Comparison of How Graphite and Shungite Affect Thermal, Mechanical, and Dielectric Properties of Dielectric Elastomer-Based Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formation of PDMS-Based Composites
2.3. Composites Characterization Methods
3. Results and Discussion
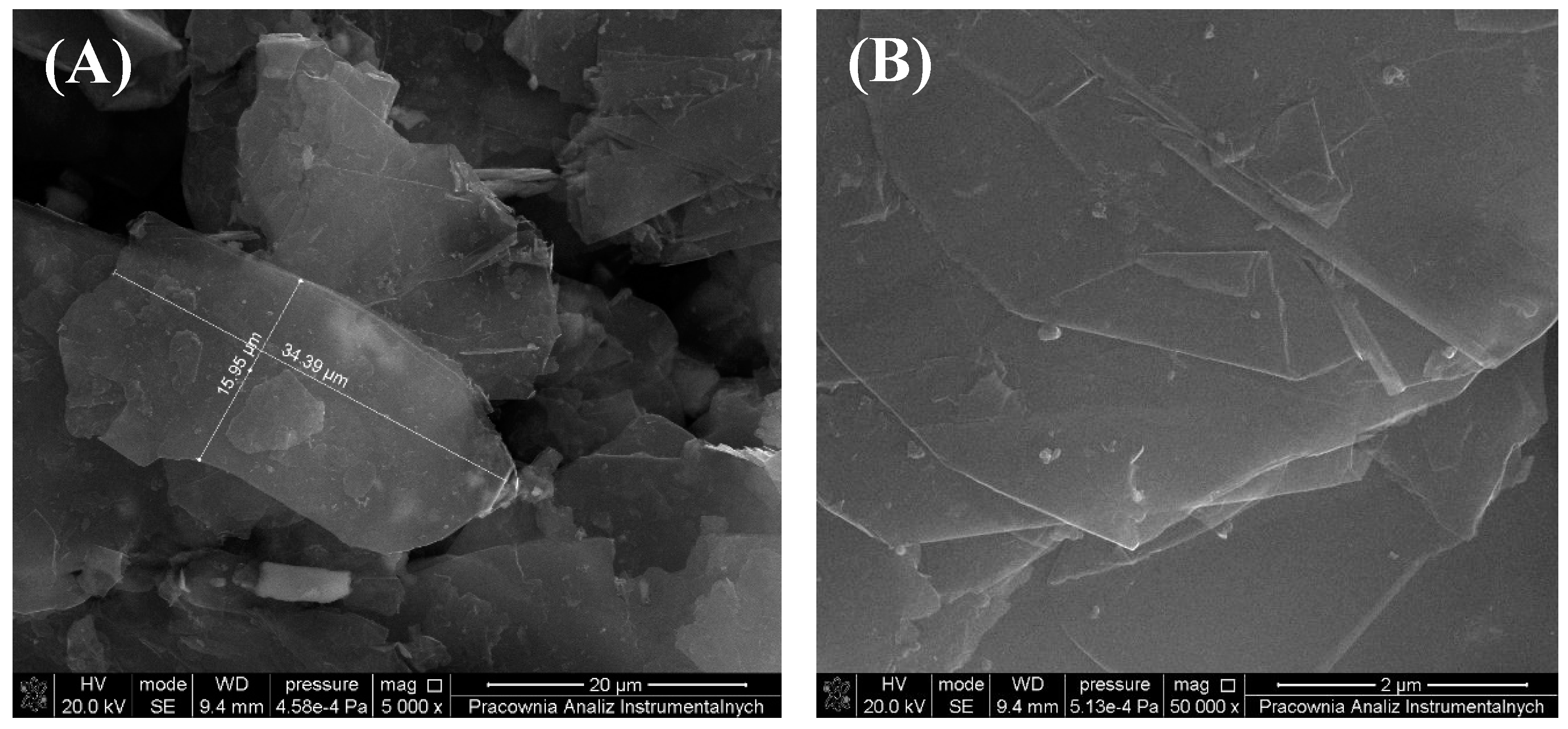
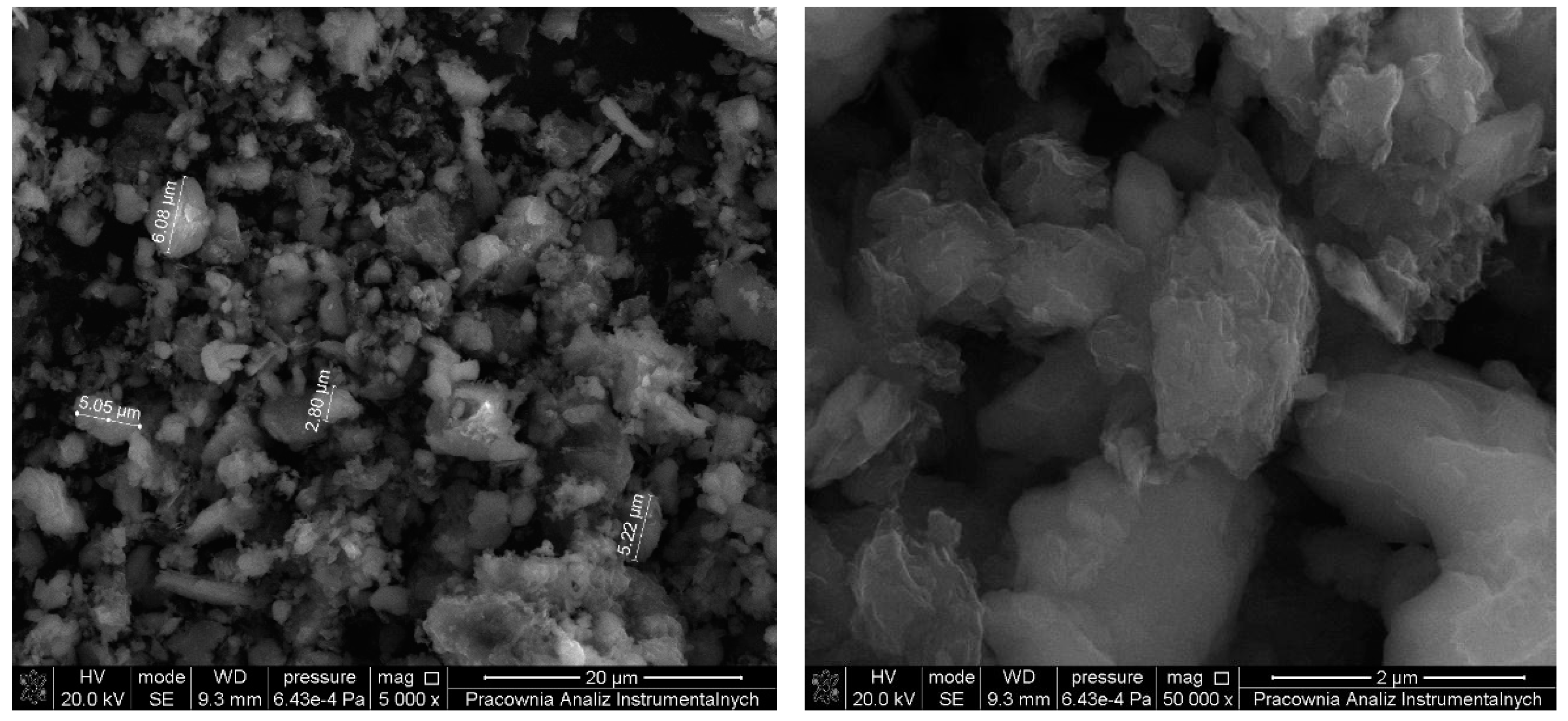
3.1. Morphology and Topography of Carbonaceous Fillers
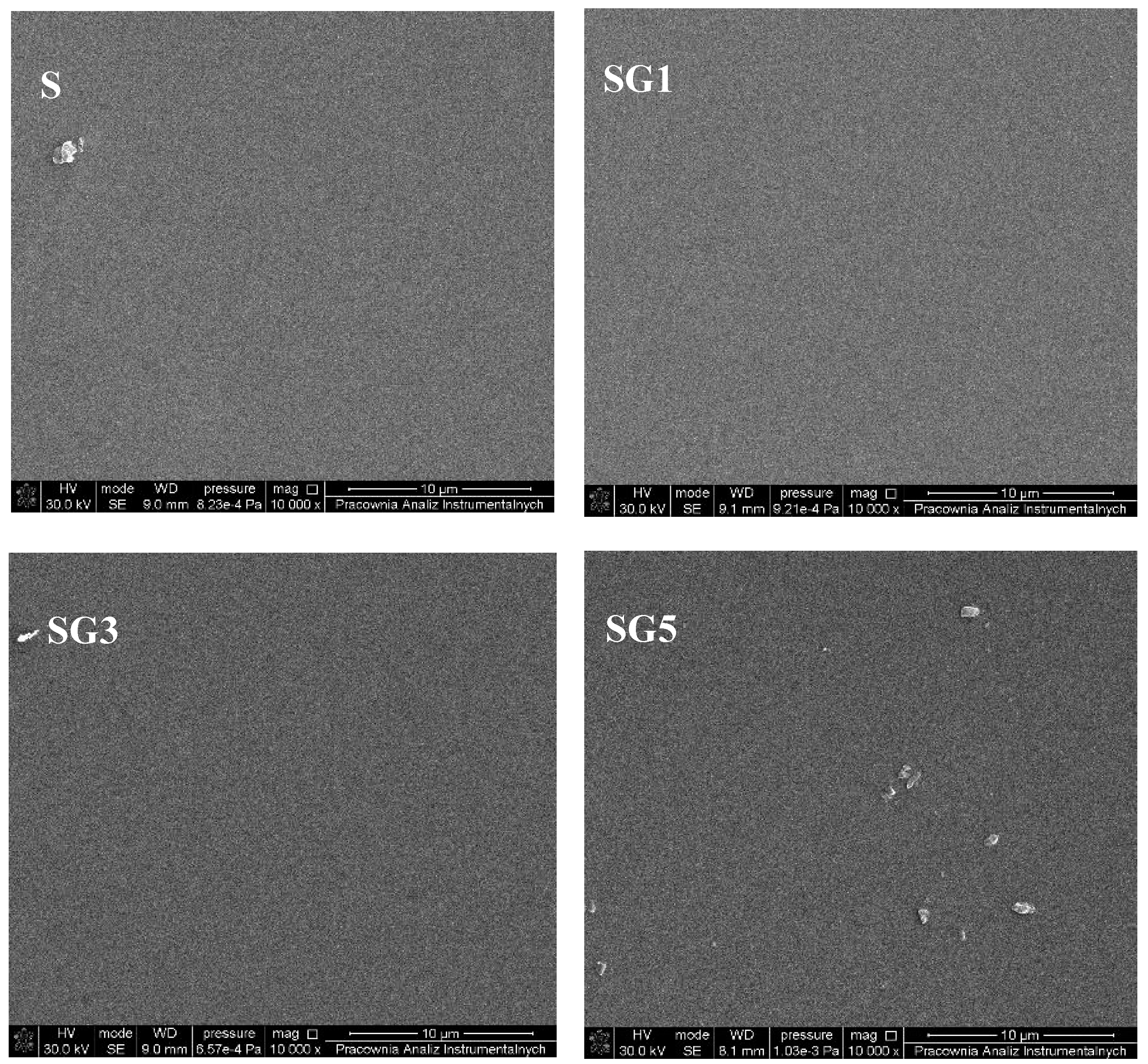
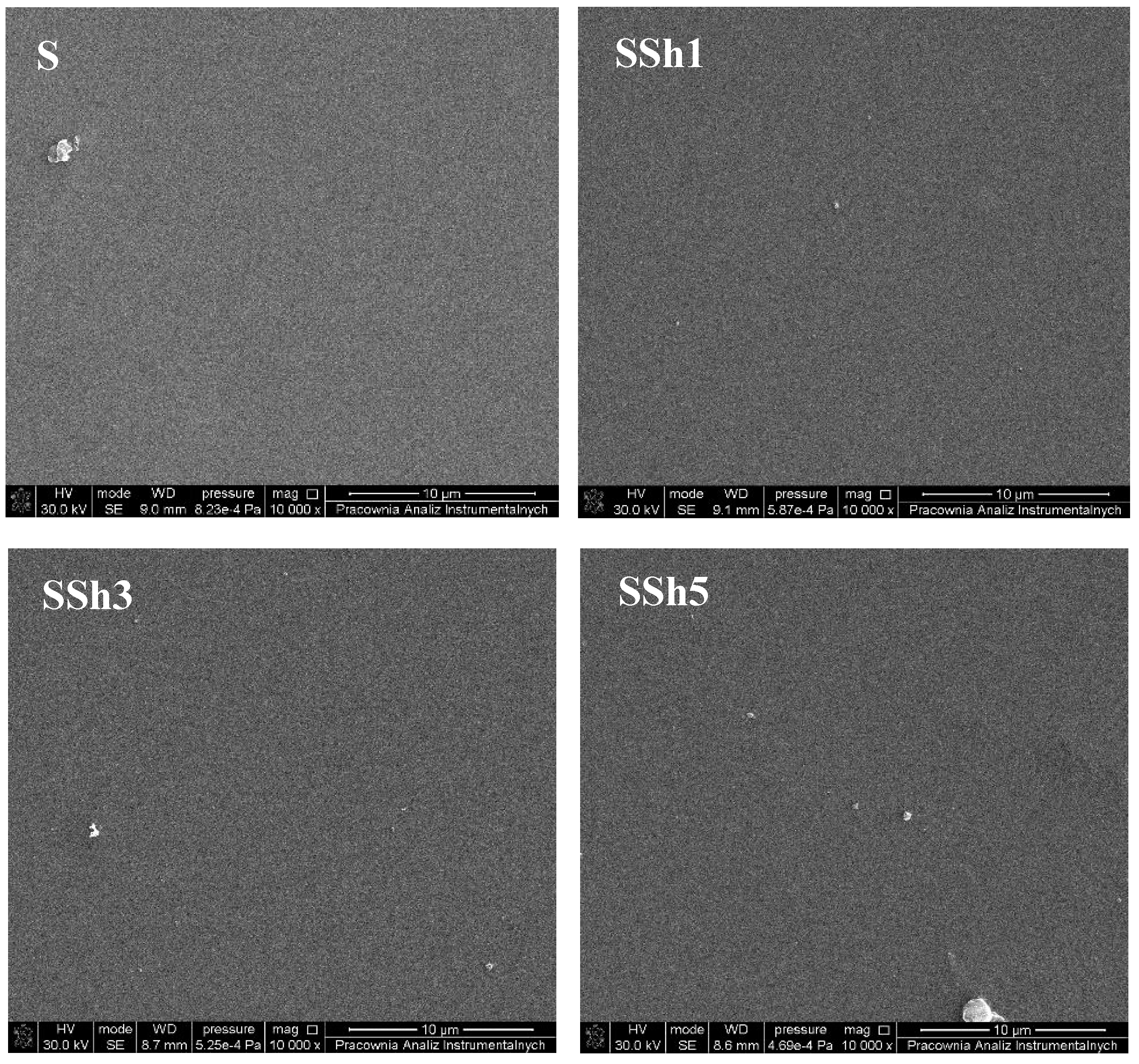
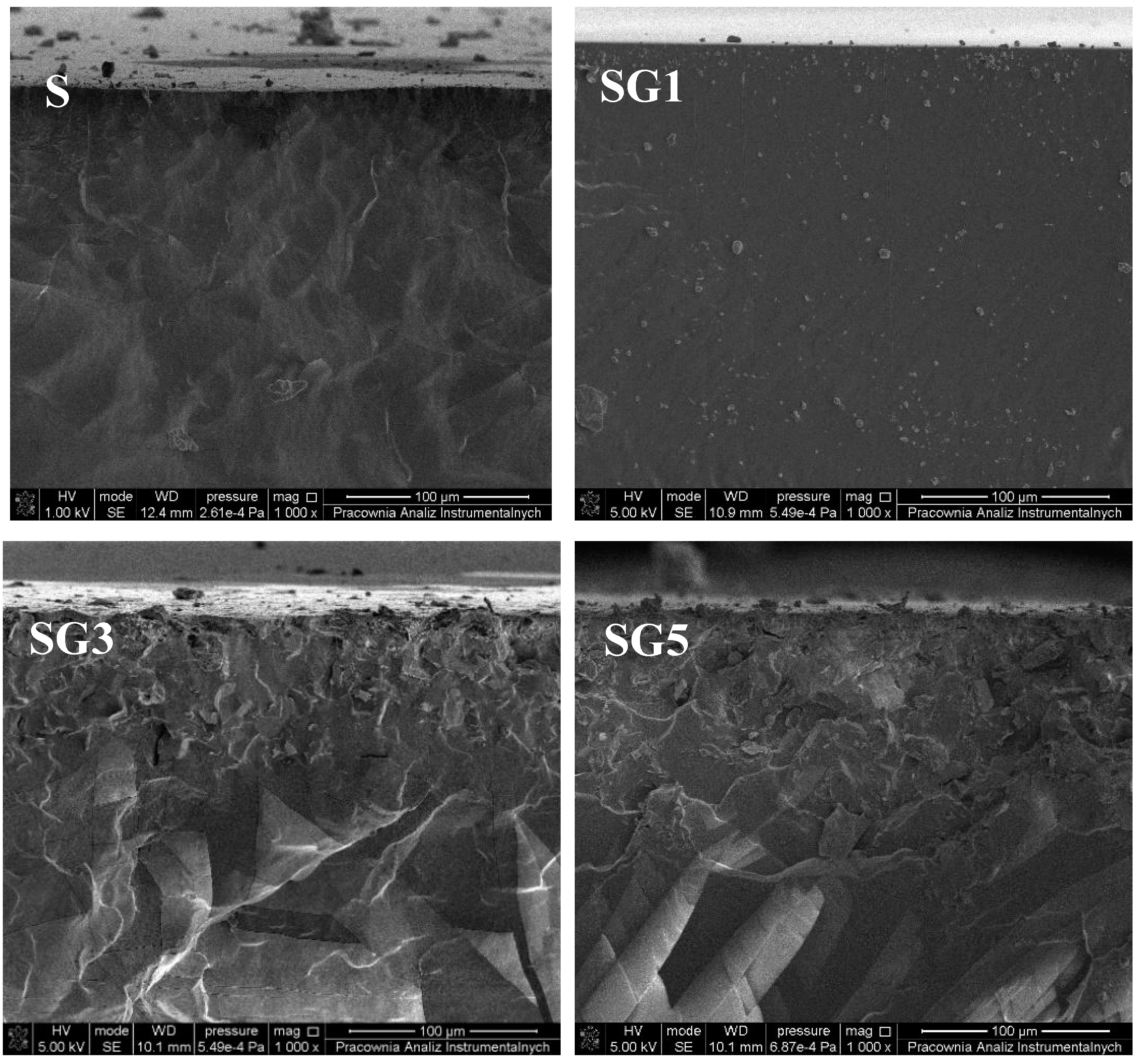
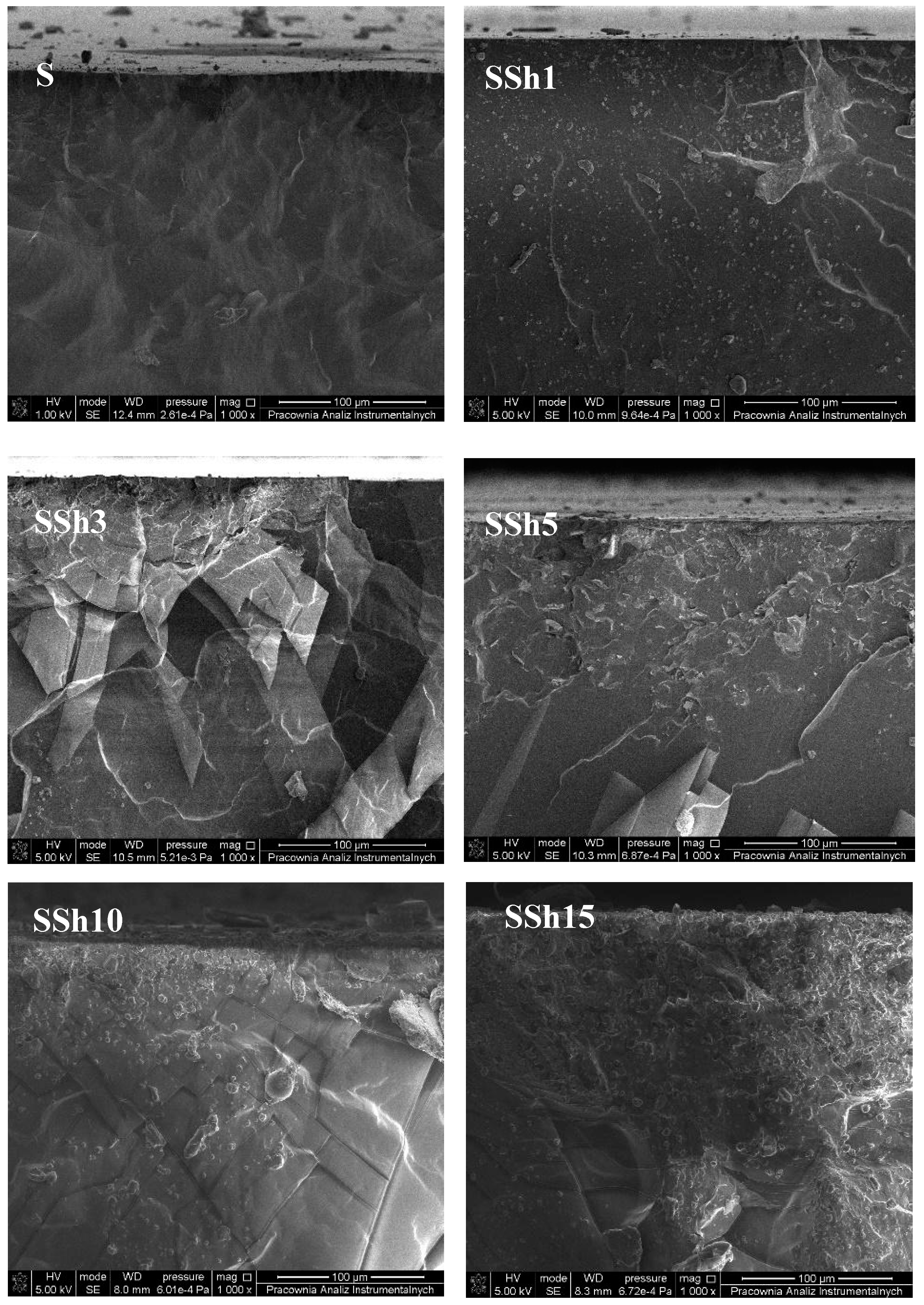
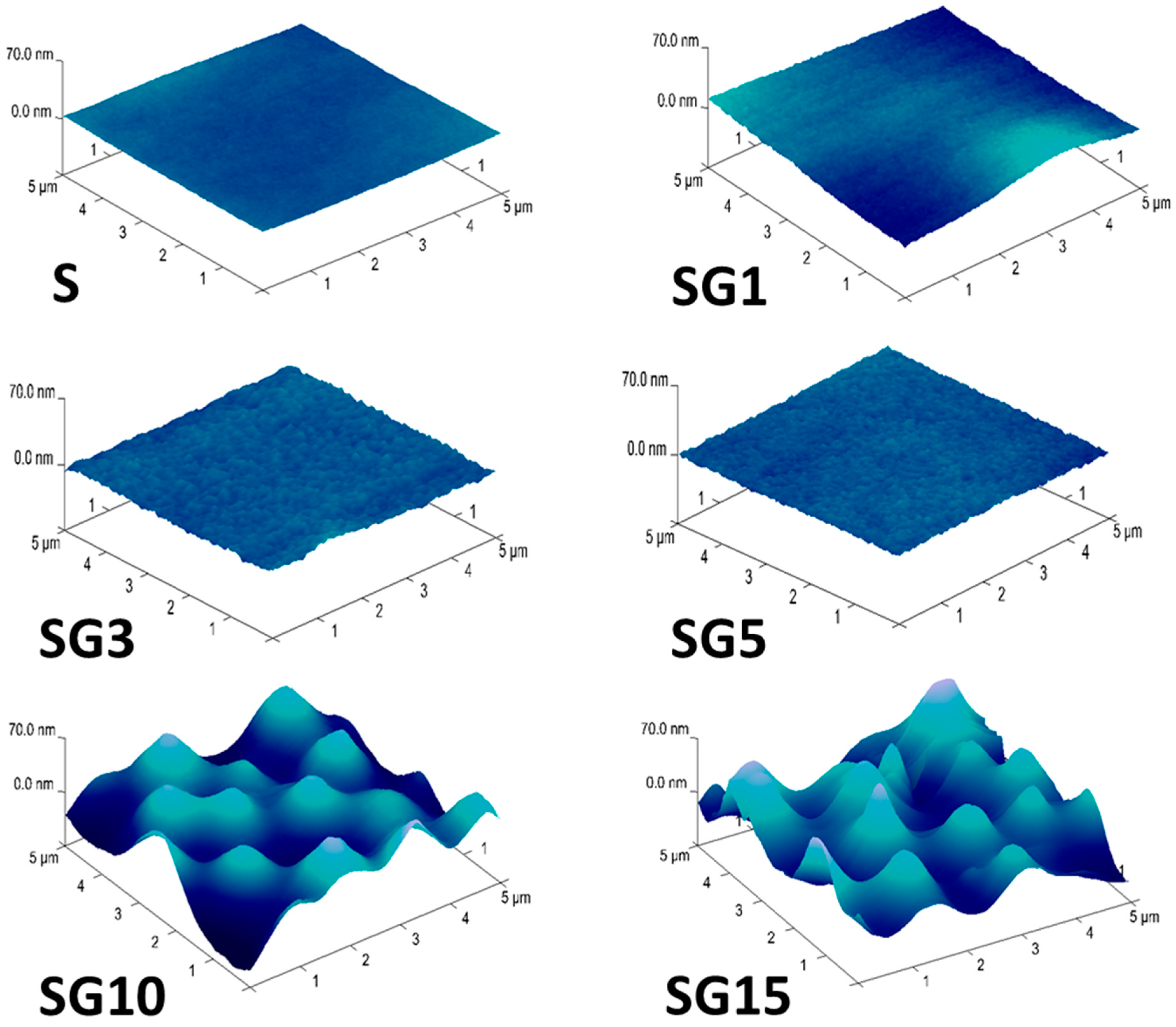
3.2. Morphology and Topography of Sylgard and Its Composites
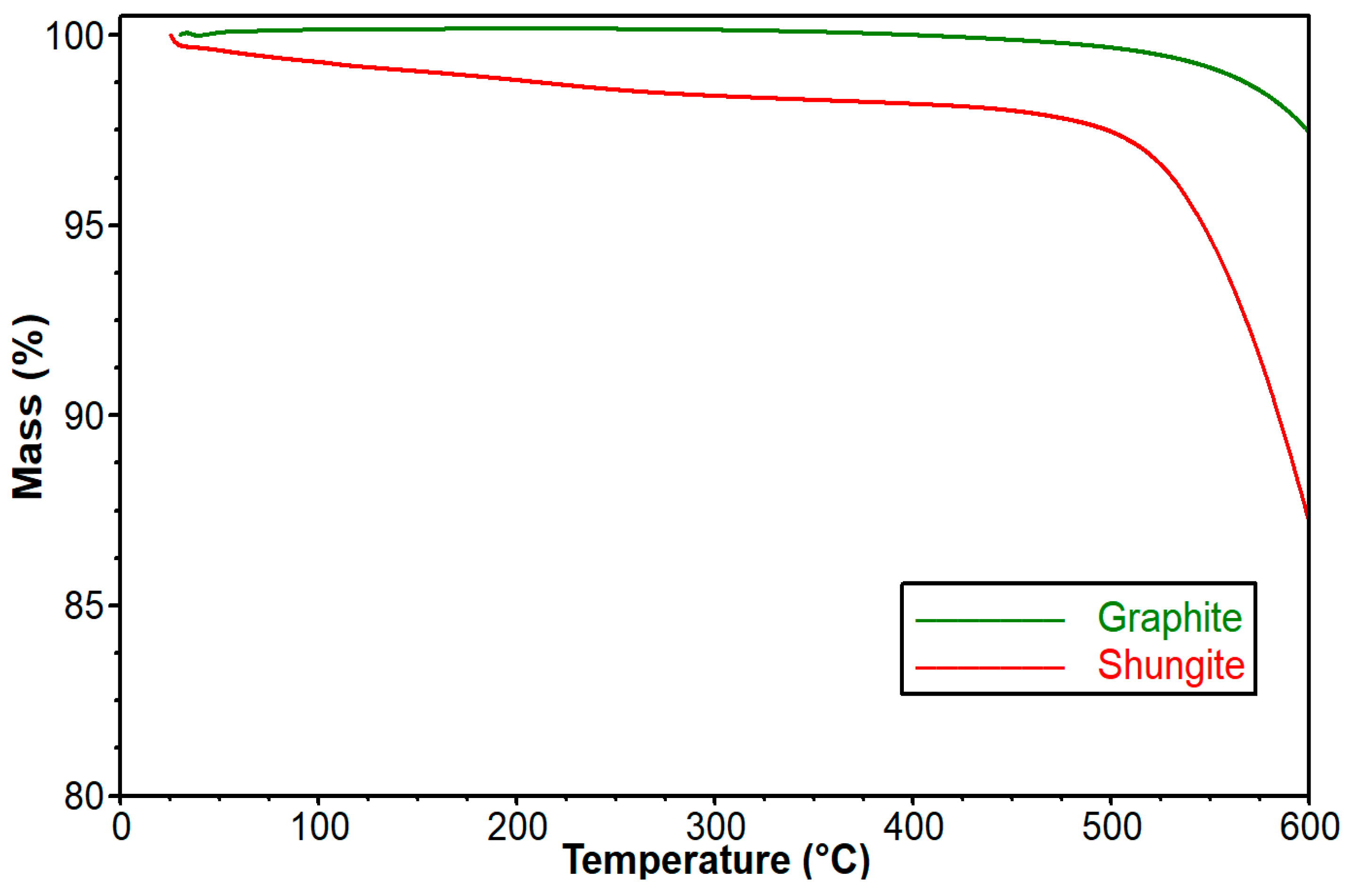
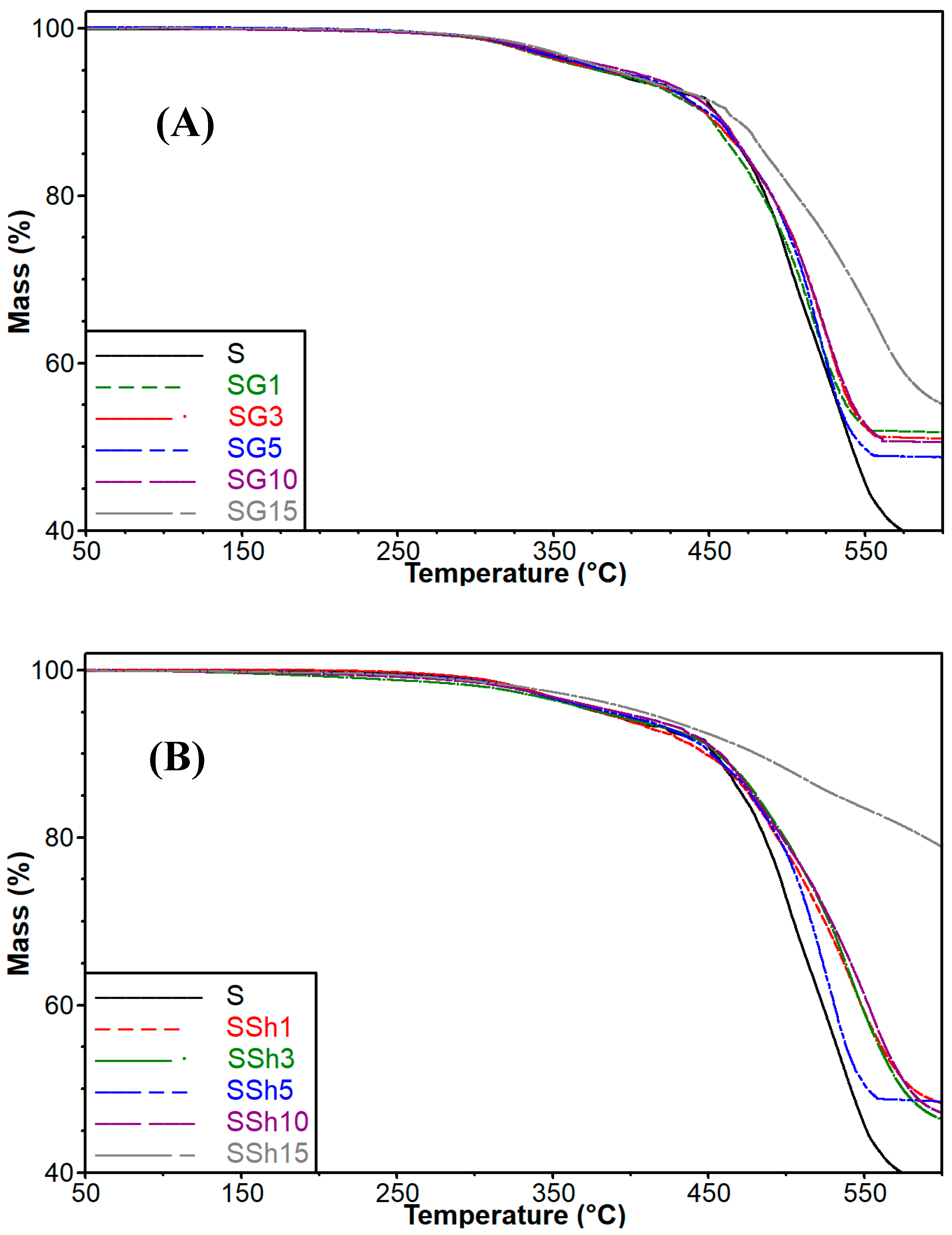
3.3. Influence of Graphite and Shungite on Thermal Stability of the Obtained Composites
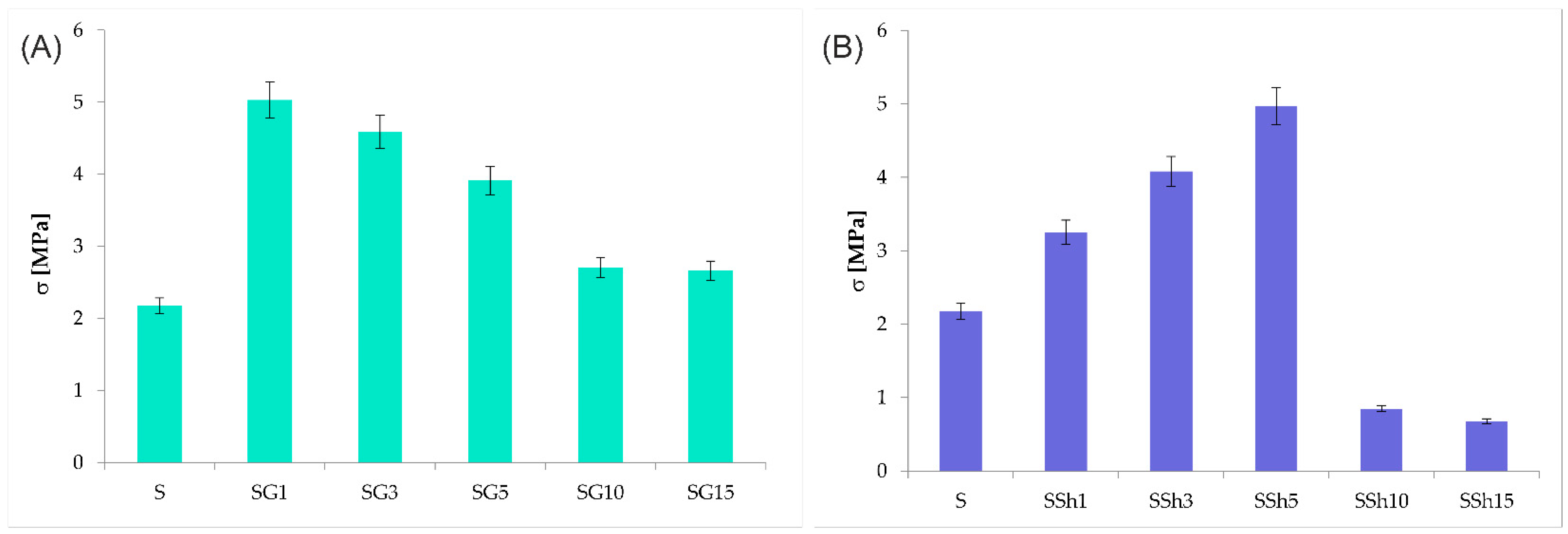
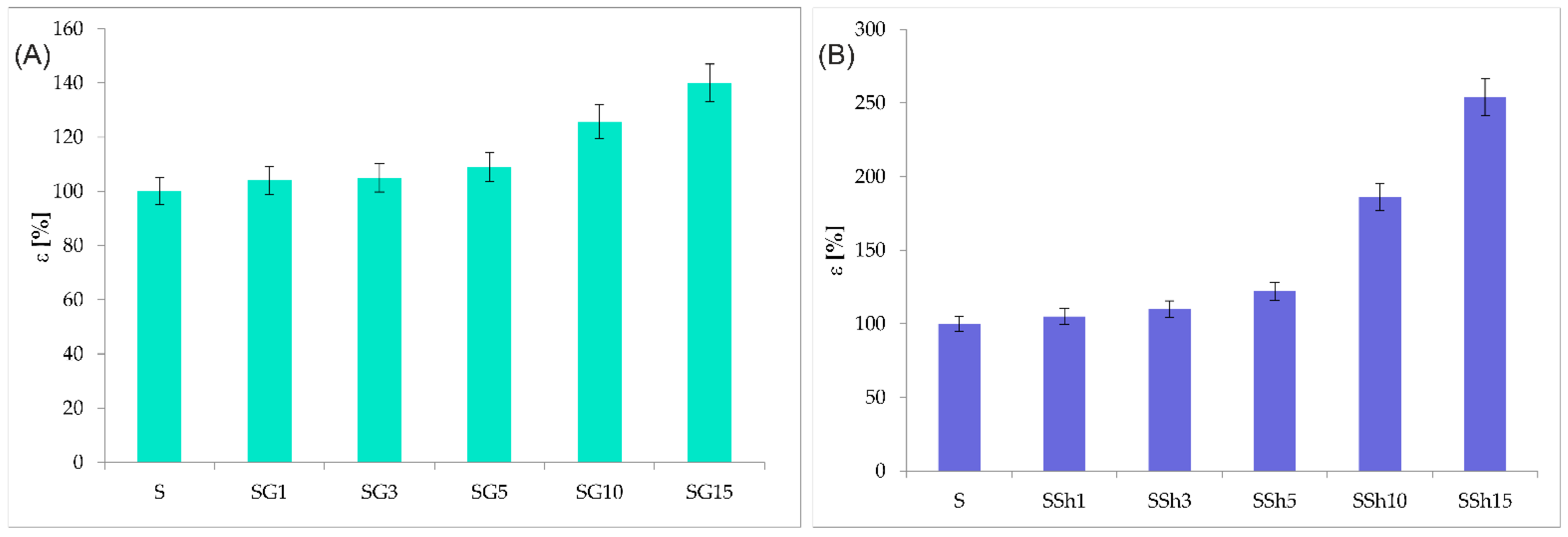
3.4. Mechanical Properties
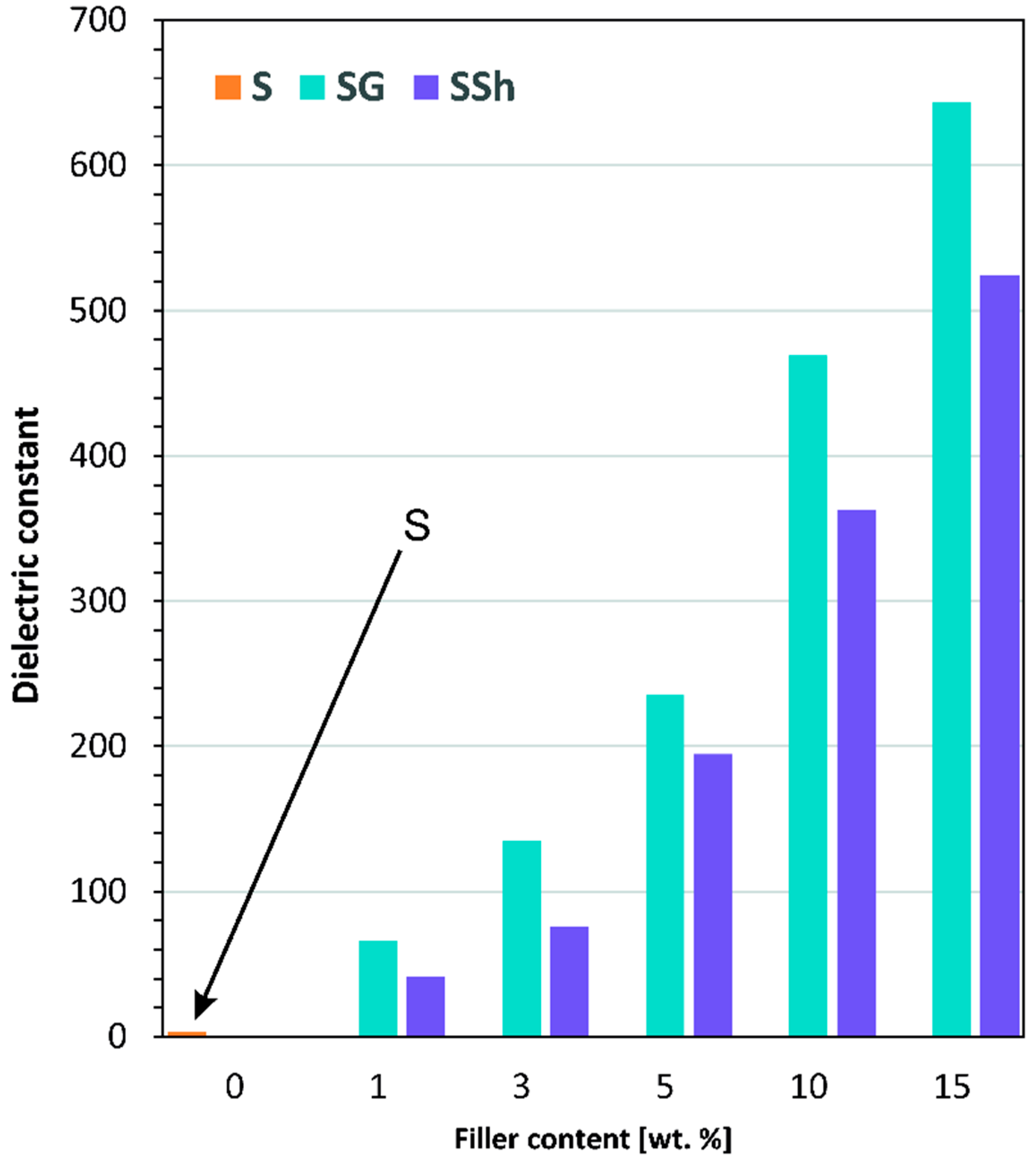
3.5. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kigozi, M.; Koech, R.K.; Kingsley, O.; Ojeaga, I.; Tebandeke, E.; Kasozi, G.N.; Onwualu, A.P. Synthesis and characterization of graphene oxide from locally mined graphite flakes and its supercapacitor applications. Results Mater. 2020, 7, 100113. [Google Scholar] [CrossRef]
- Jean-Mistral, C.; Basrour, S.; Chaillout, J.J. Comparison of electroactive polymers for energy scavenging applications. Smart Mater. Struct. 2010, 19, 12. [Google Scholar] [CrossRef]
- Cornogolub, A.; Cottinet, P.J.; Petit, L. Hybrid Energy Harvesting Using Electroactive Polymers Combined with Piezoelectric Materials. Adv. Sci. Technol. 2014, 96, 117–123. [Google Scholar] [CrossRef]
- Hassanpouryouzband, A.; Joonaki, E.; Edlmann, K.; Haszeldine, R.S. Offshore Geological Storage of Hydrogen: Is This Our Best Option to Achieve Net-Zero? ACS Energy Lett. 2021, 6, 2181–2186. [Google Scholar] [CrossRef]
- Jamal, E.M.A.; Joy, P.A.; Kurian, P.; Anantharaman, M.R. On the magnetic and dielectric properties of nickel-neoprene nanocomposites. Mater. Chem. Phys. 2010, 121, 154–160. [Google Scholar] [CrossRef]
