Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Pyrolysis Setup: Pyrolysis of Biomass in a Fixed-Bed Reactor
2.3. Material Analysis
3. Results and Discussion
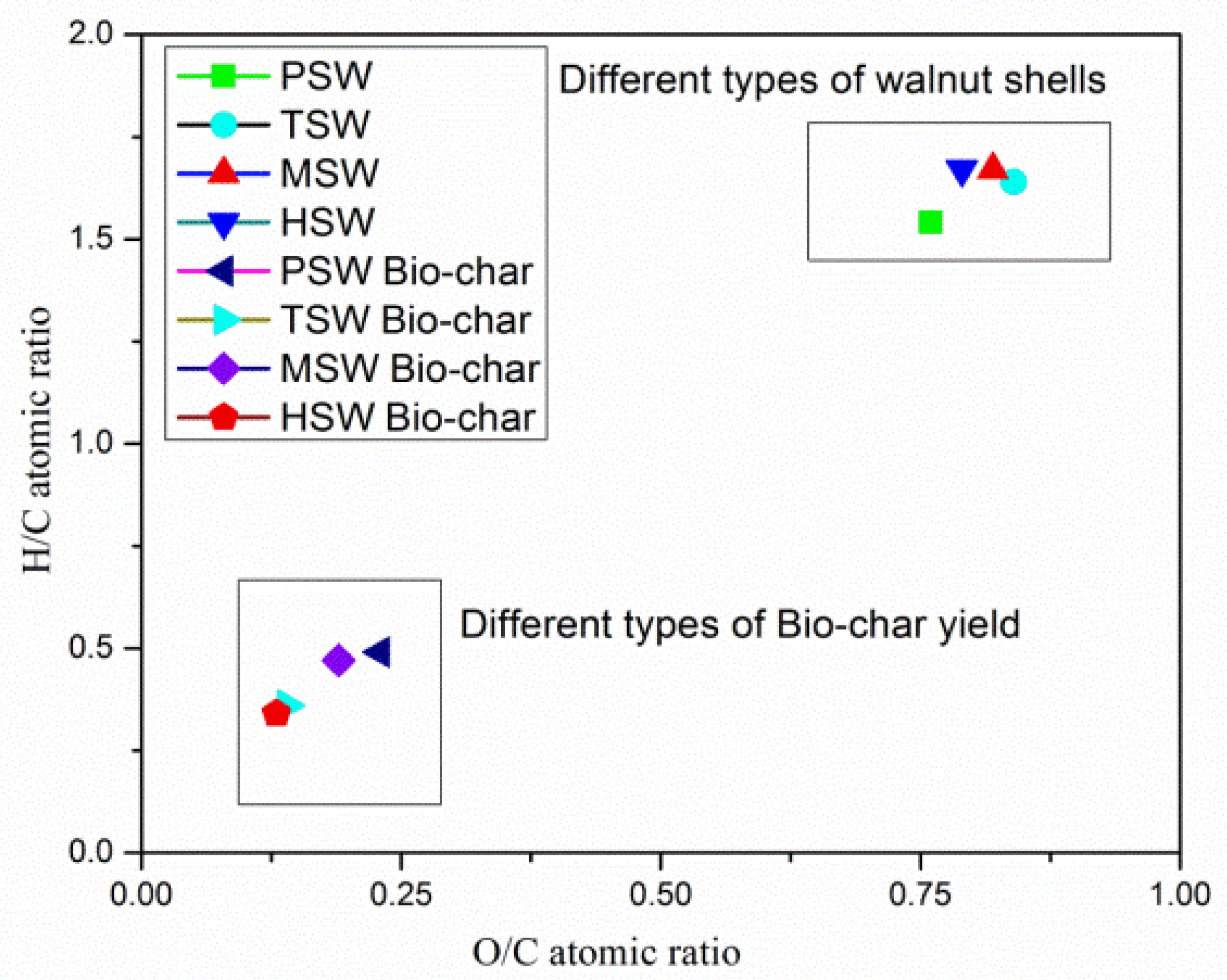
3.1. Proximate and Ultimate Analysis
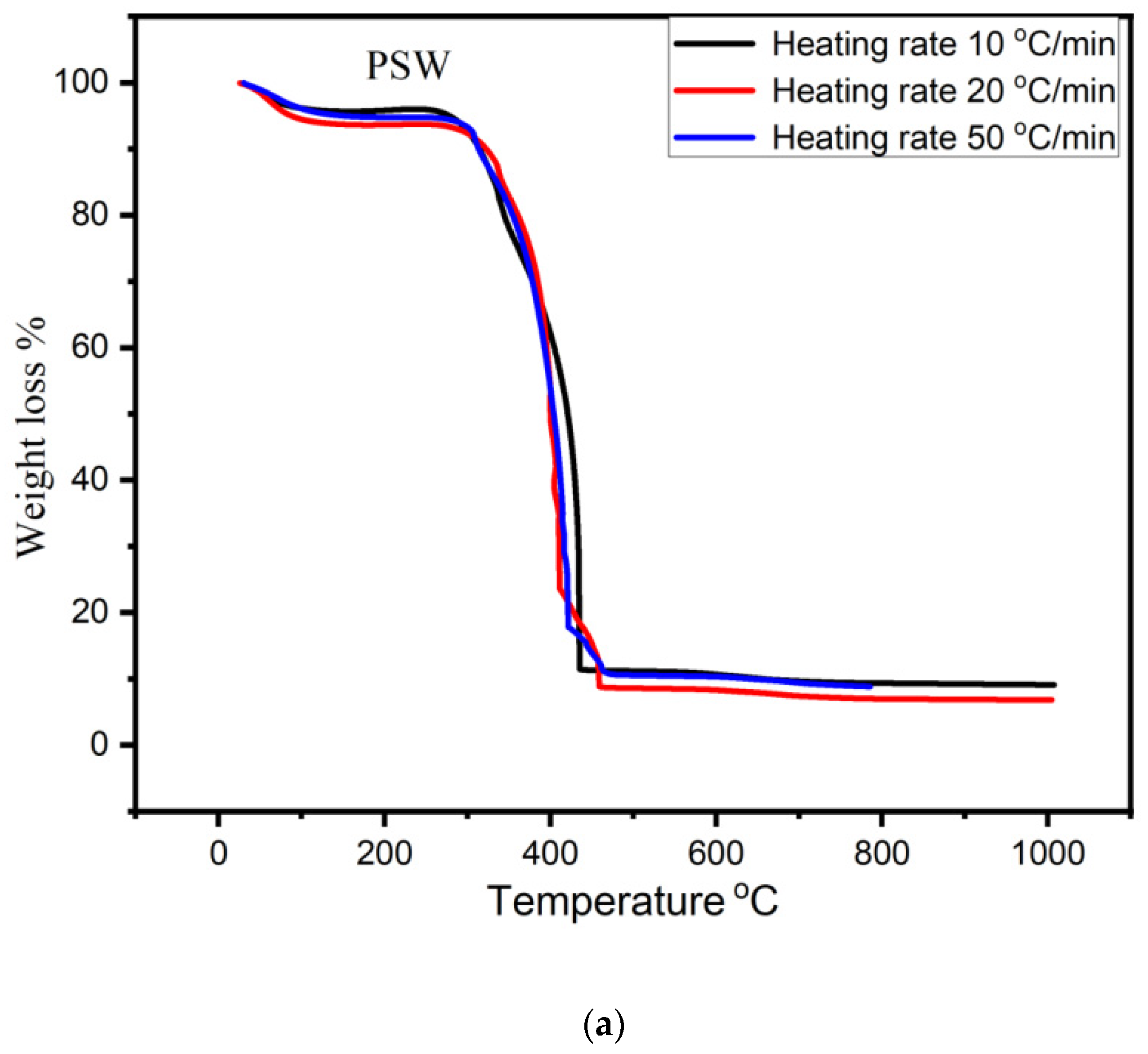
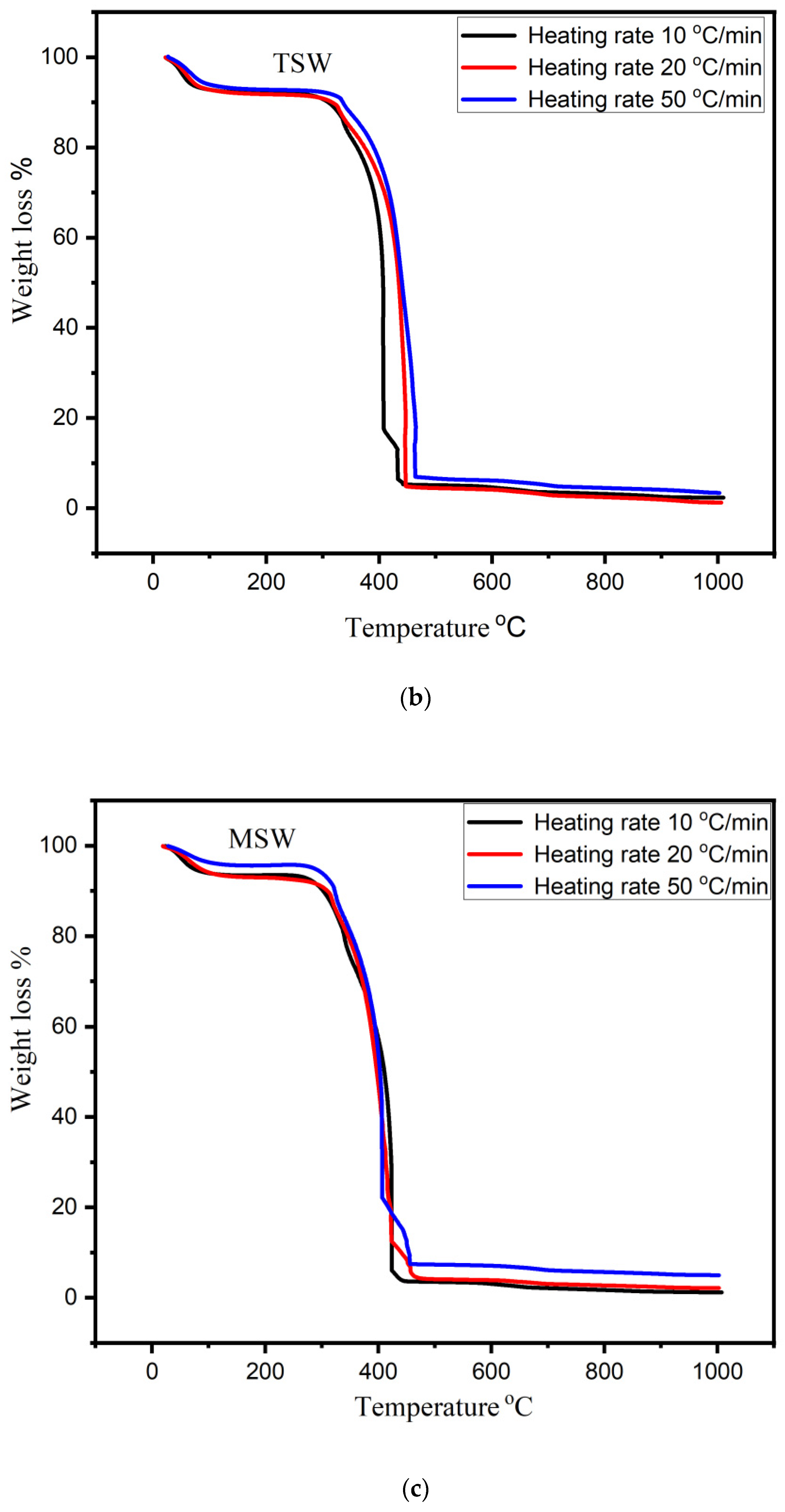
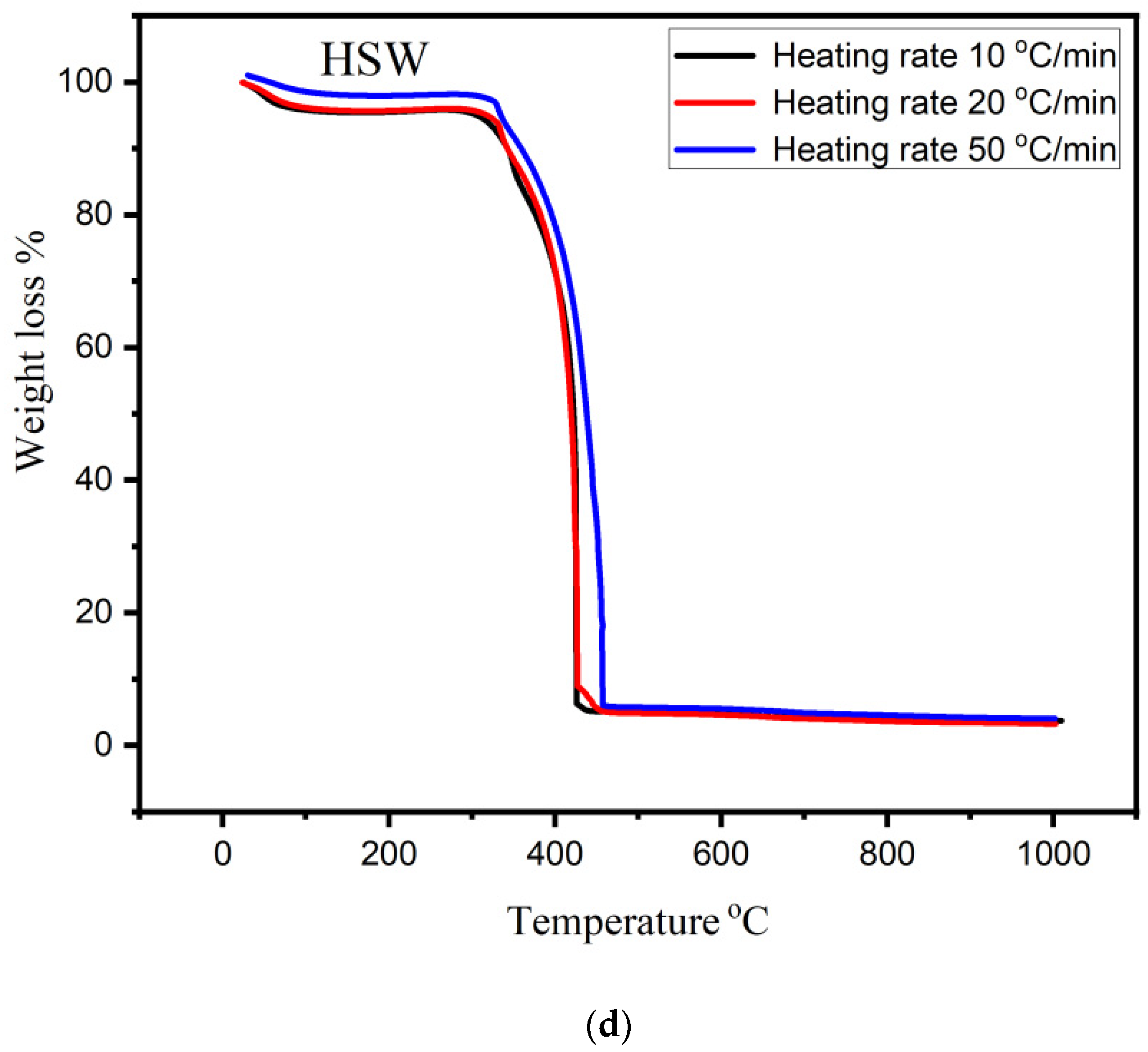
