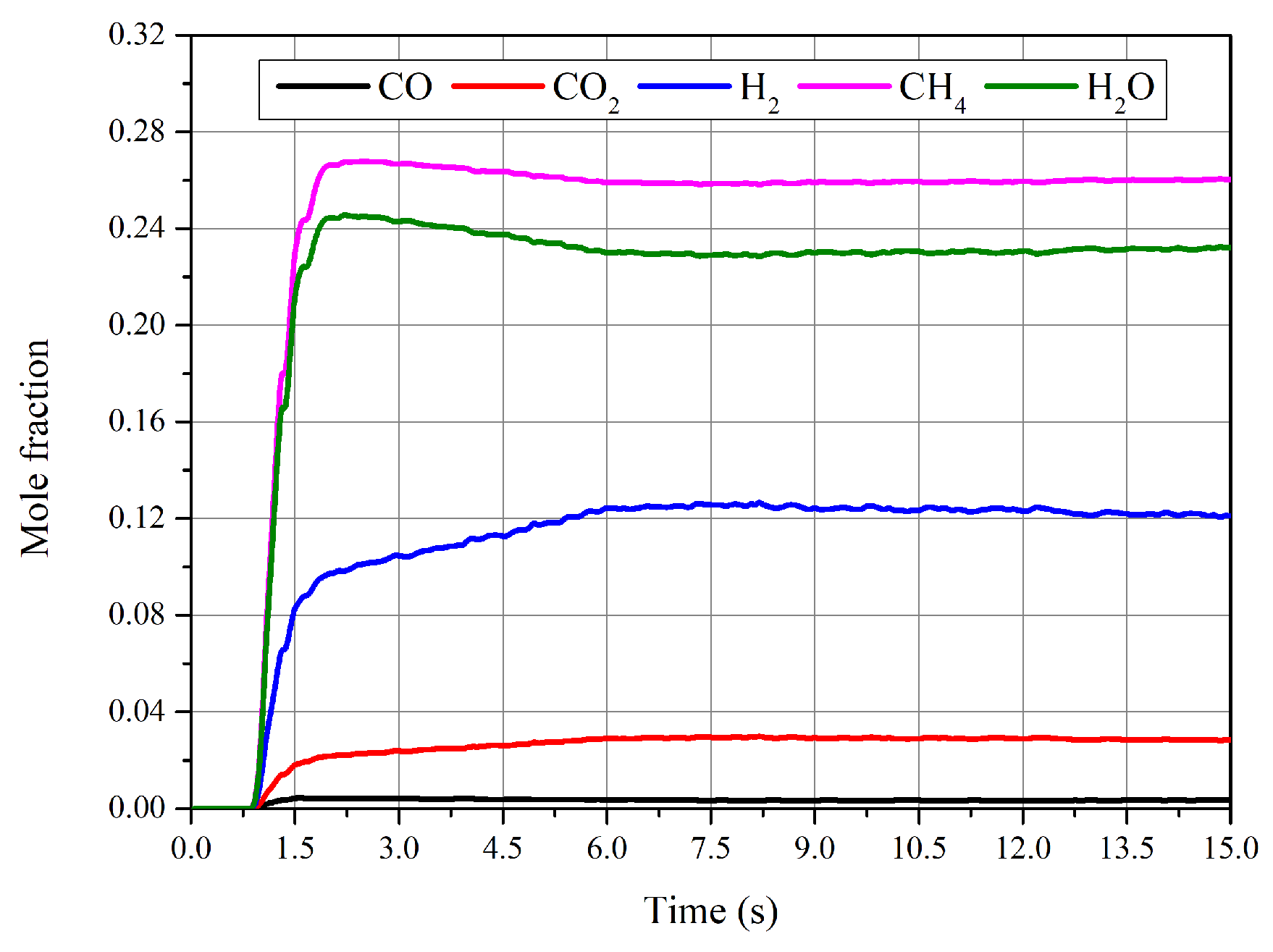
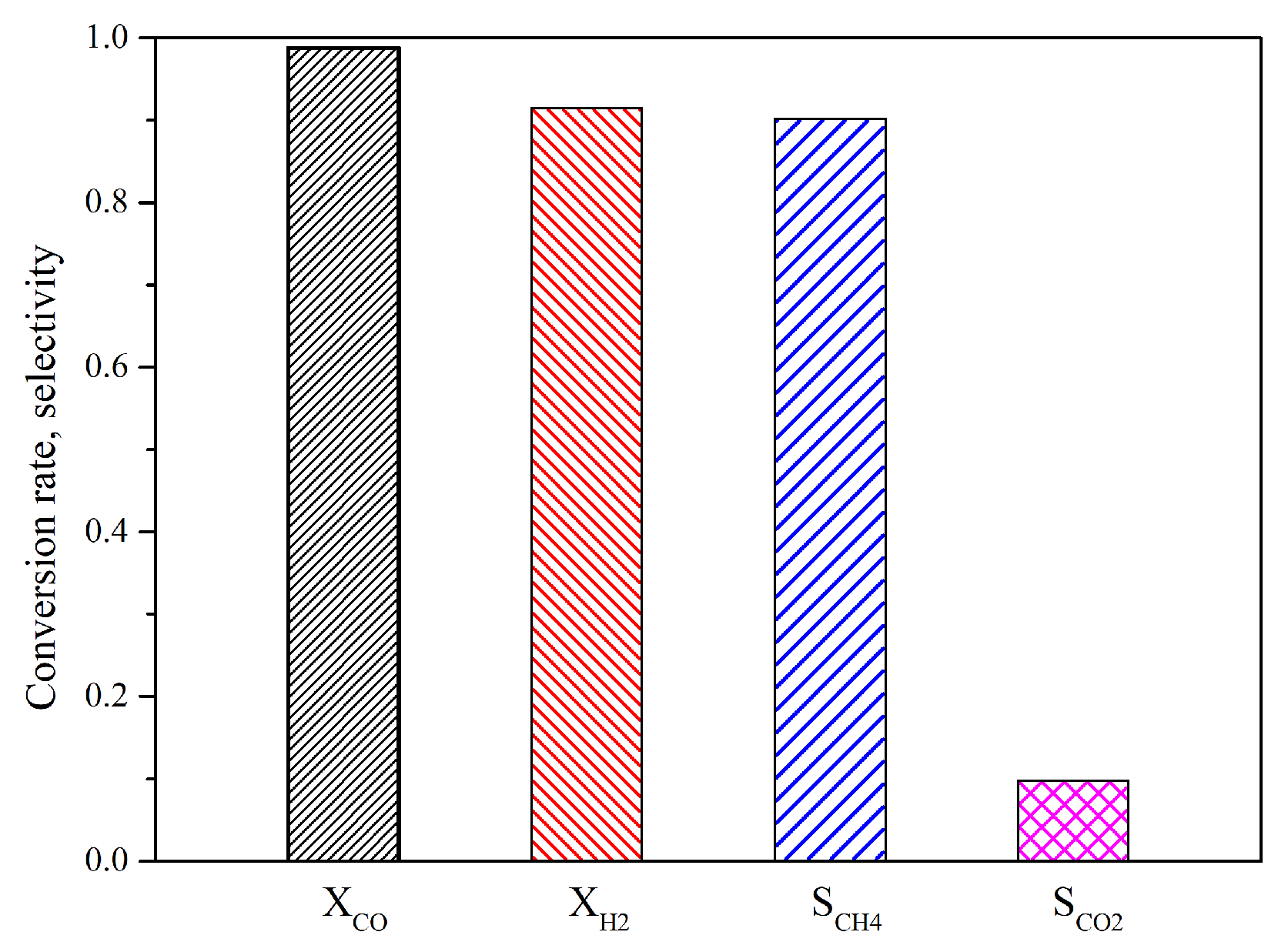
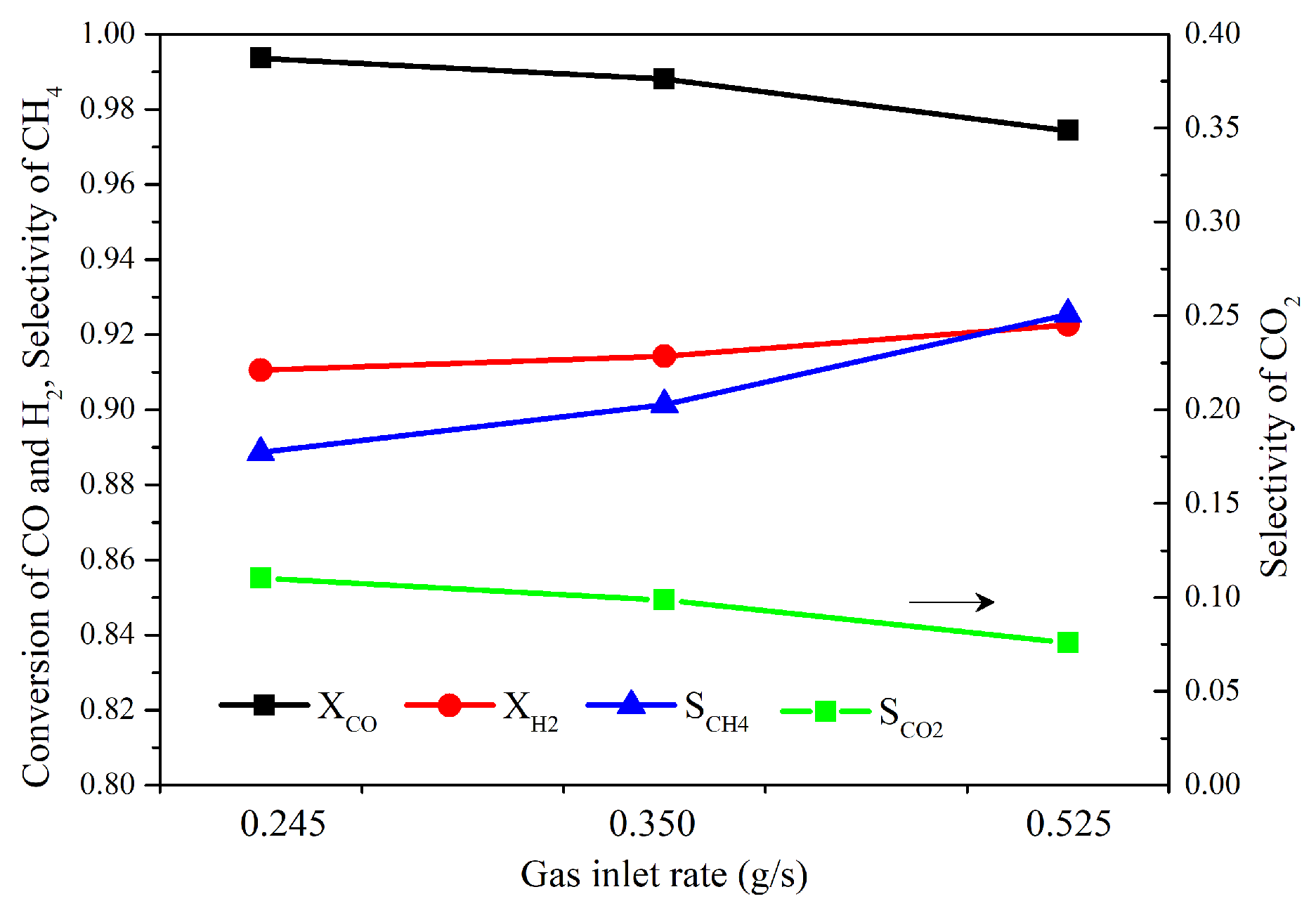
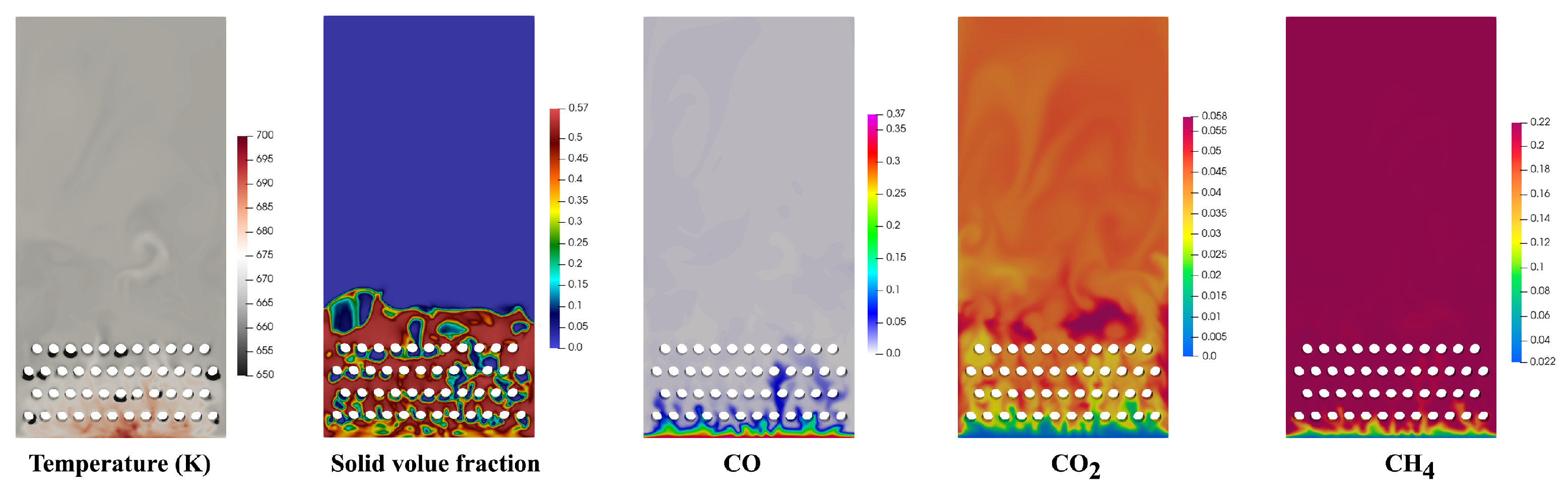
4.1. Performance of Reactor
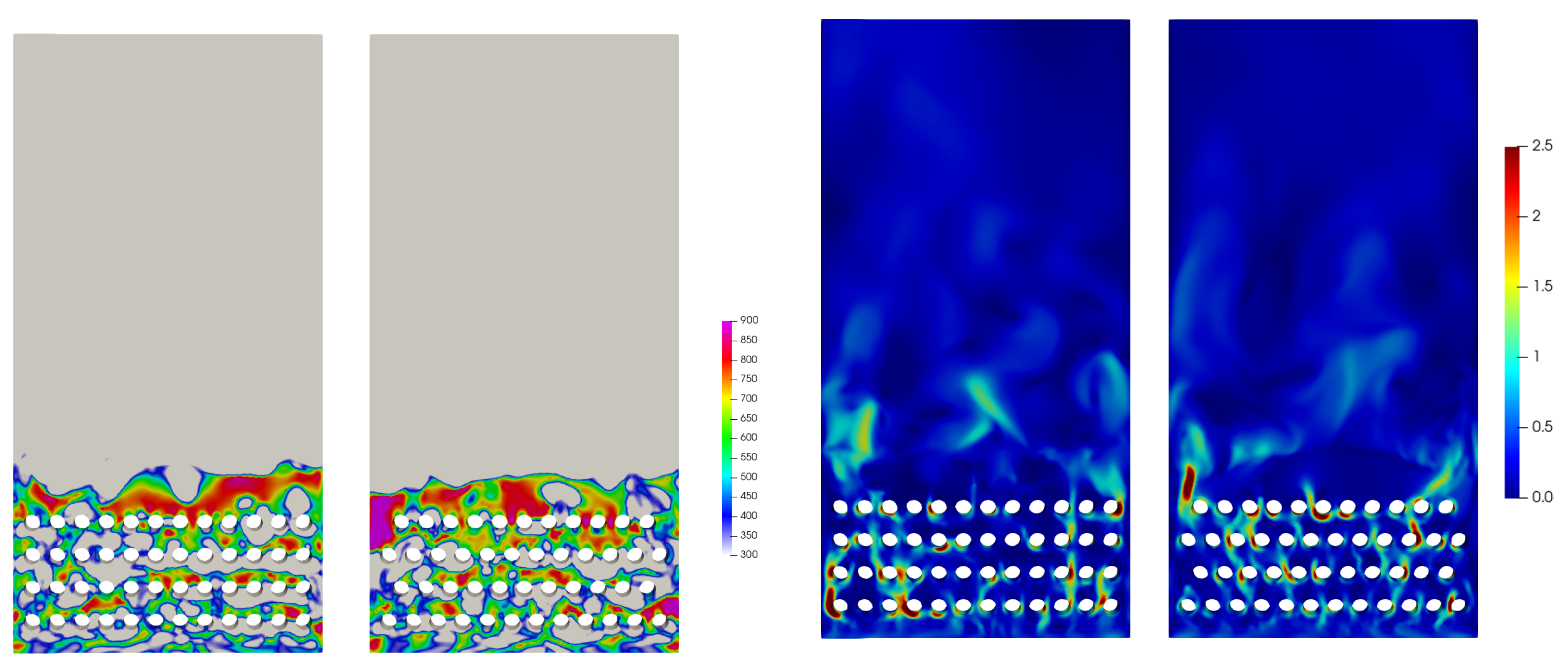
4.2. Influence of the Temperature
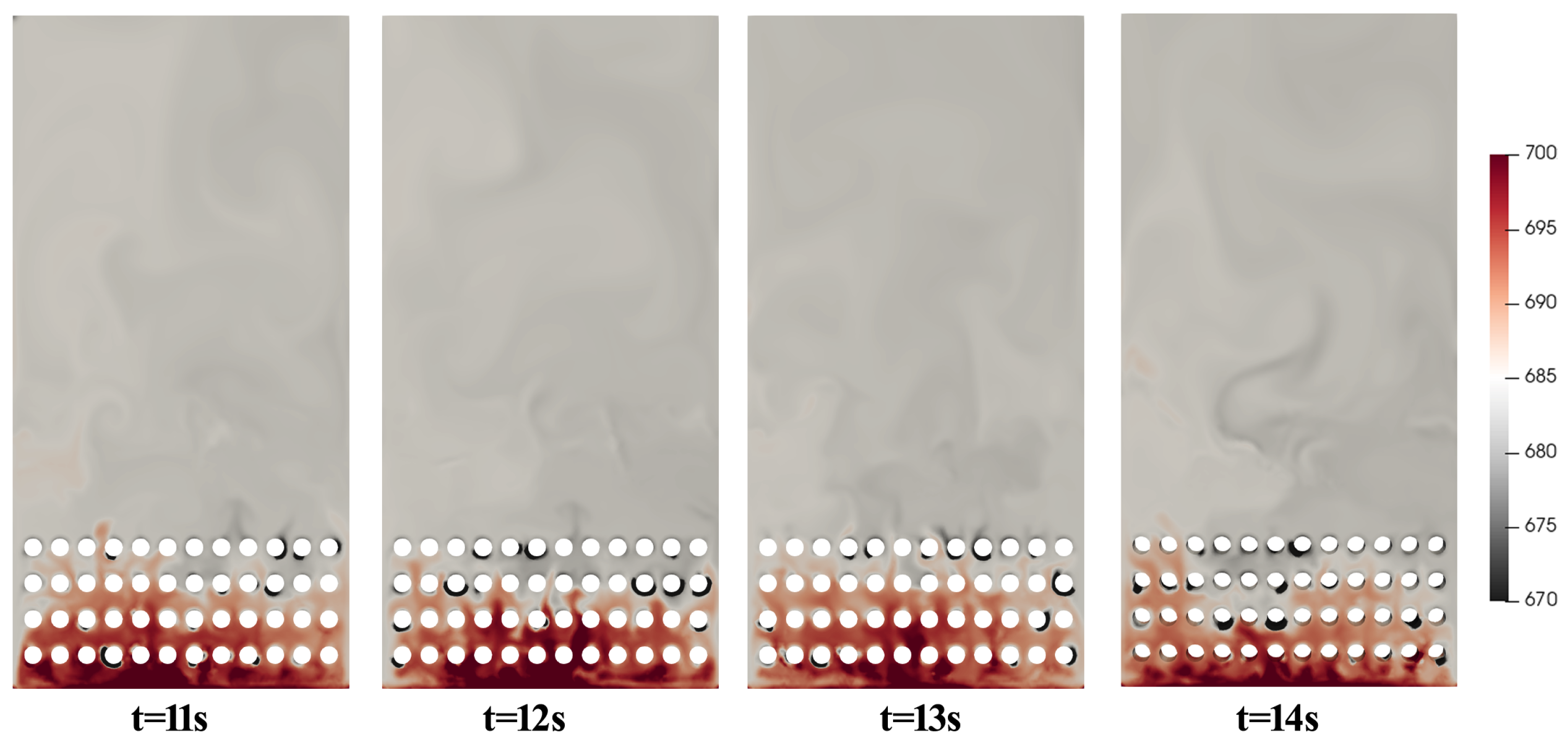