- Yang, T.I.; Brown, R.N.C.; Kempel, L.C.; Kofinas, P. Controlled synthesis of core-shell iron-silica nanoparticles and their magneto-dielectric properties in polymer composites. Nanotechnology 2011, 22, 105601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofod, G.; Risse, S.; Stoyanov, H.; McCarthy, D.N.; Sokolov, S.; Kraehnert, R. Broad-spectrum enhancement of polymer composite dielectric constant at ultralow volume fractions of silica-supported copper nanoparticles. ACS Nano 2011, 5, 1623–1629. [Google Scholar] [CrossRef] [PubMed]
- Stiubianu, G.; Bele, A.; Cazacu, M.; Racles, C.; Vlad, S.; Ignat, M. Dielectric silicone elastomers with mixed ceramic nanoparticles. Mater. Res. Bull. 2015, 71, 67–74. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Hu, Y.; Lu, H.; Song, L. Morphology, thermal, and mechanical properties of flame-retardant silicone rubber/montmorillonite nanocomposites. J. Appl. Polym. Sci. 2006, 99, 3275–3280. [Google Scholar] [CrossRef]
- Carpi, F.; De Rossi, D. Improvement of electromechanical actuating performances of a silicone dielectric elastomer by dispersion of titanium dioxide powder. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 835–843. [Google Scholar] [CrossRef]
- Zhang, X.; Wen, H.; Chen, X.; Wu, Y.; Xiao, S. Study on the thermal and dielectric properties of SrTiO3/epoxy nanocomposites. Energies 2017, 10, 692. [Google Scholar] [CrossRef] [Green Version]
- Romasanta, L.J.; Leret, P.; Casaban, L.; Hernández, M.; De La Rubia, M.A.; Fernández, J.F.; Kenny, J.M.; Lopez-Manchado, M.A.; Verdejo, R. Towards materials with enhanced electro-mechanical response: CaCu 3Ti4O12-polydimethylsiloxane composites. J. Mater. Chem. 2012, 22, 24705–24712. [Google Scholar] [CrossRef] [Green Version]
- Fang, X.; Pan, L.; Yao, J.; Yin, S.; Wang, Y.; Li, Q.; Yang, J. Controllable dielectric properties and strong electromagnetic wave absorption properties of SiC/spherical graphite-AlN microwave-attenuating composite ceramics. Ceram. Int. 2021, 47, 22636–22645. [Google Scholar] [CrossRef]
- Xi, X.; Chung, D.D.L. Dielectric behavior of graphite, with assimilation of the AC permittivity, DC polarization and DC electret. Carbon N. Y. 2021, 181, 246–259. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Weng, L.; Yu, Y.; Zhang, X.; Liu, L.; Wang, C. Dielectrical properties of graphite nanosheets/PVDF composites regulated by coupling agent. Mater. Today Commun. 2019, 21, 100705. [Google Scholar] [CrossRef]
- Kumar, P.; Penta, S.; Mahapatra, S.P. Dielectric Properties of Graphene Oxide Synthesized by Modified Hummers’ Method from Graphite Powder. Integr. Ferroelectr. 2019, 202, 41–51. [Google Scholar] [CrossRef]