3.2. TGA Analysis of Bio-Char
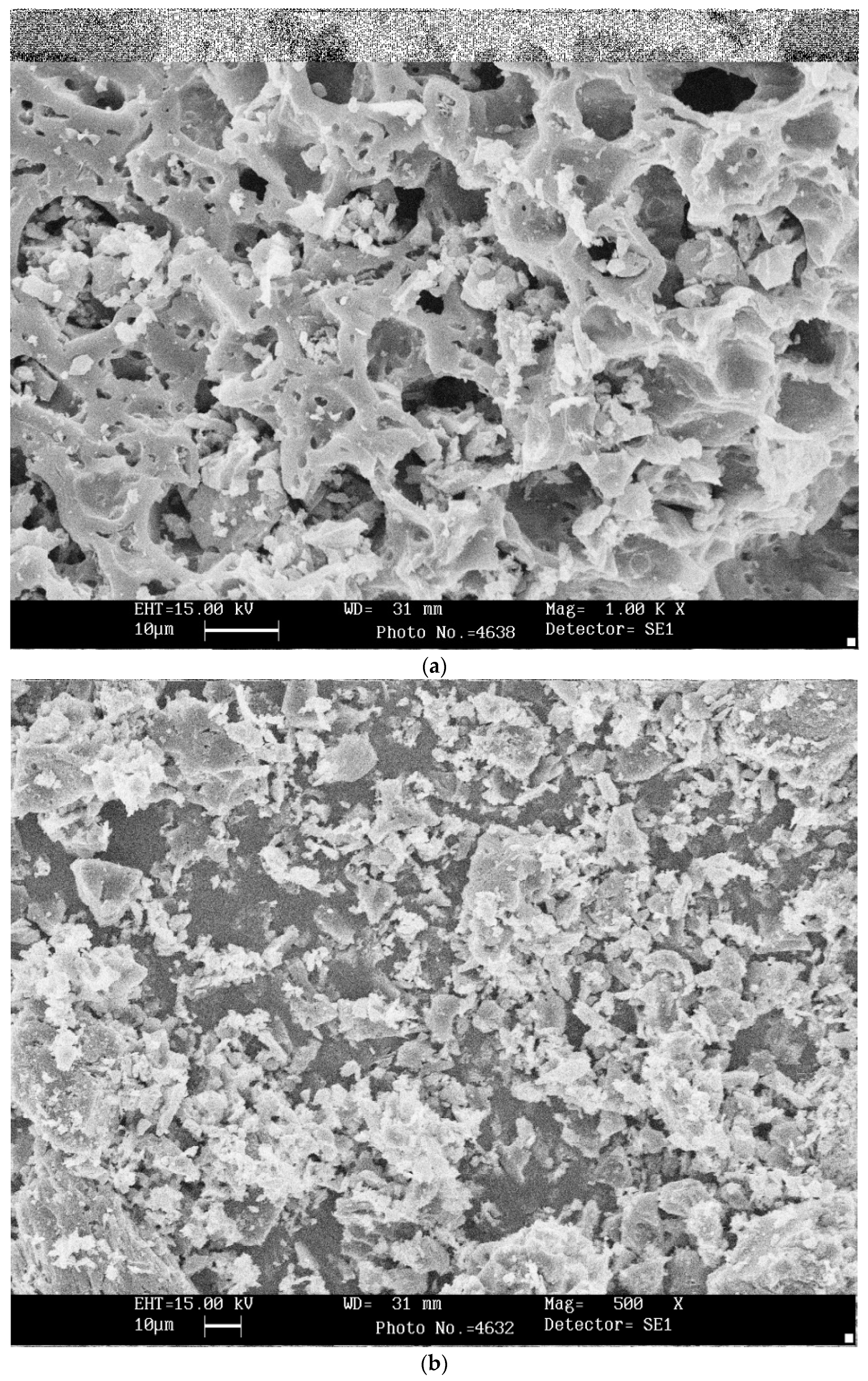
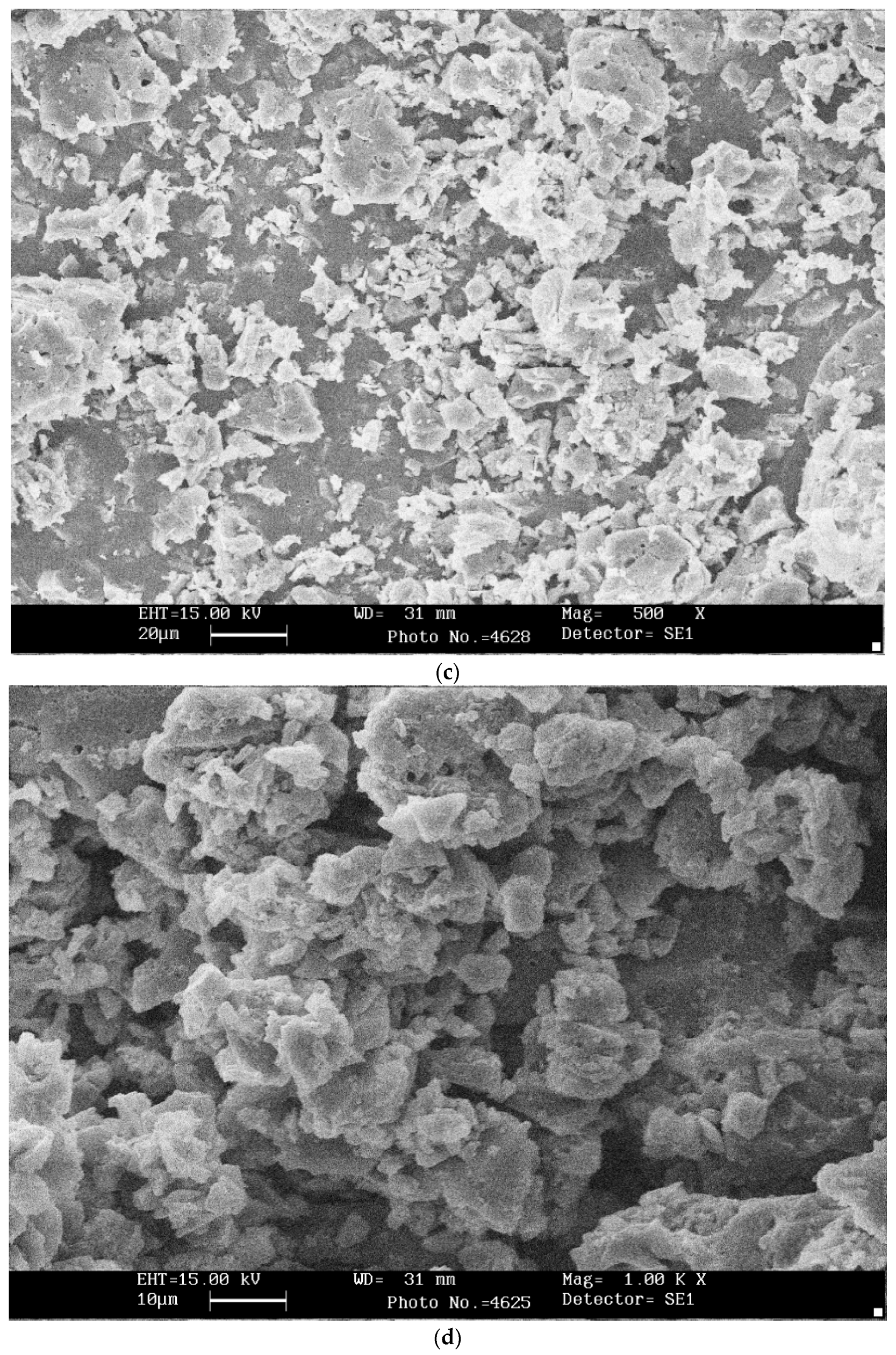
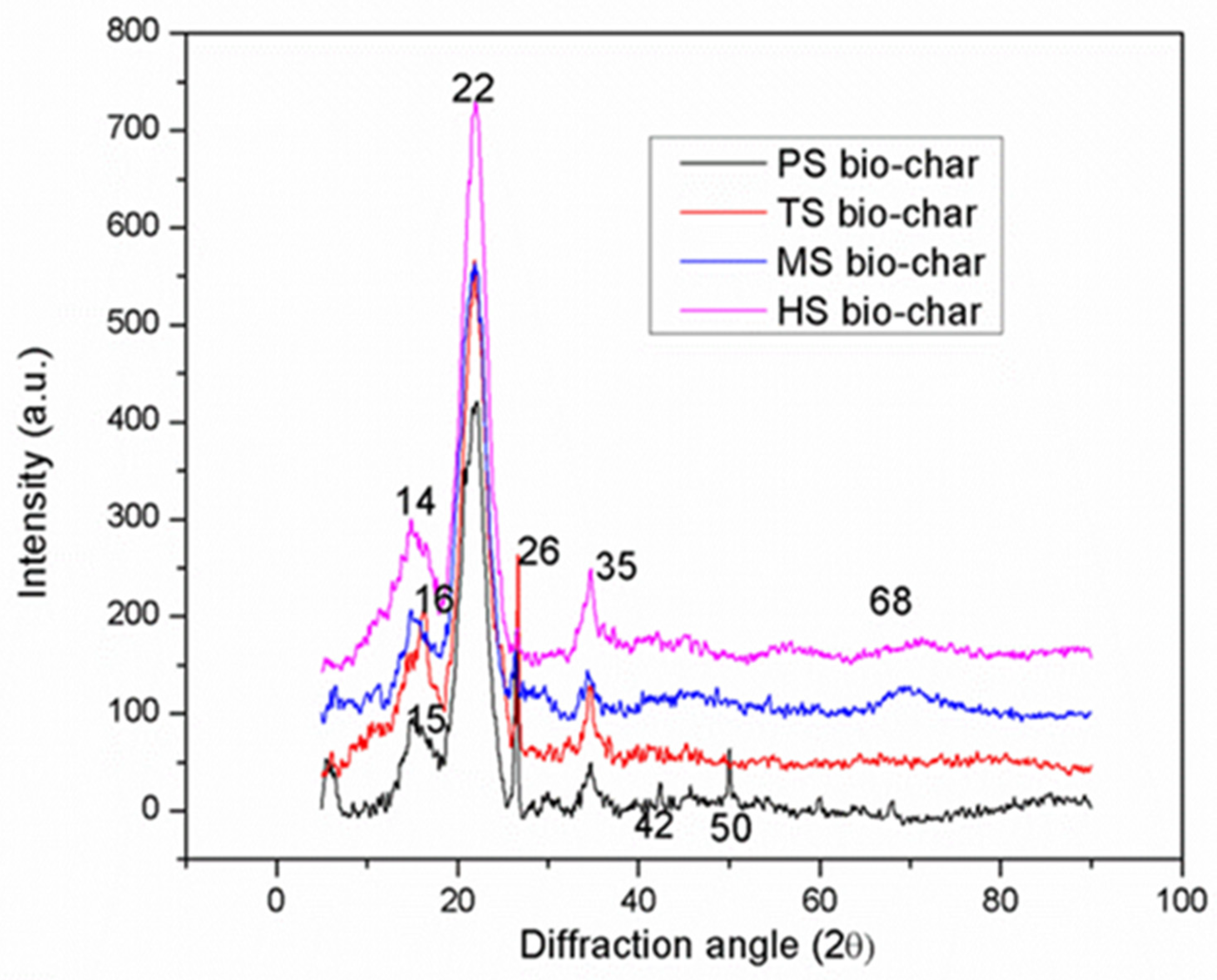
3.3. SEM and XRD Analysis of Bio-Chars
3.4. FTIR of Bio-Char
3.5. Surface Area, Total Pore Volume, Average Pore size Volume, pH and its Potential Applications
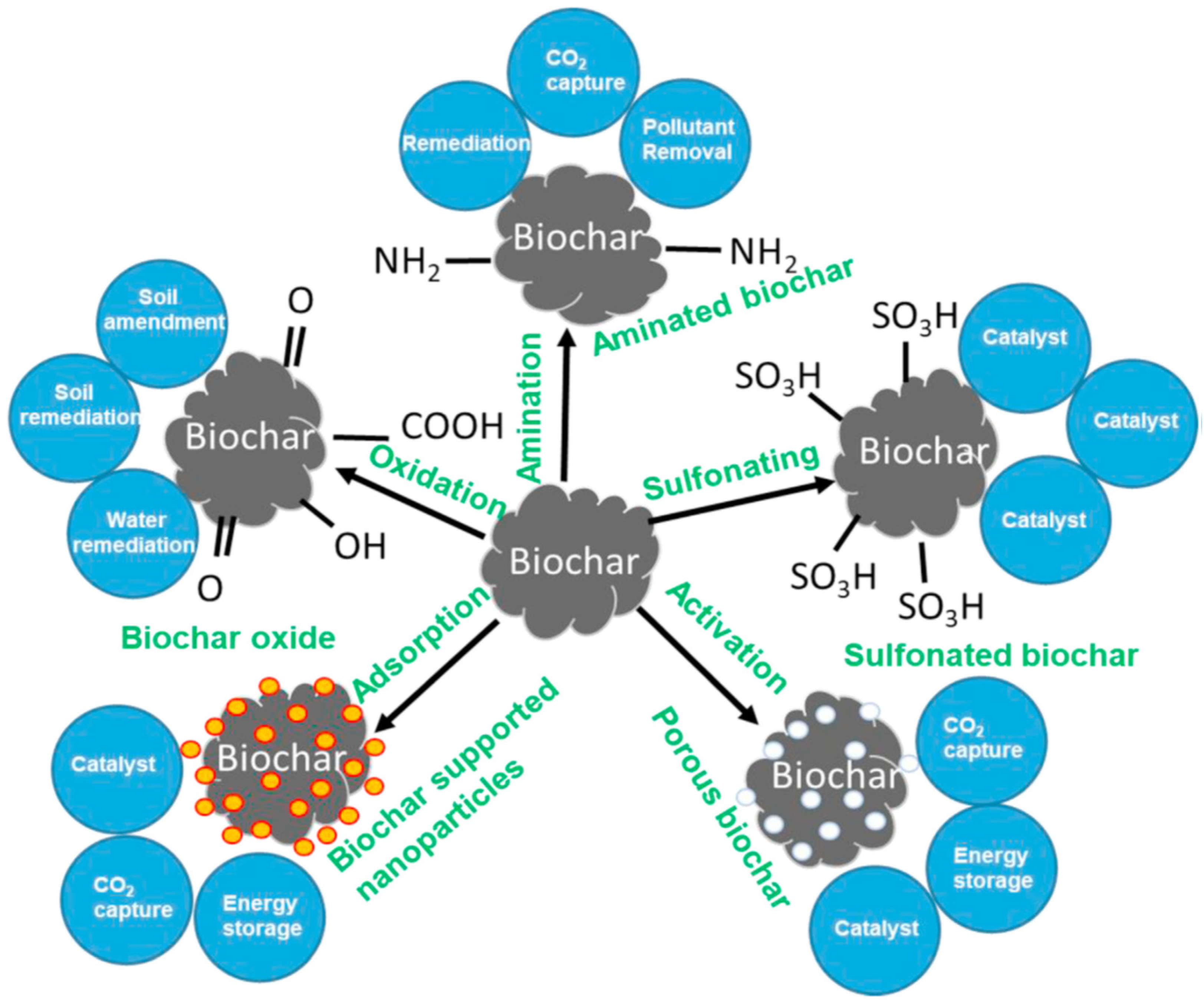
3.6. Circular Economy Models
4. Conclusions
- The pyrolytic behavior of bio-char was studied using thermogravimetric analysis (TGA) in an oxidizing atmosphere. SEM analysis confirmed morphological change and showed heterogeneous and rough texture structure.
- Crystalline nature of the bio-chars was established by X-ray powder diffraction (XRD) analysis. The maximum higher heating values (HHV), high fixed carbon content and surface area obtained for walnut shells (WS) samples were found as ~18.4 MJ kg−1, >80% and 58 m2/g, respectively.
- Improvement in HHV and decrease of O/C and H/C ratios led the bio-char samples to fall into the category of coal and confirmed their hydrophobic, carbonized and aromatized nature.
