Structural Characteristics and Environmental Applications of Covalent Organic Frameworks
Abstract
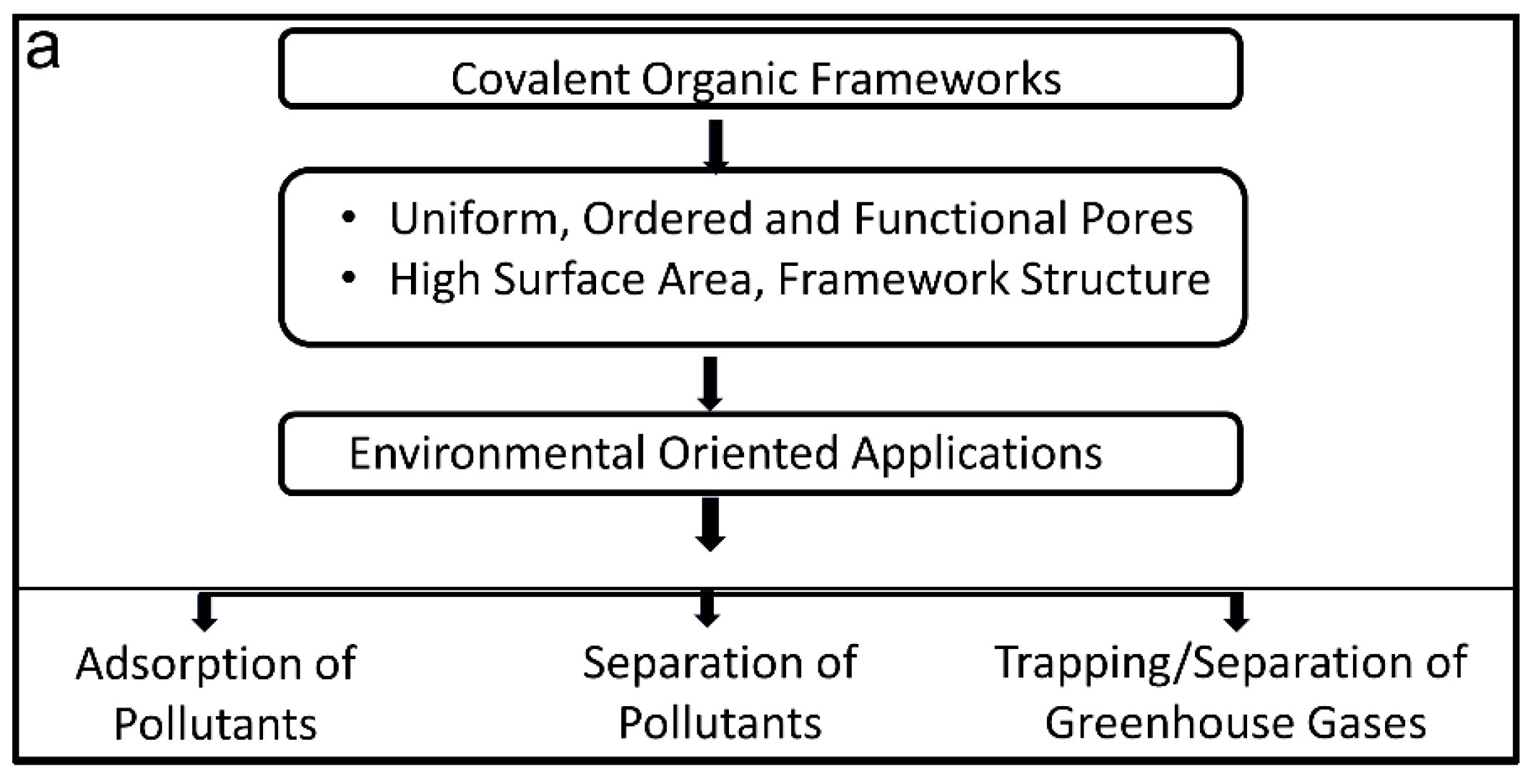
1. Introduction
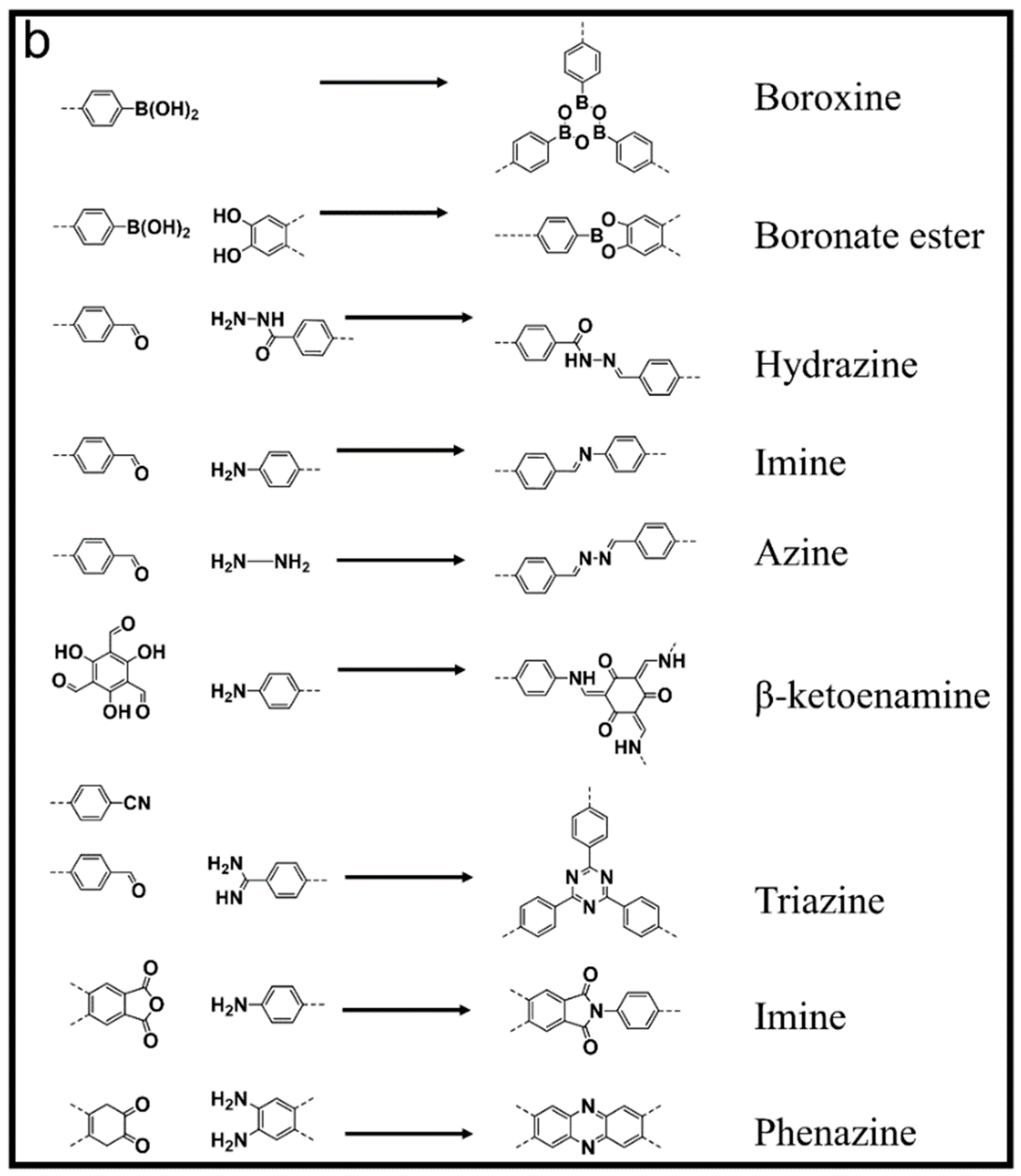
2. Evolution of COF Synthesis through Time
3. Important Aspects of COF Materials towards Cleaner Environment
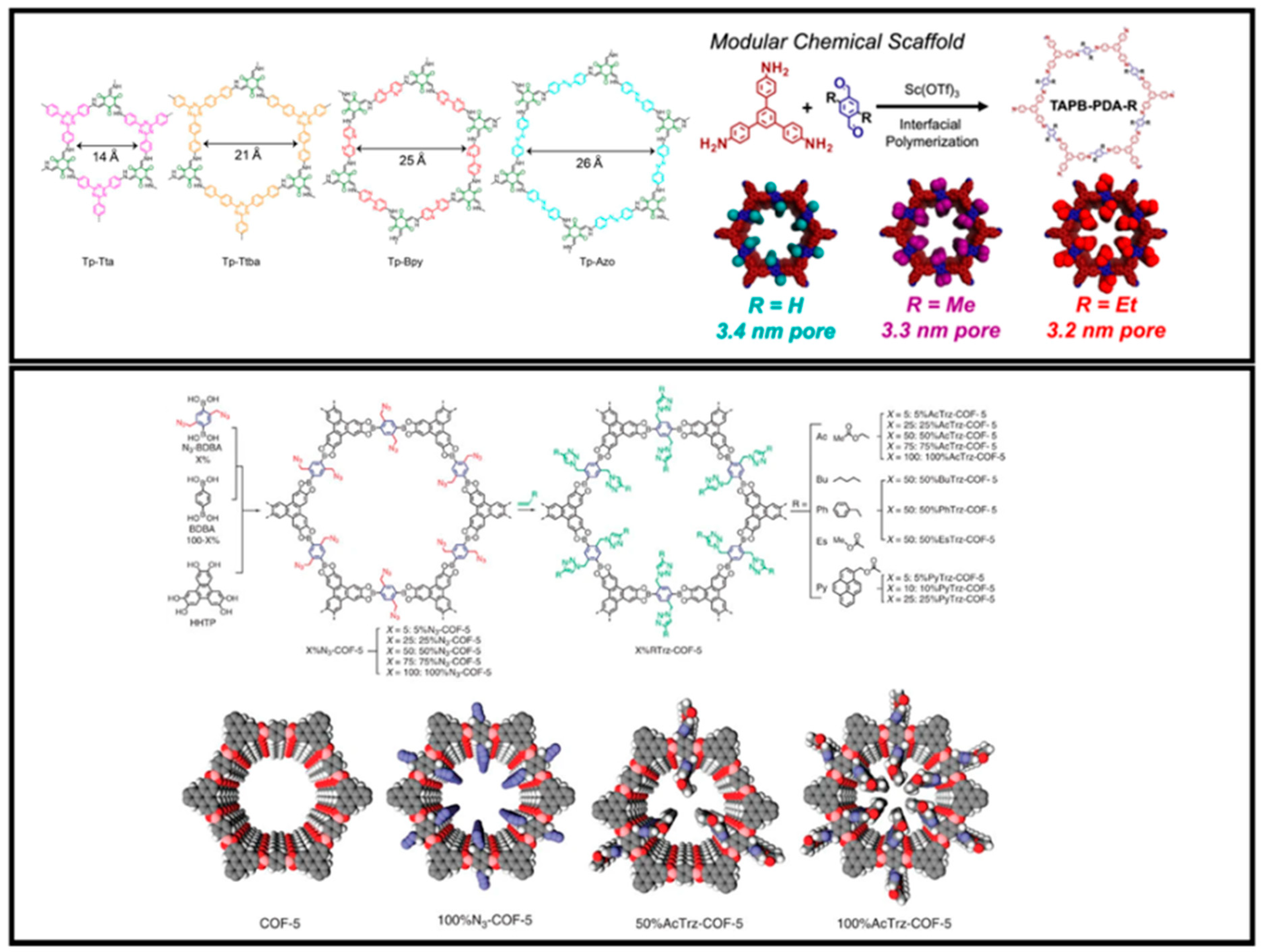
3.1. Pore Structure
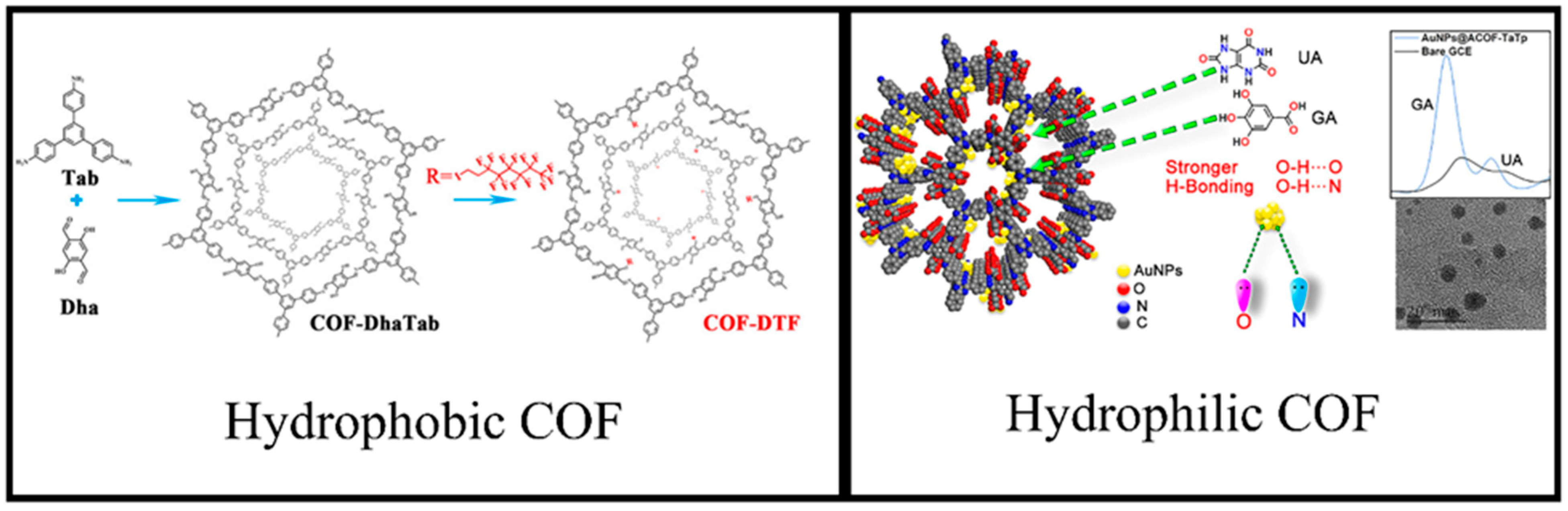
3.2. Hydrophobicity/Hydrophilicity of COFs
3.3. Structural Stability
3.4. Pore Charge
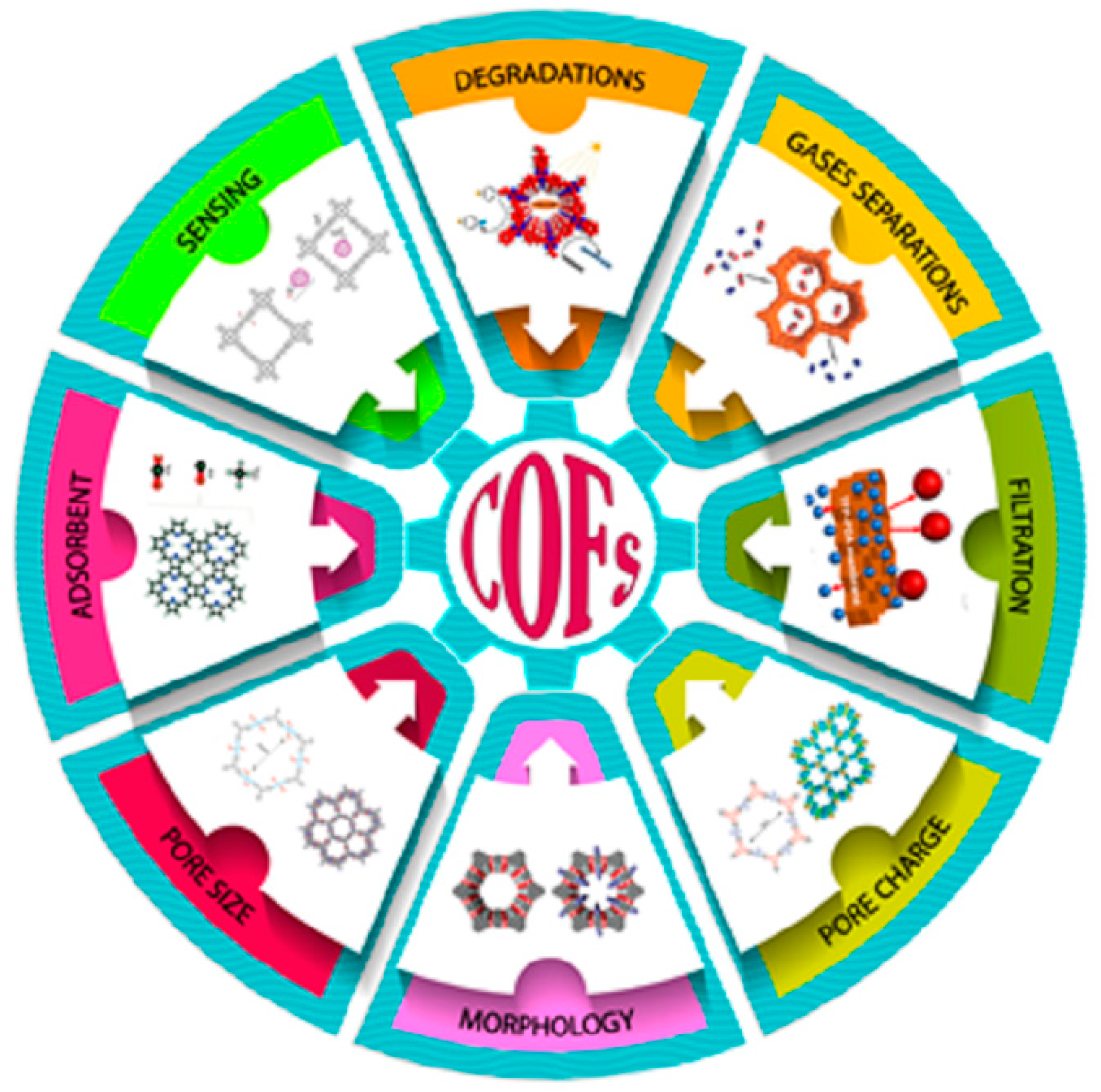
4. Applications of COFs towards Environment