- Tjong, S.C.; Li, Y.C.; Li, R.K.Y. Frequency and temperature dependences of dielectric dispersion and electrical properties of polyvinylidene fluoride/expanded graphite composites. J. Nanomater. 2010, 2010, 261748. [Google Scholar] [CrossRef] [Green Version]
- Bhuyan, M.S.A.; Uddin, M.N.; Islam, M.M.; Bipasha, F.A.; Hossain, S.S. Synthesis of graphene. Int. Nano Lett. 2016, 6, 65–83. [Google Scholar] [CrossRef] [Green Version]
- Prasanna, P.B.; Avadhani, D.N.; Muralidhara, H.B.; Revanasiddappa, M. Synthesis, Characterization and Enhanced Dielectric Constant of Polyaniline-Exfoliated Graphite Flakes Composites. IJLTEMAS 2014, III, 55–60. [Google Scholar]
- Li, Y.C.; Li, R.K.Y.; Tjong, S.C. Electrical transport properties of graphite sheets doped polyvinylidene fluoride nanocomposites. J. Mater. Res. 2010, 25, 1645–1648. [Google Scholar] [CrossRef]
- Rzeczkowski, P.; Krause, B.; Pötschke, P. Characterization of highly filled PP/graphite composites for adhesive joining in fuel cell applications. Polymers 2019, 11, 462. [Google Scholar] [CrossRef] [Green Version]
- Metz, R.; Blanc, C.; Dominguez, S.; Tahir, S.; Leparc, R.; Hassanzadeh, M. Nonlinear field dependent conductivity dielectrics made of graphite nanoplatelets filled composites. Mater. Lett. 2021, 292, 129611. [Google Scholar] [CrossRef]
- Biryan, F.; Abubakar, A.M.; Demirelli, K. Product analysis, electrical and dielectric properties depending on thermal influence of poly(N-isopropyl acrylamide)/graphite-filled composite. Thermochim. Acta 2018, 669, 66–79. [Google Scholar] [CrossRef]
- Parida, S.; Parida, R.K.; Parida, B.N.; Padhee, R.; Nayak, N.C. Structural, thermal and dielectric behaviour of exfoliated graphite nanoplatelets (xGnP) filled EVA/EOC blend composites. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2022, 275, 115497. [Google Scholar] [CrossRef]
- Torğut, G.; Biryan, F.; Demirelli, K. Effect of graphite particle fillers on dielectric and conductivity properties of poly(NIPAM-co-HEMA). Bull. Mater. Sci. 2019, 42. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Yang, D. Dielectric Properties and Thermal Conductivity of Poly(vinylidene fluoride)-Based Composites with Graphite Nanosheet and Nickel Particle. J. Nanosci. Nanotechnol. 2019, 19, 3591–3596. [Google Scholar] [CrossRef]