4.3. Influence of Flow Behavior
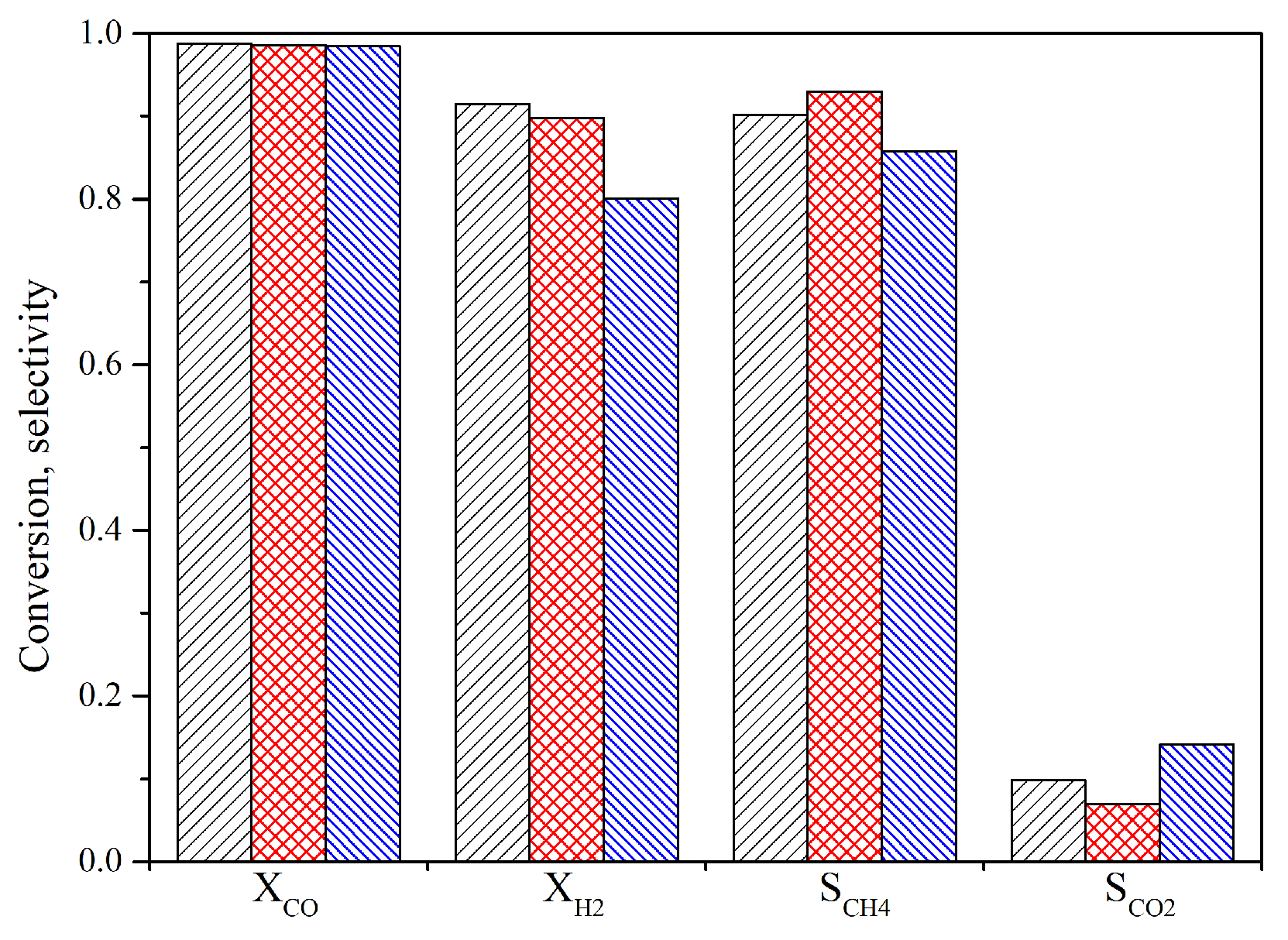
4.4. Influence of the Arrangement of Tubes
5. Conclusions
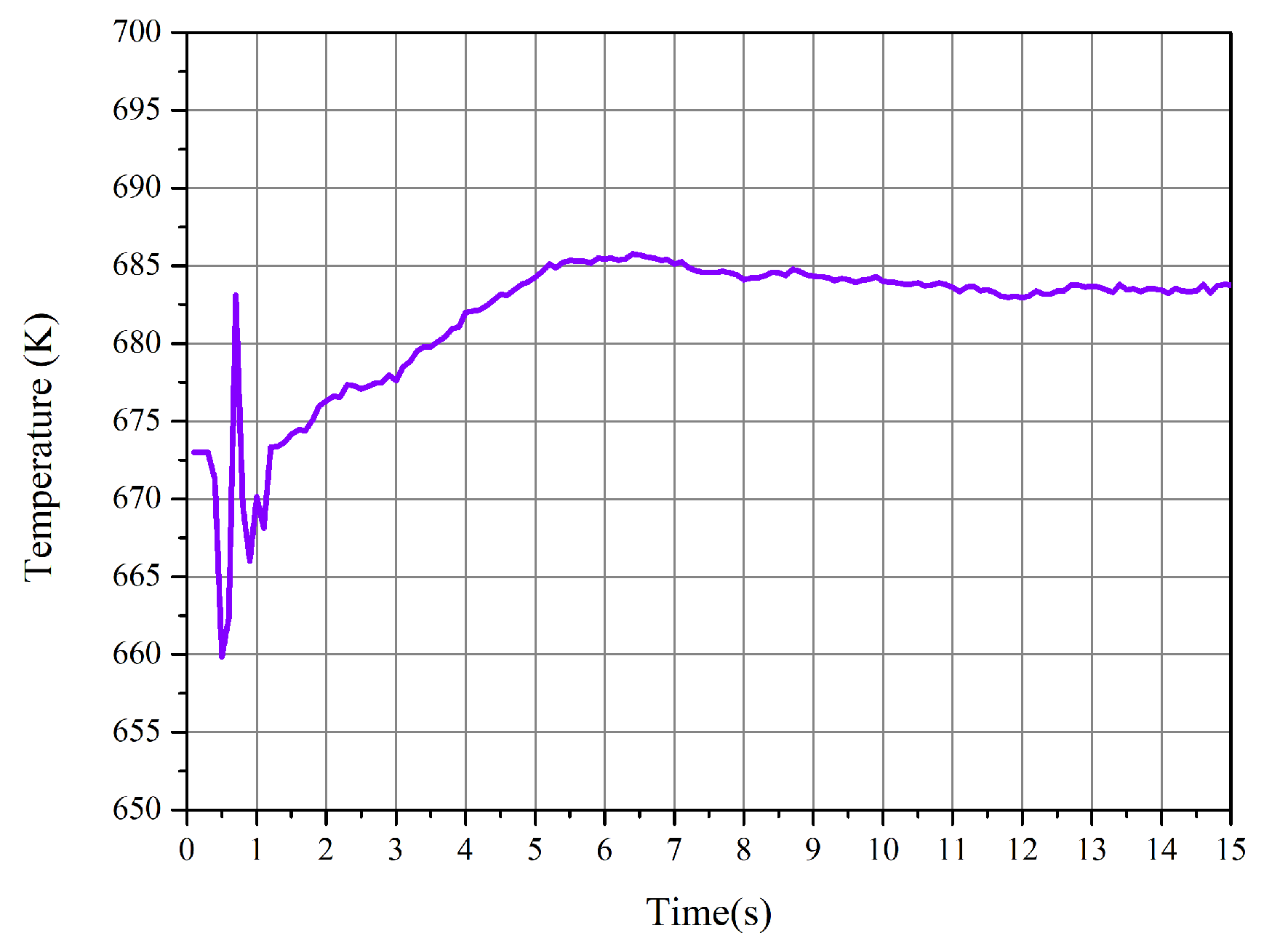
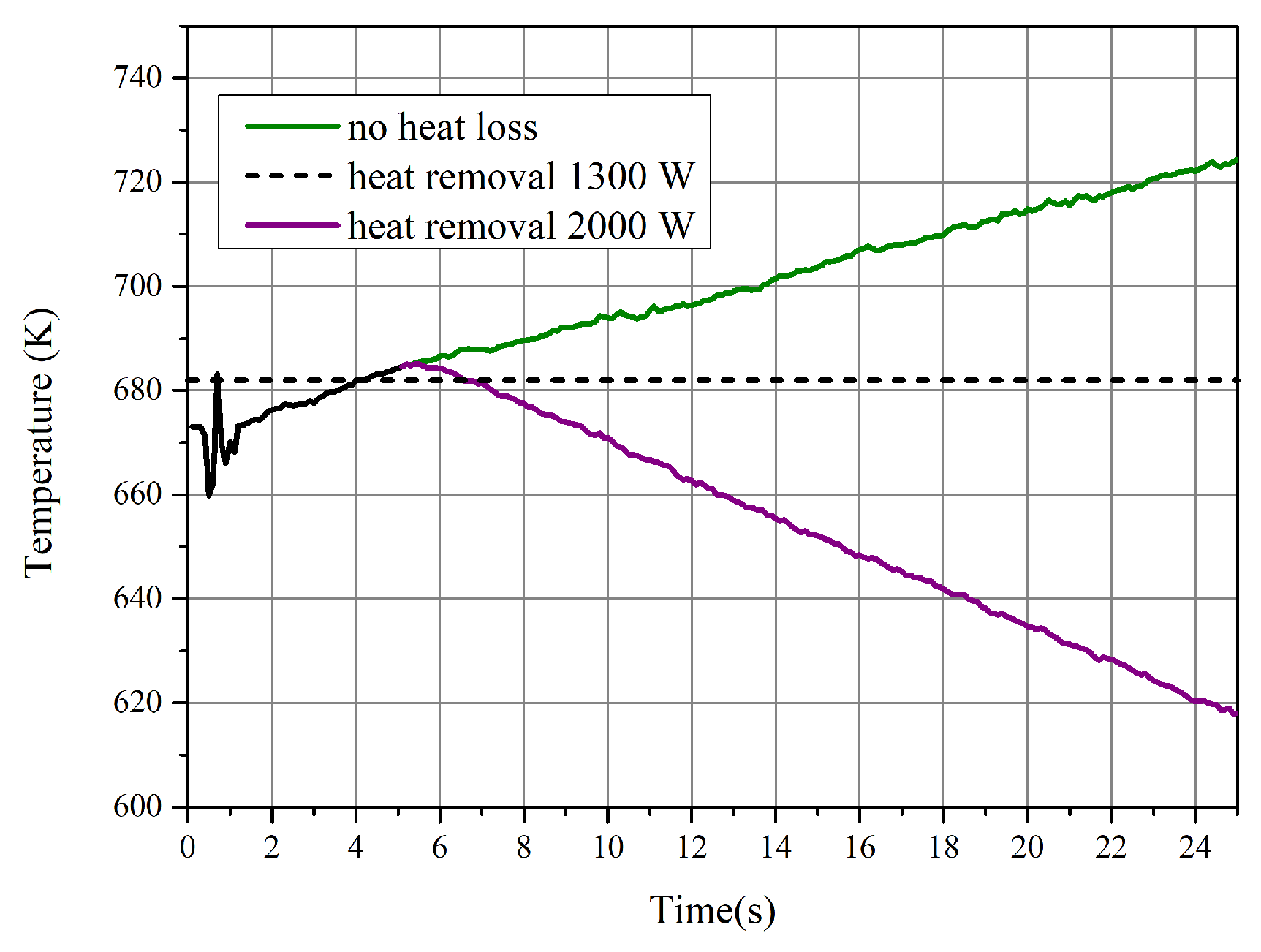
- The chemical equilibrium of the methanation process