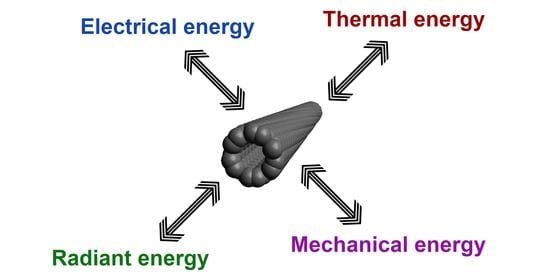
Carbon Nanotube Films for Energy Applications
Abstract
1. Introduction
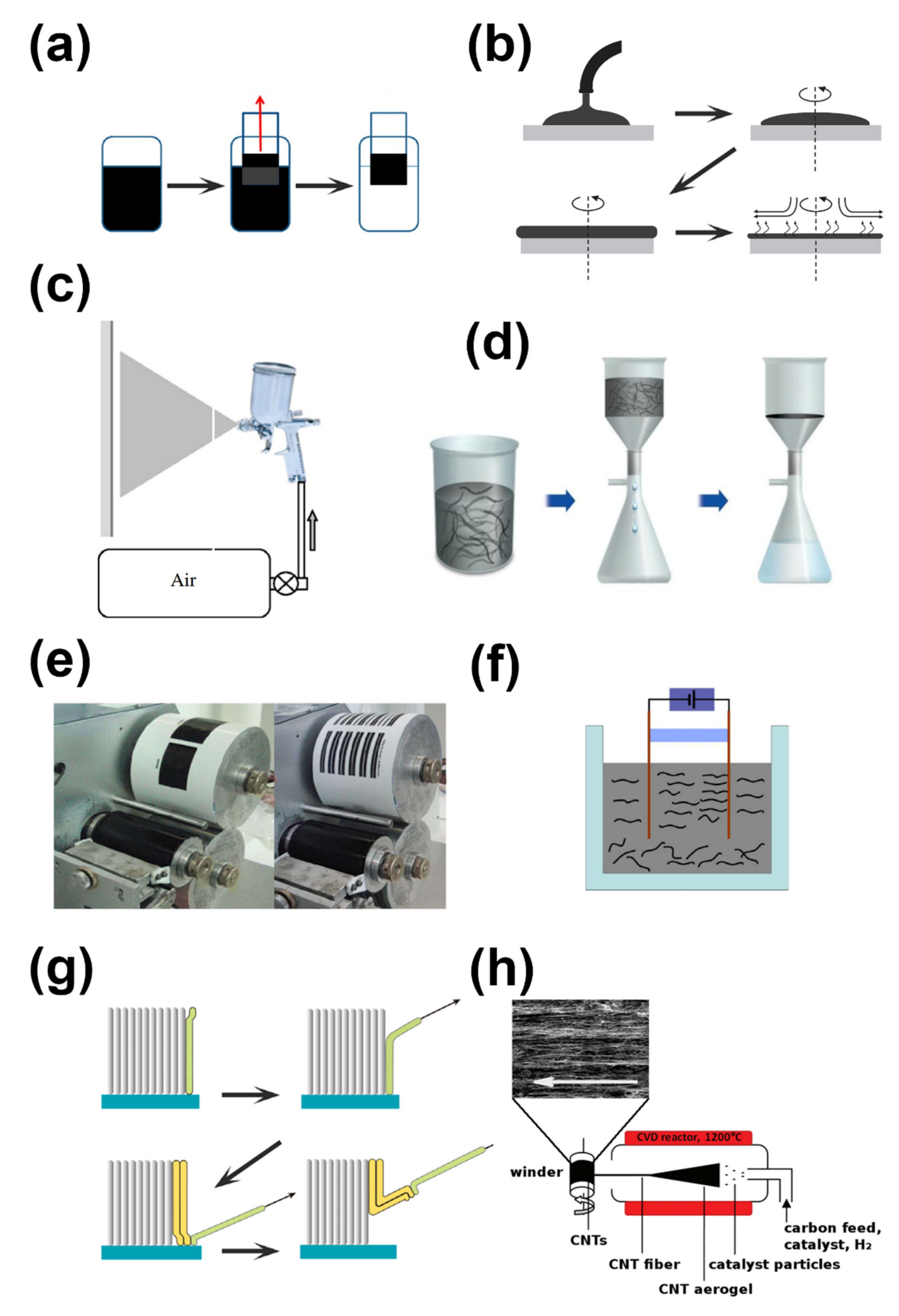
