Evaluation on Enhanced Heat Transfer Using Sonochemically Synthesized Stable Zno-Eg@Dw Nanofluids in Horizontal Calibrated Circular Flow Passage
Abstract
1. Introduction
2. Materials and Methodology
2.1. ZnO Synthesis Procedure
2.2. Two-Step Synthesis of ZnO-EG@DW Nanofluids
2.3. The Geometry of the Test Section
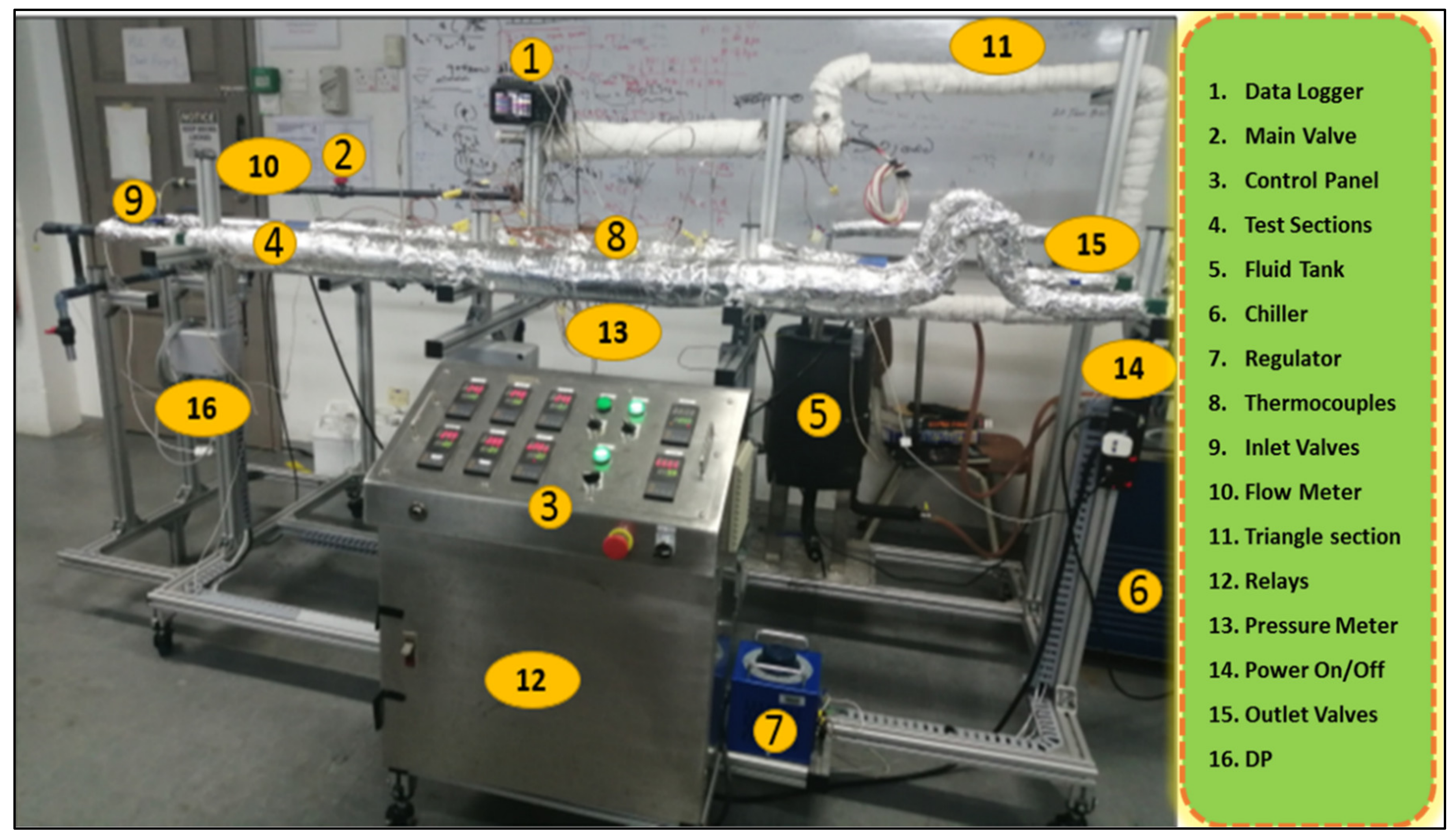
2.4. Experimental Setup