was achieved during the CFD simulation with effective heat removal by immersed pipes, and the preferable temperature was about 682 K. The results also show that the temperature has an essential impact on the production of methane.
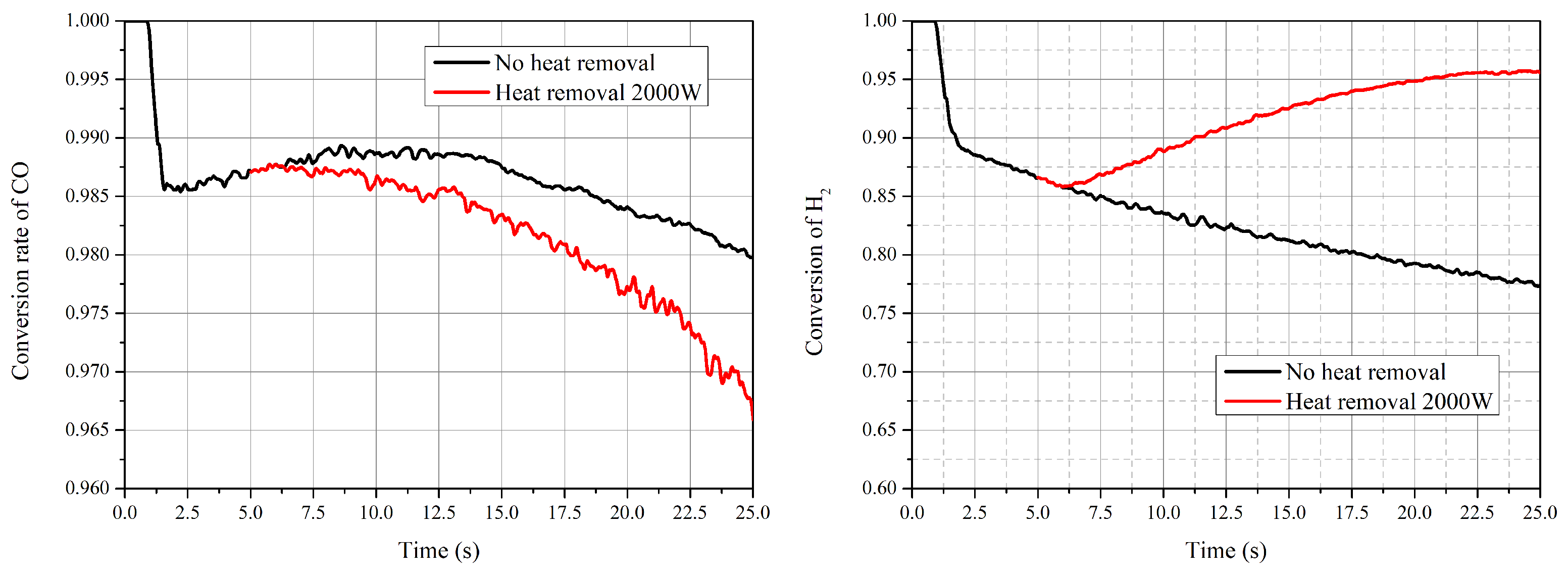