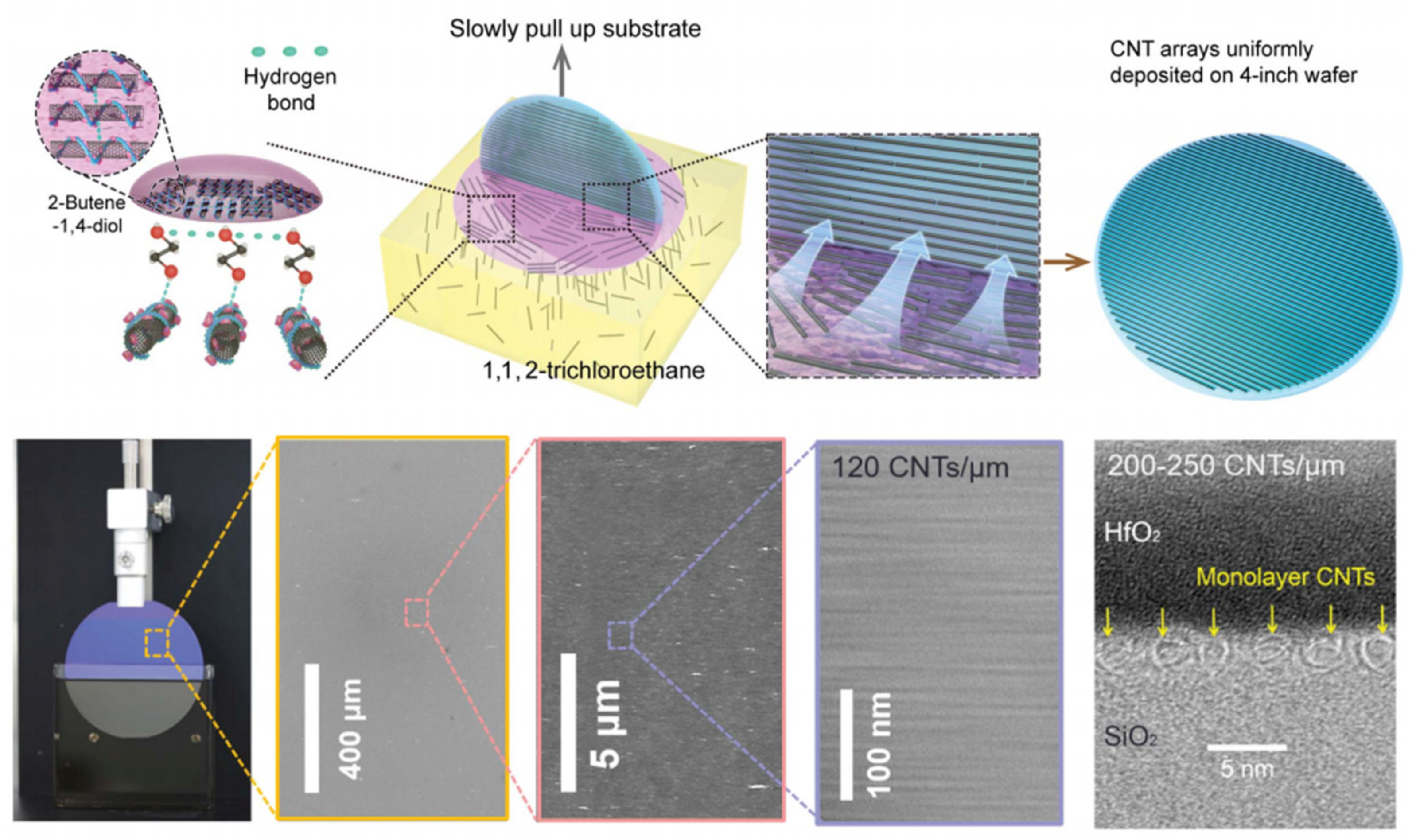
2. Synthesis of CNT Films
3. CNT Films for Energy Applications
3.1. Electrical Energy
3.1.1. Conductive Networks