4.1. Adsorption
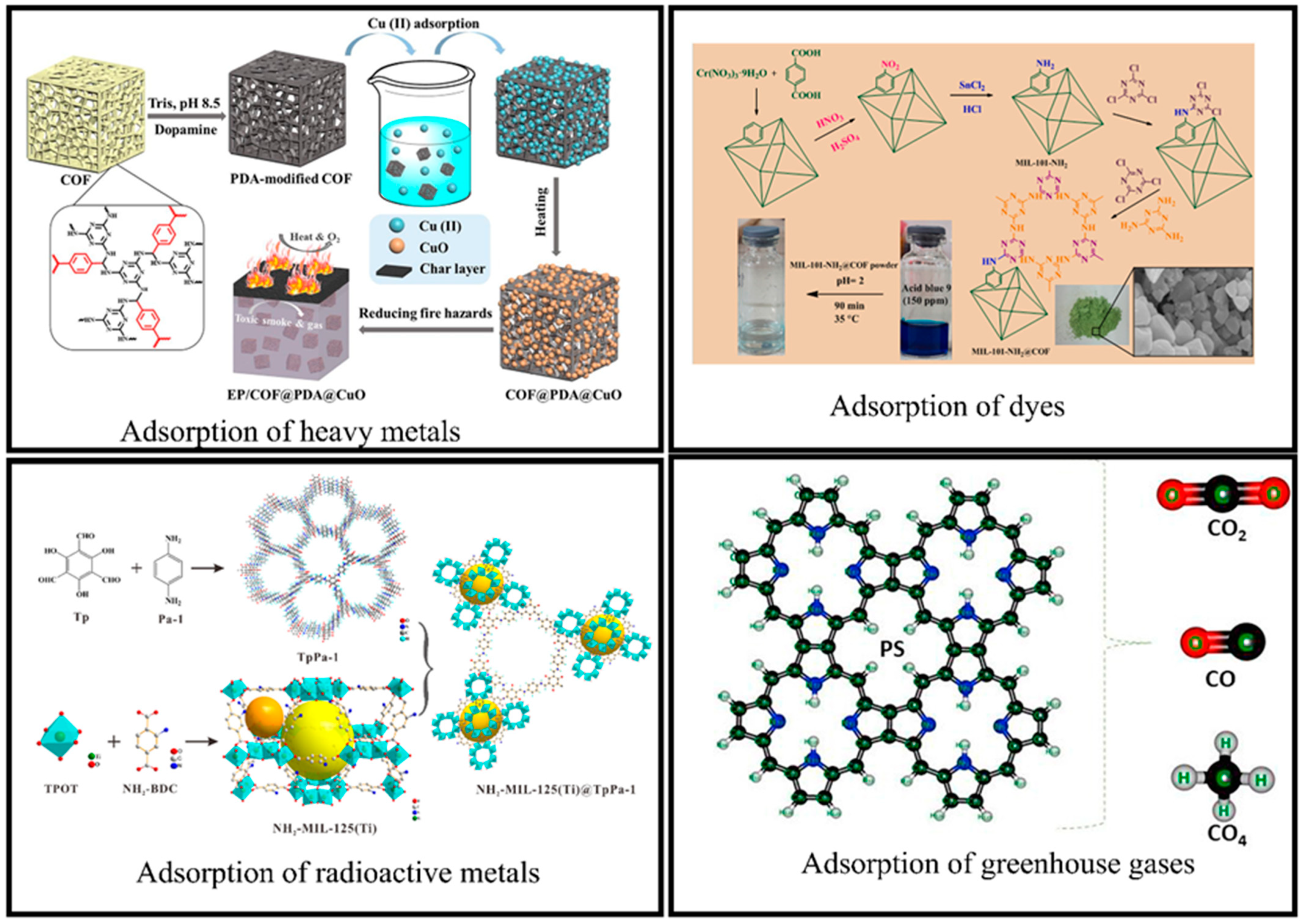
4.1.1. Adsorption of Heavy Metals
4.1.2. Adsorption of Hazardous Chemicals and Gases
4.2. COFs as Sensors for Chemicals
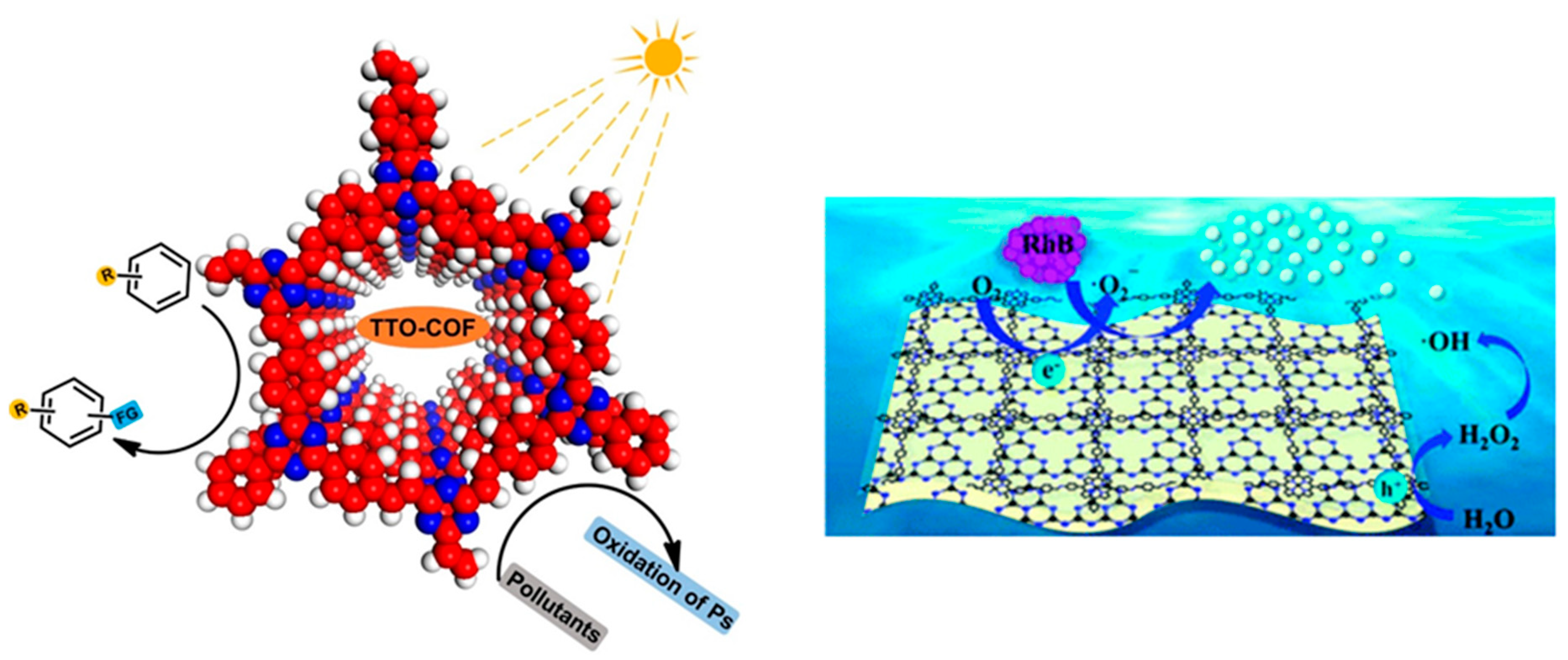
4.3. COF as Pollutant Degradation Agent for Clean Environment
4.4. COFs as Membrane-Forming Materials
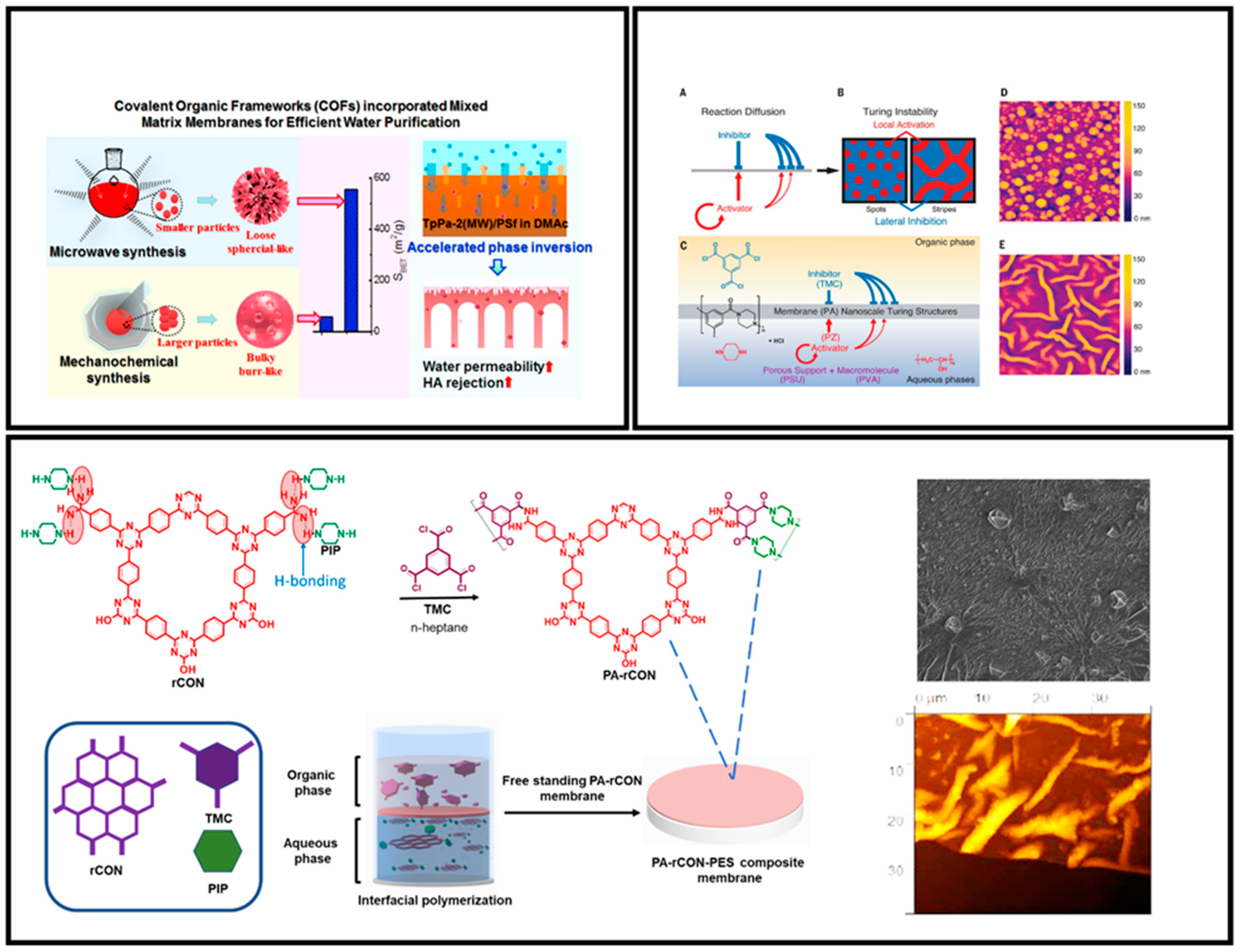
4.4.1. Mixed Matrix Membranes (MMMs)
4.4.2. Stacking of COF Nanosheets in the Membrane Form
4.4.3. Pure COF as Active Separation Layer in Membranes
5. Conclusions and Perspectives
- COFs with certain functionalities are still needed.
- Economically viable preparation methods are needed for commercialization.
- The crystallinity of the COF materials is still an issue that needs further incitement.
- Many COFs are not stable in humid conditions and this is a serious issue.
- CO2 separation in the presence of water and other acidic gases is not well explored.
- More structural performance of COFs need to be designed for environmental applications.
- The fragile nature of a pure nanosheet of COFs is challenging for industrial applications. Thus, flexible COF membranes would be an attractive approach.
