Experimental Study of Absorbent Hygiene Product Devolatilization in a Bubbling Fluidized Bed
Abstract
1. Introduction
2. Materials and Methods
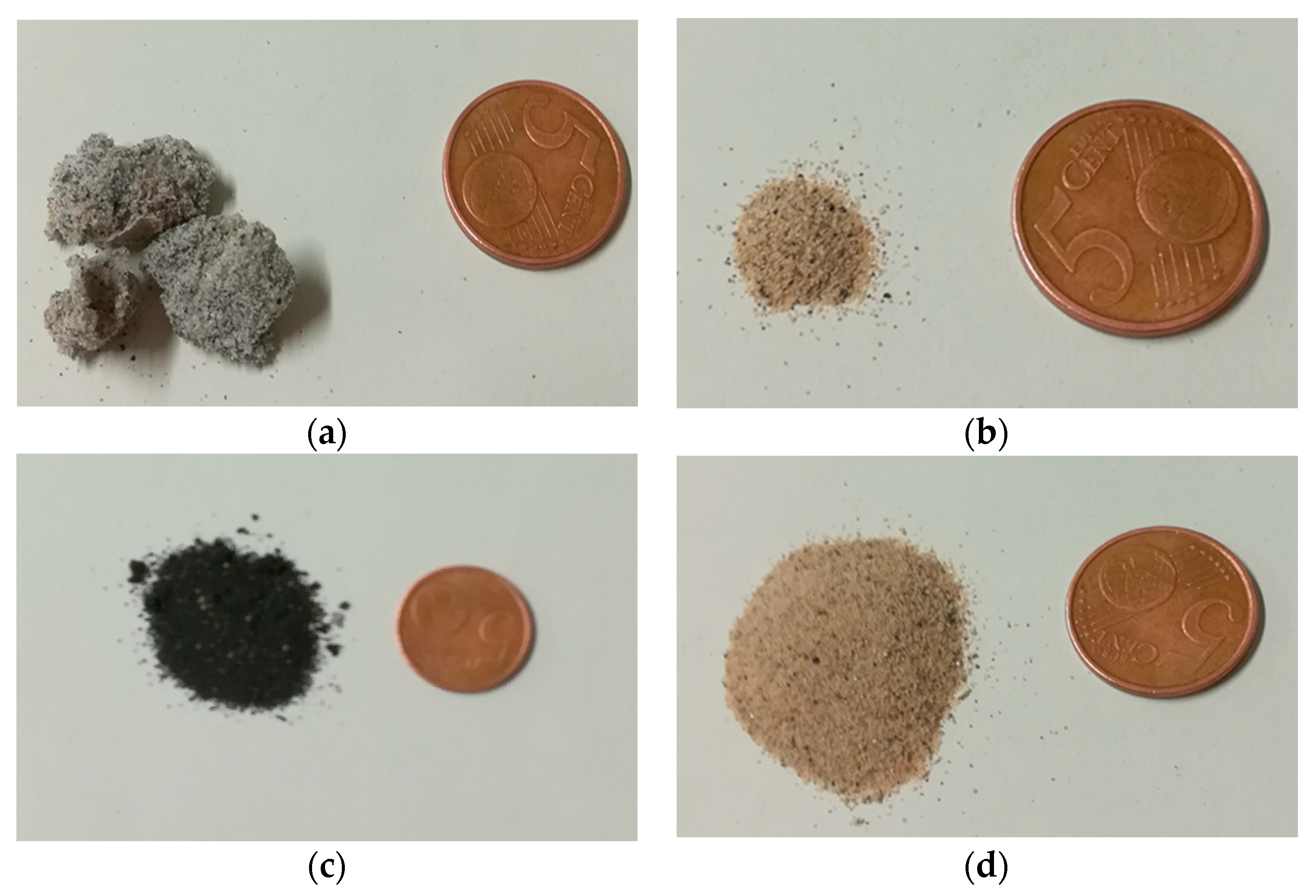
2.1. Waste Materials from AHP for Devolatilization Tests
2.2. Characterization of Waste Materials from AHP
2.3. Bed Material and Fluid-Dynamic Conditions for Devolatilization Tests