- From the Fourier transform infra-red spectroscopy (FTIR), it was observed that there was alteration in functional groups with an increase in temperature, and illustrated higher aromaticity. Therefore, it could be concluded that the bio-char obtained from walnut shell has a high potential to be used as an efficient fuel in both industrial as well as domestic furnaces for energy production.
- Further, from surface area and pH analysis of bio-chars, it was found that WS bio-chars had similar characteristics to adsorbents used for water purifications, retention of essential elements in soil and carbon sequestration.
- The improvement in characteristics of different bio-chars, as compared to their respective biomass residues, showed that it may also be used as a good adsorbent for wastewater treatment as well as for enhancing soil fertility.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, Y.; Wang, Y.; Zhang, Q.; Rong, H.; Liu, Y.; Xiao, B.; Guo, D.; Laghari, M.; Ruan, R. Gas-carrying enhances the combustion temperature of the biomass particles. Energy 2021, 239, 121956. [Google Scholar] [CrossRef]
- Hansson, A.; Haikola, S.; Fridahl, M.; Yanda, P.; Mabhuye, E.; Pauline, N. Biochar as multi-purpose sustainable technology: Experiences from projects in Tanzania. Environ. Dev. Sustain. 2021, 23, 5182–5214. [Google Scholar] [CrossRef]
- Masek, O.; Brownsort, P.; Cross, A.; Sohi, S. Influence of production conditions on the yield and environmental stability of biochar. Fuel 2013, 103, 151–155. [Google Scholar] [CrossRef] [Green Version]
- Manya, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Kane, S.N.; Mishra, A.; Dutta, A.K. Preface: International Conference on Recent Trends in Physics (ICRTP 2016). J. Phys. Conf. Ser. 2016, 755, 5. [Google Scholar] [CrossRef]
- Ciolkosz, D.; Wallace, R. A review of torrefaction for bioenergy feedstock production. Biofuels Bioprod. Biorefin. 2011, 5, 317–329. [Google Scholar] [CrossRef]
- Liu, Z.; Han, G. Production of solid fuel biochar from waste biomass by low temperature pyrolysis. Fuel 2015, 158, 159–165. [Google Scholar] [CrossRef]
- Chen, W.-H.; Lin, B.-J.; Colin, B.; Chang, J.-S.; Pétrissans, A.; Bi, X.; Pétrissans, M. Hygroscopic transformation of woody biomass torrefaction for carbon storage. Appl. Energy 2018, 231, 768–776. [Google Scholar] [CrossRef]
- Zhu, X. Effects and mechanisms of biochar-microbe interactions in soil improvement and pollution remediation: A review. Environ. Pollut. 2017, 227, 98–115. [Google Scholar] [CrossRef]
- Oliveira, F.R.; Patel, A.K.; Jaisi, D.P.; Adhikari, S.; Lu, H.; Khanal, S.K. Environmental application of biochar: Current status and perspectives. Bioresour. Technol. 2017, 246, 110–122. [Google Scholar] [CrossRef]
- Xu, G.; Lv, Y.; Sun, J.; Shao, H.; Wei, L. Recent Advances in Biochar Applications in Agricultural Soils: Benefits and Environmental Implications. Clean Soil Air Water 2012, 40, 1093–1098. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Cao, X.; Masek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Kua, H.W.; Low, C.Y. Low, Use of biochar as carbon sequestering additive in cement mortar. Cem. Concr. Compos. 2018, 87, 110–129. [Google Scholar] [CrossRef]
- Gan, Y.Y. Torrefaction of microalgal biochar as potential coal fuel and application as bio-adsorbent. Energy Convers. Manag. 2018, 165, 152–162. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.M.; Kim, S.; Ryu, C.; Park, S.H.; Park, Y.-K. Review of the use of activated biochar for energy and environmental applications. Carbon Lett. 2018, 26, 1–10. [Google Scholar]
- Chen, W.; Lee, K.T.; Chih, Y.K.; Eng, C.; Lin, H.P.; Chiou, Y.B.; Cheng, C.L.; Lin, Y.X.; Chang, J.S. Novel Renewable Double-Energy System for Activated Biochar Production and Thermoelectric Generation from Waste Heat. Energy Fuels 2020, 34, 3383–3393. [Google Scholar] [CrossRef]
- Windeatt, J.H.; Ross, A.B.; Williams, P.T.; Forster, P.M.; Nahil, M.A.; Singh, S. Characteristics of biochars from crop residues: Potential for carbon sequestration and soil amendment. J. Environ. Manag. 2014, 146, 189–197. [Google Scholar] [CrossRef]
- Moreira, R.; Vaz, J.M.; Spinace, E.V.; dos Reis Orsini, R.; Penteado, J.C. Production of Biochar, Bio-Oil and Synthesis Gas from Cashew Nut Shell by Slow Pyrolysis. Waste Biomass Valorization 2016, 8, 217–224. [Google Scholar] [CrossRef]
- Demiral, I.; Kul, S.C. Pyrolysis of apricot kernel shell in a fixed-bed reactor: Characterization of bio-oil and char. J. Anal. Appl. Pyrolysis 2014, 107, 17–24. [Google Scholar] [CrossRef]
- Jiang, K.-M.; Cheng, C.-G.; Ran, M.; Lu, Y.-G.; Wu, Q.-L. Preparation of a biochar with a high calorific value from chestnut shells. New Carbon Mater. 2018, 33, 183–187. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Varjani, S.; Saravanan, A. A critical review on the biochar production techniques, characterization, stability and applications for circular bioeconomy. Biotechnol. Rep. 2020, 28, e00570. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.R.; El Hanandeh, A. Life cycle perspective of bio-oil and biochar production from hardwood biomass; what is the optimum mix and what to do with it? J. Clean. Prod. 2019, 212, 173–189. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.; Yu, S.; Ryu, C.; Park, J. Clean and energy-efficient mass production of biochar by process integration: Evaluation of process concept. Chem. Eng. J. 2019, 355, 840–849. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Gurwick, N.P.; Moore, L.A.; Kelly, C.; Elias, P. A systematic review of biochar research, with a focus on its stability in situ and its promise as a climate mitigation strategy. PLoS ONE 2013, 8, e75932. [Google Scholar] [CrossRef] [Green Version]
- Marmiroli, M.; Bonas, U.; Imperiale, D.; Lencioni, G.; Mussi, F.; Marmiroli, N.; Maestri, E. Structural and functional features of chars from different biomasses as potential plant amendments. Front Plant Sci. 2018, 9, 1119. [Google Scholar] [CrossRef] [Green Version]
- Ghodake, G.S.; Shinde, S.K.; Kadam, A.A.; Saratale, R.G.; Saratale, G.D.; Kumar, M.; Palem, R.R.; AL-Shwaiman, H.A.; Elgorban, A.M.; Syed, A. Review on biomass feedstocks, pyrolysis mechanism and physicochemical properties of biochar: State-of-the-art framework to speed up vision of circular bioeconomy. J. Clean. Prod. 2020, 297, 126645. [Google Scholar] [CrossRef]
- Lin, Y.L.; Zheng, N.Y.; Hsu, C.H. Torrefaction of fruit peel waste to produce environmentally friendly biofuel. J. Clean. Prod. 2020, 284, 124676. [Google Scholar] [CrossRef]
- Romanowska-Duda, Z.; Szufa, S.; Grzesik, M.; Piotrowski, K.; Janas, R. The Promotive Effect of Cyanobacteria and Chlorella sp. Foliar Biofertilization on Growth and Metabolic Activities of Willow (Salix viminalis L.) Plants as Feedstock Production, Solid Biofuel and Biochar as C Carrier for Fertilizers via Torrefaction Process. Energies 2021, 14, 5262. [Google Scholar] [CrossRef]
- D’Adamo, I.; Morone, P.; Huisingh, D. Bioenergy: A Sustainable Shift. Energies 2021, 14, 5661. [Google Scholar] [CrossRef]
- Xu, T.; Bhattacharya, S. Mineral transformation and morphological change during pyrolysis and gasification of victorian brown coals in an entrained flow reactor. Energy Fuels 2019, 33, 6134–6147. [Google Scholar] [CrossRef]
- Li, J.; Du, Y.; Bao, T.; Dong, J.; Lin, M.; Shim, H.; Yang, S.-T. n-Butanol production from lignocellulosic biomass hydrolysates without detoxification by Clostridium tyrobutyricum Dack-adhE2 in a fibrous-bed bioreactor. Bioresour. Technol. 2019, 289, 121749. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, Y.J.; Chen, J.; Zhao, Y.R.; Chang, J.; Junge, H.; Beller, M.; Li, Y. Streamlined hydrogen production from biomass. Nat. Cat. 2018, 1, 332–338. [Google Scholar] [CrossRef]
- Sette, J.R.; Hansted, C.R.; Novaes, A.L.S.; Lima, E.; Rodrigues, P.A.F.; Santos, A.C.; Yamaji, D.R.S. Energy enhancement of the eucalyptus bark by briquette production. Ind. Crops. Prod. 2018, 122, 209–213. [Google Scholar] [CrossRef]
- The International Nut and Dried Fruit Council Foundation, Stastical Year Book 2017–2018. Available online: https://www.nutfruit.org/files/tech/1524481168_INC_Statistical_Yearbook_2017-2018.pdf (accessed on 20 June 2021).