- Kranauskaite, I.; Macutkevic, J.; Kuzhir, P.; Volynets, N.; Paddubskaya, A.; Bychanok, D.; Maksimenko, S.; Banys, J.; Juskenas, R.; Bistarelli, S.; et al. Dielectric properties of graphite-based epoxy composites. Phys. Status Solidi Appl. Mater. Sci. 2014, 211, 1623–1633. [Google Scholar] [CrossRef] [Green Version]
- Tolvanen, J.; Hannu, J.; Nelo, M.; Juuti, J.; Jantunen, H. Dielectric properties of novel polyurethane-PZT-graphite foam composites. Smart Mater. Struct. 2016, 25, 95039. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.H.; Tan, L.C.; Qin, S.; He, Y.; Wu, T.; Qu, J. ping Efficient fabrication of highly exfoliated and evenly dispersed high-density polyethylene/expanded graphite nanocomposite with enhanced dielectric constant and extremely low dielectric loss. Compos. Part A Appl. Sci. Manuf. 2021, 142, 106242. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, J.; Chen, P.; Bo, M.; Deng, Q. Filler-synergy route for optimising dielectric features of novel polymer-based ternary composite films bearing Fe2TiO5 pseudo-perovskite and graphite particles. Ceram. Int. 2021, 47, 8357–8364. [Google Scholar] [CrossRef]
- Obradović, N.; Gigov, M.; Ðorđević, A.; Kern, F.; Dmitrović, S.; Matović, B.; Ðorđević, A.; Tshantshapanyan, A.; Vlahović, B.; Petrović, P.; et al. Shungite—A carbon-mineral rock material: Its sinterability and possible applications. Process. Appl. Ceram. 2019, 13, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Moshnikov, I.A.; Kovalevski, V.V. Composite materials based on nanostructured shungite filler. Mater. Today Proc. 2018, 5, 25971–25975. [Google Scholar] [CrossRef]
- Sajo, M.E.J.; Kim, C.S.; Kim, S.K.; Shim, K.Y.; Kang, T.Y.; Lee, K.J. Antioxidant and Anti-Inflammatory Effects of Shungite against Ultraviolet B Irradiation-Induced Skin Damage in Hairless Mice. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef] [Green Version]
- Fujita, T.; Aoki, T.; Ponou, J.; Dodbiba, G.; He, C.; Wang, K.; Ning, S.; Chen, H.; Wei, Y. Removal of impurities from shungite via a combination of physical and chemical treatments. Minerals 2021, 11, 245. [Google Scholar] [CrossRef]
- Antonets, I.V.; Golubev, Y.A.; Shcheglov, V.I.; Sun, S. Electromagnetic shielding effectiveness of lightweight and flexible ultrathin shungite plates. Curr. Appl. Phys. 2021, 29, 97–106. [Google Scholar] [CrossRef]
- Antonets, I.V.; Golubev, Y.A.; Shcheglov, V.I. Evaluation of microstructure and conductivity of two-phase materials by the scanning spreading resistance microscopy (the case of shungite). Ultramicroscopy 2021, 222, 113212. [Google Scholar] [CrossRef] [PubMed]