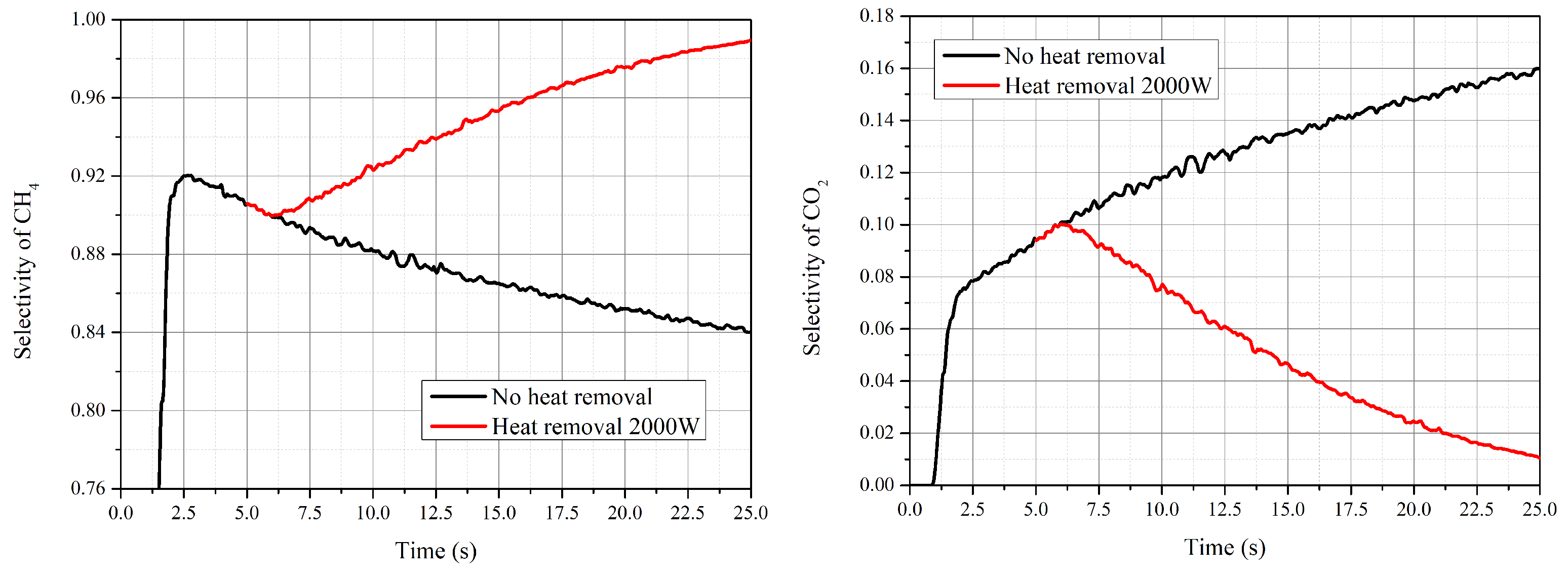
- The reaction finished near the inlet of the reactor, and then the main process was the mixing of the gas components. The CO vanished for each operating condition and the conversion rate was higher than 98%. The highest value of selectivity of methane was 92 % under current operating conditions.
- During the process of increasing temperature from the steady condition, the water–gas shift reaction and reverse reactions played a more important role for the performance of the reactor. The production rate of CH decreased and more reactants were converted into CO. Chemical equilibrium was the decisive factor during the process with increasing temperature.
- With the decrease in temperature, the effect of the reaction kinetic became the dominant factor. The selectivity of CH increased, whilst the conversion rate of CO decreased due to the low reaction rate.
- The arrangement of the tubes will influence the interaction between the fluid and tubes. The staggered tubes are beneficial for the effective removal of reaction heat. The selectivity of methane with staggered tubes was 3% higher than that with normal tubes.