- The environmental risk management data for COFs is still lacking, which needs to be addressed.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sheng, Y.; Chen, Q.; Mahurin, S.M.; Mayes, R.T.; Zhan, W.; Zhang, J.; Liu, H.; Dai, S. Fibers with Hyper-Crosslinked Functional Porous Frameworks. Macromol. Rapid Commun. 2018, 39, 1700767. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, B.; Mahurin, S.M.; Chai, S.-H.; Nelson, K.M.; Baker, D.C.; Liu, H.; Dai, S. Carbohydrate based hyper-crosslinked organic polymers with -OH functional groups for CO2 separation. J. Mater. Chem. A 2015, 3, 20913–20918. [Google Scholar] [CrossRef]
- Dawson, R.; Cooper, A.I.; Adams, D.J. Nanoporous organic polymer networks. Prog. Polym. Sci. 2012, 37, 530–563. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Li, A.; Sun, H.-X.; Tan, D.-Z.; Fan, W.-J.; Wen, S.-H.; Qing, X.-J.; Li, G.-X.; Li, S.-Y.; Deng, W.-Q. Superhydrophobic conjugated microporous polymers for separation and adsorption. Energy Environ. Sci. 2011, 4, 2062–2065. [Google Scholar] [CrossRef]
- Liang, B.; Wang, H.; Shi, X.; Shen, B.; He, X.; Ghazi, Z.A.; Khan, N.A.; Sin, H.; Khattak, A.M.; Li, L.; et al. Microporous membranes comprising conjugated polymers with rigid backbones enable ultrafast organic-solvent nanofiltration. Nat. Chem. 2018, 10, 961–967. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Sin, H.; Liang, B.; Ghazi, Z.A.; Khattak, A.M.; Khan, N.A.; Alanagh, H.R.; Li, L.; Lu, X.; Tang, Z. Controlling the Selectivity of Conjugated Microporous Polymer Membrane for Efficient Organic Solvent Nanofiltration. Adv. Funct. Mater. 2019, 29, 1900134. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Dillon, A.C.; Jones, K.M.; Bekkedahl, T.A.; Kiang, C.H.; Bethune, D.S.; Heben, M.J. Storage of hydrogen in single-walled carbon nanotubes. Nature 1997, 386, 377–379. [Google Scholar] [CrossRef]
- Adio, S.O.; Ganiyu, S.A.; Usman, M.; Abdulazeez, I.; Alhooshani, K. Facile and efficient nitrogen modified porous carbon derived from sugarcane bagasse for CO2 capture: Experimental and DFT investigation of nitrogen atoms on carbon frameworks. Chem. Eng. J. 2020, 382, 122964. [Google Scholar] [CrossRef]
- Yildirim, O.; Bonomo, M.; Barbero, N.; Atzori, C.; Civalleri, B.; Bonino, F.; Viscardi, G.; Barolo, C. Application of Metal-Organic Frameworks and Covalent Organic Frameworks as (Photo)Active Material in Hybrid Photovoltaic Technologies. Energies 2020, 13, 5602. [Google Scholar] [CrossRef]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef]
- Usman, M.; Ali, M.; Al-Maythalony, B.A.; Ghanem, A.S.; Saadi, O.W.; Ali, M.; Jafar Mazumder, M.A.; Abdel-Azeim, S.; Habib, M.A.; Yamani, Z.H.; et al. Highly Efficient Permeation and Separation of Gases with Metal–Organic Frameworks Confined in Polymeric Nanochannels. ACS Appl. Mater. Interfaces 2020, 12, 49992–50001. [Google Scholar] [CrossRef]
- Ghanem, A.S.; Ba-Shammakh, M.; Usman, M.; Khan, M.F.; Dafallah, H.; Habib, M.A.; Al-Maythalony, B.A. High gas permselectivity in ZIF-302/polyimide self-consistent mixed-matrix membrane. J. Appl. Polym. Sci. 2020, 137, 48513. [Google Scholar] [CrossRef]
- Boyd, G.E.; Adamson, A.W.; Myers, L.S. The exchange adsorption of ions from aqueous solutions by organic zeolites 2. J. Am. Chem. Soc. 1947, 69, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Imran, A.; Bramer, E.A.; Seshan, K.; Brem, G. Catalytic Flash Pyrolysis of Biomass Using Different Types of Zeolite and Online Vapor Fractionation. Energies 2016, 9, 187. [Google Scholar] [CrossRef]
- Luis Miguez, J.; Porteiro, J.; Perez-Orozco, R.; Angel Gomez, M. Technology Evolution in Membrane-Based CCS. Energies 2018, 11, 3153. [Google Scholar] [CrossRef]
- Usman, M.; Zhu, J.; Chuiyang, K.; Arslan, M.T.; Khan, A.; Galadima, A.; Muraza, O.; Khan, I.; Helal, A.; Al-Maythalony, B.A.; et al. Propene Adsorption-Chemisorption Behaviors on H-SAPO-34 Zeolite Catalysts at Different Temperatures. Catalysts 2019, 9, 919. [Google Scholar] [CrossRef]
- Côté, A.P.; Benin, A.I.; Ockwig, N.W.; O’Keeffe, M.; Matzger, A.J.; Yaghi, O.M. Porous, Crystalline, Covalent Organic Frameworks. Science 2005, 310, 1166–1170. [Google Scholar] [CrossRef]
- Kandambeth, S.; Mallick, A.; Lukose, B.; Mane, M.V.; Heine, T.; Banerjee, R. Construction of Crystalline 2D Covalent Organic Frameworks with Remarkable Chemical (Acid/Base) Stability via a Combined Reversible and Irreversible Route. J. Am. Chem. Soc. 2012, 134, 19524–19527. [Google Scholar] [CrossRef] [PubMed]
- Katekomol, P.; Roeser, J.; Bojdys, M.; Weber, J.; Thomas, A. Covalent Triazine Frameworks Prepared from 1,3,5-Tricyanobenzene. Chem. Mater. 2013, 25, 1542–1548. [Google Scholar] [CrossRef]
- Furukawa, H.; Yaghi, O.M. Storage of Hydrogen, Methane, and Carbon Dioxide in Highly Porous Covalent Organic Frameworks for Clean Energy Applications. J. Am. Chem. Soc. 2009, 131, 8875–8883. [Google Scholar] [CrossRef]
- Alahakoon, S.B.; Thompson, C.M.; Occhialini, G.; Smaldone, R.A. Design Principles for Covalent Organic Frameworks in Energy Storage Applications. Chemsuschem 2017, 10, 2116–2129. [Google Scholar] [CrossRef]
- Khattak, A.M.; Ghazi, Z.A.; Liang, B.; Khan, N.A.; Iqbal, A.; Li, L.; Tang, Z. A redox-active 2D covalent organic framework with pyridine moieties capable of faradaic energy storage. J. Mater. Chem. A 2016, 4, 16312–16317. [Google Scholar] [CrossRef]
- Royuela, S.; Gil-San Millan, R.; Mancheno, M.J.; Mar Ramos, M.; Segura, J.L.; Navarro, J.A.R.; Zamora, F. Catalytically Active Imine-based Covalent Organic Frameworks for Detoxification of Nerve Agent Simulants in Aqueous Media. Materials 2019, 12, 1974. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Gao, J.; Wang, Q.; Zhang, Y.; Song, W.-G.; Su, C.-Y.; Wang, W. Construction of Covalent Organic Framework for Catalysis: Pd/COF-LZU1 in Suzuki-Miyaura Coupling Reaction. J. Am. Chem. Soc. 2011, 133, 19816–19822. [Google Scholar] [CrossRef] [PubMed]
- Calik, M.; Auras, F.; Salonen, L.M.; Bader, K.; Grill, I.; Handloser, M.; Medina, D.D.; Dogru, M.; Loebermann, F.; Trauner, D.; et al. Extraction of Photogenerated Electrons and Holes from a Covalent Organic Framework Integrated Heterojunction. J. Am. Chem. Soc. 2014, 136, 17802–17807. [Google Scholar] [CrossRef] [PubMed]
- DeBlase, C.R.; Silberstein, K.E.; Thanh-Tam, T.; Abruna, H.D.; Dichtel, W.R. beta-Ketoenamine-Linked Covalent Organic Frameworks Capable of Pseudocapacitive Energy Storage. J. Am. Chem. Soc. 2013, 135, 16821–16824. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Q.; Shao, P.; Han, Y.; Gao, X.; Ma, L.; Yuan, S.; Ma, X.; Zhou, J.; Feng, X.; et al. Exfoliation of Covalent Organic Frameworks into Few-Layer Redox-Active Nanosheets as Cathode Materials for Lithium-Ion Batteries. J. Am. Chem. Soc. 2017, 139, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Xie, J.; Guo, W.; Li, D.-S.; Zhang, Q. Covalent-Organic Frameworks: Advanced Organic Electrode Materials for Rechargeable Batteries. Adv. Energy Mater. 2020, 10, 1904199. [Google Scholar] [CrossRef]
- Ghazi, Z.A.; Zhu, L.; Wang, H.; Naeem, A.; Khattak, A.M.; Liang, B.; Khan, N.A.; Wei, Z.; Li, L.; Tang, Z. Efficient Polysulfide Chemisorption in Covalent Organic Frameworks for High-Performance Lithium-Sulfur Batteries. Adv. Energy Mater. 2016, 6, 1601250. [Google Scholar] [CrossRef]
- Delrue, F.; Alvarez-Diaz, P.D.; Fon-Sing, S.; Fleury, G.; Sassi, J.-F. The Environmental Biorefinery: Using Microalgae to Remediate Wastewater, a Win-Win Paradigm. Energies 2016, 9, 132. [Google Scholar] [CrossRef]
- Song, W.; Deng, X. Effects of Urbanization-Induced Cultivated Land Loss on Ecosystem Services in the North China Plain. Energies 2015, 8, 5678–5693. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Jarup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef]
- Raju, K.A.; Ramakrishna, C. The effects of heavy metals on the anatomical structures of Avicennia marina (Forssk.) Vierh. Braz. J. Bot. 2021, 54, 1–9. [Google Scholar]
- Abrham, F.; Gholap, A.V. Analysis of heavy metal concentration in some vegetables using atomic absorption spectroscopy. Pollution 2021, 7, 205–216. [Google Scholar]