- Golubev, Y.A.; Antonets, I.V.; Shcheglov, V.I. Static and dynamic conductivity of nanostructured carbonaceous shungite geomaterials. Mater. Chem. Phys. 2019, 226, 195–203. [Google Scholar] [CrossRef]
- Barashkova, I.I.; Komova, N.N.; Motyakin, M.V.; Potapov, E.E.; Wasserman, A.M. Shungite-elastomer interface layers. Dokl. Phys. Chem. 2014, 456, 83–85. [Google Scholar] [CrossRef]
- Solov’eva, A.B.; Neschadina, L.E.; Rozhkova, N.N.; Zaidenberg, A.Z. Shungite effect on some properties of elastomers. Ext. Abstr. 23rd Bienn. Conf. Carbon. Pennsylvania. USA 1997, 2, 248–249. [Google Scholar]
- Frąc, M.; Szudek, W.; Szołdra, P.; Pichór, W. The applicability of shungite as an electrically conductive additive in cement composites. J. Build. Eng. 2022, 45, 103469. [Google Scholar] [CrossRef]
- Zenkiewicz, M.; Richert, J.; Rytlewski, P.; Richert, A. Comparative analysis of shungite and graphite effects on some properties of polylactide composites. Polym. Test. 2011, 30, 429–435. [Google Scholar] [CrossRef]
- Olewnik-Kruszkowska, E.; Brzozowska, W.; Adamczyk, A.; Gierszewska, M.; Wojtczak, I.; Sprynskyy, M. Effect of diatomaceous biosilica and talc on the properties of dielectric elastomer based composites. Energies 2020, 13, 828. [Google Scholar] [CrossRef]
- Andryushchenko, N.D.; Safonov, A.V.; Babich, T.L.; Ivanov, P.V.; Konevnik, Y.V.; Kondrashova, A.A.; Proshin, I.M.; Zakharova, E.V. Sorption characteristics of materials of the filtration barrier in upper aquifers contaminated with radionuclides. Radiochemistry 2017, 59, 414–424. [Google Scholar] [CrossRef]
- Diyuk, V.E.; Ishchenko, O.V.; Loginova, O.B.; Melnik, L.M.; Kisterska, L.D.; Garashchenko, V.V.; Lisovenko, S.O.; Beda, O.A.; Tkachuk, N.A.; Shevchenko, O.Y.; et al. Recovery of Adsorption Properties of Shungite. J. Superhard Mater. 2019, 41, 221–228. [Google Scholar] [CrossRef]
- Siburian, R.; Sihotang, H.; Lumban Raja, S.; Supeno, M.; Simanjuntak, C. New route to synthesize of graphene nano sheets. Orient. J. Chem. 2018, 34, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Ogbu, C.I.; Feng, X.; Dada, S.N.; Bishop, G.W. Screen-printed soft-nitrided carbon electrodes for detection of hydrogen peroxide. Sensors 2019, 19, 3741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voigt, B.; McQueen, D.H.; Pelišková, M.; Rozhkova, N. Electrical and mechanical properties of melamine-formaldehyde-based laminates with shungite filler. Polym. Compos. 2005, 26, 552–562. [Google Scholar] [CrossRef]