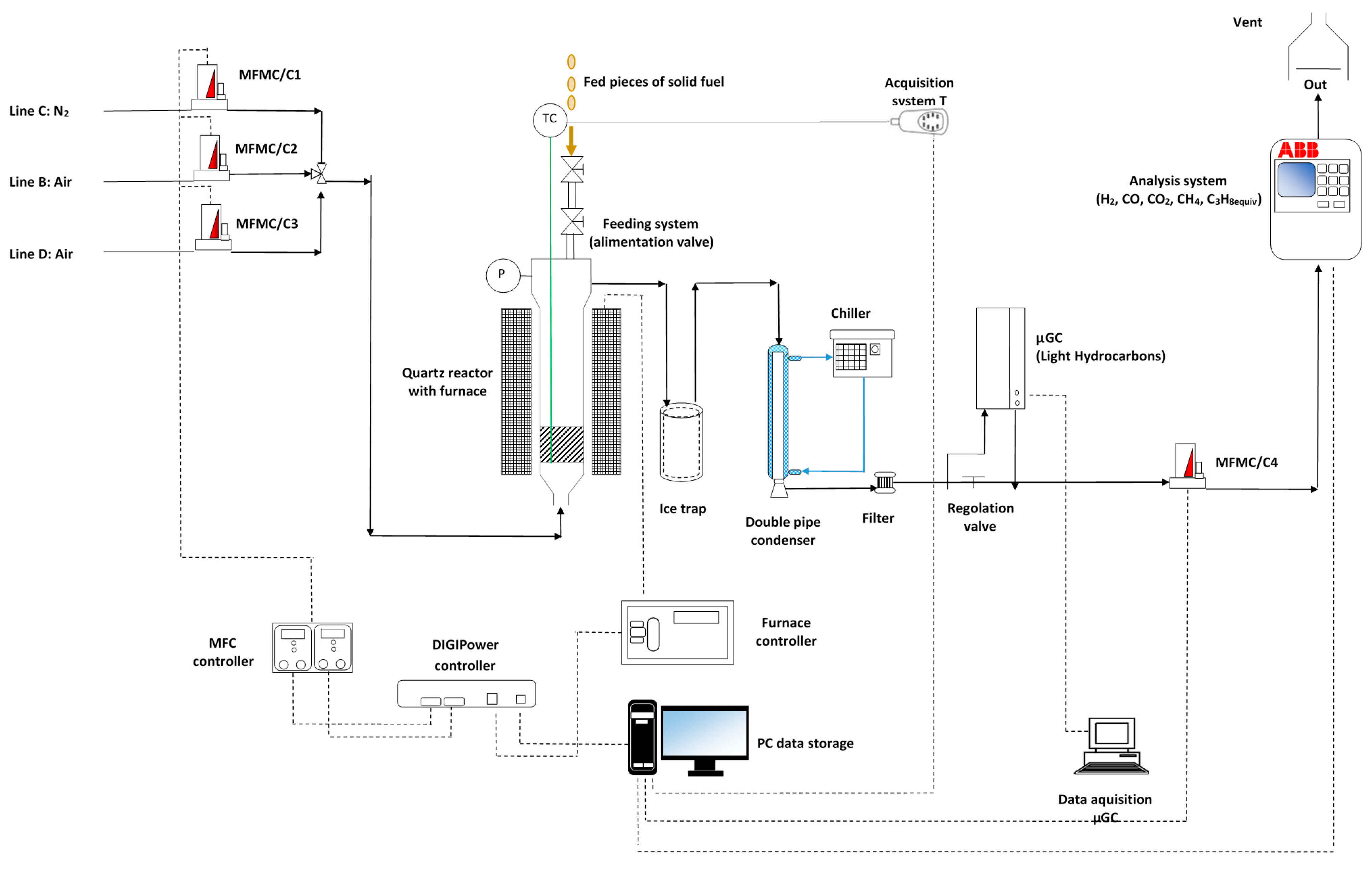
2.4. Devolatilization Tests: Experimental Rig and Procedure
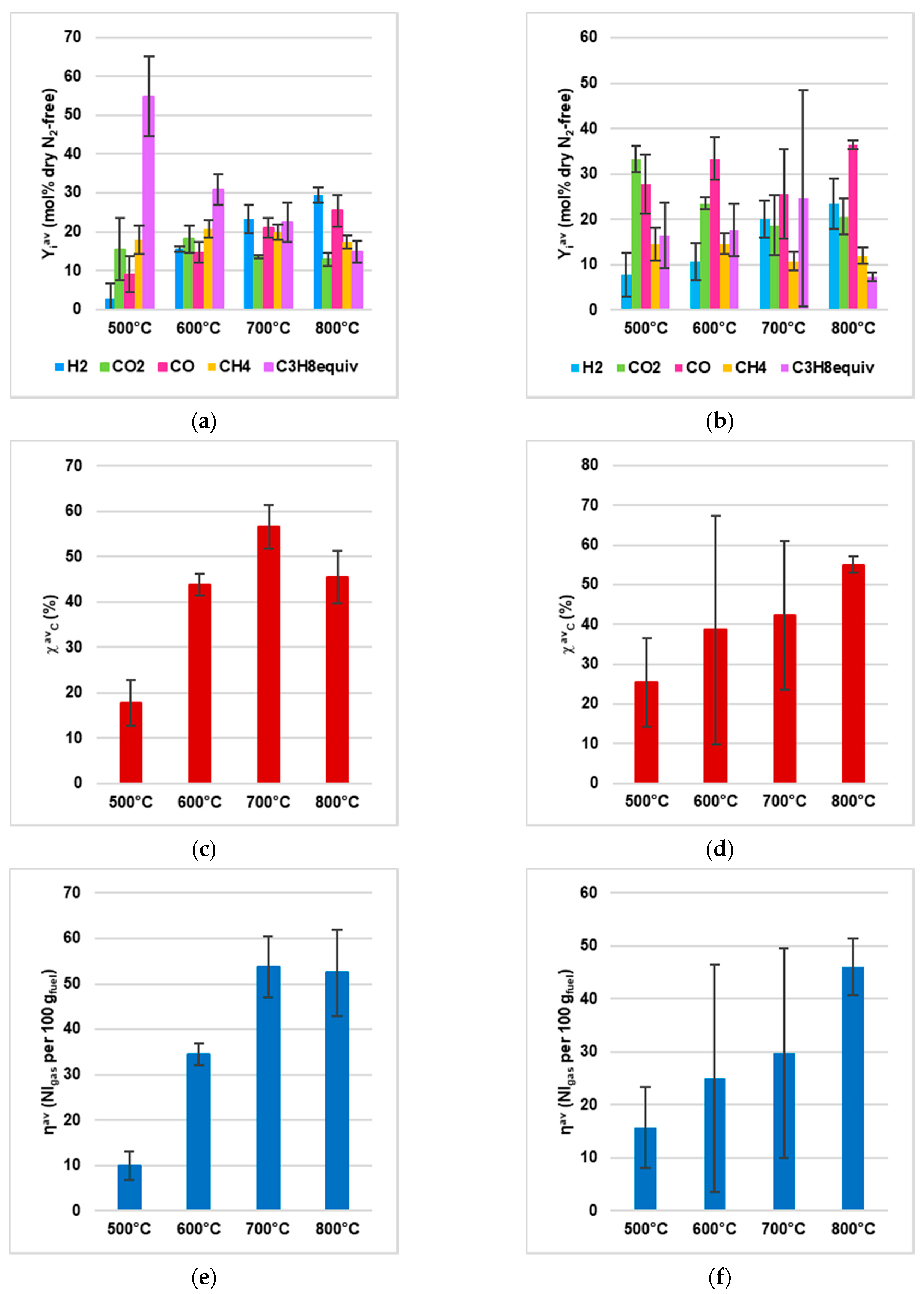
- Gas yield (ηav, Equation (1));
- Percentage of i on dry and dilution free basis (Yiav, Equation (2));
- Carbon conversion (χavC, Equation (3), with nj as the number of C atoms in C-containing species j).