- Shah, M.A.; Khan, M.N.S.; Kumar, V. Biomass residue characterization for their potential application as biofuels. J. Therm. Anal. Calorim. 2018, 134, 2137–2145. [Google Scholar] [CrossRef]
- ASTM E 871-82; Moisture Analysis of Particulate Wood Fuels; ASTM: West Conshohocken, PA, USA, 2014. [CrossRef]
- ASTM D1102-84; Standard Test Method for Ash in Wood; ASTM: West Conshohocken, PA, USA, 2013. [CrossRef]
- EN 14175-3:2003; Fume Hoods—Type Test Methods; National Standards Association Ireland: Dublin, Ireland, 2003. [CrossRef]
- Chowdhury, Z.Z.; Pal, K.; Johan, R.B.; Yehye, W.; Ali, M.E. Comparative Evaluation of Physiochemical Properties of a Solid Fuel Derived from Adansonia digitata Trunk Using Torrefaction. Bioresources 2017, 12, 3816–3833. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Quek, A.; Kent, H.S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- ASTM D5373-08; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Laboratory Samples of Coal; ASTM: West Conshohocken, PA, USA, 2012. [CrossRef]
- ASTM D2015-85; Standard Test Method for Gross Calorific Value of Coal and Coke by the Adiabatic Bomb Calorimeter; ASTM: West Conshohocken, PA, USA, 1975.
- Gonzalez, J.F.; Ramiro, A.; Gonzalez-Garcia, C.M.; Ganan, J.; Encinar, J.M.; Sabio, E.; Rubiales, J. Pyrolysis of almond shells. Energy applications of fractions. Ind. Eng. Chem. Res. 2005, 44, 3003–3012. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of their Candidacy for Alternate Renewable Fuels. Bioenergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 1): Overview of biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef]
- Yang, Y.; Brammer, J.G.; Mahmood, A.S.N.; Hornung, A. Intermediate pyrolysis of biomass energy pellets for producing sustainable liquid, gaseous and solid fuels. Bioresour. Technol. 2014, 169, 794–799. [Google Scholar] [CrossRef] [Green Version]
- Onay, O.; Beis, S.H.; Kockar, O.M. Pyrolysis of walnut shell in a well-swept fixed-bed reactor. Energy Sources 2004, 26, 771–782. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Zhang, X.; Silva, L.C.R.; Six, J.; Parikh, S.J. Use of chemical and physical characteristics to investigate trends in biochar feedstocks. J. Agric. Food Chem. 2013, 61, 2196–2204. [Google Scholar] [CrossRef]
- Rather, M.A.; Khan, N.S.; Gupta, R. Hydrothermal carbonization of macrophyte Potamogeton lucens for solid biofuel production: Production of solid biofuel from macrophyte Potamogeton lucens. Eng. Sci. Technol. Int. J. 2017, 20, 168–174. [Google Scholar]
- Varma, A.K.; Mondal, P. Physicochemical characterization and kinetic study of pine needle for pyrolysis process. J. Therm. Anal. Calorim. 2016, 124, 487–497. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of sugarcane bagasse in semi batch reactor: Effects of process parameters on product yields and characterization of products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Maiti, S.; Dey, S.; Purakayastha, S.; Ghosh, B. Physical and thermochemical characterization of rice husk char as a potential biomass energy source. Bioresour. Technol. 2006, 97, 2065–2070. [Google Scholar] [CrossRef] [PubMed]
- Crombie, K.; Mašek, O.; Brownsort, P.; Sohi, S.P.; Cross, A. The effect of pyrolysis conditions on biochar stability as determined by three methods. GCB Bioenergy 2012, 5, 122–131. [Google Scholar] [CrossRef] [Green Version]
- Souza, B.S.; Moreira, A.P.D.; Teixeira, A.M.R.F. TG-FTIR coupling to monitor the pyrolysis products from agricultural residues. J. Therm. Anal. Calorim. 2009, 97, 637–642. [Google Scholar] [CrossRef]
- Bisht, A.S.; Kumar, S.R. Use of pine needle in energy generation. Int. J. Res. Appl. Sci. Eng. Technol. 2014, 2, 59–63. [Google Scholar]
- Cai, J.M.; Bi, L.S. Kinetic analysis of wheat straw pyrolysis using isoconversional methods. J. Therm. Anal. Calorim. 2009, 98, 325–330. [Google Scholar] [CrossRef]
- Chutia, R.S.; Kataki, R.; Bhaskar, T. Thermogravimetric and decomposition kinetic studies of Mesua ferrea L. deoiled cake. Bioresour. Technol. 2013, 139, 66–72. [Google Scholar] [CrossRef]
- Mishra, G.; Bhaskar, T. Non isothermal model free kinetics for pyrolysis of rice straw. Bioresour. Technol. 2014, 169, 614–621. [Google Scholar] [CrossRef]
- AcIkalIn, K. Pyrolytic characteristics and kinetics of pistachio shell by thermogravimetric analysis. J. Therm. Anal. Calorim. 2012, 109, 227–235. [Google Scholar] [CrossRef]
- Lopez-Velazquez, M.A.; Santes, V.; Balmaseda, J.; Torres-Garcia, E. Pyrolysis of orange waste: A thermo-kinetic study. J. Anal. Appl. Pyrolysis 2013, 99, 170–177. [Google Scholar] [CrossRef]
- Lapuerta, M.; Hernández, J.J.; Rodríguez, J. Kinetics of devolatilisation of forestry wastes from thermogravimetric analysis. Biomass Bioenergy 2004, 27, 385–391. [Google Scholar] [CrossRef]
- Guerrero, M.; Ruiz, M.P.; Millera, A.; Alzueta, M.U.; Bilbao, R. Characteriza-tion of biomass chars formed under different devolatilization conditions: Differences between rice husk and eucalyptus. Energy Fuels 2008, 22, 1275–1284. [Google Scholar] [CrossRef]