- Johnson, R.T.; Biefeld, R.M.; Sayre, J.A. High-temperature electrical conductivity and thermal decomposition of Sylgard® 184 and mixtures containing hollow microspherical fillers. Polym. Eng. Sci. 1984, 24, 435–441. [Google Scholar] [CrossRef]
- Sarath, P.S.; Samson, S.V.; Reghunath, R.; Pandey, M.K.; Haponiuk, J.T.; Thomas, S.; George, S.C. Fabrication of exfoliated graphite reinforced silicone rubber composites-Mechanical, tribological and dielectric properties. Polym. Test. 2020, 89. [Google Scholar] [CrossRef]
- Mohanavel, V.; Rajan, K.; Suresh Kumar, S.; Vijayan, G.; Vijayanand, M.S. Study on mechanical properties of graphite particulates reinforced aluminium matrix composite fabricated by stir casting technique. Mater. Today Proc. 2018, 5, 2945–2950. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Fukushima, H.; Drzal, L.T. Mechanical properties and morphological characterization of exfoliated graphite-polypropylene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2007, 38, 1675–1682. [Google Scholar] [CrossRef]
- Garishin, O.K.; Shadrin, V.V.; Belyaev, A.Y.; Kornev, Y.V. Micro and nanoshungites-Perspective mineral fillers for rubber composites used in the tires. Mater. Phys. Mech. 2018, 40, 56–62. [Google Scholar] [CrossRef]
- Ravikumar, K.; Palanivelu, K.; Ravichandran, K. Dielectric properties of natural rubber composites filled with graphite. Mater. Today Proc. 2019, 16, 1338–1343. [Google Scholar] [CrossRef]
- Khan, J.A.; Qasim, M.; Singh, B.R.; Singh, S.; Shoeb, M.; Khan, W.; Das, D.; Naqvi, A.H. Synthesis and characterization of structural, optical, thermal and dielectric properties of polyaniline/CoFe2O4 nanocomposites with special reference to photocatalytic activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 109, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Li, X.; Li, Q.; Xia, X.; He, C.; Feng, Y.; Peng, C. Well-balanced high permittivity and low dielectric loss obtained in PVDF/graphite/BN ternary composites by depressing interfacial leakage conduction. Microelectron. Eng. 2020, 231, 111404. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Dong, M.; Zuo, G. Study on changing the dielectric constant and radar reflection of foamed polyurethane by adding graphite. J. Phys. Conf. Ser. 2021, 1980. [Google Scholar] [CrossRef]