3. Characterizations of Sonochemically Synthesized ZnO Nanoparticles
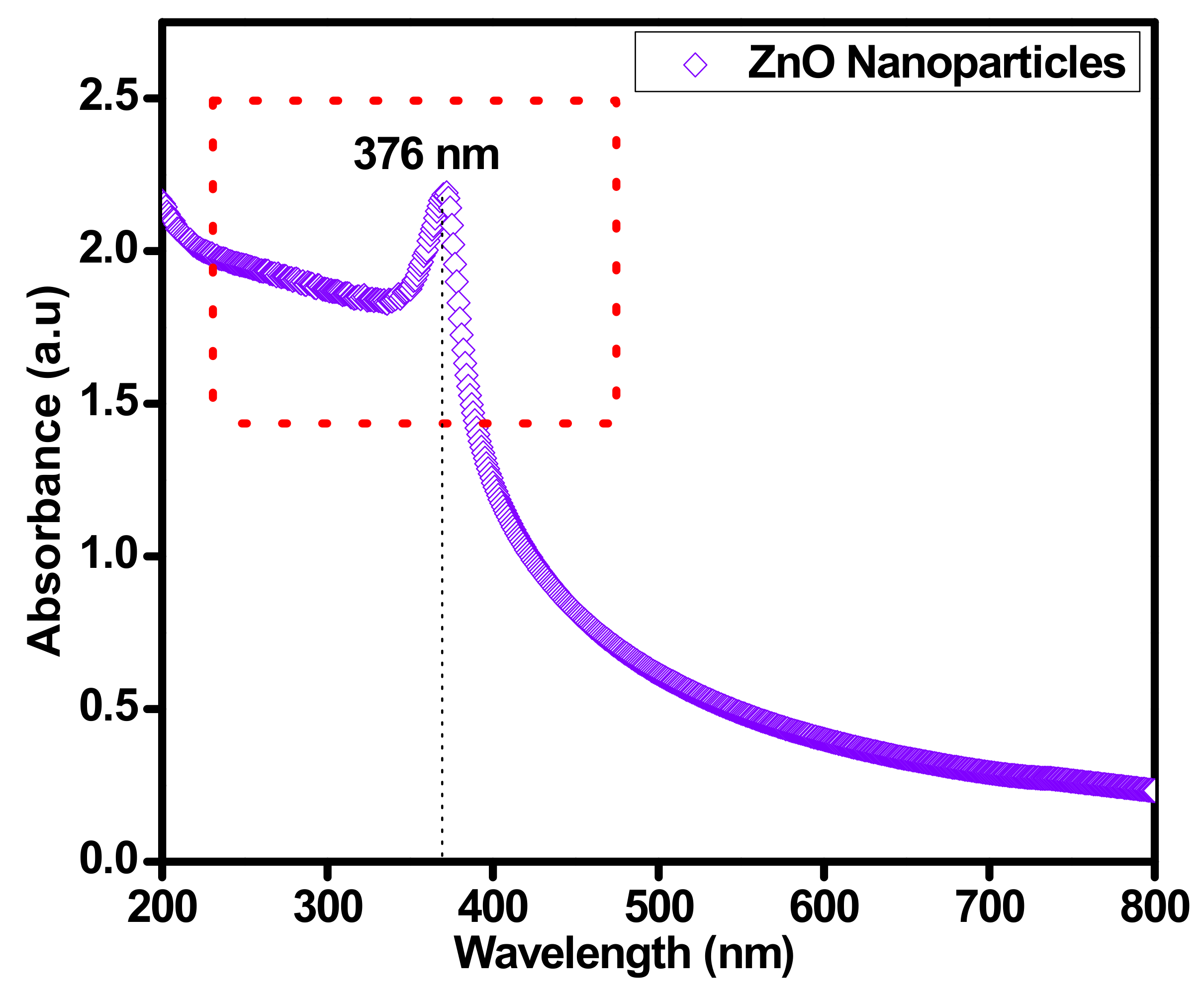
3.1. UV–VIS Spectrum
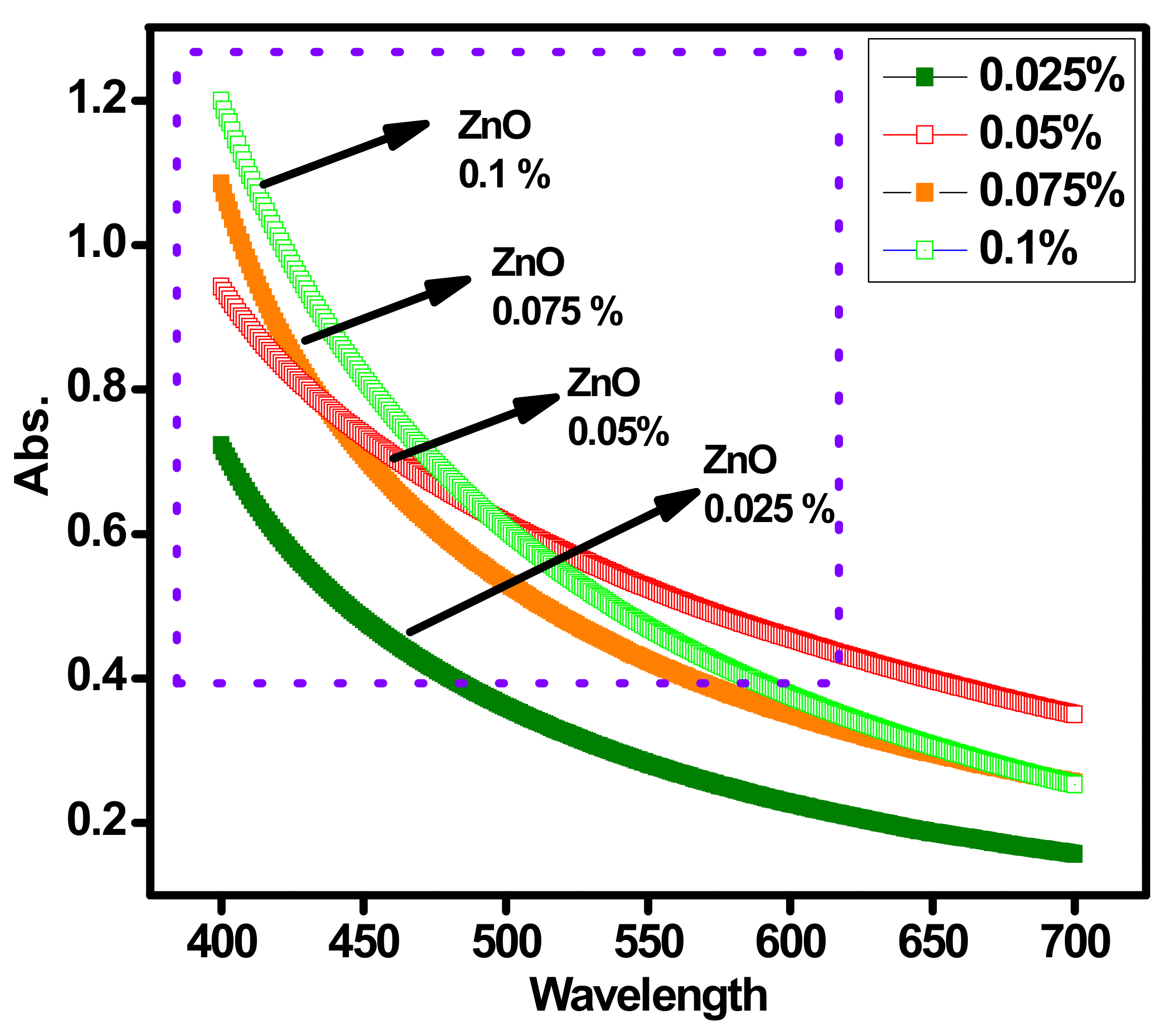
3.2. UV–VIS Absorption of Different Concentrations
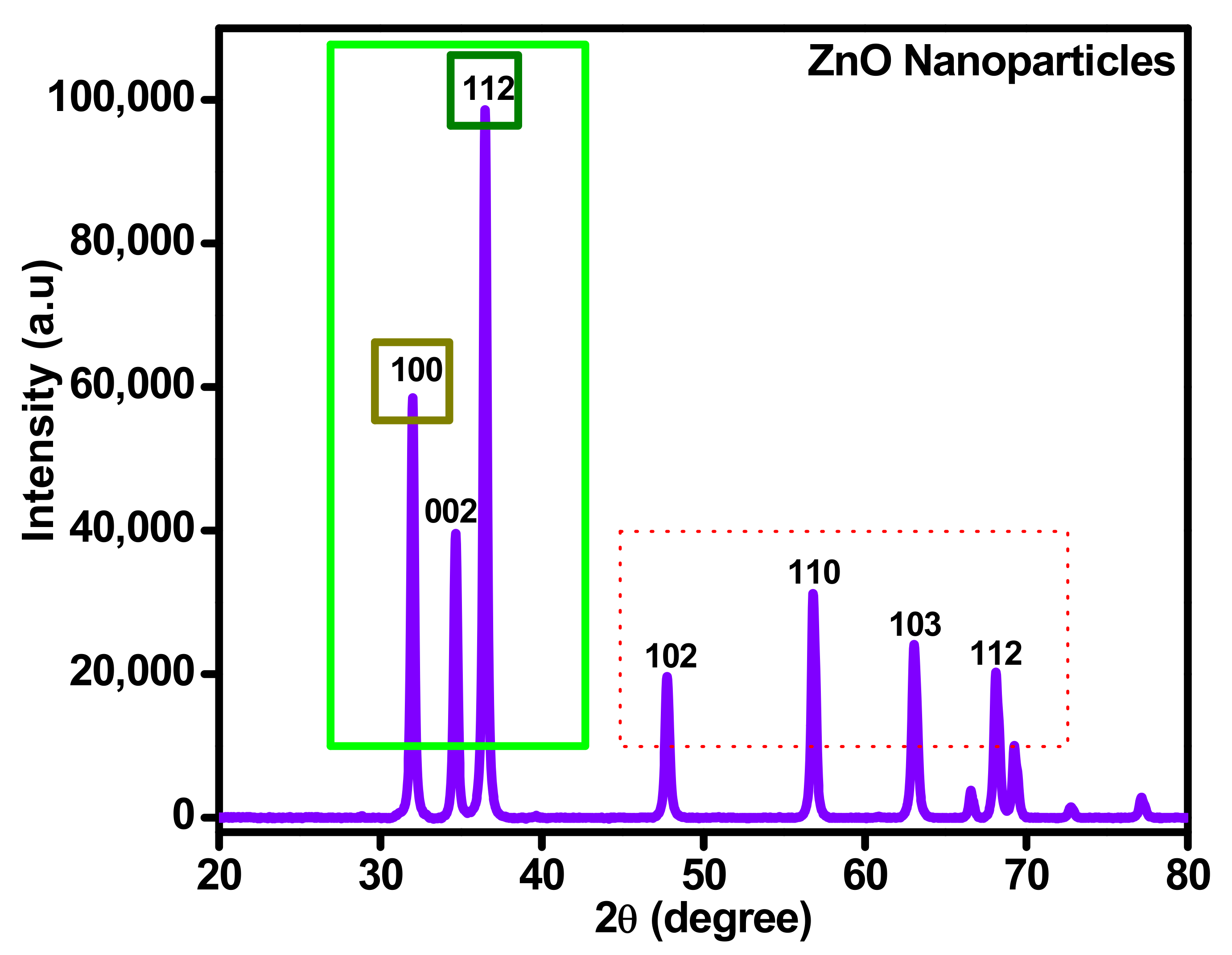
3.3. X-ray Diffraction (XRD) Analysis of ZnO Nanoparticles
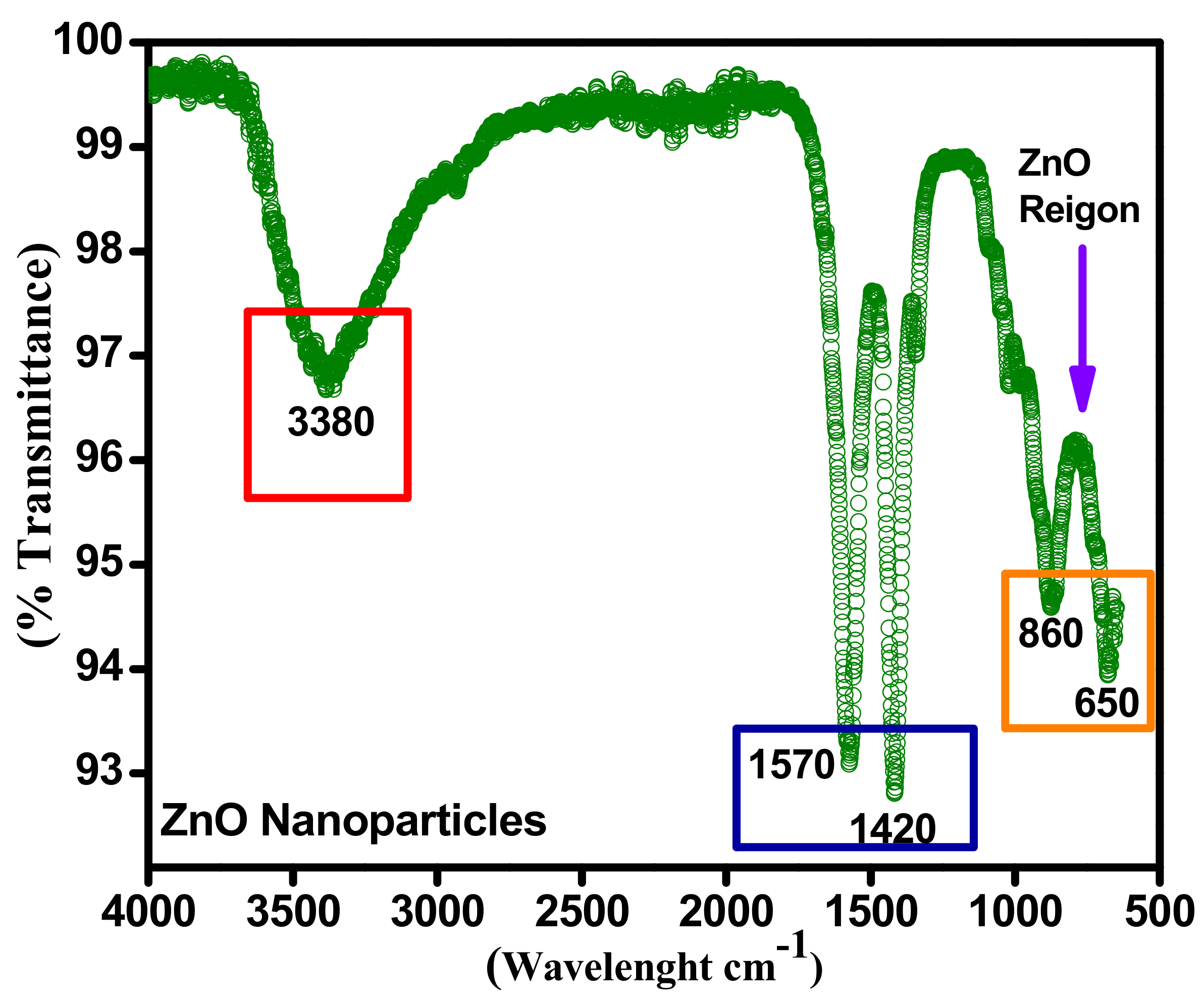
3.4. FTIR of ZnO Nanoparticles
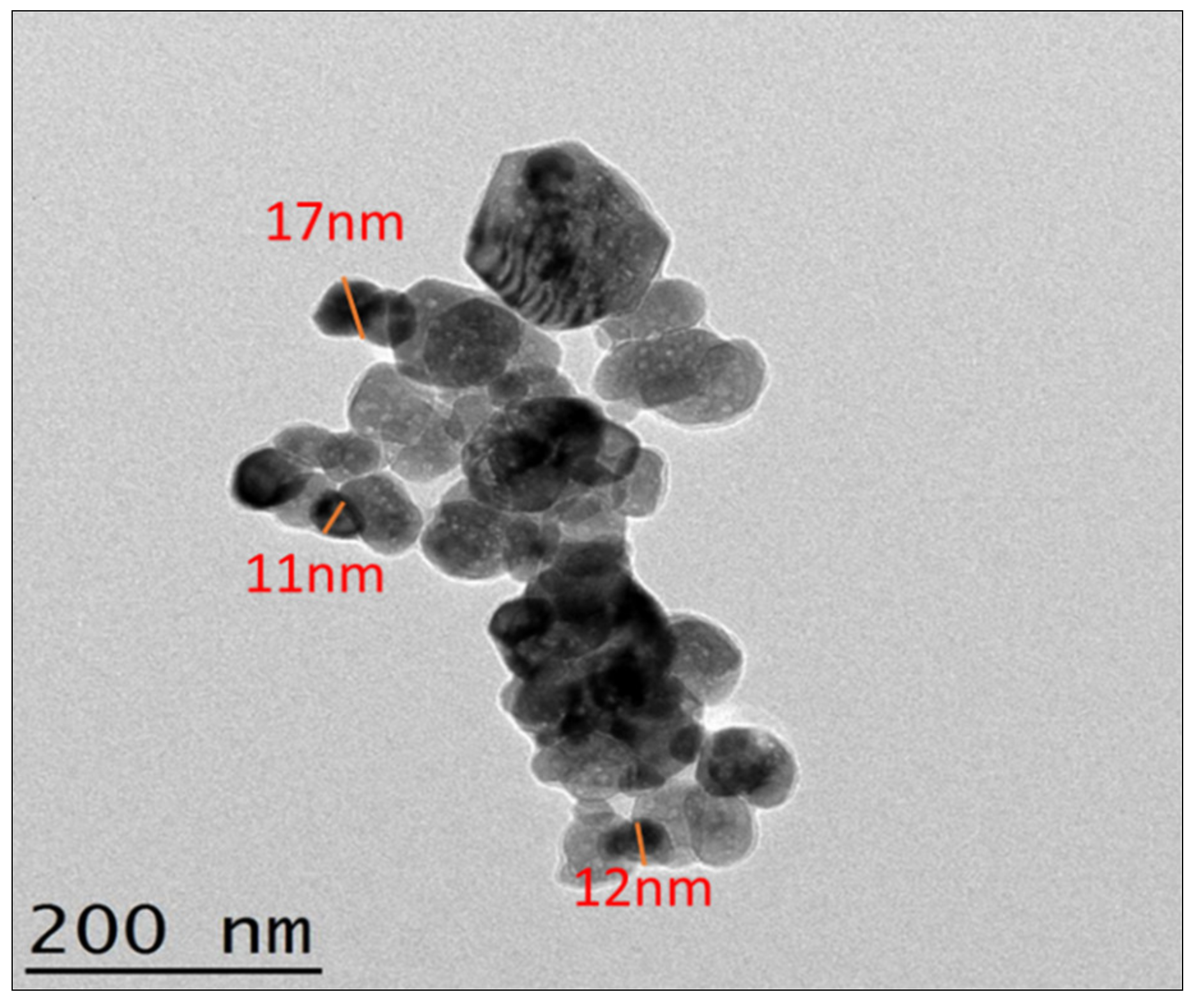