2.5. Characterization of the Fluidized Bed after Devolatilization Tests
3. Results
3.1. Physical-Chemical Characterizations of AHPam and AHPus
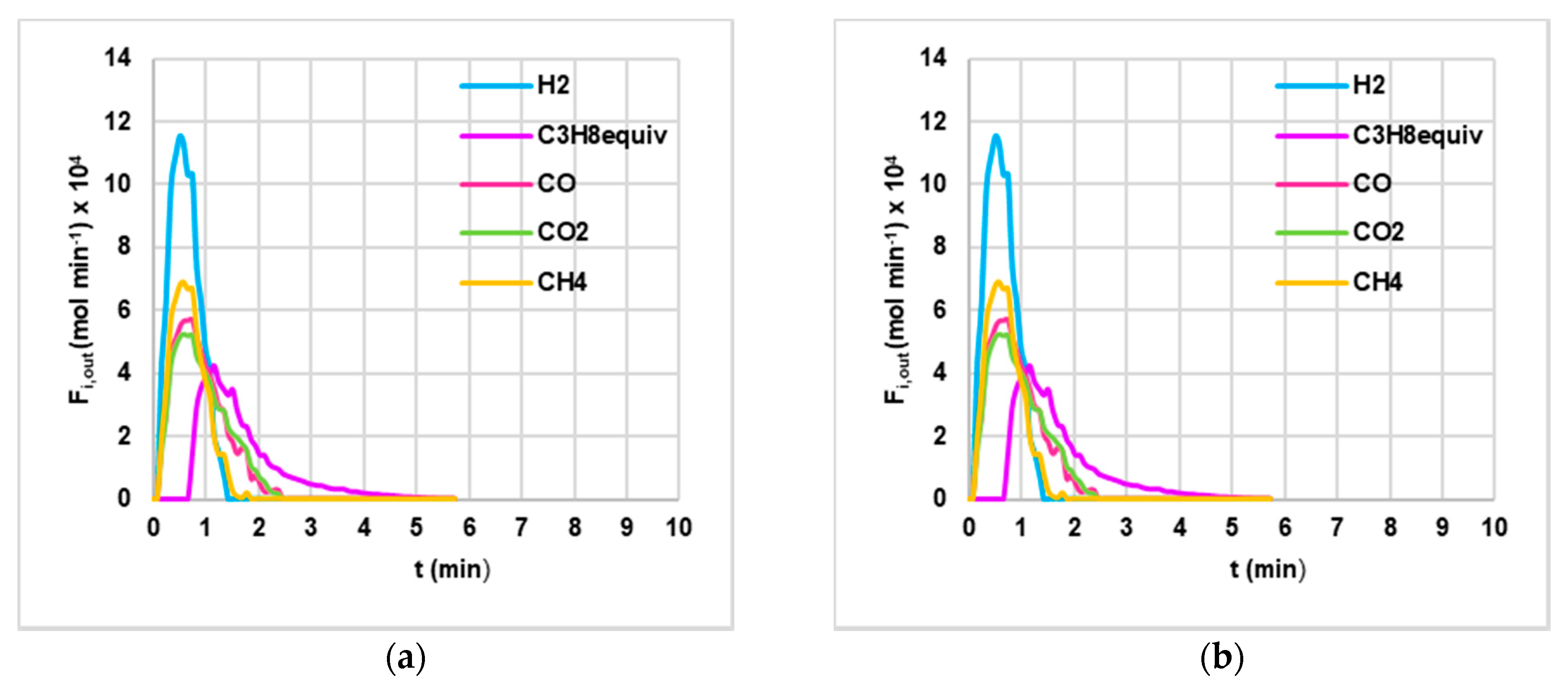
3.2. Devolatilization Tests
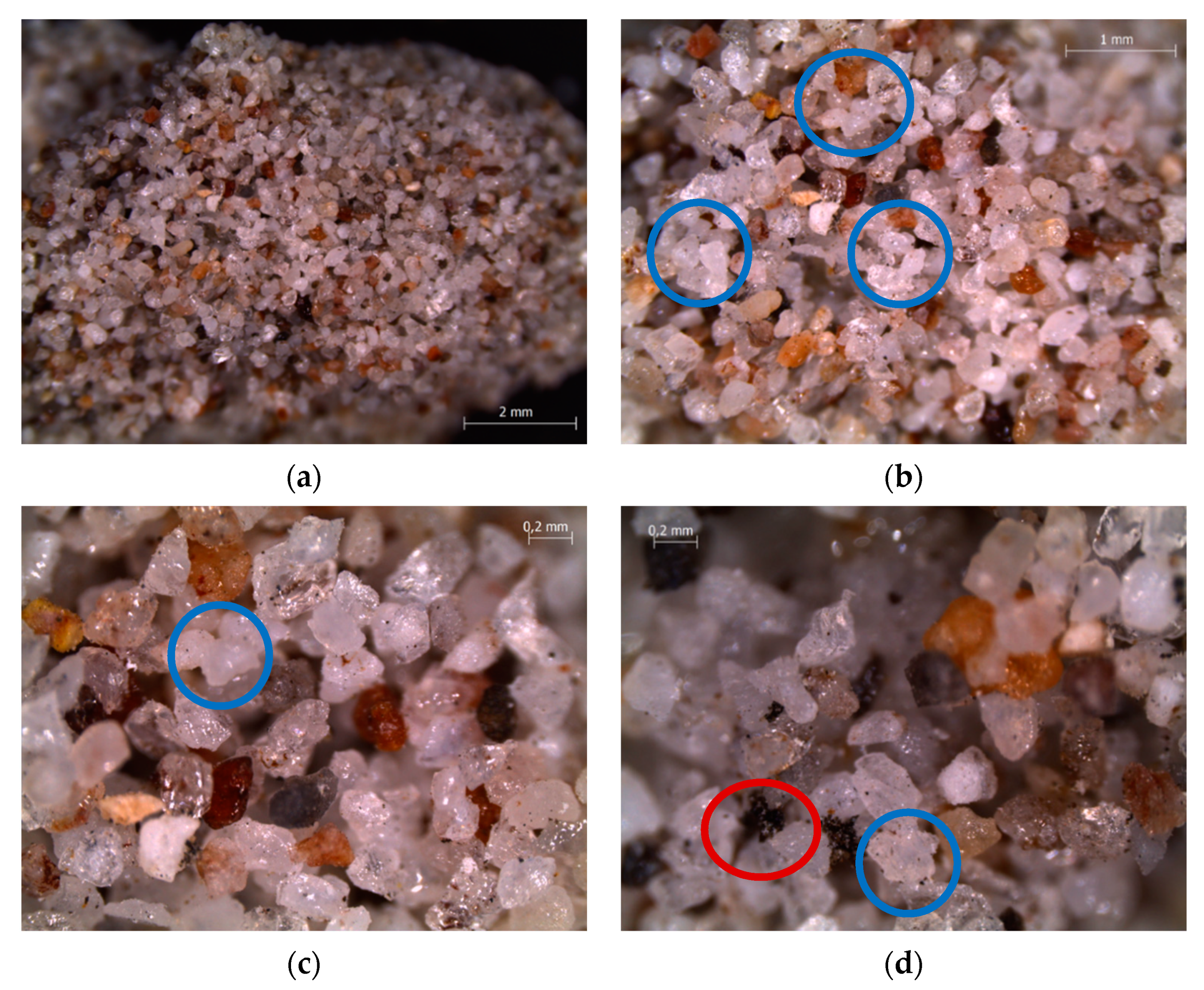
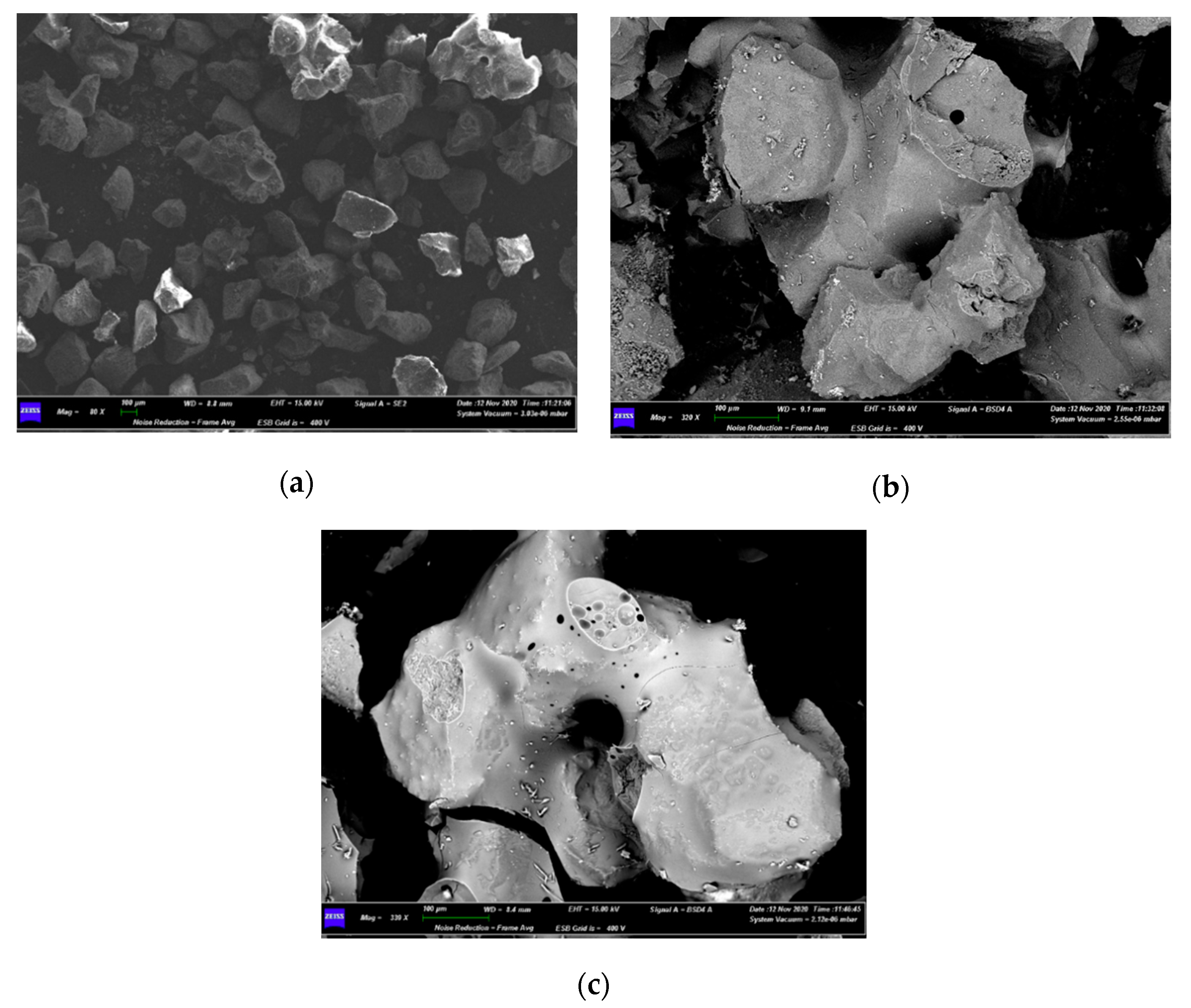
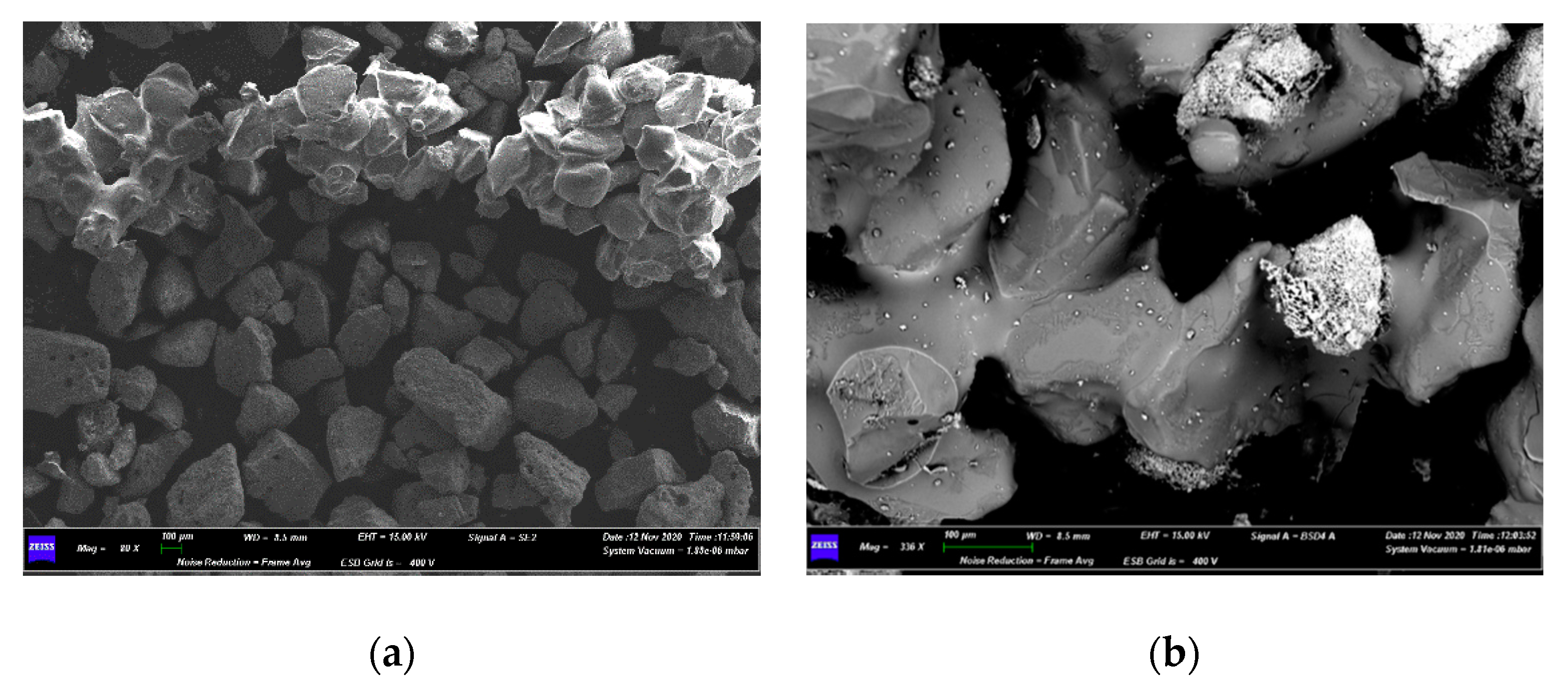
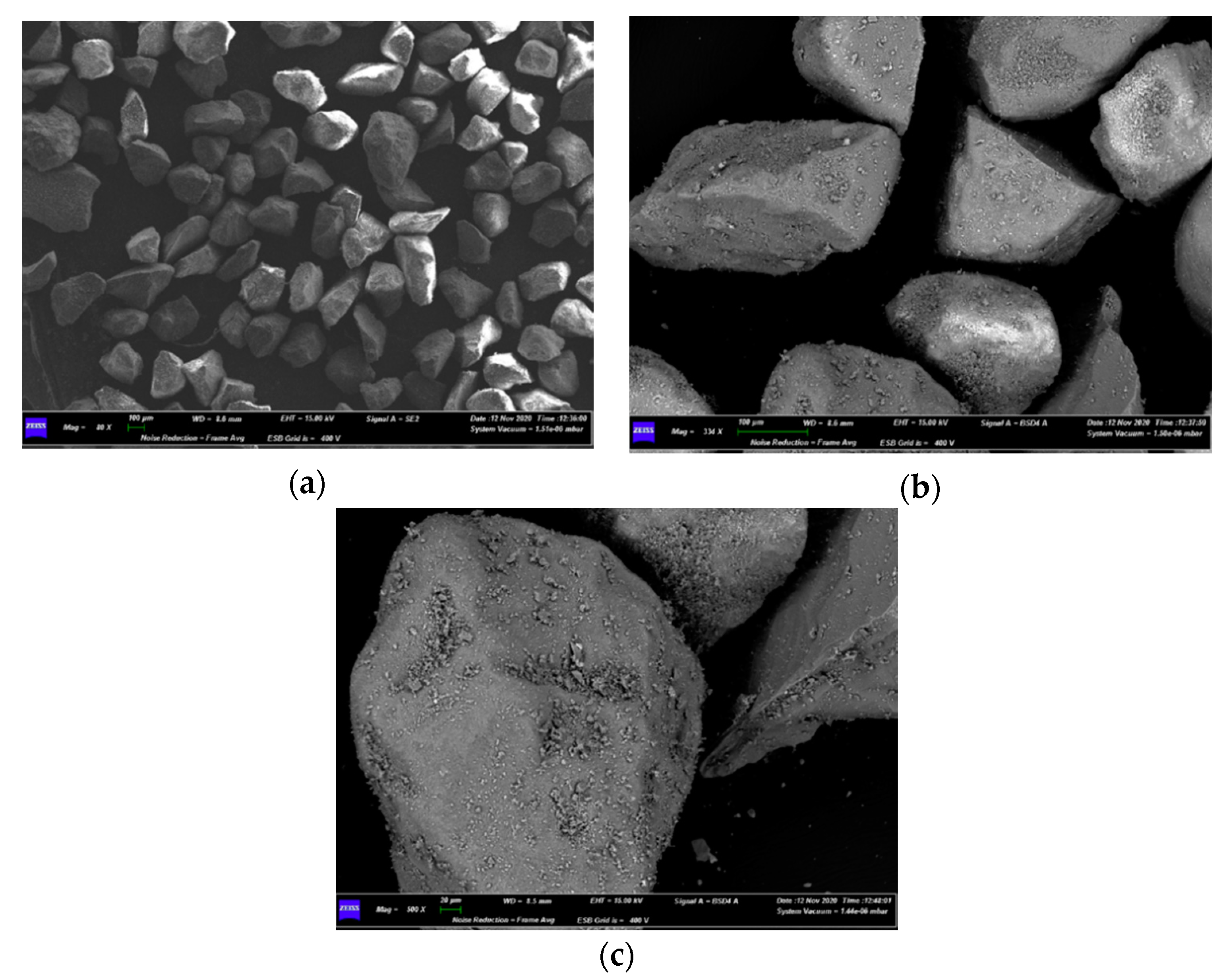
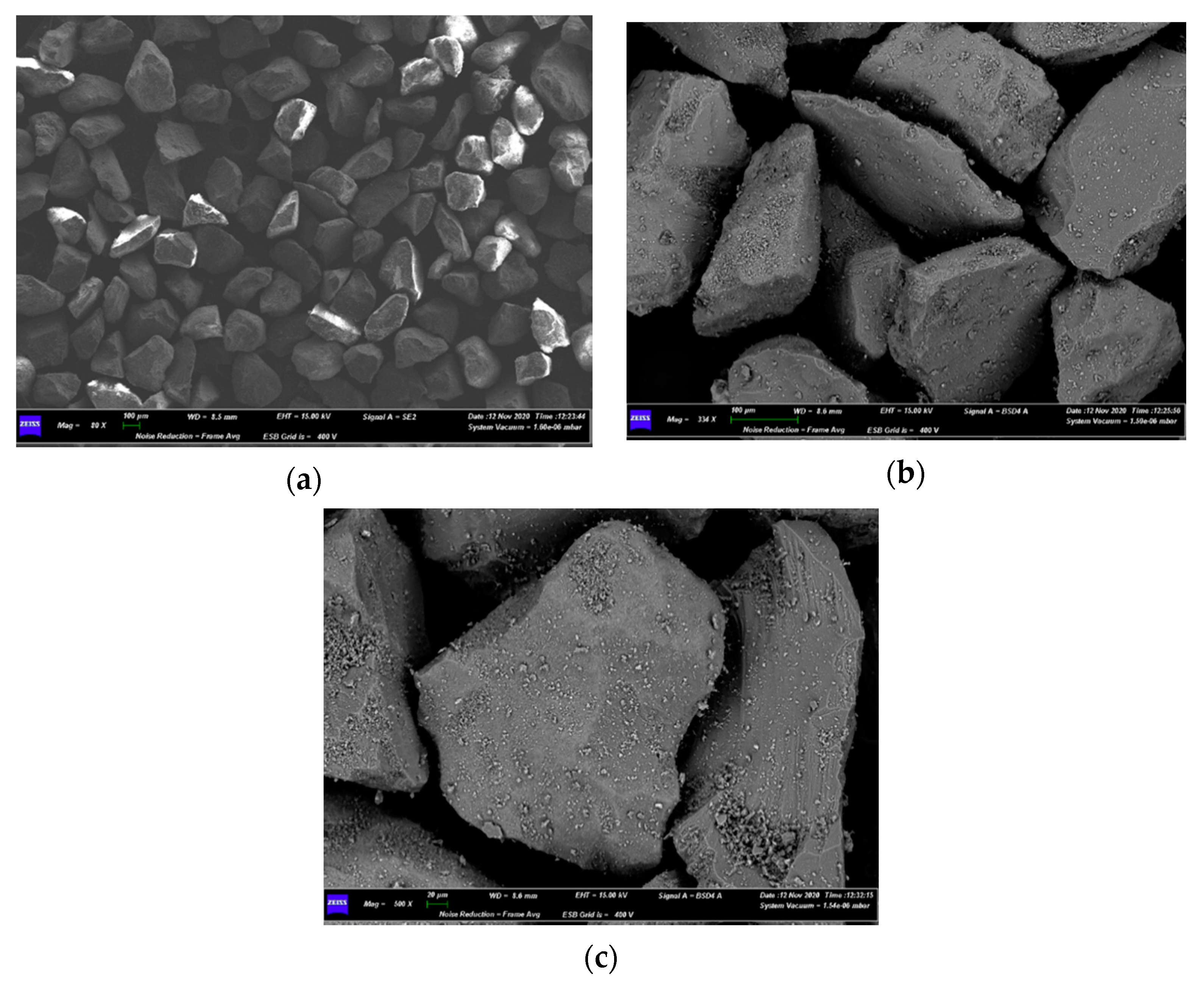
3.3. Characterizations of Spent Fluidized Bed
4. Discussion
4.1. Physical-Chemical Characterizations
4.2. Experimental Data from Devolatilization Tests
4.3. Characterization of Spent Fluidized Bed
- A priori, it was expected to find a greater number of melted particles/agglomerates in the upper part of the bed, because it is the zone of the first contact between its free surface and the falling pieces of waste materials from AHP; however, the exact opposite was found, as the smaller particles had moved to the top of the bed, while the larger ones, allegedly formed on the top, were found on the bottom. This is proof of the good mixing within the fluidized bed;