- Azadmanjiri, J.; Berndt, C.C.; Wang, J.; Kapoor, A.; Srivastava, V.K.; Wen, C. A review on hybrid nanolaminate materials synthesized by deposition techniques for energy storage applications. J. Mater. Chem. A 2014, 2, 3695–3708. [Google Scholar] [CrossRef]
- Robertson, J. High dielectric constant oxides. Eur. Phys. J. Appl. Phys. 2004, 28, 265–291. [Google Scholar] [CrossRef] [Green Version]
- Xi, X.; Chung, D.D.L. Role of grain boundaries in the dielectric behavior of graphite. Carbon N. Y. 2021, 173, 1003–1019. [Google Scholar] [CrossRef]
- Pal, R.; Akhtar, M.J.; Kar, K.K. Study on dielectric properties of synthesized exfoliated graphite reinforced epoxy composites for microwave processing. Polym. Test. 2018, 70, 8–17. [Google Scholar] [CrossRef]
- Liu, L.; Lei, Y.; Zhang, Z.; Liu, J.; Lv, S.; Guo, Z. Fabrication of PDA@SiO2@rGO/PDMS dielectric elastomer composites with good electromechanical properties. React. Funct. Polym. 2020, 154, 104656. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.K.Y.; Tjong, S.C. Fabrication and properties of PVDF/expanded graphite nanocomposites. E-Polymers 2009, 19, 1–13. [Google Scholar] [CrossRef]





| Sample | Content [%] | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C | O | Si | Fe | Al | Ca | S | Mg | Cu | |
| Graphite | 82.86 | 11.98 | 1.81 | 0.91 | 1.22 | 0.28 | 0.33 | 0.34 | 0.27 |
| Shungite | 15.19 | 50.76 | 24.71 | 2.28 | 3.72 | 1.26 | 0.84 | 0.80 | 0.44 |
| Sample | Rq [nm] | Ra [nm] | Rmax [nm] |
|---|---|---|---|
| S | 1.14 | 0.86 | 7.94 |
| SG1 | 1.22 | 0.98 | 8.95 |
| SG3 | 1.73 | 1.32 | 17.42 |
| SG5 | 5.04 | 4.01 | 29.91 |
| SG10 | 18.20 | 14.40 | 107.30 |
| SG15 | 23.50 | 18.40 | 149.20 |
| SSh1 | 1.87 | 1.41 | 20.20 |
| SSh3 | 2.20 | 1.69 | 20.60 |
| SSh5 | 2.26 | 1.73 | 54.40 |
| SSh10 | 6.81 | 5.42 | 66.20 |
| SSh15 | 19.06 | 12.11 | 231.00 |
| Sample | Temperature [°C] at Mass Loss | |||
|---|---|---|---|---|
| 5% | 10% | 30% | 50% | |
| Graphite | >600 | >600 | >600 | >600 |
| Shungite | 546.7 | 584.7 | >600 | >600 |
| Sample | Temperature [°C] at Mass Loss | |||
|---|---|---|---|---|
| 5% | 10% | 30% | 50% | |
| S | 375.8 | 453.1 | 504.5 | 541.6 |
| SG1 | 377.0 | 449.8 | 508.3 | >600 |
| SG3 | 381.3 | 450.6 | 511.6 | >600 |
| SG5 | 383.6 | 450.5 | 513.2 | >600 |
| SG10 | 386.0 | 454.0 | 514.5 | >600 |
| SG15 | 392.2 | 462.6 | 541.5 | >600 |