3.5. Transmission Electron Microscopy (TEM)
4. Results and Discussions
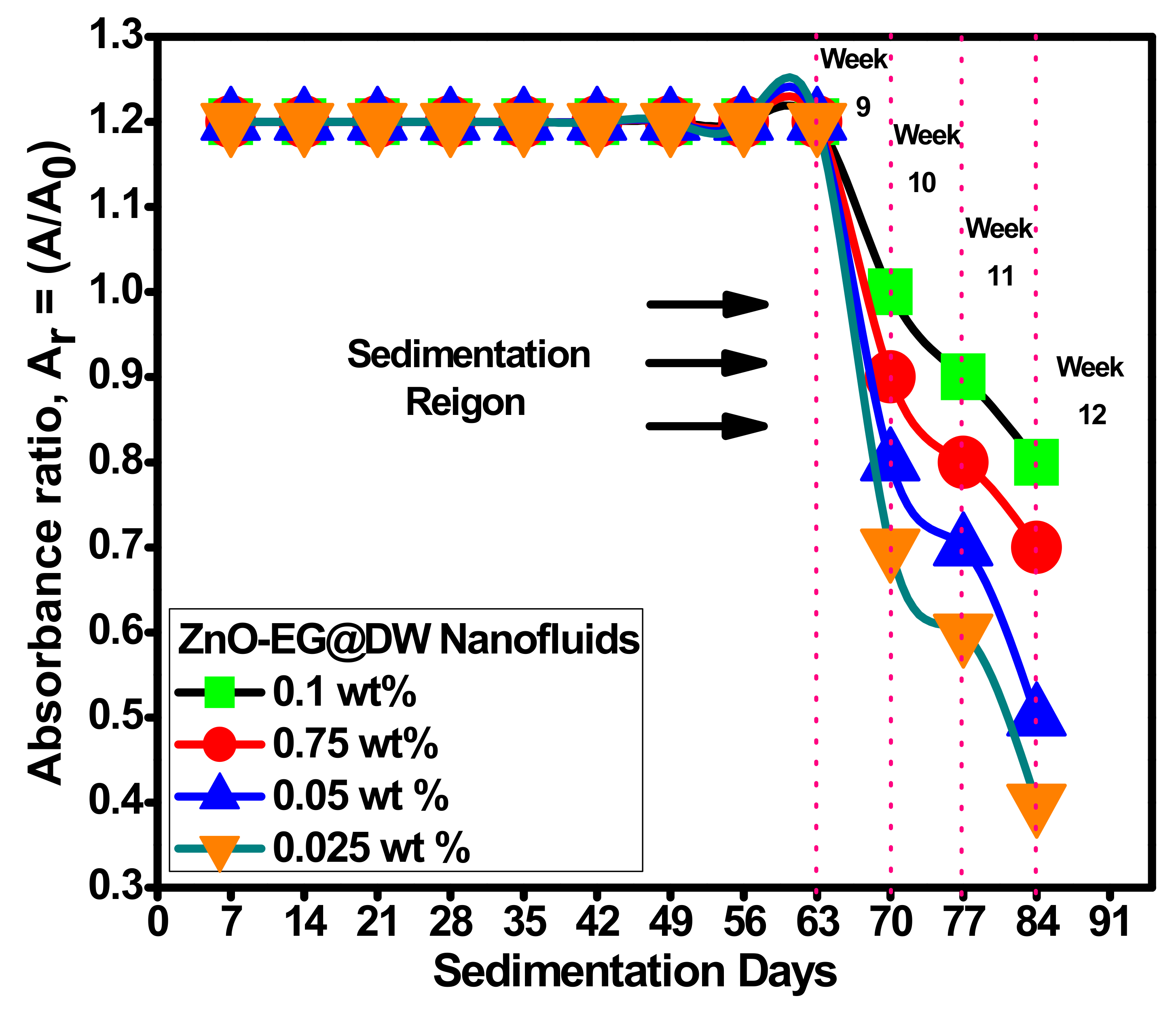
4.1. Homogeneous Dispersion and Stability of ZnO Nanoparticles in EG@DW Base Fluid
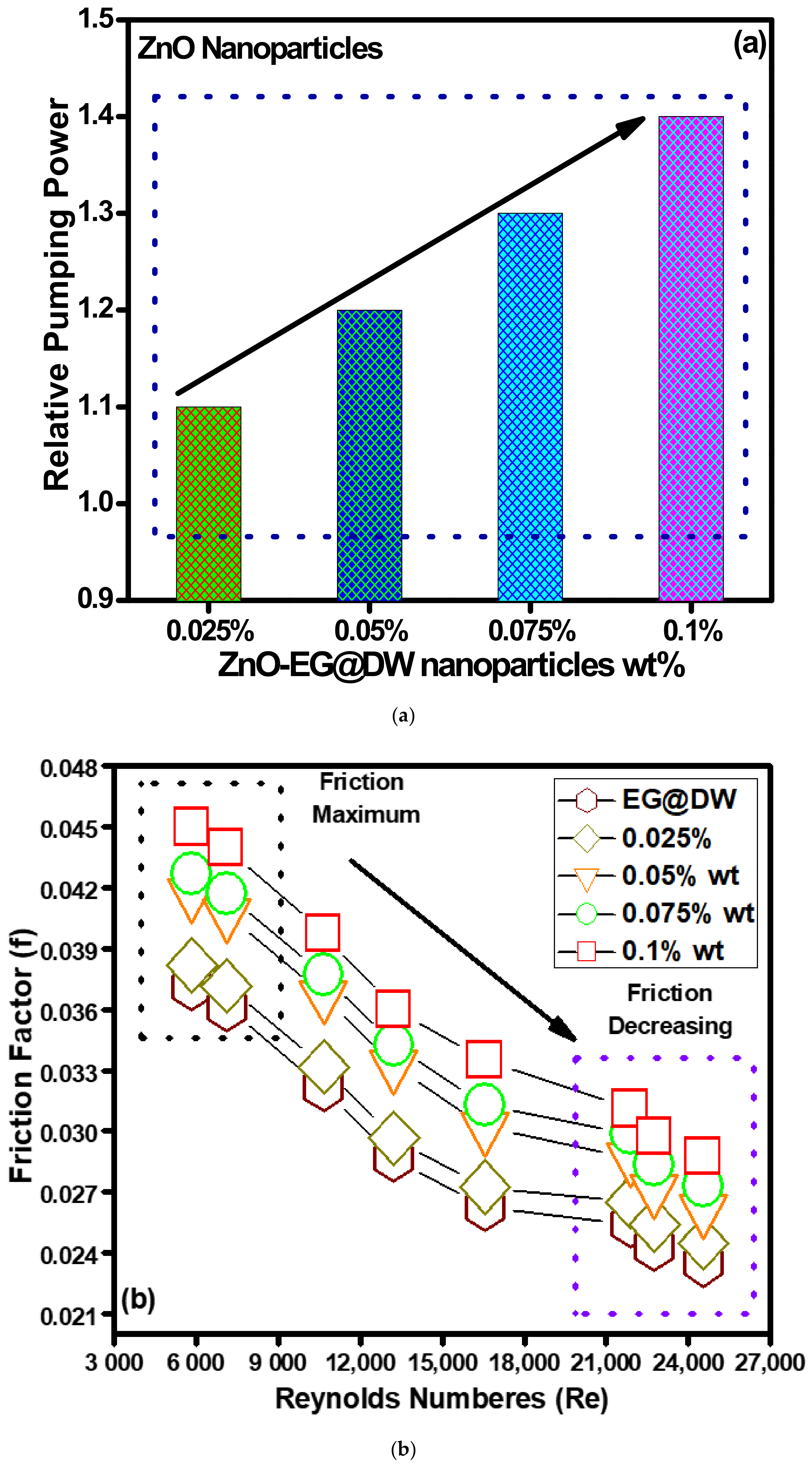
4.2. Hydrodynamic Properties
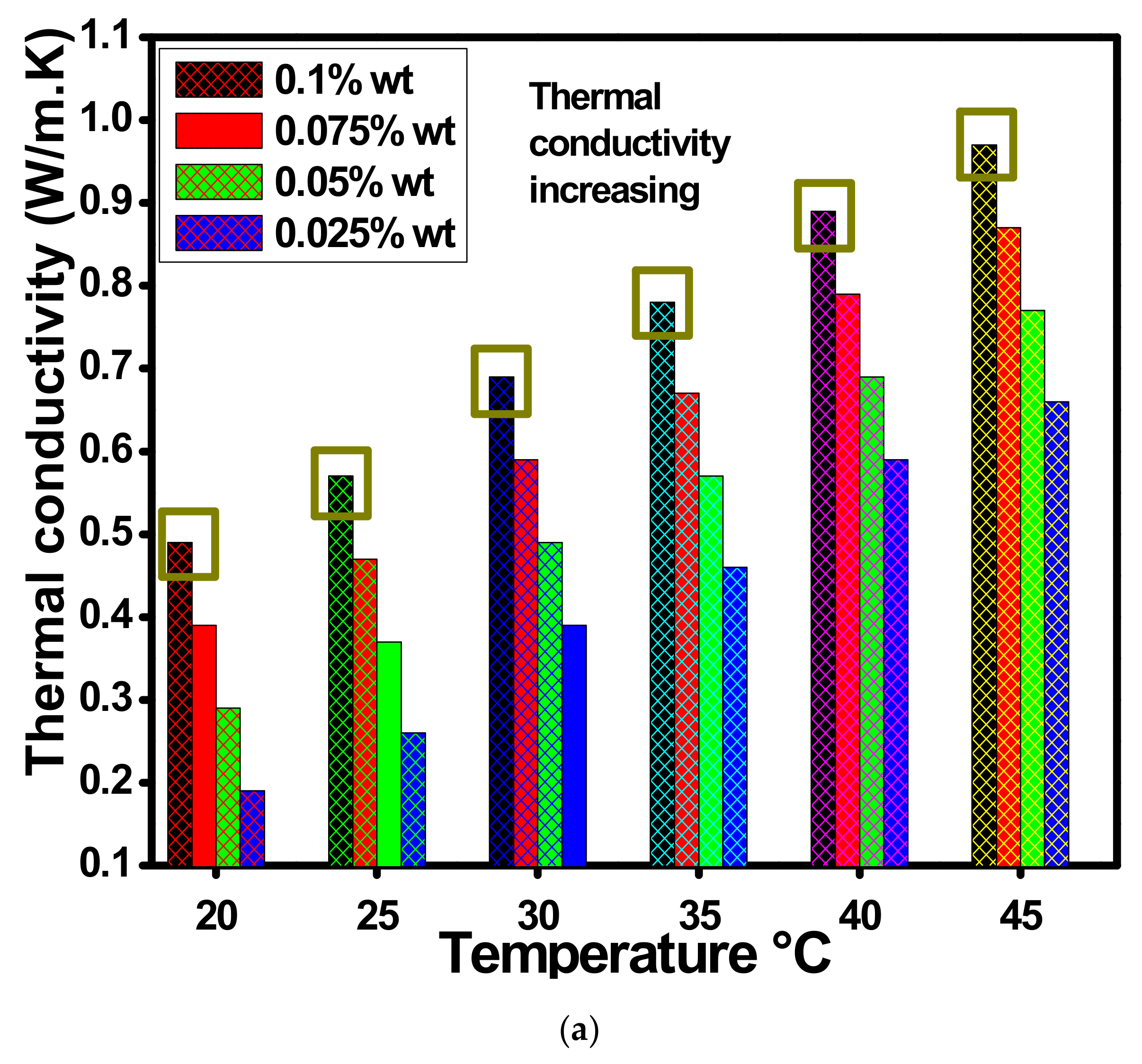
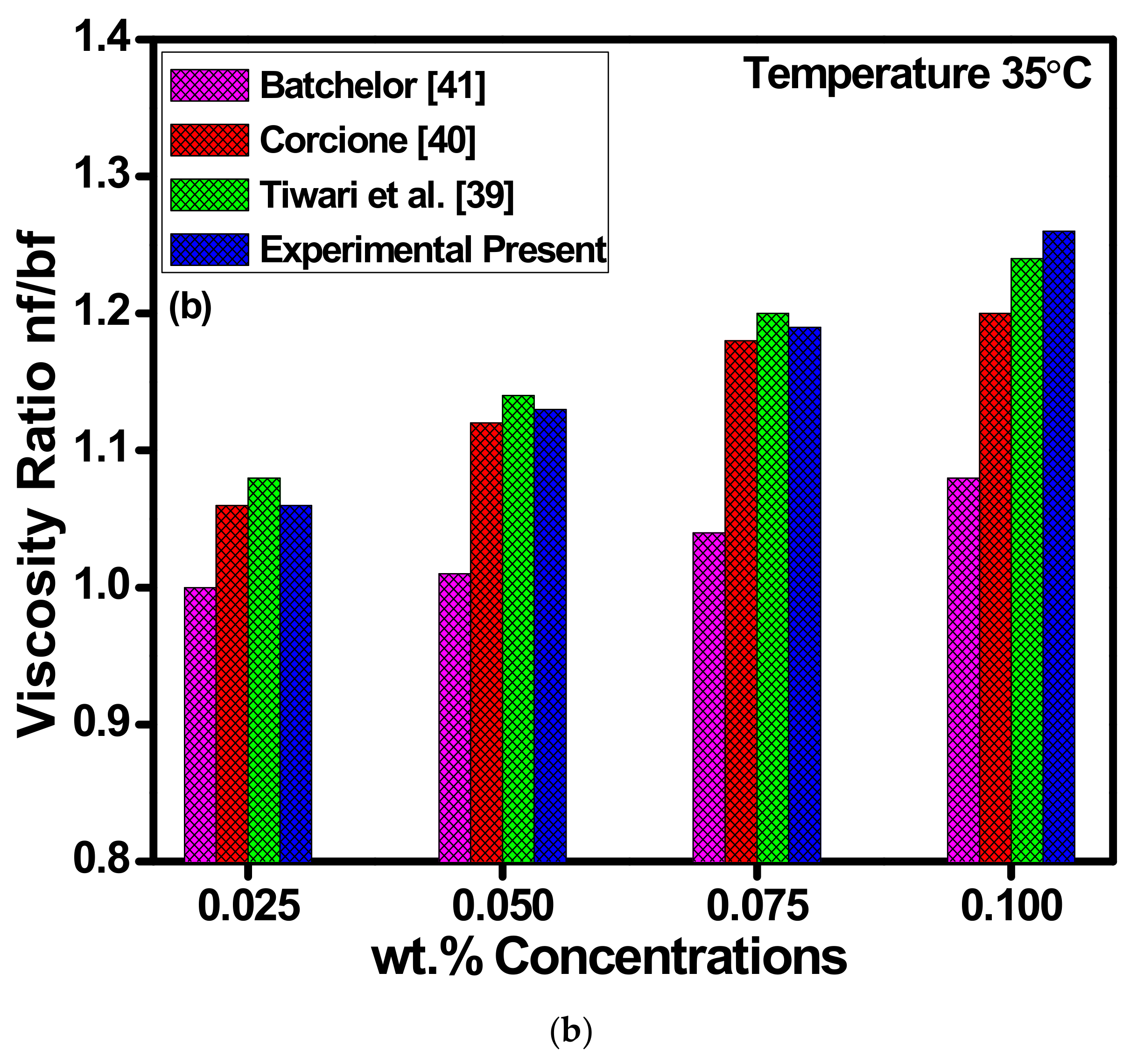
4.3. Thermal Conductivity and Viscosity Analysis