- Agglomerations did not diffusely involve the whole bed inventory. This suggested that only where the bed had experienced locally high Na concentrations the eutectic had formed [35], even though the quantity of Na in the fed fuel was low (2–3 wt%) compared to Na eutectic concentration in Si (45 wt%) [35].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sample | ηav | χavC | YavH2 | YavC3H8equiv | YavCO | YavCO2 | YavCH4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Nl Per 100 gfuel) | (%) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | ||||||||
| av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | |
| 500 °C | ||||||||||||||
| AHPam | 10.0 | 3.11 | 17.8 | 5.1 | 2.80 | 3.90 | 54.8 | 10.2 | 9.00 | 4.62 | 15.5 | 7.91 | 17.8 | 3.63 |
| AHPus | 15.8 | 7.67 | 25.4 | 11.1 | 7.90 | 4.80 | 16.5 | 7.26 | 27.8 | 6.50 | 33.3 | 3.00 | 14.5 | 9.96 |
| 600 °C | ||||||||||||||
| AHPam | 34.5 | 2.43 | 43.8 | 2.5 | 15.6 | 0.63 | 30.8 | 3.96 | 14.7 | 2.71 | 18.1 | 3.50 | 20.8 | 2.24 |
| AHPus | 25.0 | 21.4 | 38.6 | 28.7 | 10.7 | 4.07 | 17.7 | 5.86 | 33.4 | 4.66 | 23.5 | 1.40 | 14.7 | 0.38 |
| 700 °C | ||||||||||||||
| AHPam | 53.7 | 6.66 | 56.5 | 4.9 | 23.3 | 3.72 | 22.5 | 5.03 | 21.0 | 2.55 | 13.5 | 0.38 | 19.8 | 2.02 |