- Morin, M.; Pécate, S.; Hémati, M.; Kara, Y. Pyrolysis of biomass in a batch fluidized bed reactor: Effect of the pyrolysis conditions and the nature of the biomass on the physicochemical properties and the reactivity of char. J. Anal. Appl. Pyrolysis 2016, 122, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G.; Morgan, T.J. An overview of the organic and inorganic phase composition of biomass. Fuel 2012, 94, 1–33. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Li, P.; Zhang, J.; Zheng, C. Pyrolysis of maize stalk on the characterization of chars formed under different devolatilization conditions. Energy Fuels 2009, 23, 4605–4611. [Google Scholar] [CrossRef]
- Sotoudehnia, F.; Baba, R.A.; Alayat, A.; McDonald, A.G. Characterization of bio-oil and biochar from pyrolysis of waste corrugated cardboard. J. Anal. Appl. Pyrolysis 2020, 145, 104722. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Yang, H.; Lu, Q.; Li, D.; Yang, Q.; Chen, H. Study on pyrolysis behaviors of non-woody lignins with TG-FTIR and Py-GC / MS. J. Anal. Appl. Pyrolysis 2015, 113, 499–507. [Google Scholar] [CrossRef]
- Xu, F.; Yu, J.; Wang, D.; Tesso, T. Qualitative and quantitative analysis of lignocellulosic biomass using infrared techniques: A mini-review. Appl. Energy 2013, 104, 801–809. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, D.; Singh, R.K.; Bendu, H.; Mund, R. Pyrolysis of Mahua seed (Madhuca indica)—Production of biofuel and its characterization. Energy Convers. Manag. 2016, 108, 529–538. [Google Scholar] [CrossRef]
- Tushar, H.K.; Mahinpey, M.S.; Khan, N.A.; Ibrahim, H.; Kumar, P.; Idem, R. Production, characterization and reactivity studies of chars produced by the isothermal pyrolysis of flax straw. Biomass Bioenergy 2012, 37, 97–105. [Google Scholar] [CrossRef]
- Angın, D. Effect of pyrolysis temperature and heating rate on biochar obtainedfrom pyrolysis of safflower seed press cake. Bioresour. Technol. 2013, 128, 593–597. [Google Scholar] [CrossRef]
- Fu, P.; Hu, S.; Xiang, J.; Sun, L.; Su, S.; Wang, J. Evaluation of the porous structure development of chars from pyrolysis of rice straw: Effects of pyrolysis temperature and heating rate. J. Anal. Appl. Pyrolysis 2012, 98, 177–183. [Google Scholar] [CrossRef]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and characterization of slow pyrolysis biochar: Influence of feedstock type and pyrolysis conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Lee, Y.; Park, J.; Ryu, C.; Gang, K.S.; Yang, W.; Park, Y.; Jung, J.; Hyun, S. Comparison of biochar properties from biomass residues produced by slow pyrolysis at 500 °C. Bioresour. Technol. 2013, 148, 196–201. [Google Scholar] [CrossRef]
- Nzihou, A.; Stanmore, B.; Lyczko, N.; Minh, D.P. The catalytic effect of inherent and adsorbed metals on the fast/flash pyrolysis of biomass: A review. Energy 2019, 170, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Bordoloi, N.; Narzari, R.; Chutia, R.S.; Bhaskar, T.; Kataki, R. Pyrolysis of Mesua ferrea and Pongamia glabra seed cover: Characterization of bio-oil and its sub-fractions. Bioresour. Technol. 2015, 178, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, Z.Z.; Karim, Z.M.; Ashraf, M.A.; Khalid, K. Influence of carbonization temperature on physicochemical properties of biochar derived from slow pyrolysis of durian wood (Durio zibethinus) sawdust. BioResources 2016, 11, 3356–3372. [Google Scholar] [CrossRef]
- Wu, Q.; Xian, Y.; He, Z.; Zhang, Q.; Wu, J.; Yang, G. Adsorption characteristics of Pb(II) using biochar derived from spent mushroom substrate. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y. Structural and adsorption characteristics of potassium carbonate activated biochar. RSC Adv. 2018, 8, 21012–21019. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, M.; Kalderis, D. A complementary analysis of the porous structure of biochars obtained from biomass. Carbon Lett. 2020, 30, 325–329. [Google Scholar] [CrossRef]
- Batista, E.M.C.C.; Shultz, J.; Matos, T.T.S.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Tan, E.C.D. Sustainable Biomass Conversion Process Assessment. In Process Intensification and Integration for Sustainable Design; Foo, D.C.Y., El-Halwagi, M.M., Eds.; John Wiley and Sons, Ltd: Weinheim, Germany, 2021; pp. 301–318. [Google Scholar]
- Dewulf, J.; Benini, L.; Mancini, L.; Sala, S.; Blengini, G.A.; Ardente, F.; Recchioni, M.; Maes, J.; Pant, R.; Pennington, D. Rethinking the area of protection “natural resources” in life cycle assessment. Environ. Sci. Technol. 2015, 49, 5310–5317. [Google Scholar] [CrossRef]
- Gallie, W.B. IX—Essentially contested concepts. Proc. Arist. Soc. 1956, 56, 167–198. [Google Scholar] [CrossRef]
- Korhonen, J.; Nuur, C.; Feldmann, A.; Birkie, S.E. Circular economy as an essentially contested concept. J. Clean. Prod. 2018, 175, 544–552. [Google Scholar] [CrossRef]
- European Commission. Bioeconomy. 2013. Available online: https://ec.europa.eu/programmes/horizon2020/en/h2020-section/bioeconomy (accessed on 20 November 2013).
- Bocken, N.M.P.; de Pauw, I.; Bakker, C.; van der Grinten, B. Product design and business model strategies for a circular economy. J. Ind. Prod. Eng. 2016, 33, 308–320. [Google Scholar] [CrossRef] [Green Version]
- Tan, E.C.D.; Lamers, P. Circular Bioeconomy Concepts—A Perspective. Front. Sustain. 2021, 2, 701509. [Google Scholar] [CrossRef]

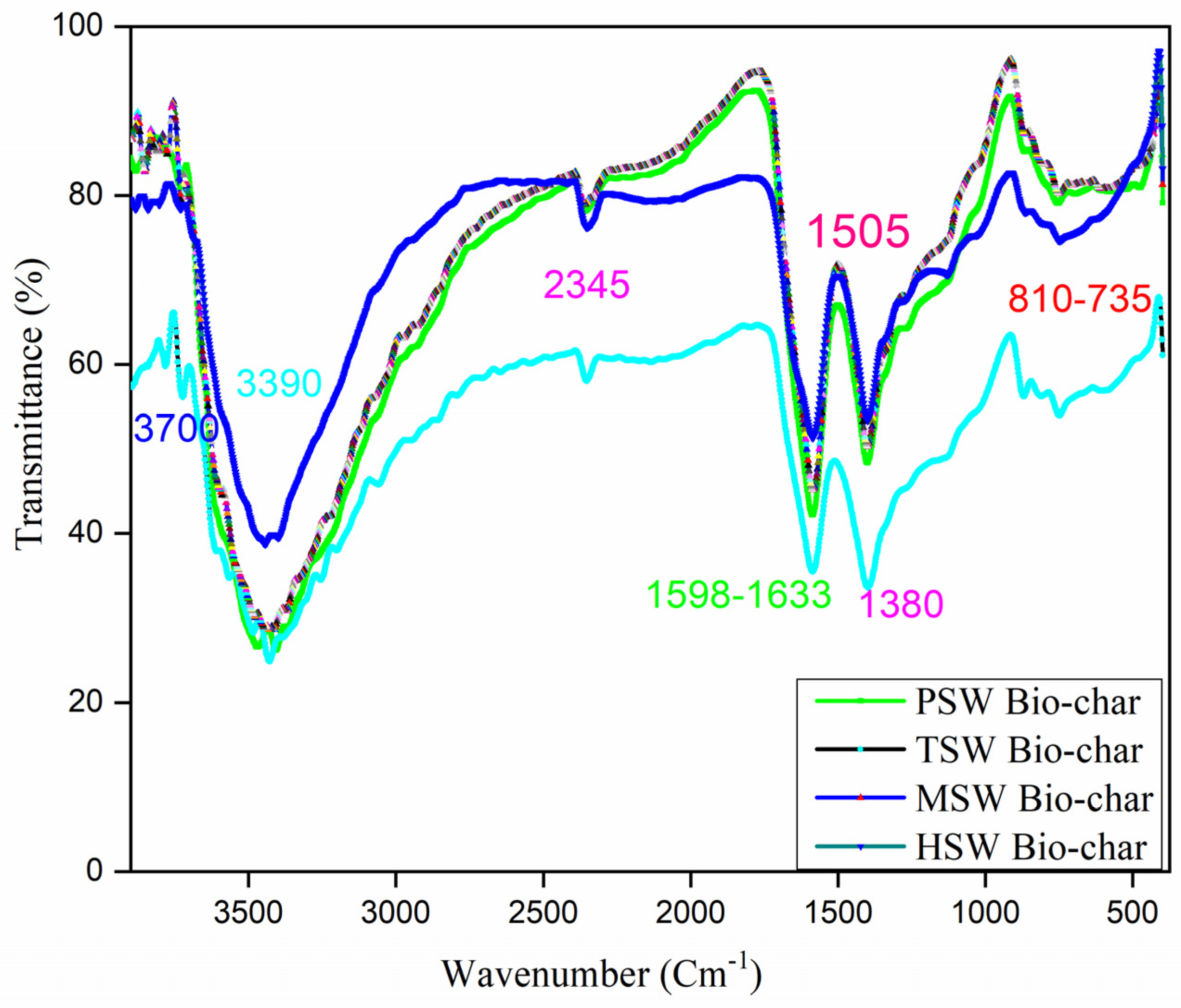