- Ghazi, Z.A.; Khattak, A.M.; Iqbal, R.; Ahmad, R.; Khan, A.A.; Usman, M.; Nawaz, F.; Ali, W.; Felegari, Z.; Jan, S.U.; et al. Adsorptive removal of Cd2+ from aqueous solutions by a highly stable covalent triazine-based framework. New J. Chem. 2018, 42, 10234–10242. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.-X.; Wang, X.; Xu, G.-J.; Zhao, Y.-F.; Wang, M.-L.; Lin, J.-M.; Zhao, R.-S.; Wu, Y. Triazine-cored covalent organic framework for ultrasensitive detection of polybrominated diphenyl ethers from real samples: Experimental and DFT study. J. Hazard. Mater. 2021, 403, 123917. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Chang, J.; Guan, X.; Tang, L.; Fang, Q.; Valtchev, V.; Yan, Y.; Qiu, S. 3D Thioether-Based Covalent Organic Frameworks for Selective and Efficient Mercury Removal. Small 2021, e2006112. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Shen, R.; Liu, R.; Xu, S.; Shuai, Q. Facile fabrication of magnetic covalent organic frameworks for magnetic solid-phase extraction of diclofenac sodium in milk. Food Chem. 2021, 347, 129002. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Lin, Y.-L.; Li, N.; Wang, Z.-W.; Liu, M.; Zhao, R.-S.; Lin, J.-M. Application of magnetic N-doped carbon nanotubes in solid-phase extraction of trace bisphenols from fruit juices. Food Chem. 2018, 269, 413–418. [Google Scholar] [CrossRef]
- Li, X.; Cui, Y.-Y.; Yang, C.-X. Covalent coupling fabrication of microporous organic network bonded capillary columns for gas chromatographic separation. Talanta 2021, 224, 121914. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, C.; Jin, Z.; Wang, J.; He, L.; Mu, X.; Song, L.; Hu, Y. Functional covalent organic framework for exceptional Fe2+, Co2+ and Ni2+ removal: An upcycling strategy to achieve water decontamination and reutilization as smoke suppressant and flame retardant simultaneously. Chem. Eng. J. 2020, 127837. [Google Scholar] [CrossRef]
- Xin, J.; Zhou, Y.; Wang, X.; Xu, G.; Xie, M.; Liu, L.; Zhao, R.; Wu, Y.; Wang, M. Room-temperature synthesis of magnetic covalent organic frameworks for analyzing trace benzoylurea insecticide residue in tea beverages. Food Chem. 2021, 347, 129075. [Google Scholar] [CrossRef] [PubMed]
- Garba, M.D.; Usman, M.; Mazumder, M.A.J.; Al-Ahmed, A. Inamuddin, Complexing agents for metal removal using ultrafiltration membranes: A review. Environ. Chem. Lett. 2019, 17, 1195–1208. [Google Scholar] [CrossRef]
- Turangan, N.; Xu, Y.; Spratt, H.; Rintoul, L.; Bottle, S.; MacLeod, J. Self-supporting covalent organic framework membranes synthesized through two different processes: Solvothermal annealing and solvent vapor annealing. Nanotechnology 2021, 32, 075604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, Y.; Wang, Y.; Ma, S.; Ou, J.; Shen, Y.; Ye, M.; Uyama, H. Integration of covalent organic frameworks into hydrophilic membrane with hierarchical porous structure for fast adsorption of metal ions. J. Hazard. Mater. 2021, 407, 124390. [Google Scholar] [CrossRef]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Structural and electronic properties of oxygen defective and Se-doped p-type BiVO4 (001) thin film for the applications of photocatalysis. Appl. Catal. B 2018, 224, 895–903. [Google Scholar] [CrossRef]
- Ullah, H.; Tahir, A.A.; Mallick, T.K. Polypyrrole/TiO2 composites for the application of photocatalysis. Sens. Actuators B 2017, 241, 1161–1169. [Google Scholar] [CrossRef]
- Ullah, H.; Tahir, A.A.; Bibi, S.; Mallick, T.K.; Karazhanov, S.Z. Electronic properties of β-TaON and its surfaces for solar water splitting. Appl. Catal. B 2018, 229, 24–31. [Google Scholar] [CrossRef]
- Ullah, H.; Loh, A.; Trudgeon, D.P.; Li, X. Density Functional Theory Study of NiFeCo Trinary Oxy-Hydroxides for an Efficient and Stable Oxygen Evolution Reaction Catalyst. ACS Omega 2020, 5, 20517–20524. [Google Scholar] [CrossRef]
- Ullah, H.; Bibi, S.; Tahir, A.A.; Mallick, T.K. Donor-acceptor polymer for the design of all-solid-state dye-sensitized solar cells. J. Alloy. Compd. 2017, 696, 914–922. [Google Scholar] [CrossRef]
- Ullah, H.; Bibi, S.; Tahir, A.A.; Mallick, T.K. Density functional theory study of selenium-substituted low-bandgap donor–acceptor–donor polymer. J. Phys. Chem. C 2016, 120, 27200–27211. [Google Scholar] [CrossRef]
- Nasir, S.N.F.M.; Ullah, H.; Ebadi, M.; Tahir, A.A.; Sagu, J.S.; Mat Teridi, M.A. New insights into Se/BiVO4 heterostructure for photoelectrochemical water splitting: A combined experimental and DFT study. J. Phys. Chem. C 2017, 121, 6218–6228. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Cao, J.; Pi, W.; Yuan, Y.; Ali, S.; Tahir, A.A.; Yue, P.; Khan, A.; Zheng, Z. Experimental and DFT Studies of Au Deposition Over WO 3/gC 3 N 4 Z-Scheme Heterojunction. Nano-Micro Lett. 2020, 12, 1–18. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Ullah, H.; Tahir, A.A.; Li, X.; Ng, Y.H.; Sufian, S. Superior photoelectrocatalytic performance of ternary structural BiVO4/GQD/g-C3N4 heterojunction. J. Colloid Interface Sci. 2021, 586, 785–796. [Google Scholar] [CrossRef]
- Samsudin, M.F.R.; Ullah, H.; Bashiri, R.; Mohamed, N.M.; Sufian, S.; Ng, Y.H. Experimental and DFT insights on microflower g-C3N4/BiVO4 photocatalyst for enhanced photoelectrochemical hydrogen generation from lake water. ACS Sustain. Chem. Eng. 2020, 8, 9393–9403. [Google Scholar] [CrossRef]
- Noh, M.F.M.; Ullah, H.; Arzaee, N.A.; Ab Halim, A.; Rahim, M.A.F.A.; Mohamed, N.A.; Safaei, J.; Nasir, S.N.F.M.; Wang, G.; Teridi, M.A.M. Rapid fabrication of oxygen defective α-Fe2O3 (110) for enhanced photoelectrochemical activities. Dalton Trans. 2020, 49, 12037–12048. [Google Scholar]
- Mohamed, N.A.; Ullah, H.; Safaei, J.; Ismail, A.F.; Mohamad Noh, M.F.; Soh, M.F.; Ibrahim, M.A.; Ludin, N.A.; Mat Teridi, M.A. Efficient Photoelectrochemical Performance of γ Irradiated g-C3N4 and Its g-C3N4@BiVO4 Heterojunction for Solar Water Splitting. J. Phys. Chem. C 2019, 123, 9013–9026. [Google Scholar] [CrossRef]
- Safaei, J.; Ullah, H.; Mohamed, N.A.; Mohamad Noh, M.F.; Soh, M.F.; Tahir, A.A.; Ahmad Ludin, N.; Ibrahim, M.A.; Wan Isahak, W.N.R.; Mat Teridi, M.A. Enhanced photoelectrochemical performance of Z-scheme g-C3N4/BiVO4 photocatalyst. Appl. Catal. B 2018, 234, 296–310. [Google Scholar] [CrossRef]
- Ola, O.; Ullah, H.; Chen, Y.; Thummavichai, K.; Wang, N.; Zhu, Y. DFT and Experimental Studies of Iron Oxide-based Nanocomposites for Efficient Electrocatalysis. J. Mater. Chem. C 2021. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, P.; Loh, A.; Wan, L.; Habib, U.; Xu, Z.; Li, X.; Wang, B. Assembling flower-on-sheet CoP–NiCoP nanohybrids as efficient self-supported electrocatalysts for hydrogen evolution reaction in both acidic and alkaline media. J. Mater. Sci. 2021, 56, 3375–3386. [Google Scholar] [CrossRef]
- Sookhakian, M.; Ullah, H.; Teridi, M.A.M.; Tong, G.B.; Basirun, W.J.; Alias, Y. Boron-doped graphene-supported manganese oxide nanotubes as an efficient non-metal catalyst for the oxygen reduction reaction. Sustain. Energy Fuels 2020, 4, 737–749. [Google Scholar] [CrossRef]
- Kim, H.P.; Vasilopoulou, M.; Ullah, H.; Bibi, S.; Gavim, A.E.X.; Macedo, A.G.; da Silva, W.J.; Schneider, F.K.; Tahir, A.A.; Teridi, M.A.M. A hysteresis-free perovskite transistor with exceptional stability through molecular cross-linking and amine-based surface passivation. Nanoscale 2020, 12, 7641–7650. [Google Scholar] [CrossRef]
- Bin Mohd Yusoff, A.R.; Mahata, A.; Vasilopoulou, M.; Ullah, H.; Hu, B.; da Silva, W.J.; Schneider, F.K.; Gao, P.; Ievlev, A.V.; Liu, Y. Observation of large Rashba spin–orbit coupling at room temperature in compositionally engineered perovskite single crystals and application in high performance photodetectors. Mater. Today 2021, in press. [Google Scholar]
- Ding, S.-Y.; Dong, M.; Wang, Y.-W.; Chen, Y.-T.; Wang, H.-Z.; Su, C.-Y.; Wang, W. Thioether-Based Fluorescent Covalent Organic Framework for Selective Detection and Facile Removal of Mercury(II). J. Am. Chem. Soc. 2016, 138, 3031–3037. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Covalent organic frameworks (COFs) for environmental applications. Coord. Chem. Rev. 2019, 400, 213046. [Google Scholar] [CrossRef]