| AHPus | 29.8 | 19.8 | 42.2 | 18.7 | 20.1 | 4.07 | 24.7 | 23.8 | 25.7 | 9.79 | 18.7 | 6.62 | 10.8 | 4.00 |
| 800 °C | ||||||||||||||
| AHPam | 52.5 | 9.51 | 45.5 | 5.8 | 29.5 | 2.05 | 14.8 | 2.80 | 25.4 | 4.04 | 12.9 | 1.74 | 17.5 | 1.73 |
| AHPus | 46.0 | 5.40 | 55.0 | 2.1 | 23.5 | 5.53 | 7.40 | 0.98 | 36.4 | 0.89 | 20.7 | 3.95 | 12.0 | 1.36 |
| Sample | ηav | χavC | YavH2 | YavC3H8equiv | YavCO | YavCO2 | YavCH4 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (Nl Per 100 gfuel) | (%) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | (mol% Dry N2-Free) | ||||||||
| av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | av3 | sd3 | |
| 500 °C | ||||||||||||||
| AHPam | 19.2 | 2.53 | 31.9 | 6.9 | 0.00 | 0.00 | 45.0 | 13.3 | 15.7 | 5.90 | 24.6 | 9.00 | 14.8 | 4.40 |
| AHPus | 17.2 | 11.2 | 28.4 | 13.2 | 0.00 | 0.00 | 18.4 | 13.6 | 28.6 | 5.00 | 41.0 | 8.50 | 12.0 | 0.80 |
| 600 °C | ||||||||||||||
| AHPam | 29.6 | 2.14 | 35.7 | 2.00 | 11.1 | 2.1 | 25.0 | 0.60 | 18.2 | 1.90 | 25.0 | 2.10 | 20.6 | 1.20 |
| AHPus | 25.5 | 14.6 | 38.0 | 16.3 | 7.3 | 3.3 | 16.9 | 16.2 | 29.1 | 8.70 | 33.4 | 8.60 | 13.3 | 2.10 |
| 700 °C | ||||||||||||||
| AHPam | 38.0 | 7.11 | 49.1 | 6.00 | 11.3 | 2.30 | 30.5 | 5.03 | 17.9 | 1.70 | 19.7 | 0.60 | 30.5 | 0.80 |