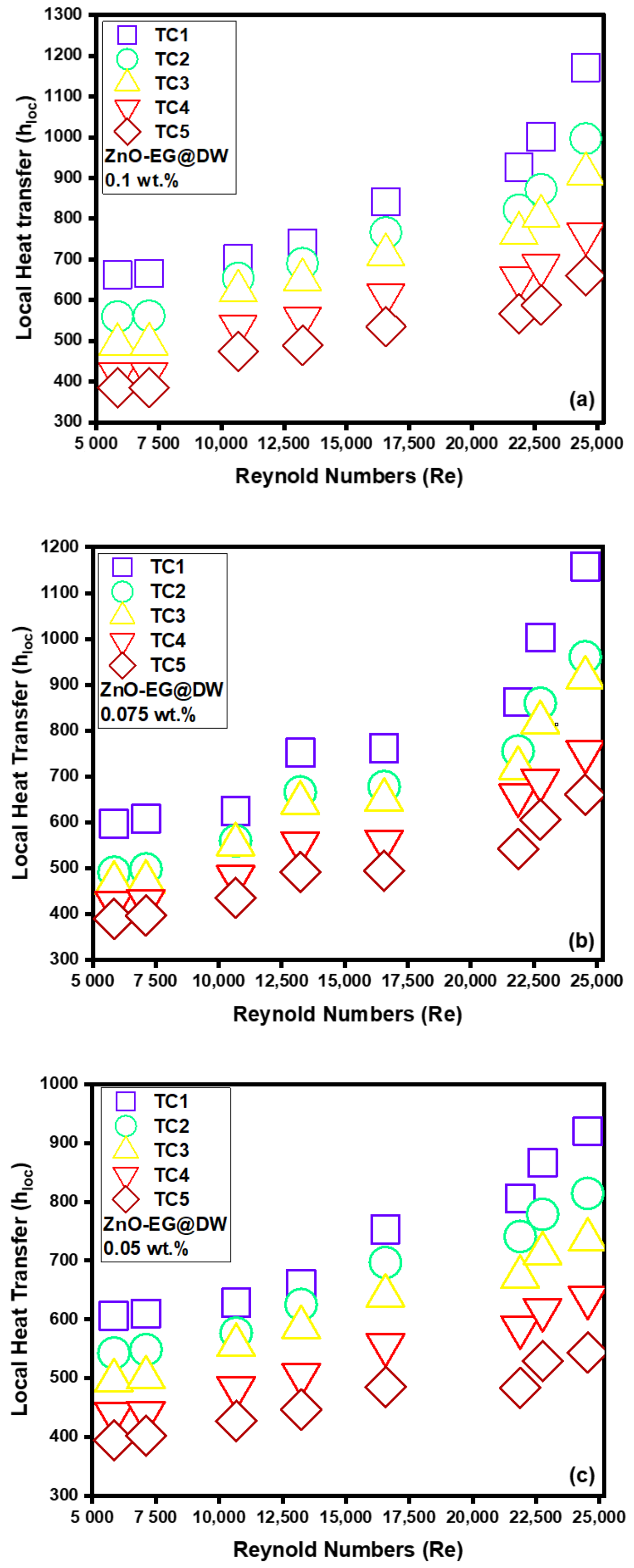
4.4. Average and Local Heat Transfer (h)
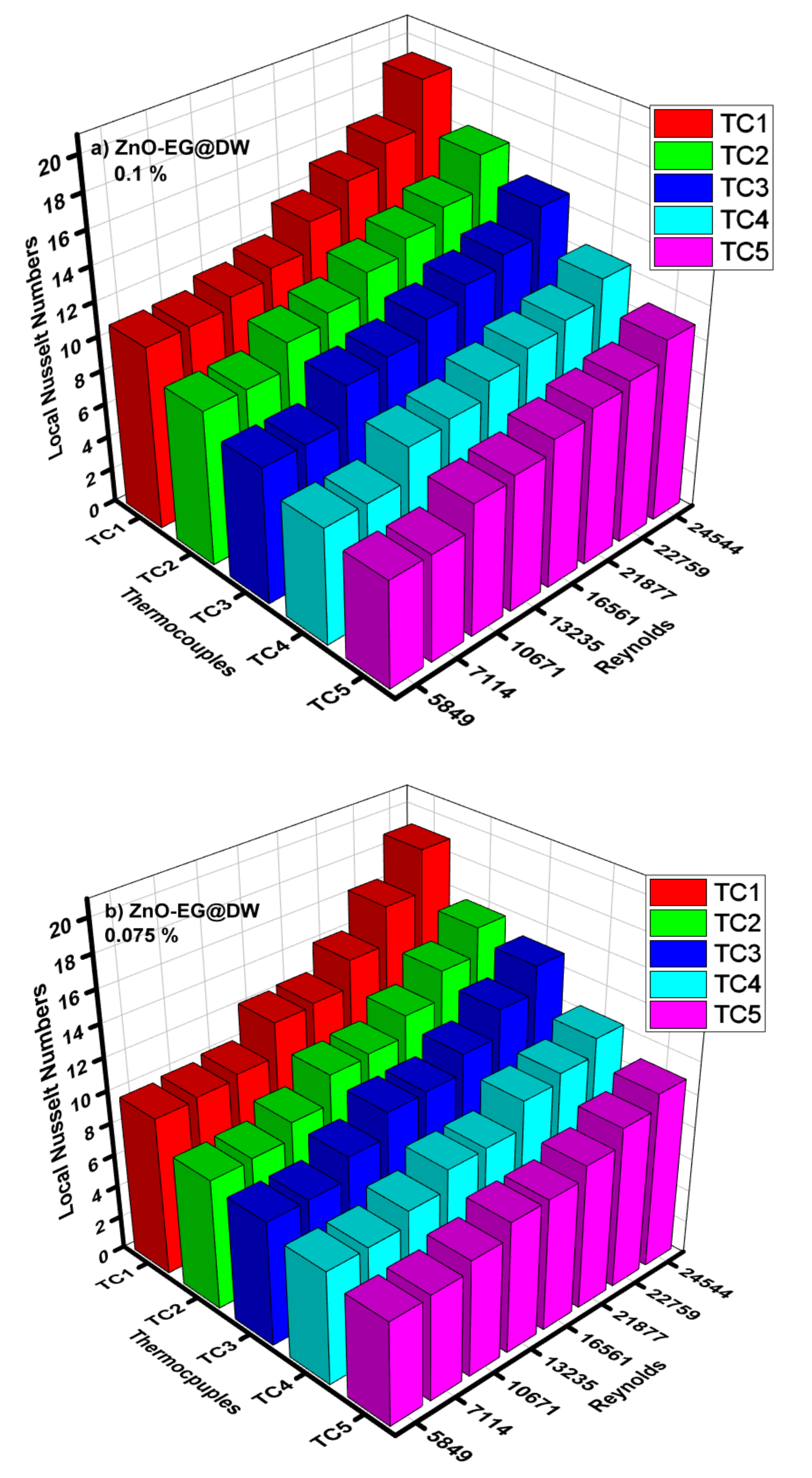
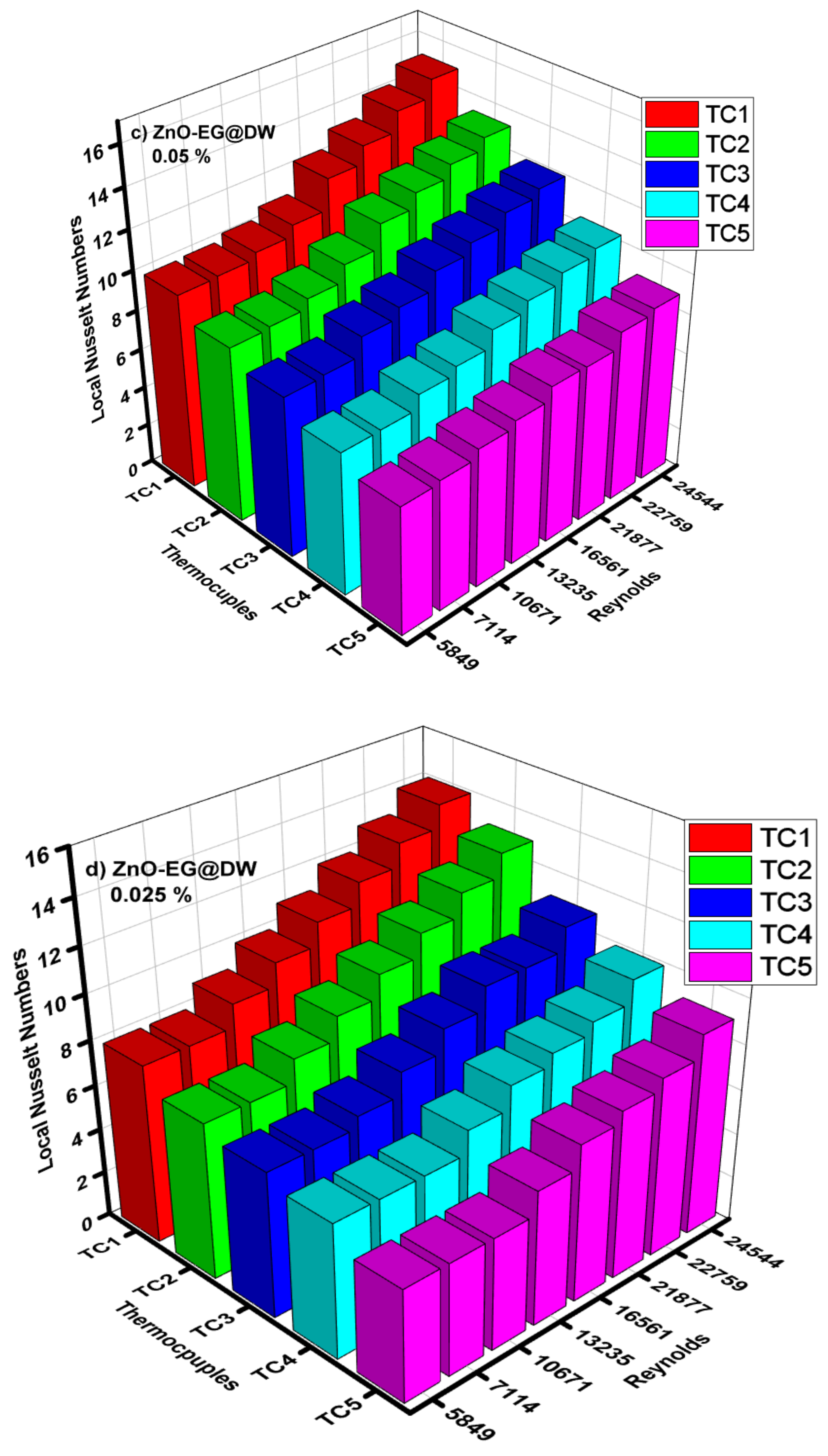
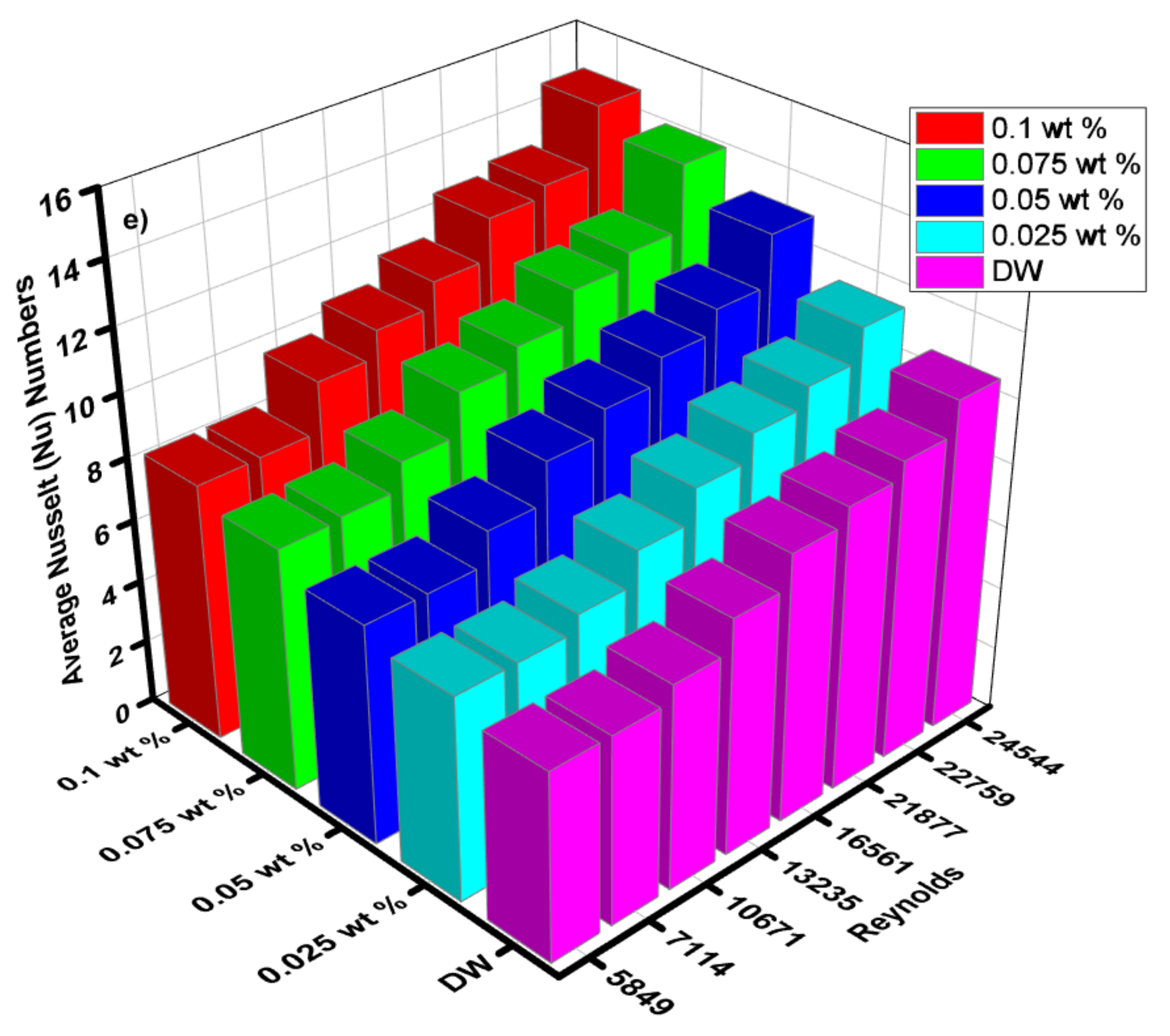
4.5. Average and Local Nusselt Numbers
4.6. Uncertainties in Experimental Test Setup
5. Conclusions
- ZnO solid nanoparticles synthesized by standard single-pot sonochemical technique provided ZnO nanoparticles having sizes from 12 to 19 nm. A two-step method was used to produce relevant nanofluids with four different wt.% of the ZnO nanoparticles.
- The proper synthesis of ZnO nanoparticles was confirmed with different characterization X-ray diffraction (XRD), Fourier-transform infrared spectroscopy (FTIR), transmission electron microscopy (TEM), elements detection (EDX) mapping and UV–VIS spectrum analysis.
- In the investigation, the ZnO-EG@DW nanofluids were varied from 0.1, 0.075, 0.05 and 0.025 wt.% concentrations, the Reynolds number ranged from 5849 to 24,544.
- The significant thermal conductivities of ZnO-EG@DW-based nanofluids were found higher than that of the water data and further improvement was noticed with the rise of nanofluid concentrations.