- Uribe-Romo, F.J.; Hunt, J.R.; Furukawa, H.; Klock, C.; O’Keeffe, M.; Yaghi, O.M. A Crystalline Imine-Linked 3-D Porous Covalent Organic Framework. J. Am. Chem. Soc. 2009, 131, 4570–4571. [Google Scholar] [CrossRef]
- Kuhn, P.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar] [CrossRef]
- Uribe-Romo, F.J.; Doonan, C.J.; Furukawa, H.; Oisaki, K.; Yaghi, O.M. Crystalline Covalent Organic Frameworks with Hydrazone Linkages. J. Am. Chem. Soc. 2011, 133, 11478–11481. [Google Scholar] [CrossRef]
- Karak, S.; Kandambeth, S.; Biswal, B.P.; Sasmal, H.S.; Kumar, S.; Pachfule, P.; Banerjee, R. Constructing Ultraporous Covalent Organic Frameworks in Seconds via an Organic Terracotta Process. J. Am. Chem. Soc. 2017, 139, 1856–1862. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.; Pal, M.; Rout, K.C.; Kunjattu, H.S.; Das, A.; Mukherjee, R.; Kharul, U.K.; Banerjee, R. Selective Molecular Separation by Interfacially Crystallized Covalent Organic Framework Thin Films. J. Am. Chem. Soc. 2017, 139, 13083–13091. [Google Scholar] [CrossRef]
- Khan, N.A.; Zhang, R.; Wu, H.; Shen, J.; Yuan, J.; Fan, C.; Cao, L.; Olson, M.A.; Jiang, Z. Solid–Vapor Interface Engineered Covalent Organic Framework Membranes for Molecular Separation. J. Am. Chem. Soc. 2020, 142, 13450–13458. [Google Scholar] [CrossRef]
- Fan, H.; Gu, J.; Meng, H.; Knebel, A.; Caro, J. High-Flux Membranes Based on the Covalent Organic Framework COF-LZU1 for Selective Dye Separation by Nanofiltration. Angew. Chem. Int. Ed. 2018, 57, 4083–4087. [Google Scholar] [CrossRef] [PubMed]
- Diaz de Grenu, B.; Torres, J.; Garcia-Gonzalez, J.; Munoz-Pina, S.; de los Reyes, R.; Costero, A.M.; Amoros, P.; Ros-Lis, J.V. Microwave-Assisted Synthesis of Covalent Organic Frameworks: A Review. ChemSusChem 2021, 14, 208–233. [Google Scholar] [CrossRef]
- Liu, X.-H.; Guan, C.-Z.; Ding, S.-Y.; Wang, W.; Yan, H.-J.; Wang, D.; Wan, L.-J. On-Surface Synthesis of Single-Layered Two-Dimensional Covalent Organic Frameworks via Solid–Vapor Interface Reactions. J. Am. Chem. Soc. 2013, 135, 10470–10474. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhou, X.; Tian, W.Q.; Gao, T.; Zhang, Y.F.; Lei, S.; Liu, Z.F. Surface-Confined Single-Layer Covalent Organic Framework on Single-Layer Graphene Grown on Copper Foil. Angew. Chem. Int. Ed. 2014, 53, 9564–9568. [Google Scholar] [CrossRef]
- Khan, N.A.; Yuan, J.; Wu, H.; Huang, T.; You, X.; Olson, M.A.; Azad, C.S.; Rahman, A.U.; Jiang, Z. Covalent Organic Framework Nanosheets as Reactive Fillers to Fabricate Free-Standing Polyamide Membranes for Efficient Desalination. ACS Appl. Mater. Interfaces 2020, 12, 27777–27785. [Google Scholar] [CrossRef]
- Bian, G.; Yin, J.; Zhu, J. Recent Advances on Conductive 2D Covalent Organic Frameworks. Small 2021, e2006043. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, W.; Teo, W.L.; Wang, K.; Zhang, L.; Zeng, Y.; Zhao, Y. Covalent-Organic-Framework-Based Composite Materials. Chem 2020, 6, 3172–3202. [Google Scholar] [CrossRef]
- Ren, D.; Ren, S.; Lin, Y.; Xu, J.; Wang, X. Recent developments of organic solvent resistant materials for membrane separations. Chemosphere 2020, 271, 129425. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, X.; Ma, X.; Wang, Y. Chiral porous organic frameworks and their application in enantioseparation. Anal. Methods 2021, 13, 8–33. [Google Scholar] [CrossRef] [PubMed]
- Diercks, C.S.; Yaghi, O.M. The atom, the molecule, and the covalent organic framework. Science 2017, 355, eaal1585. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Feng, X.; Ding, X.; Jiang, D. Covalent organic frameworks. Chem. Soc. Rev. 2012, 41, 6010–6022. [Google Scholar] [CrossRef]
- Huang, N.; Wang, P.; Jiang, D. Covalent organic frameworks: A materials platform for structural and functional designs. Nat. Rev. Mater. 2016, 1, 1–19. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheno, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gandara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef]
- Ashraf, M.; Khan, I.; Usman, M.; Khan, A.; Shah, S.S.; Khan, A.Z.; Saeed, K.; Yaseen, M.; Ehsan, M.F.; Tahir, M.N.; et al. Hematite and Magnetite Nanostructures for Green and Sustainable Energy Harnessing and Environmental Pollution Control: A Review. Chem. Res. Toxicol. 2020, 33, 1292–1311. [Google Scholar] [CrossRef] [PubMed]
- Halder, A.; Karak, S.; Addicoat, M.; Bera, S.; Chakraborty, A.; Kunjattu, S.H.; Pachfule, P.; Heine, T.; Banerjee, R. Ultrastable Imine-Based Covalent Organic Frameworks for Sulfuric Acid Recovery: An Effect of Interlayer Hydrogen Bonding. Angew. Chem. Int. Ed. 2018, 57, 5797–5802. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Fazal, A.; Hamid, S.; Arfan, M.; Khan, I.; Usman, M.; Shafiee, A.; Ashiq, M.N. CoFe2O4 decorated g-C3N4 nanosheets: New insights into superoxide anion mediated photomineralization of methylene blue. J. Environ. Chem. Eng. 2020, 8, 104556. [Google Scholar] [CrossRef]
- Ehsan, M.F.; Shafiq, M.; Hamid, S.; Shafiee, A.; Usman, M.; Khan, I.; Ashiq, M.N.; Arfan, M. Reactive oxygen species: New insights into photocatalytic pollutant degradation over g-C3N4/ZnSe nanocomposite. Appl. Surf. Sci. 2020, 532, 147418. [Google Scholar] [CrossRef]
- Corcos, A.R.; Levato, G.A.; Jiang, Z.; Evans, A.M.; Livingston, A.G.; Mariñas, B.J.; Dichtel, W.R. Reducing the Pore Size of Covalent Organic Frameworks in Thin-Film Composite Membranes Enhances Solute Rejection. ACS Mater. Lett. 2019, 1, 440–446. [Google Scholar] [CrossRef]
- Nagai, A.; Guo, Z.; Feng, X.; Jin, S.; Chen, X.; Ding, X.; Jiang, D. Pore surface engineering in covalent organic frameworks. Nat. Commun. 2011, 2, 536. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, Y.; Wang, J. Covalent organic frameworks as efficient adsorbent for sulfamerazine removal from aqueous solution. J. Hazard. Mater. 2020, 383, 121126. [Google Scholar] [CrossRef]
- Mullangi, D.; Shalini, S.; Nandi, S.; Choksi, B.; Vaidhyanathan, R. Super-hydrophobic covalent organic frameworks for chemical resistant coatings and hydrophobic paper and textile composites. J. Mater. Chem. A 2017, 5, 8376–8384. [Google Scholar] [CrossRef]
- Liu, Z.; Su, Q.; Ju, P.; Li, X.; Li, G.; Wu, Q.; Yang, B. A hydrophilic covalent organic framework for photocatalytic oxidation of benzylamine in water. Chem. Commun. 2020, 56, 766–769. [Google Scholar] [CrossRef]
- Han, N.; Zhang, Z.; Gao, H.; Qian, Y.; Tan, L.; Yang, C.; Zhang, H.; Cui, Z.; Li, W.; Zhang, X. Superhydrophobic Covalent Organic Frameworks Prepared via Pore Surface Modifications for Functional Coatings under Harsh Conditions. ACS Appl. Mater. Interfaces 2020, 12, 2926–2934. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Deng, Y.; He, Y.; Chen, J.; Hu, S. Construction of hydrophilic N, O-rich carboxylated triazine-covalent organic frameworks for the application in selective simultaneous electrochemical detection. Appl. Surf. Sci. 2021, 545, 149047. [Google Scholar] [CrossRef]
- Yuan, S.; Li, X.; Zhu, J.; Zhang, G.; Van Puyvelde, P.; Van der Bruggen, B. Covalent organic frameworks for membrane separation. Chem. Soc. Rev. 2019, 48, 2665–2681. [Google Scholar] [CrossRef]
- Kandambeth, S.; Shinde, D.B.; Panda, M.K.; Lukose, B.; Heine, T.; Banerjee, R. Enhancement of Chemical Stability and Crystallinity in Porphyrin-Containing Covalent Organic Frameworks by Intramolecular Hydrogen Bonds. Angew. Chem. Int. Ed. 2013, 52, 13052–13056. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Gao, J.; Jiang, D. Stable, crystalline, porous, covalent organic frameworks as a platform for chiral organocatalysts. Nat. Chem. 2015, 7, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Xia, H.; Mu, Y.; Liu, X. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 2016, 52, 6613–6616. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, L.; Zhao, H.; Li, B.; Ma, H. A two-dimensional cationic covalent organic framework membrane for selective molecular sieving. J. Mater. Chem. A 2018, 6, 13331–13339. [Google Scholar] [CrossRef]