| AHPus | 31.6 | 5.14 | 45.1 | 5.50 | 13.6 | 0.30 | 11.1 | 2.20 | 28.6 | 2.50 | 34.2 | 4.80 | 11.1 | 1.00 |
| 800 °C | ||||||||||||||
| AHPam | 51.5 | 17.6 | 45.1 | 7.90 | 23.3 | 5.00 | 13.8 | 7.10 | 19.6 | 1.20 | 27.0 | 7.90 | 16.3 | 3.10 |
| AHPus | 38.7 | 10.9 | 51.1 | 11.8 | 14.7 | 0.40 | 7.80 | 2.60 | 29.3 | 3.80 | 38.2 | 7.10 | 10.0 | 1.70 |
References
- Perea-Moreno, M.A.; Samerón-Manzano, E.; Perea-Moreno, A.J. Biomass as renewable energy: Worldwide research trends. Sustainability 2019, 11, 863. [Google Scholar] [CrossRef]
- Bongaarts, J. Development: Slow down population growth. Nature 2016, 530, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Ellen MacArthur Foundation Circular Economy-UK, USA, Europe, Asia & South America-The Ellen MacArthur Foundation. Available online: https://www.ellenmacarthurfoundation.org/ (accessed on 23 March 2021).
- The World Bank What a Waste: An Updated Look into the Future of Solid Waste Management. Available online: https://www.worldbank.org/en/news/immersive-story/2018/09/20/what-a-waste-an-updated-look-into-the-future-of-solid-waste-management (accessed on 11 March 2021).