| SSh1 | 378.2 | 448.8 | 524.5 | 582.0 |
| SSh3 | 379.5 | 454.5 | 524.4 | 579.6 |
| SSh5 | 384.4 | 452.9 | 518.7 | 556.2 |
| SSh10 | 389.9 | 458.2 | 528.8 | 579.3 |
| SSh15 | 408.1 | 481.4 | >600 | >600 |
| Sample | Frequency [Hz] | |||||||
|---|---|---|---|---|---|---|---|---|
| 102 | 103 | 104 | 105 | |||||
| φ | φ | φ | φ | |||||
| S | 3.277 | 0.005 | 3.214 | 0.017 | 3.120 | 0.017 | 3.056 | 0.010 |
| SG1 | 70.730 | 0.200 | 65.852 | 0.100 | 62.145 | 0.100 | 59.511 | 0.300 |
| SG3 | 141.261 | 0.200 | 135.090 | 0.600 | 108.485 | 0.200 | 101.360 | 0.500 |
| SG5 | 241.261 | 0.300 | 235.090 | 1.000 | 208.458 | 0.400 | 201.360 | 0.300 |
| SG10 | 475.863 | 0.000 | 469.088 | 0.300 | 468.524 | 0.000 | 464.572 | 0.000 |
| SG15 | 656.215 | 0.300 | 643.091 | 1.000 | 636.290 | 1.000 | 628.057 | 0.600 |
| SSh1 | 42.660 | 0.600 | 41.430 | 0.300 | 33.509 | 0.300 | 30.303 | 0.000 |
| SSh3 | 80.730 | 0.550 | 75.852 | 0.250 | 72.145 | 0.250 | 69.511 | 0.000 |
| SSh5 | 198.399 | 0.000 | 194.641 | 0.200 | 192.761 | 0.000 | 190.732 | 0.000 |
| SSh10 | 364.516 | 0.300 | 362.763 | 1.000 | 359.258 | 1.000 | 356.102 | 0.600 |
| SSh15 | 535.144 | 0.400 | 524.441 | 0.000 | 519.242 | 0.000 | 511.597 | 0.1000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olewnik-Kruszkowska, E.; Adamczyk, A.; Gierszewska, M.; Grabska-Zielińska, S. Comparison of How Graphite and Shungite Affect Thermal, Mechanical, and Dielectric Properties of Dielectric Elastomer-Based Composites. Energies 2022, 15, 152. https://doi.org/10.3390/en15010152
Olewnik-Kruszkowska E, Adamczyk A, Gierszewska M, Grabska-Zielińska S. Comparison of How Graphite and Shungite Affect Thermal, Mechanical, and Dielectric Properties of Dielectric Elastomer-Based Composites. Energies. 2022; 15(1):152. https://doi.org/10.3390/en15010152
Chicago/Turabian StyleOlewnik-Kruszkowska, Ewa, Arkadiusz Adamczyk, Magdalena Gierszewska, and Sylwia Grabska-Zielińska. 2022. "Comparison of How Graphite and Shungite Affect Thermal, Mechanical, and Dielectric Properties of Dielectric Elastomer-Based Composites" Energies 15, no. 1: 152. https://doi.org/10.3390/en15010152
APA StyleOlewnik-Kruszkowska, E., Adamczyk, A., Gierszewska, M., & Grabska-Zielińska, S. (2022). Comparison of How Graphite and Shungite Affect Thermal, Mechanical, and Dielectric Properties of Dielectric Elastomer-Based Composites. Energies, 15(1), 152. https://doi.org/10.3390/en15010152