- Duong, P.H.H.; Kuehl, V.A.; Mastorovich, B.; Hoberg, J.O.; Parkinson, B.A.; Li-Oakey, K.D. Carboxyl-functionalized covalent organic framework as a two-dimensional nanofiller for mixed-matrix ultrafiltration membranes. J. Membr. Sci. 2019, 574, 338–348. [Google Scholar] [CrossRef]
- Kuehl, V.A.; Yin, J.; Duong, P.H.H.; Mastorovich, B.; Newell, B.; Li-Oakey, K.D.; Parkinson, B.A.; Hoberg, J.O. A Highly Ordered Nanoporous, Two-Dimensional Covalent Organic Framework with Modifiable Pores, and Its Application in Water Purification and Ion Sieving. J. Am. Chem. Soc. 2018, 140, 18200–18207. [Google Scholar] [CrossRef]
- Xiao, Y.; Ma, C.; Jin, Z.; Wang, C.; Wang, J.; Wang, H.; Mu, X.; Song, L.; Hu, Y. Functional covalent organic framework illuminate rapid and efficient capture of Cu (II) and reutilization to reduce fire hazards of epoxy resin. Sep. Purif. Technol. 2021, 259, 118119. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, F.; Liu, N.; Liu, W.; Tong, M. Facile synthesis of sulfhydryl modified covalent organic frameworks for high efficient Hg(II) removal from water. J. Hazard. Mater. 2021, 405, 124190. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, Y.; Yuan, B.; Tian, Y.; Shang, J.; Tsang, D.C.W.; Liu, M.; Gan, L.; Mao, S.; Li, L. Thio-groups decorated covalent triazine frameworks for selective mercury removal. J. Hazard. Mater. 2021, 403, 123702. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Aguila, B.; Perman, J.; Earl, L.D.; Abney, C.W.; Cheng, Y.; Wei, H.; Nguyen, N.; Wojtas, L.; Ma, S. Postsynthetically Modified Covalent Organic Frameworks for Efficient and Effective Mercury Removal. J. Am. Chem. Soc. 2017, 139, 2786–2793. [Google Scholar] [CrossRef]
- Zhong, X.; Liu, Y.; Liang, W.; Zhu, Y.; Hu, B. Construction of Core-Shell MOFs@COF Hybrids as a Platform for the Removal of UO22+ and Eu3+ Ions from Solution. ACS Appl. Mater. Interfaces 2021, 13, 13883–13985. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Fu, Q.-B.; Wang, M.-L.; Lin, J.-M.; Zhao, R.-S. Determination of trace bisphenols in functional beverages through the magnetic solid-phase extraction with MOF-COF composite. Food Chem. 2021, 345, 128841. [Google Scholar] [CrossRef]
- Xu, G.; Hou, L.; Li, B.; Wang, X.; Liu, L.; Li, N.; Wang, M.-L.; Zhao, R.-S. Facile preparation of hydroxyl bearing covalent organic frameworks for analysis of phenoxy carboxylic acid pesticide residue in plant-derived food. Food Chem. 2021, 345, 128749. [Google Scholar] [CrossRef] [PubMed]
- Dinari, M.; Jamshidian, F. Preparation of MIL-101-NH2 MOF/triazine based covalent organic framework hybrid and its application in acid blue 9 removals. Polymer 2021, 215, 123383. [Google Scholar] [CrossRef]
- Suresh, R.; Vijayakumar, S. Adsorption of greenhouse gases on the surface of covalent organic framework of porphyrin—An ab initio study. Phys. E Low-Dimens. Syst. Nanostruct. 2021, 126, 114448. [Google Scholar] [CrossRef]
- Bhunia, A.; Boldog, I.; Moeller, A.; Janiak, C. Highly stable nanoporous covalent triazine-based frameworks with an adamantane core for carbon dioxide sorption and separation. J. Mater. Chem. A 2013, 1, 14990–14999. [Google Scholar] [CrossRef]
- Wang, K.; Huang, H.; Liu, D.; Wang, C.; Li, J.; Zhong, C. Covalent Triazine-Based Frameworks with Ultramicropores and High Nitrogen Contents for Highly Selective CO2 Capture. Environ. Sci. Technol. 2016, 50, 4869–4876. [Google Scholar] [CrossRef]
- Wang, L.; Yang, Y.; Liang, H.; Wu, N.; Peng, X.; Wang, L.; Song, Y. A novel N,S-rich COF and its derived hollow N,S-doped carbon@Pd nanorods for electrochemical detection of Hg2+ and paracetamol. J. Hazard. Mater. 2021, 409, 124528. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Biswal, B.P.; Kandambeth, S.; Venkatesh, V.; Kaur, G.; Addicoat, M.; Heine, T.; Verma, S.; Banerjee, R. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem. Sci. 2015, 6, 3931–3939. [Google Scholar] [CrossRef]
- Dalapati, S.; Jin, E.Q.; Addicoat, M.; Heine, T.; Jiang, D.L. Highly Emissive Covalent Organic Frameworks. J. Am. Chem. Soc. 2016, 138, 5797–5800. [Google Scholar] [CrossRef]
- Xiang, Z.H.; Cao, D.P. Synthesis of Luminescent Covalent-Organic Polymers for Detecting Nitroaromatic Explosives and Small Organic Molecules. Macromol. Rapid Commun. 2012, 33, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.N.; Zhan, C.X.; Cao, D.P. Highly sensitive and selective detection of 2,4,6-trinitrophenol using covalent-organic polymer luminescent probes. J. Mater. Chem. A 2015, 3, 92–96. [Google Scholar] [CrossRef]
- Zhang, W.; Qiu, L.G.; Yuan, Y.P.; Xie, A.J.; Shen, Y.H.; Zhu, J.F. Microwave-assisted synthesis of highly fluorescent nanoparticles of a melamine-based porous covalent organic framework for trace-level detection of nitroaromatic explosives. J. Hazard. Mater. 2012, 221, 147–154. [Google Scholar] [CrossRef]
- Cui, C.; Wang, Q.; Xin, C.; Liu, Q.; Deng, X.; Liu, T.; Xu, X.; Zhang, X. Covalent organic framework with bidentate ligand sites as reliable fluorescent sensor for Cu2+. Microporous Mesoporous Mater. 2020, 299, 110122. [Google Scholar] [CrossRef]
- Acharyya, K.; Mukherjee, P.S. A fluorescent organic cage for picric acid detection. Chem. Commun. 2014, 50, 15788–15791. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.L.; Sun, Y.; Huo, R.; Xin, Y.; Bai, F.Y.; Xing, Y.H.; Sun, L.X. Cu-MOF Material Constructed with a Triazine Polycarboxylate Skeleton: Multifunctional Identify and Microdetecting of the Aromatic Diamine Family (o, m, p-Phenylenediamine) Based on the Luminescent Response. Inorg. Chem. 2021, 60, 2829–2838. [Google Scholar] [CrossRef]
- Sun, X.; Wang, N.; Xie, Y.; Chu, H.; Wang, Y.; Wang, Y. In-situ anchoring bimetallic nanoparticles on covalent organic framework as an ultrasensitive electrochemical sensor for levodopa detection. Talanta 2021, 225, 122072. [Google Scholar] [CrossRef] [PubMed]
- Garba, M.D.; Usman, M.; Khan, S.; Shehzad, F.; Galadima, A.; Ehsan, M.F.; Ghanem, A.S.; Humayun, M. CO2 towards fuels: A review of catalytic conversion of carbon dioxide to hydrocarbons. J. Environ. Chem. Eng. 2020, 9, 104756. [Google Scholar] [CrossRef]
- Yaseen, M.; Humayun, M.; Khan, A.; Usman, M.; Ullah, H.; Tahir, A.A. Preparation, Functionalization, Modification, and Applications of Nanostructured Gold: A Critical Review. Energies 2021, 14, 1278. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, H.; Xu, L.; Zhang, H.; Cai, Y. Triazine functionalized fully conjugated covalent organic framework for efficient photocatalysis. Appl. Catal. B Environ. 2020, 269, 118799. [Google Scholar] [CrossRef]
- Hou, Y.; Cui, C.-X.; Zhang, E.; Wang, J.-C.; Li, Y.; Zhang, Y.; Zhang, Y.; Wang, Q.; Jiang, J. A hybrid of g-C3N4 and porphyrin-based covalent organic frameworks via liquid-assisted grinding for enhanced visible-light-driven photoactivity. Dalton Trans. 2019, 48, 14989–14995. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Li, H.; Guan, X.; Zhu, L.; Xue, M.; Yan, Y.; Valtchev, V.; Qiu, S.; Fang, Q. Ambient aqueous-phase synthesis of covalent organic frameworks for degradation of organic pollutants. Chem. Sci. 2019, 10, 10815–10820. [Google Scholar] [CrossRef]
- Lv, H.; Zhao, X.; Niu, H.; He, S.; Tang, Z.; Wu, F.; Giesy, J.P. Ball milling synthesis of covalent organic framework as a highly active photocatalyst for degradation of organic contaminants. J. Hazard. Mater. 2019, 369, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.W.; Liu, J.M.; Li, C.Y.; Zhao, N.; Wang, Z.H.; Wang, S. Two novel MOFs@COFs hybrid-based photocatalytic platforms coupling with sulfate radical-involved advanced oxidation processes for enhanced degradation of bisphenol A. Chemosphere 2020, 243, 125378. [Google Scholar] [CrossRef]
- Preet, K.; Gupta, G.; Kotal, M.; Kansal, S.K.; Salunke, D.B.; Sharma, H.K.; Chandra Sahoo, S.; Van Der Voort, P.; Roy, S. Mechanochemical Synthesis of a New Triptycene-Based Imine-Linked Covalent Organic Polymer for Degradation of Organic Dye. Cryst. Growth Des. 2019, 19, 2525–2530. [Google Scholar] [CrossRef]