- The magnitude of the convective heat transfer (h) was enhanced from 600 to 1200 W/m2k. Nusselt (Nu) number also increased from 6 to 17 with the variation of nanoparticle concentrations. ZnO-EG@DW-based nanofluids grew Nusselt number (Nu) values from 79% as compared to the base fluid data in the specific inspected range of Reynolds number (Re).
- Thus, the improvement in thermal conductivities and convective heat transfer (h) properties with improved Nusselt (Nu) numbers can initiate the application of the stated nanofluid as a promising choice for heat exchanging fluid for several industries and household applications.
Author Contributions
Acknowledgments
Conflicts of Interest
Nomenclature
| EG | Ethylene glycol |
| k | Thermal conductivity (W/m.K) |
| DW | Distilled water |
| A | Pipe inner area |
| Tout | Outlet temperature |
| Tin | Inlet temperature |
| Cp | Heat capacity for nanofluid |
| ZnO | zinc oxide |
| h | Heat transfer coefficient |
| Greek Symbols | |
| ρ | Fluid density (kg/m3) |
| µ | Viscosity (MPa.s) |
| Φ | Percentage volume concentration |
| ω | Percentage of weight concentration |
| Ts | Surface outer temperature |
| Tb | Bulk temperature |
| f | Friction |
| V | Velocity |
| Re | Reynolds numbers |
| ρ | Fluid density (kg/m^3) |
| PP | Pumping power |
| Nu | Nusselt numbers |
| Subscripts | |
| np | Nanoparticles |
| nf | Nanofluid |
| bf | Base fluid |
References
- Dehghan, P.; Azari, A.; Azin, R. Measurement and correlation for CO2 mass diffusivity in various metal oxide nanofluids. J. Environ. Chem. Eng. 2020, 8, 103598. [Google Scholar] [CrossRef]
- Khanafer, K.; Vafai, K. A critical synthesis of thermophysical characteristics of nanofluids. Int. J. Heat Mass Transf. 2011, 54, 4410–4428. [Google Scholar] [CrossRef]
- Lynn, T.Z.; Baheta, A.T.; Akilu, S. Experimental Investigation of the Effect of Inclination Angle on Heat Pipe Thermal Performance Using Cu-Nanofluids. In Advances in Material Sciences and Engineering; Springer: Berlin/Heidelberg, Germany, 2020; pp. 445–452. [Google Scholar] [CrossRef]
- Umer Ilyas, S.; Pendyala, R.; Shuib, A.; Marneni, N. A review on the viscous and thermal transport properties of nanofluids. In Advanced Materials Research; Trans Tech Publications Ltd.: Baech, Switzerland, 2014; Volume 917, pp. 18–27. [Google Scholar] [CrossRef]
- Nagarajan, F.C.; Kannaiyan, S.; Boobalan, C. Intensification of heat transfer rate using alumina-silica nanocoolant. Int. J. Heat Mass Transf. 2020, 149, 119127. [Google Scholar] [CrossRef]
- Dincer, I. Renewable energy and sustainable development: A crucial review. Renew. Sustain. Energy Rev. 2000, 4, 157–175. [Google Scholar] [CrossRef]
- Sven, E.; Reinhard, H. Specific heat capacity enhancement studied in silica doped potassium nitrate via molecular dynamics simulation. Sci. Rep. (Nat. Publ. Group) 2019, 9. [Google Scholar] [CrossRef]
- Bhunia, M.M.; Das, S.; Chattopadhyay, K.K.; Chattopadhyay, P. Enhanced heat transfer properties of RGO-TiO2 based Ethylene Glycol Nanofluids. Mater. Today Proc. 2019, 18, 1096–1107. [Google Scholar] [CrossRef]
- Razavi, R.; Sabaghmoghadam, A.; Bemani, A.; Baghban, A.; Chau, K.W.; Salwana, E. Application of ANFIS and LSSVM strategies for estimating thermal conductivity enhancement of metal and metal oxide based nanofluids. Eng. Appl. Comput. Fluid Mech. 2019, 13, 560–578. [Google Scholar] [CrossRef]
- Barai, D.P.; Bhanvase, B.A.; Saharan, V.K. Reduced Graphene Oxide-Fe3O4 Nanocomposite Based Nanofluids: Study on Ultrasonic Assisted Synthesis, Thermal Conductivity, Rheology and Convective Heat Transfer. Ind. Eng. Chem. Process Des. Dev. 2019. [Google Scholar] [CrossRef]
- Li, Y.; Fernández-Seara, J.; Du, K.; Pardiñas, Á.Á.; Latas, L.L.; Jiang, W. Experimental investigation on heat transfer and pressure drop of ZnO/ethylene glycol-water nanofluids in transition flow. Appl. Therm. Eng. 2016, 93, 537–548. [Google Scholar] [CrossRef]
- Kumar, A.; Hassan, M.A.; Chand, P. Heat transport in nanofluid coolant car radiator with louvered fins. Powder Technol. 2020, 376, 631–642. [Google Scholar] [CrossRef]
- Radkar, R.N.; Bhanvase, B.A.; Barai, D.P.; Sonawane, S.H. Intensified convective heat transfer using ZnO nanofluids in heat exchanger with helical coiled geometry at constant wall temperature. Mater. Sci. Energy Technol. 2019, 2, 161–170. [Google Scholar] [CrossRef]
- Islam, R.; Shabani, B. Prediction of electrical conductivity of TiO2 water and ethylene glycol-based nanofluids for cooling application in low temperature PEM fuel cells. Energy Procedia 2019, 160, 550–557. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; He, Y.; Hu, Y.; Zhu, J.; Jiang, B. Experimental investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluids. Appl. Therm. Eng. 2015, 88, 363–368. [Google Scholar] [CrossRef]
- Chaurasia, P.; Kumar, A.; Yadav, A.; Rai, P.K.; Kumar, V.; Prasad, L. Heat transfer augmentation in automobile radiator using Al2O3–water based nanofluid. SN Appl. Sci. 2019, 1, 257. [Google Scholar] [CrossRef]
- Pastoriza-Gallego, M.J.; Lugo, L.; Legido, J.L.; Piñeiro, M.M. Thermal conductivity and viscosity measurements of ethylene glycol-based Al2O3 nanofluids. Nanoscale Res. Lett. 2011, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Vasheghani, M.; Marzbanrad, E.; Zamani, C.; Aminy, M.; Raissi, B. Thermal Conductivity and Viscosity of TiO2–Engine Oil Nanofluids. Nanosci. Technol. Int. J. 2013, 4. [Google Scholar] [CrossRef]
- Yu, W.; Xie, H.; Chen, L.; Li, Y. Investigation of thermal conductivity and viscosity of ethylene glycol based ZnO nanofluid. Thermochim. Acta 2009, 491, 92–96. [Google Scholar] [CrossRef]
- Zhu, D.; Wang, L.; Yu, W.; Xie, H. Intriguingly high thermal conductivity increment for CuO nanowires contained nanofluids with low viscosity. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Sun, B.; Yang, A.; Yang, D. Experimental study on the heat transfer and flow characteristics of nanofluids in the built-in twisted belt external thread tubes. Int. J. Heat Mass Transf. 2017, 107, 712–722. [Google Scholar] [CrossRef]
- Sun, B.; Zhang, Z.; Yang, D. Improved heat transfer and flow resistance achieved with drag reducing Cu nanofluids in the horizontal tube and built-in twisted belt tubes. Int. J. Heat Mass Transf. 2016, 95, 69–82. [Google Scholar] [CrossRef]
- Yang, J.C.; Li, F.C.; He, Y.R.; Huang, Y.M.; Jiang, B.C. Experimental study on the characteristics of heat transfer and flow resistance in turbulent pipe flows of viscoelastic-fluid-based Cu nanofluid. Int. J. Heat Mass Transf. 2013, 62, 303–313. [Google Scholar] [CrossRef]
- Poongavanam, G.K.; Kumar, B.; Duraisamy, S.; Panchabikesan, K.; Ramalingam, V. Heat transfer and pressure drop performance of solar glycol/activated carbon based nanofluids in shot peened double pipe heat exchanger. Renew. Energy 2019, 140, 580–591. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Gao, N.; Chen, G. Prediction of thermal conductivity of various nanofluids with ethylene glycol using artificial neural network. J. Therm. Sci. 2019, 1–9. [Google Scholar] [CrossRef]
- Das, S.K.; Putra, N.; Thiesen, P.; Roetzel, W. Temperature dependence of thermal conductivity enhancement for nanofluids. J. Heat Transf. 2003, 125, 567–574. [Google Scholar] [CrossRef]