| Types of Bio-Char | Proximate Analysis (wt% daf) | |||||||
|---|---|---|---|---|---|---|---|---|
| M | VM | A | FC | HHV (Mj/Kg) | ED | EY | References | |
| PSW | 0.8 | 14.4 | 5.5 | 78.5 | 14.8 | 1.08 | 72.6 | Present work |
| TSW | 0.6 | 12.1 | 4.4 | 78.4 | 15.2 | 1.11 | 72.6 | |
| MSW | 0.3 | 11.7 | 3.2 | 84.2 | 16.6 | 1.15 | 69.7 | |
| HSW | 0.2 | 8.7 | 2.02 | 85.6 | 18.4 | 1.26 | 71.9 | |
| Almond shells char | - | 21.2 | 1.9 | 76.9 | 28.2 | - | - | [45] |
| Palm shell char | 2.2 | 11.5 | 6.7 | 88.5 | 33.6 | - | - | [17] |
| Coconut shell | 7.1 | 8.1 | 4.1 | 91.9 | 33.7 | - | - | |
| Wheat straw bio-char (WSB) | 0.6 ± 0.01 | 7.3 | 8.2 ± 0.2 | 83.9 | 22.0±0.7 | - | - | [46] |
| Coal (lignite) | 34 | 43.93 | 9 | 46.96 | 9.3-19.3 * | - | - | [47] |
| Coal (bituminous) | 11 | 39.93 | 10.11 | 50.56 | 27.9–34.89 ** | - | - | |
| Type of Bio-Chars | Ultimate Analysis (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| C | H | N | O | H/C | O/C | References | |
| PSW | 73.4 | 3.0 | 0.8 | 22.7 | 0.49 | 0.3 | Present work |
| TSW | 76.6 | 2.4 | 0.8 | 15.2 | 0.31 | 0.19 | |
| MSW | 81.8 | 3.0 | 0.6 | 19.6 | 0.36 | 0.23 | |
| HSW | 82.7 | 2.38 | 0.8 | 14.0 | 0.28 | 0.17 | |
| Walnut shell | 55.3 | 0.89 | 0.47 | 1.6 | [35] | ||
| Apricot Kernel shell char | 72.72 | 3.17 | 1.27 | 19.84 | 0.50 | - | [19] |
| Barley straw | 74.83 | 3.51 | 0.10 | 8.46 | [48] | ||
| Wheat straw bio-char (WSB) | 64.8 | 3.1 | 0.8 | 23.0 | 0.6 | 0.3 | [46] |
| Coal (lignite) | 56.4 | 4.2 | 1.6 | 18.4 | - | - | [47] |
| Coal (bituminous) | 73.1 | 5.5 | 1.4 | 8.7 | - | - | |
| Band Assignment | Band Frequency (cm−1) | |||
|---|---|---|---|---|
| PSW | TSW | MSW | HSW | |
| O–H stretching | 3465 | 3428 | 3411 | 3442 |
| C–H stretching | - | 3050, 2849 | 3075, 2925 | - |
| Aromatic vibrations of C–C/C = C stretching | 1587 | 1585 | 1585 | 1586 |
| C–O–H in-plane bending and aromatic vibrations | 1401 | 1398 | 1400 | 1399 |
| OH bending and CH deformation vibrations | 1265 | - | 1265 | 1265 |
| C–H in-plane deformation and C–OH stretch in syringyl | 1121 | 1124 | 1124 | 1127 |
| O–CH3 and C–OH stretching | - | - | 1021 | 1015 |
| C–H out-of-plane stretching | 868 | 870, 806 | 865, 803 | 868 |
| 750 | 750 | 747 | 750 | |
| Bio-Char Types (Pyrolysis Temperature) | BET Surface Areas (m2/g) | Total Pore Volume cm3/g | Average Pore Size (Å) | pH | Applications | References |
|---|---|---|---|---|---|---|
| PSW | 42 | 0.012 | 17 | 8.4 | Fuel, energy storage, soil conditioner, building sector, drinking and wastewater treatments, biogas production, exhaust filters, industrial materials, electronics semiconductors, cosmetics, paints and coloring | Present work |
| TSW | 44 | 0.013 | 18 | 8.2 | ||
| MSW | 48 | 0.014 | 19 | 8.1 | ||
| HSW | 58 | 0.016 | 19.5 | 8.3 | ||
| Sugarcane bagasse (500 °C) | 10.85 | 0.011 | 43.7 | 8.1 | Solid fuel, adsorbent, soil amendment | [53] |
| Apricot kernel shell (550 °C) | 195 | 0.1124 | - | - | Activated carbon, fuel applications, water purification, adsorption | [19] |
| Rice straw (600 °C) | 4.76 | 0.0023 | 18.74 | - | Adsorption Water purification, activated carbon, fuel applications | [74] |
| Coconut fiber (600 °C) | 23.2 | 0.04 | 9.6 | Sequester carbon in soils, improving soil quality and plant growth | [17] | |
| Flax straw (550 °C) | 28.7 | 0.009 | 37.5 | - | Soil amendment, carbon sequestration, activated chars. | [72] |
| Wood (450 °C) | 23 | 6.7 | Soil reduced the C-mineralization rate compared against the control soil samples | [75] | ||
| Paddy Straw (500 °C) | 45.8 | 10.5 | Fertilizer consumption reduced, and sequestrate carbon | [76] | ||
| Durian wood sawdust (450 °C) | 45.78 | 5.786 | 80.98 | 6.4 | Provide suitable proportions for developing clusters of microorganisms, water retention capacity in soil and enhances soil fertility. | [79] |
| Spent P. ostreatus (500 °C) | 18.05 | 0.061 | 136 | 10.37 | Potential adsorbent for removing heavy metals from wastewater | [80] |
| Corn stalk (450 °C) | 57.80 | 0.081 | 49.1 | Adsorption characteristics and mechanism of bio-char on nonpolar pollutants | [81] | |
| Rice husk (500 °C) | 92.6 | 0.076 | 22.0 | Adsorptive properties | [82] | |
| Charcoal fines (500 °C) | 43 ± 3 | 0.035 | Water retention capacities and cation exchange capacity | [83] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfattani, R.; Shah, M.A.; Siddiqui, M.I.H.; Ali, M.A.; Alnaser, I.A. Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel. Energies 2022, 15, 1. https://doi.org/10.3390/en15010001
Alfattani R, Shah MA, Siddiqui MIH, Ali MA, Alnaser IA. Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel. Energies. 2022; 15(1):1. https://doi.org/10.3390/en15010001
Chicago/Turabian StyleAlfattani, Rami, Mudasir Akbar Shah, Md Irfanul Haque Siddiqui, Masood Ashraf Ali, and Ibrahim A. Alnaser. 2022. "Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel" Energies 15, no. 1: 1. https://doi.org/10.3390/en15010001
APA StyleAlfattani, R., Shah, M. A., Siddiqui, M. I. H., Ali, M. A., & Alnaser, I. A. (2022). Bio-Char Characterization Produced from Walnut Shell Biomass through Slow Pyrolysis: Sustainable for Soil Amendment and an Alternate Bio-Fuel. Energies, 15(1), 1. https://doi.org/10.3390/en15010001