- Wang, R.-L.; Li, D.-P.; Wang, L.-J.; Zhang, X.; Zhou, Z.-Y.; Mu, J.-L.; Su, Z.-M. The preparation of new covalent organic framework embedded with silver nanoparticles and its applications in degradation of organic pollutants from waste water. Dalton Trans. 2019, 48, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Hu, Y.; Hu, H.; Chen, L.; Yu, M.; Gao, M.; Wang, S. Metal-free catalysts of graphitic carbon nitride–covalent organic frameworks for efficient pollutant destruction in water. J. Colloid Interface Sci. 2019, 554, 376–387. [Google Scholar] [CrossRef]
- Ma, R.; Liu, N.; Lin, T.-T.; Zhao, T.; Huang, S.-L.; Yang, G.-Y. Anderson polyoxometalate built-in covalent organic frameworks for enhancing catalytic performances. J. Mater. Chem. A 2020, 8, 8548–8553. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, F.; Hao, J.; Ling, Y.; Gong, Y.; Wang, S.; Wei, J.; Yang, Z. Boosting the photocatalytic performances of covalent organic frameworks enabled by spatial modulation of plasmonic nanocrystals. Appl. Catal. B Environ. 2020, 272, 119035. [Google Scholar] [CrossRef]
- Kang, Z.X.; Peng, Y.W.; Qian, Y.H.; Yuan, D.Q.; Addicoat, M.A.; Heine, T.; Hu, Z.G.; Tee, L.; Guo, Z.G.; Zhao, D. Mixed Matrix Membranes (MMMs) Comprising Exfoliated 2D Covalent Organic Frameworks (COFs) for Efficient CO2 Separation. Chem. Mater. 2016, 28, 1277–1285. [Google Scholar] [CrossRef]
- Biswal, B.P.; Chaudhari, H.D.; Banerjee, R.; Kharul, U.K. Chemically Stable Covalent Organic Framework (COF)-Polybenzimidazole Hybrid Membranes: Enhanced Gas Separation through Pore Modulation. Chem. Eur. J. 2016, 22, 4695–4699. [Google Scholar] [CrossRef]
- Liu, Y.T.; Wu, H.; Wu, S.Q.; Song, S.Q.; Guo, Z.Y.; Ren, Y.X.; Zhao, R.; Yang, L.X.; Wu, Y.Z.; Jiang, Z.Y. Multifunctional covalent organic framework (COF)-Based mixed matrix membranes for enhanced CO2 separation. J. Membr. Sci. 2021, 618, 9. [Google Scholar] [CrossRef]
- Xiong, S.H.; Li, L.; Dong, L.; Tang, J.T.; Yu, G.P.; Pan, C.Y. Covalent-organic frameworks (COFs)-based membranes for CO2 separation. J. CO2 Util. 2020, 41, 12. [Google Scholar] [CrossRef]
- Wang, X.; Shi, X.; Wang, Y. In Situ Growth of Cationic Covalent Organic Frameworks (COFs) for Mixed Matrix Membranes with Enhanced Performances. Langmuir 2020, 36, 10970–10978. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Wu, H.; Yao, Z.Q.; Shi, B.B.; Xu, Z.; Cheng, X.X.; Pan, F.S.; Liu, G.H.; Jiang, Z.Y.; Cao, X.Z. Functionally graded membranes from nanoporous covalent organic frameworks for highly selective water permeation. J. Mater. Chem. A 2018, 6, 583–591. [Google Scholar] [CrossRef]
- Xu, L.; Xu, J.; Shan, B.T.; Wang, X.L.; Gao, C.J. TpPa-2-incorporated mixed matrix membranes for efficient water purification. J. Membr. Sci. 2017, 526, 355–366. [Google Scholar] [CrossRef]
- Xu, L.; Yang, T.; Li, M.; Chang, J.; Xu, J. Thin-film nanocomposite membrane doped with carboxylated covalent organic frameworks for efficient forward osmosis desalination. J. Membr. Sci. 2020, 610, 118111. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Yuan, J.; Wu, H.; Cao, L.; Zhang, R.; Liu, Y.; Li, L.; Rahman, A.U.; Kasher, R.; Jiang, Z. Mixed Nanosheet Membranes Assembled from Chemically Grafted Graphene Oxide and Covalent Organic Frameworks for Ultra-high Water Flux. ACS Appl. Mater. Interfaces 2019, 11, 28978–28986. [Google Scholar] [CrossRef] [PubMed]
- Berlanga, I.; Ruiz-González, M.L.; González-Calbet, J.M.; Fierro, J.L.G.; Mas-Ballesté, R.; Zamora, F. Delamination of Layered Covalent Organic Frameworks. Small 2011, 7, 1207–1211. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Kandambeth, S.; Biswal, B.P.; Lukose, B.; Kunjir, S.M.; Chaudhary, M.; Babarao, R.; Heine, T.; Banerjee, R. Chemically Stable Multilayered Covalent Organic Nanosheets from Covalent Organic Frameworks via Mechanical Delamination. J. Am. Chem. Soc. 2013, 135, 17853–17861. [Google Scholar] [CrossRef]
- Yang, H.; Yang, L.; Wang, H.; Xu, Z.; Zhao, Y.; Luo, Y.; Nasir, N.; Song, Y.; Wu, H.; Pan, F.; et al. Covalent organic framework membranes through a mixed-dimensional assembly for molecular separations. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Yao, J.; Liu, C.; Liu, X.; Guo, J.; Zhang, S.; Zheng, J.; Li, S. Azobenzene-assisted exfoliation of 2D covalent organic frameworks into large-area, few-layer nanosheets for high flux and selective molecular separation membrane. J. Membr. Sci. 2020, 601, 117864. [Google Scholar] [CrossRef]
- Li, G.L.; Wang, W.; Fang, Q.L.; Liu, F. Covalent triazine frameworks membrane with highly ordered skeleton nanopores for robust and precise molecule/ion separation. J. Membr. Sci. 2020, 595, 9. [Google Scholar] [CrossRef]
| COFs as Adsorbent Materials | ||||
| Abbreviation | Adsorbed Material | Capacity | Reference | |
| COF@PDA | Cu(II) | 109.2 mg/g | [109] | |
| COF-SH | Hg(II) | 1283 mg/g | [110] | |
| MSCTF-1, MSCTF-2, xSCTF-2 | Hg+2 | 840.5 mg/g | [111] | |
| COF-S-SH | Hg+2 and Hg0 | 1350 and 863 mg/g | [112] | |
| NH2-MIL-125(Ti)@TpPa-1 | UO22+ and Eu3+ | 536.73 and 593.97 mg/g | [113] | |
| Fe3O4@TAPB-COF@ZIF-8 | Bisphenol | Detection limit (0.04 ng/mL) | [114] | |
| TAPT-DHTA | Phenoxy carboxylic acids (PCAs) pesticides | Detection limit (0.007 ng/g) | [115] | |
| MIL-101-NH2@COF | Blue 9 dye | 256 mg/g | [116] | |
| Porphyrin sheets | Greenhouse gases | NA | [117] | |
| PCTF5 | CO2 | CO2 uptake of 58.1 cm3/g at 273 K | [118] | |
| CTF-FUM-350 | CO2 | 57.2 cm3/g at 298 K | [119] | |
| COFs as Sensor Materials | ||||
| Abbreviation | Sensing Materials | Reference | ||
| COF-LZU8 | Hg+2 | [68] | ||
| COFBTT-TZT | Paracetamol | [120] | ||
| TpBDH and TfpBDH | Nitrophenols | [121] | ||
| TPE-Ph COF | Ammonia | [122] | ||
| COP-3 and COP-4 | Nitroaromatic explosives | [123] | ||
| COF-Cage 4 | Picric acid | [127] | ||
| ACOF-TaTp | Gallic acid and uric acid | [128] | ||
| AgCo/TAPB-DMTP-COF | Levodopa | [129] | ||
| COFs as Fillers in Mixed Matrix Membranes | ||||
| Abbreviation | Application | Reference | ||
| COF-Ultem and COF-PBI | CO2 separation | [142] | ||
| TpPa-1@PBI-BuI and TpBD@PBI-BuI | Greenhouse gases separation | [143] | ||
| COF-MMM | CO2/CH4 separation | [144] | ||
| TpEB | BSA removal | [146] | ||
| COF TpHZ | Water/ethanol separation | [147] | ||
| COF (TpPa-2) | Organic foulant removal | [148] | ||
| COF (COF-COOH) | Desalination | [149] | ||
| PA-rCON | Desalination | [80] | ||
| GO-CTN | Dye removal | [151] | ||
| COFs as Active Layers in Separation Membranes | ||||
| Abbreviation | Method of Preparation | Application | Reference | |
| Tp-PA1, TpPA-2 etc | Liquid-liquid interfacial polymerization | Dye separation | [74] | |
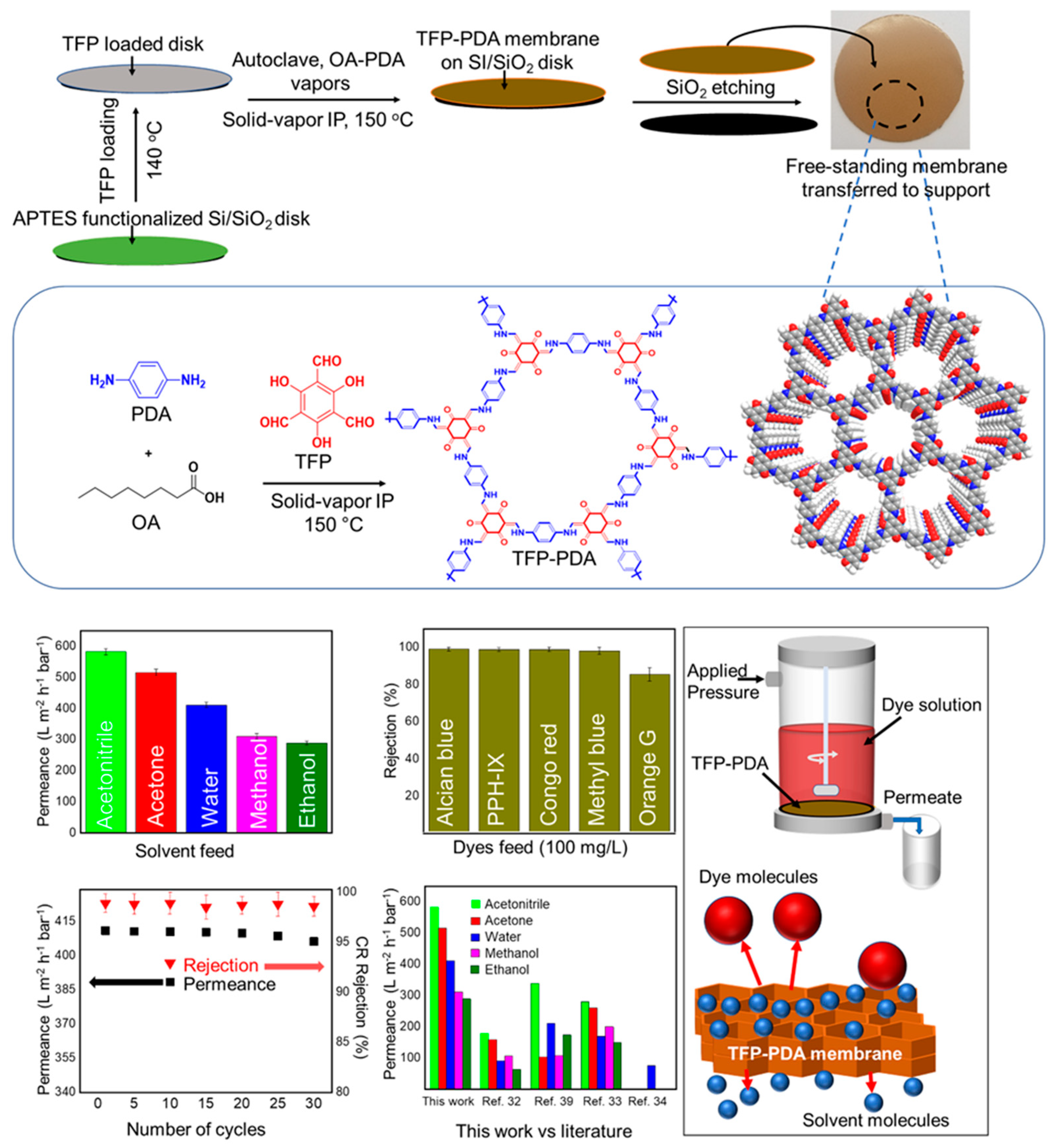
| TFP-PDA | Solid-vapor interfacial polymerization | Dye separation | [75] | |
| COF-LZU1 | In-situ polymerization | Dye separation | [76] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, N.A.; Humayun, M.; Usman, M.; Ghazi, Z.A.; Naeem, A.; Khan, A.; Khan, A.L.; Tahir, A.A.; Ullah, H. Structural Characteristics and Environmental Applications of Covalent Organic Frameworks. Energies 2021, 14, 2267. https://doi.org/10.3390/en14082267
Khan NA, Humayun M, Usman M, Ghazi ZA, Naeem A, Khan A, Khan AL, Tahir AA, Ullah H. Structural Characteristics and Environmental Applications of Covalent Organic Frameworks. Energies. 2021; 14(8):2267. https://doi.org/10.3390/en14082267
Chicago/Turabian StyleKhan, Niaz Ali, Muhammad Humayun, Muhammad Usman, Zahid Ali Ghazi, Abdul Naeem, Abbas Khan, Asim Laeeq Khan, Asif Ali Tahir, and Habib Ullah. 2021. "Structural Characteristics and Environmental Applications of Covalent Organic Frameworks" Energies 14, no. 8: 2267. https://doi.org/10.3390/en14082267
APA StyleKhan, N. A., Humayun, M., Usman, M., Ghazi, Z. A., Naeem, A., Khan, A., Khan, A. L., Tahir, A. A., & Ullah, H. (2021). Structural Characteristics and Environmental Applications of Covalent Organic Frameworks. Energies, 14(8), 2267. https://doi.org/10.3390/en14082267