- Motevasel, M.; Nazar, A.R.S.; Jamialahmadi, M. Experimental study on turbulent convective heat transfer of water-based nanofluids containing alumina, copper oxides and silicon carbide nanoparticles. J. Therm. Anal. Calorim. 2019, 135, 133–143. [Google Scholar] [CrossRef]
- Zing, C.; Mahjoob, S.; Vafai, K. Analysis of porous filled heat exchangers for electronic cooling. Int. J. Heat Mass Transf. 2019, 133, 268–276. [Google Scholar] [CrossRef]
- Xie, H.Q.; Wang, J.C.; Xi, T.G.; Liu, Y. Thermal conductivity of suspensions containing nanosized SiC particles. Int. J. Thermophys. 2002, 23, 571–580. [Google Scholar] [CrossRef]
- Kherbeet, A.S.; Mohammed, H.A.; Salman, B.H. The effect of nanofluids flow on mixed convection heat transfer over microscale backward-facing step. Int. J. Heat Mass Transf. 2012, 55, 5870–5881. [Google Scholar] [CrossRef]
- Teng, T.P.; Hsiao, T.C.; Chung, C.C. Characteristics of carbon-based nanofluids and their application in a brazed plate heat exchanger under laminar flow. Appl. Therm. Eng. 2019, 146, 160–168. [Google Scholar] [CrossRef]
- Suganthi, K.S.; Rajan, K.S. ZnO propylene glycol water nanofluids with improved properties for potential applications in renewable energy and thermal management. Coll. Surf. Physicochem. Eng. Asp. 2016, 506, 63–73. [Google Scholar] [CrossRef]
- Hilo, A.; Talib, A.A.; Nfawa, S.R.; Sultan, M.H.; Hamid, M.F.A.; Bheekhun, M.N. Heat transfer and thermal conductivity enhancement using graphene nanofluid: A review. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 55, 74–87. [Google Scholar]
- Krishnakumar, T.S.; Sheeba, A.; Mahesh, V.; Prakash, M.J. Heat transfer studies on ethylene glycol/water nanofluid containing TiO2 nanoparticles. Int. J. Refrig. 2019, 102, 55–61. [Google Scholar] [CrossRef]
- Bao, Y.; Gao, L.; Feng, C.; Ma, J.; Zhang, W.; Liu, C.; Simion, D. Sonochemical synthesis of flower-like ZnO assembled by hollow cones toward water vapor permeability and water resistance enhancement of waterborne film. J. Ind. Eng. Chem. 2020, 82, 180–189. [Google Scholar] [CrossRef]
- Noman, M.T.; Petru, M.; Militký, J.; Azeem, M.; Ashraf, M.A. One-Pot Sonochemical Synthesis of ZnO Nanoparticles for Photocatalytic Applications, Modelling and Optimization. Materials 2020, 13, 14. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Reddy, T.M.; Gopal, T.V.; Venkataprasad, G.; Kotakadi, V.S.; Palakollu, V.N.; Karpoormath, R. A simple sonochemical assisted synthesis of nanocomposite (ZnO/MWCNTs) for electrochemical sensing of Epinephrine in human serum and pharmaceutical formulation. Coll. Surf. Physicochem. Eng. Asp. 2020, 584, 124038. [Google Scholar] [CrossRef]
- Ahmed, W.; Chowdhury, Z.Z.; Kazi, S.N.; Johan, M.R.; Akram, N.; Oon, C.S. Effect of ZnO-water based nanofluids from sonochemical synthesis method on heat transfer in a circular flow passage. Int. Commun. Heat Mass Transf. 2020, 114, 104591. [Google Scholar] [CrossRef]
- Ahmed, W.; Chowdhury, Z.Z.; Kazi, S.N.; Johan, M.R.; Akram, N.; Oon, C.S.; Abdelrazek, A.H. Characteristics investigation on heat transfer growth of sonochemically synthesized ZnO-DW based nanofluids inside square heat exchanger. J. Therm. Anal. Calorim. 2020, 1–18. [Google Scholar] [CrossRef]
- Ahmed, W.; Kazi, S.N.; Chowdhury, Z.Z.; Johan, M.R. One-pot sonochemical synthesis route for the synthesis of ZnO@ TiO 2/DW hybrid/composite nanofluid for enhancement of heat transfer in a square heat exchanger. J. Therm. Anal. Calorim. 2020, 1–17. [Google Scholar] [CrossRef]
- Ahmed, W.; Kazi, S.N.; Chowdhury, Z.Z.; Johan, M.R.B.; Akram, N.; Mujtaba, M.A.; Gul, M.; Oon, C.S. Experimental investigation of convective heat transfer growth on ZnO@ TiO 2/DW binary composites/hybrid nanofluids in a circular heat exchanger. J. Therm. Anal. Calorim. 2020, 1–20. [Google Scholar] [CrossRef]
- Fernandez-Seara, J.; Uhía, F.J.; Sieres, J.; Campo, A. A general review of the Wilson plot method and its modifications to determine convection coefficients in heat exchange devices. Appl. Therm. Eng. 2007, 27, 2745–2757. [Google Scholar] [CrossRef]
- Mansour, R.B.; Galanis, N.; Nguyen, C.T. Effect of uncertainties in physical properties on forced convection heat transfer with nanofluids. Appl. Therm. Eng. 2007, 27, 240–249. [Google Scholar] [CrossRef]
- Batchelor, G.K. The effect of Brownian motion on the bulk stress in a suspension of spherical particles. J. Fluid Mech. 1977, 83, 97–117. [Google Scholar] [CrossRef]
- Corcione, M. Empirical correlating equations for predicting the effective thermal conductivity and dynamic viscosity of nanofluids. Energy Convers. Manag. 2011, 52, 789–793. [Google Scholar] [CrossRef]
- Gnielinski, V. New equations for heat and mass transfer in the turbulent flow in pipes and channels. STIA 1975, 41, 8–16. [Google Scholar]
- Petukhov, B.S. Heat transfer and friction in turbulent pipe flow with variable physical properties. Adv. Heat Transf. 1970, 6, i565. [Google Scholar]




| S. No | wt.% Concentration | Base Fluid in Liter | DW in Liter | EG in Liter | Nanoparticles in Grams |
|---|---|---|---|---|---|
| 1 | 0.1 | 7 | 3.5 | 3.5 | 7 |
| 2 | 0.075 | 7 | 3.5 | 3.5 | 5.25 |
| 3 | 0.05 | 7 | 3.5 | 3.5 | 3.5 |
| 4 | 0.025 | 7 | 3.5 | 3.5 | 1.75 |
| S. No | Parameters | Symbols | Uncertainty % |
|---|---|---|---|
| 1 | NF inlet temperature | Tin | ±0.141 |
| 2 | NF outlet temperature | Tout | ±0.141 |
| 3 | Ambient temperature | Te | ±0.140 |
| 4 | NF mass flow rate | v | ±1.92 |
| 5 | NF differential pressure | hh | ±2.22 |
| 6 | NF thermal conductivity | knf | ±4.42 |
| 7 | NF viscosity measurement | µnf | ±3.6 |
| 8 | NF specific heat measurement | Cpnf | ±4.21 |
| 9 | Voltage | V | ± 0.15 |
| 10 | Current | I | ± 0.152 |
| 11 | Heat flux | Q | ± 0.15 |
| 12 | Power | P | ± 0.151 |
| 13 | Reynolds numbers | Re | ± 4.2 |
| 14 | Convective heat transfer | h | ± 3.33 |
| 15 | Nusselt numbers | Nu | ± 4.31 |
| 16 | Pumping power | PP | ± 4.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, W.; Zaman Chowdhury, Z.; Kazi, S.N.; Johan, M.R.B.; Badruddin, I.A.; Soudagar, M.E.M.; Kamangar, S.; Mujtaba, M.A.; Gul, M.; Khan, T.M.Y. Evaluation on Enhanced Heat Transfer Using Sonochemically Synthesized Stable Zno-Eg@Dw Nanofluids in Horizontal Calibrated Circular Flow Passage. Energies 2021, 14, 2400. https://doi.org/10.3390/en14092400
Ahmed W, Zaman Chowdhury Z, Kazi SN, Johan MRB, Badruddin IA, Soudagar MEM, Kamangar S, Mujtaba MA, Gul M, Khan TMY. Evaluation on Enhanced Heat Transfer Using Sonochemically Synthesized Stable Zno-Eg@Dw Nanofluids in Horizontal Calibrated Circular Flow Passage. Energies. 2021; 14(9):2400. https://doi.org/10.3390/en14092400
Chicago/Turabian StyleAhmed, Waqar, Zaira Zaman Chowdhury, Salim Newaz Kazi, Mohd. Rafie Bin Johan, Irfan Anjum Badruddin, Manzoore Elahi M Soudagar, Sarfaraz Kamangar, Muhammad Abbas Mujtaba, Mustabshirha Gul, and T.M. Yunus Khan. 2021. "Evaluation on Enhanced Heat Transfer Using Sonochemically Synthesized Stable Zno-Eg@Dw Nanofluids in Horizontal Calibrated Circular Flow Passage" Energies 14, no. 9: 2400. https://doi.org/10.3390/en14092400
APA StyleAhmed, W., Zaman Chowdhury, Z., Kazi, S. N., Johan, M. R. B., Badruddin, I. A., Soudagar, M. E. M., Kamangar, S., Mujtaba, M. A., Gul, M., & Khan, T. M. Y. (2021). Evaluation on Enhanced Heat Transfer Using Sonochemically Synthesized Stable Zno-Eg@Dw Nanofluids in Horizontal Calibrated Circular Flow Passage. Energies, 14(9), 2400. https://doi.org/10.3390/en14092400