- EC European Commission–Environment–Circular Economy Strategy. Available online: http://ec.europa.eu/environment/circular-economy/index_en.htm (accessed on 11 March 2021).
- Obama, B. The irreversible momentum of clean energy: Private-sector efforts help drive decoupling of emissions and economic growth. Science 2017, 355, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Marris, E. Why young climate activists have captured the world’s attention. Nature 2019, 573, 471–473. Available online: https://www.nature.com/articles/d41586-019-02696-0 (accessed on 11 March 2021). [CrossRef] [PubMed]
- United Nations Climate Change The Paris Agreement|UNFCCC. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 11 March 2021).
- COP 24|UNFCCC. Available online: https://unfccc.int/process-and-meetings/conferences/past-conferences/katowice-climate-change-conference-december-2018/sessions-of-negotiating-bodies/cop-24 (accessed on 11 March 2021).
- A European Green Deal|European Commission. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 3 January 2021).
- Johnston, R.B. Arsenic and the 2030 Agenda for sustainable development. Arsen. Res. and Glob. Sustain. As 2016, 12–14. [Google Scholar] [CrossRef]
- Arena, U. Process and technological aspects of municipal solid waste gasification. A review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, L.; Carnevale, E.; Corti, A. A review of technologies and performances of thermal treatment systems for energy recovery from waste. Waste Manag. 2015, 37, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Materazzi, M.; Foscolo, P.U. The role of waste and renewable gas to decarbonize the energy sector. In Substitute Natural Gas from Waste: Technical Assessment and Industrial Applications of Biochemical and Thermochemical Processes; Academic Press: London, UK, 2019; ISBN 9780128155547. [Google Scholar]
- Lig2Liq–Cost Effective Conversion of Lignite and Waste to Liquid Fuels. Available online: https://www.lig2liq.eu/ (accessed on 3 January 2021).
- Weber, G.; Di Giuliano, A.; Rauch, R.; Hofbauer, H. Developing a simulation model for a mixed alcohol synthesis reactor and validation of experimental data in IPSEpro. Fuel Process. Technol. 2016, 141, 167–176. [Google Scholar] [CrossRef]
- Rapagnà, S.; Gallucci, K.; Foscolo, P.U. Olivine, dolomite and ceramic filters in one vessel to produce clean gas from biomass. Waste Manag. 2018, 71, 792–800. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Savuto, E.; Di Carlo, A.; Gallucci, K.; Di Giuliano, A.; Rapagnà, S. Steam gasification of lignite and solid recovered fuel (SRF) in a bench scale fluidized bed gasifier. Waste Manag. 2020, 114, 341–350. [Google Scholar] [CrossRef] [PubMed]
- European Comission Innovation and Networks Executive Agency. Available online: https://ec.europa.eu/inea/en/connecting-europe-facility/cef-transport/projects-by-country/multi-country/2014-eu-tm-0196-s (accessed on 23 March 2021).
- Treatment of Absorbent Hygiene Products for Improved Recycling of Materials|Green Best Practice Community. Available online: https://greenbestpractice.jrc.ec.europa.eu/node/131#bemp_general_title_id (accessed on 23 March 2021).
- Waldheim, L. Gasification of Waste for Energy Carriers–A Review. 2018. Available online: https://www.etipbioenergy.eu/databases/reports/353-gasification-of-waste-for-energy-carriers-a-review (accessed on 23 March 2021).
- Deliverable Number 1.7–Blueprint for the Replication of the AHP Pretreatment Technol; Establishing a Multi-Purpose Biorefinery for the Recycling of the Organic Content of AHP Waste in Circular Economy Domain Grant Agreement No.: 745746. Available online: https://www.embraced.eu/repository/EMBRACED_D1.7_Blueprint_DEF.pdf (accessed on 23 March 2021).
- Mendoza, J.M.F.; D’Aponte, F.; Gualtieri, D.; Azapagic, A. Disposable baby diapers: Life cycle costs, eco-efficiency and circular economy. J. Clean. Prod. 2019, 211, 455–467. [Google Scholar] [CrossRef]
- Kashyap, P.; Ko Win, T.; Visvanathan, C. Absorbent Hygiene Products-An emerging urban waste management issue. In Proceedings of the Asia-Pacific Conference on Biotechnology for Waste Conversion 2016, Hong Kong, China, 5–8 December 2016. [Google Scholar]
- Arena, U.; Ardolino, F.; Gregorio, F.D.I. Sustainability of an Integrated Recycling Process of Absorbent Hygiene Products. In Proceedings of the 15th International Waste Management and Landfill Symposium, Cagliari, Italy, 5–9 October 2015. [Google Scholar]
- Gerina-Ancane, A.; Eiduka, A. Research and analysis of Absorbent Hygiene Product (AHP) recycling. In Proceedings of the Engineering for Rural Development, Jelgava, Latvia, 25–27 May 2016; Volume 2016, pp. 904–910. [Google Scholar]
- Khanyile, A.; Caws, G.C.; Nkomo, S.L.; Mkhize, N.M. Characterisation study of various disposable diaper brands. Sustainability 2020, 12, 437. [Google Scholar] [CrossRef]
- Khoo, S.C.; Phang, X.Y.; Ng, C.M.; Lim, K.L.; Lam, S.S.; Ma, N.L. Recent technologies for treatment and recycling of used disposable baby diapers. Process Saf. Environ. Prot. 2019, 123, 116–129. [Google Scholar] [CrossRef]
- Roddy, D.J.; Manson-Whitton, C. Biomass Gasification and Pyrolysis. In Comprehensive Renewable Energy-Biomass & Biofuels 2012; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, ISBN 9780080878737. [Google Scholar]
- Bassano, C.; Deiana, P.; Ricci, G.; Veca, E.; Rse, R. Analisi Termogravimetrica su Campioni di Carbone. 2009. Available online: https://docplayer.it/2987461-Analisi-termogravimetrica-su-campioni-di-carbone.html (accessed on 23 March 2021).
- BS EN 15414-3:2011Solid Recovered Fuels. Determination of Moisture Content Using the Oven Dry Method. Moisture in General Analysis Sample. Available online: https://www.en-standard.eu/din-en-15414-3-solid-recovered-fuels-determination-of-moisture-content-using-the-oven-dry-method-part-3-moisture-in-general-analysis-sample/ (accessed on 23 March 2021).
- BS 1016-104.4-1998: Methods for Analysis and Testing of Coal and Coke. Proximate Analysis. Determination of ash. Available online: https://shop.bsigroup.com/ProductDetail/?pid=000000000030013765 (accessed on 23 March 2021).