- The effect of the structures of the tubes, including the diameter, distance between tubes, and the amount of tubes, should be studied to determine the construction at the next stage.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Governing Equations
References
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Employing Catalyst Fluidization to Enable Carbon Management in the Synthetic Natural Gas Production from Biomass. Chem. Eng. Technol. 2009, 32, 343–347. [Google Scholar] [CrossRef]
- Hussain, I.; Jalil, A.; Hassan, N.; Farooq, M.; Mujtaba, M.; Hamid, M.; Sharif, H.; Nabgan, W.; Aziz, M.; Owgi, A. Contemporary thrust and emerging prospects of catalytic systems for substitute natural gas production by CO methanation. Fuel 2021, 122604. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Q.; Gu, F.; Liu, B.; Zhong, Z.; Su, F. Recent advances in methanation catalysts for the production of synthetic natural gas. RSC Adv. 2015, 5, 22759–22776. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, B.; Li, S.; Shi, H.; Ma, M.; Lu, J.; Yang, F.; Deng, X.; Jia, X.; Ma, X.; et al. Influence of the Microstructure of Ni–Co Bimetallic Catalyst on CO Methanation. Ind. Eng. Chem. Res. 2020, 59, 1845–1854. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation – From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Methanation in a fluidized bed reactor with high initial CO partial pressure: Part II— Modeling and sensitivity study. Chem. Eng. Sci. 2011, 66, 1612–1621. [Google Scholar] [CrossRef]
- Eri, Q.; Peng, J.; Zhao, X. CFD simulation of biomass steam gasification in a fluidized bed based on a multi-composition multi-step kinetic model. Appl. Therm. Eng. 2018, 129, 1358–1368. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M. Methanation in a fluidized bed reactor with high initial CO partial pressure: Part I—Experimental investigation of hydrodynamics, mass transfer effects, and carbon deposition. Chem. Eng. Sci. 2011, 66, 924–934. [Google Scholar] [CrossRef]
- Kopyscinski, J.; Schildhauer, T.J.; Biollaz, S.M.A. Fluidized-Bed Methanation: Interaction between Kinetics and Mass Transfer. Ind. Eng. Chem. Res. 2011, 50, 2781–2790. [Google Scholar] [CrossRef]
- Cobb, J.T.; Streeter, R.C. Evaluation of Fluidized-Bed Methanation Catalysts and Reactor Modeling. Ind. Eng. Chem. Process Des. Dev. 1979, 18, 672–679. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, H.; Buahom, P.; Liu, J.; Xia, X.; Park, C.B. Prediction of thermal conductivity of micro/nano porous dielectric materials: Theoretical model and impact factors. Energy 2021, 233, 121140. [Google Scholar] [CrossRef]
- Li, J.; Yang, B. Multi-scale CFD simulations of bubbling fluidized bed methanation process. Chem. Eng. J. 2019, 377, 119818. [Google Scholar] [CrossRef]
- Chein, R.Y.; Yu, C.T.; Wang, C.C. Numerical simulation on the effect of operating conditions and syngas compositions for synthetic natural gas production via methanation reaction. Fuel 2016, 185, 394–409. [Google Scholar] [CrossRef]
- Liu, Y.; Hinrichsen, O. CFD Simulation of Hydrodynamics and Methanation Reactions in a Fluidized-Bed Reactor for the Production of Synthetic Natural Gas. Ind. Eng. Chem. Res. 2014, 53, 9348–9356. [Google Scholar] [CrossRef]
- Li, J.; Yang, B. Bubbling fluidized bed methanation study with resolving the mesoscale structure effects. AIChE J. 2019, 65, 16561. [Google Scholar] [CrossRef]
- Ich Ngo, S.; Lim, Y.I.; Lee, D.; Won Seo, M.; Kim, S. Experiment and numerical analysis of catalytic CO2 methanation in bubbling fluidized bed reactor. Energy Convers. Manag. 2021, 233, 113863. [Google Scholar] [CrossRef]
- Zhang, W.; Machida, H.; Takano, H.; Izumiya, K.; Norinaga, K. Computational fluid dynamics simulation of CO2 methanation in a shell-and-tube reactor with multi-region conjugate heat transfer. Chem. Eng. Sci. 2020, 211, 115276–115285. [Google Scholar] [CrossRef]
- Li, J.; Agarwal, R.K.; Yang, B. Two-Dimensional Computational Fluid Dynamics Simulation of Heat Removal in Fluidized Bed Methanation Reactors from Coke Oven Gas Using Immersed Horizontal Tubes. Ind. Eng. Chem. Res. 2020, 59, 981–991. [Google Scholar] [CrossRef]
- Zhang, Q.; Cao, Z.; Ye, S.; Sha, Y.; Chen, B.; Zhou, H. Mathematical modeling for bubbling fluidized bed CO-methanation reactor incorporating the effect of circulation and particle flows. Chem. Eng. Sci. 2022, 249, 117305. [Google Scholar] [CrossRef]
- Sun, L.; Luo, K.; Fan, J. 3D Unsteady Simulation of a Scale-Up Methanation Reactor with Interconnected Cooling Unit. Energies 2021, 14, 7095. [Google Scholar] [CrossRef]



| Parameter | A | Unit | E | Unit |
|---|---|---|---|---|
| 3.711 × 10 | mol · s kg Pa | 240,100 | J/mol | |
| 5.431 | mol · s kg Pa | 67,130 | J/mol | |
| 1.198 × 10 | Pa | 26,830 | J/mol | |
| 1.767 × 10 | −4400 | J/mol | ||
| 6.65 × 10 | Pa | −38,280 | J/mol | |
| 8.23 × 10 | Pa | −70,650 | J/mol | |
| 6.12 × 10 | Pa | −82,900 | J/mol | |
| 1.77 × 10 | 88,680 | J/mol |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.; Lin, J.; Kong, D.; Luo, K.; Fan, J. Three-Dimensional Computation Fluid Dynamics Simulation of CO Methanation Reactor with Immersed Tubes. Energies 2022, 15, 321. https://doi.org/10.3390/en15010321
Sun L, Lin J, Kong D, Luo K, Fan J. Three-Dimensional Computation Fluid Dynamics Simulation of CO Methanation Reactor with Immersed Tubes. Energies. 2022; 15(1):321. https://doi.org/10.3390/en15010321
Chicago/Turabian StyleSun, Liyan, Junjie Lin, Dali Kong, Kun Luo, and Jianren Fan. 2022. "Three-Dimensional Computation Fluid Dynamics Simulation of CO Methanation Reactor with Immersed Tubes" Energies 15, no. 1: 321. https://doi.org/10.3390/en15010321
APA StyleSun, L., Lin, J., Kong, D., Luo, K., & Fan, J. (2022). Three-Dimensional Computation Fluid Dynamics Simulation of CO Methanation Reactor with Immersed Tubes. Energies, 15(1), 321. https://doi.org/10.3390/en15010321