- Ambiente Italia s.r.l. Analisi Ambientale della Raccolta e del Riciclo di Prodotti Sanitari Assorbenti Risultati Sintetici. Available online: https://www.senato.it/application/xmanager/projects/leg17/attachments/documento_evento_procedura_commissione/files/000/000/636/Documentazione_FATER.pdf (accessed on 23 March 2021).
- Okamoto, H. Supplemental Literature Review of Binary Phase Diagrams: Ag-Nd, Ag-Zr, Al-Nb, B-Re, B-Si, In-Pt, Ir-Y, Na-Si, Na-Zn, Nb-P, Nd-Pt, and Th-Zr. J. Phase Equilibria Diffus. 2014, 35, 636–648. [Google Scholar] [CrossRef]
- Di Giuliano, A.; Lucantonio, S.; Gallucci, K. Devolatilization of Residual Biomasses for Chemical Looping Gasification in Fluidized Beds Made Up of Oxygen-Carriers. Energies 2021, 14, 311. [Google Scholar] [CrossRef]
- Scala, F. Particle agglomeration during fluidized bed combustion: Mechanisms, early detection and possible countermeasures. Fuel Process. Technol. 2018, 171, 31–38. [Google Scholar] [CrossRef]
- Gibilaro, L.G. Fluidization-Dynamic; Butterworth-Heinemann: Oxford, UK, 2001; ISBN 978-0-7506-5003-8. Available online: https://books.google.co.jp/books?hl=zh-CN&lr=&id=RlZutdBFy4sC&oi=fnd&pg=PP1&dq=Gibilaro,+L.G.+Fluidization-Dynamic%3B+Butterworth-Heinemann:+2001%3B+ISBN+978-0-7506-5003-8,+doi:10.1016/B978-0-7506-5003-8.X5000-9.&ots=Jn_kGOQKRB&sig=IisegBeCakEykL43CA6MirBVotk&redir_esc=y#v=onepage&q&f=false (accessed on 23 March 2021). [CrossRef]
- Basu, P. Combustion and Gasification in Fluidized Beds; CRC Press/Taylor & Francis: Abingdon, UK, 2006; ISBN 9780849333965. [Google Scholar]
- Green, D.W.; Robert, H.P. Perry’s Chemical Engineers’ Handbook, 8th ed.; McGraw-Hill: New York, NY, USA, 2017; ISBN 978007142294. [Google Scholar]
- Namkung, H.; Xu, L.H.; Kim, C.H.; Yuan, X.; Kang, T.J.; Kim, H.T. Effect of mineral components on sintering of ash particles at low temperature fouling conditions. Fuel Process. Technol. 2016, 141, 82–92. [Google Scholar] [CrossRef]



| Bed Material | Sand |
|---|---|
| dp (μm) | 212–250 |
| ρp (kg m−3) | 2587 |
| T (°C) | umf (cm s−1) |
| 500 | 2.9 |
| 600 | 2.7 |
| 700 | 2.5 |
| 800 | 2.3 |
| Gas | LHVg,i [MJ kmol−1] | Ref |
|---|---|---|
| CO | 282.99 | [39] |
| CO2 | 0 | |
| H2 | 241.83 | [39] |
| CH4 | 802.34 | [39] |
| C3H8 | 46.2 | [40] |
| Material | LHVp [MJ kg−1] | |
| AHPam | 23.60 | [34] |
| AHPus | 10.36 | [34] |
| wt% ar | ||
| AHPam | AHPus | |
| Dry weight | 94.2 | 93.4 |
| Moisture | 5.8 | 6.6 |
| Ashes | 7.5 | 10.7 |
| Volatile matter | 82.8 | 81.5 |
| Fixed carbon | 3.9 | 1.2 |
| wt% db | ||
| C | 65.3 | 43.5 |
| H | 10.3 | 6.6 |
| N | 0.0 | 0.1 |
| S | 1.1 | 1.8 |
| O | 23.3 | 48.0 |
| Element | AHPam | AHPus | ||
|---|---|---|---|---|
| (wt %) | abs. err. (wt %) | (wt %) | abs. err. (wt %) | |
| Mg | 1.007 | 0.031 | <0.0020 | 0.0 |
| Al | <0.0020 | 0.0 | <0.0020 | 0.0 |
| Si | 0.1016 | 0.0024 | 1.272 | 0.008 |
| P | 0.0581 | 0.001 | 0.5238 | 0.0034 |
| S | 0.03947 | 0.00049 | 4.467 | 0.005 |
| Cl | 0.0079 | 0.00016 | 1.425 | 0.002 |
| K | 0.04641 | 0.00069 | 2.935 | 0.003 |
| Ca | 11.99 | 0.01 | 1.157 | 0.002 |
| Ti | 1.402 | 0.001 | 0.08108 | 0.00029 |
| V | 0.05397 | 0.0006 | 0.00465 | 0.00013 |
| Cr | <0.00010 | 0.0 | 0.00796 | 0.00006 |
| Mn | 0.01207 | 0.00008 | 0.00906 | 0.00007 |
| Fe | 0.3068 | 0.0009 | 0.2159 | 0.0006 |
| Heating Rates (°C min−1) | 5 | 10 | 20 |
| Temperature of decomposition end for AHPam (°C) | 690 | 660 | 540 |
| Temperature of decomposition end for AHPus (°C) | 530 | 520 | 540 |
| T | Tests with N2 | Tests with N2 Plus Air | ||
|---|---|---|---|---|
| (°C) | AHPam (%) | AHPus (%) | AHPam (%) | AHPus (%) |
| 500 | 2.6 | 16.5 | 6.8 | 13.8 |
| 600 | 27.6 | 27.1 | 14.3 | 23.9 |
| 700 | 29.1 | 32.2 | 18.6 | 29.7 |
| 800 | 28.8 | 51.6 | 23.8 | 33.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malsegna, B.; Di Giuliano, A.; Gallucci, K. Experimental Study of Absorbent Hygiene Product Devolatilization in a Bubbling Fluidized Bed. Energies 2021, 14, 2399. https://doi.org/10.3390/en14092399
Malsegna B, Di Giuliano A, Gallucci K. Experimental Study of Absorbent Hygiene Product Devolatilization in a Bubbling Fluidized Bed. Energies. 2021; 14(9):2399. https://doi.org/10.3390/en14092399
Chicago/Turabian StyleMalsegna, Barbara, Andrea Di Giuliano, and Katia Gallucci. 2021. "Experimental Study of Absorbent Hygiene Product Devolatilization in a Bubbling Fluidized Bed" Energies 14, no. 9: 2399. https://doi.org/10.3390/en14092399
APA StyleMalsegna, B., Di Giuliano, A., & Gallucci, K. (2021). Experimental Study of Absorbent Hygiene Product Devolatilization in a Bubbling Fluidized Bed. Energies, 14(9), 2399. https://doi.org/10.3390/en14092399