3.1.2. Electrodes for Electrochemistry
3.1.3. Charge Storage
3.2. Thermal Energy
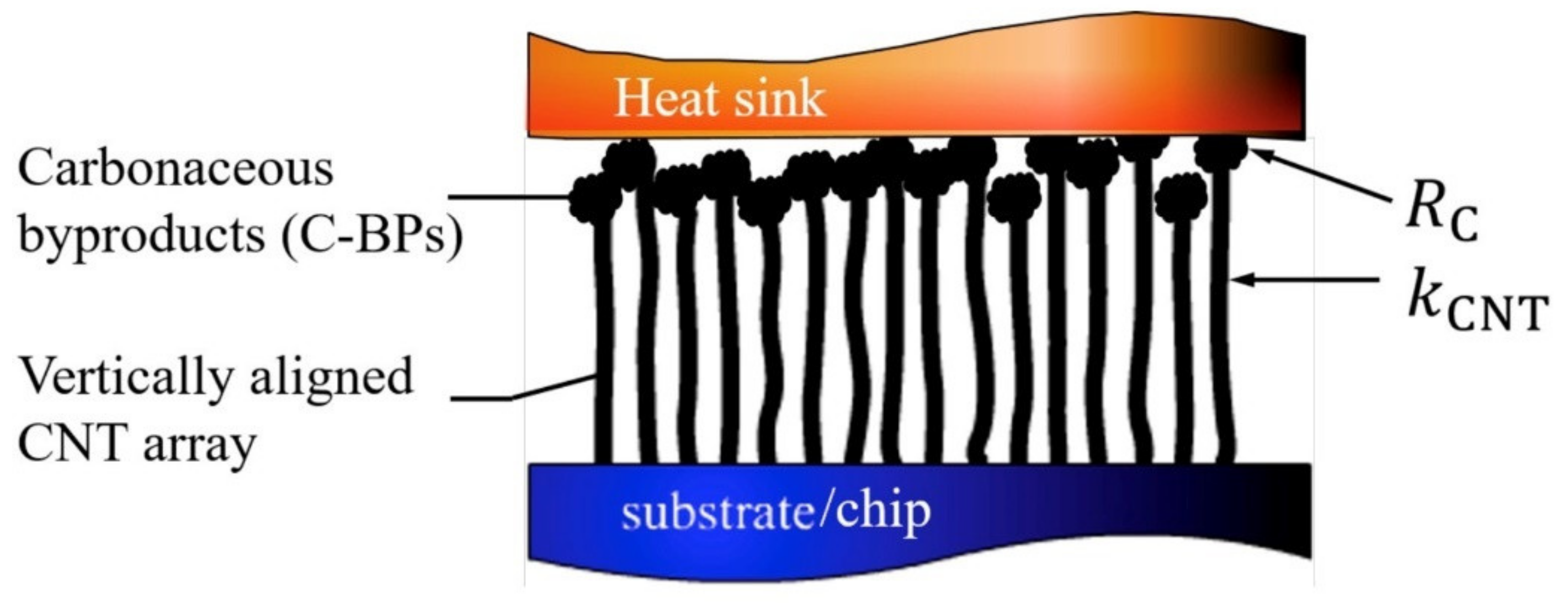
3.2.1. Heat Dissipation
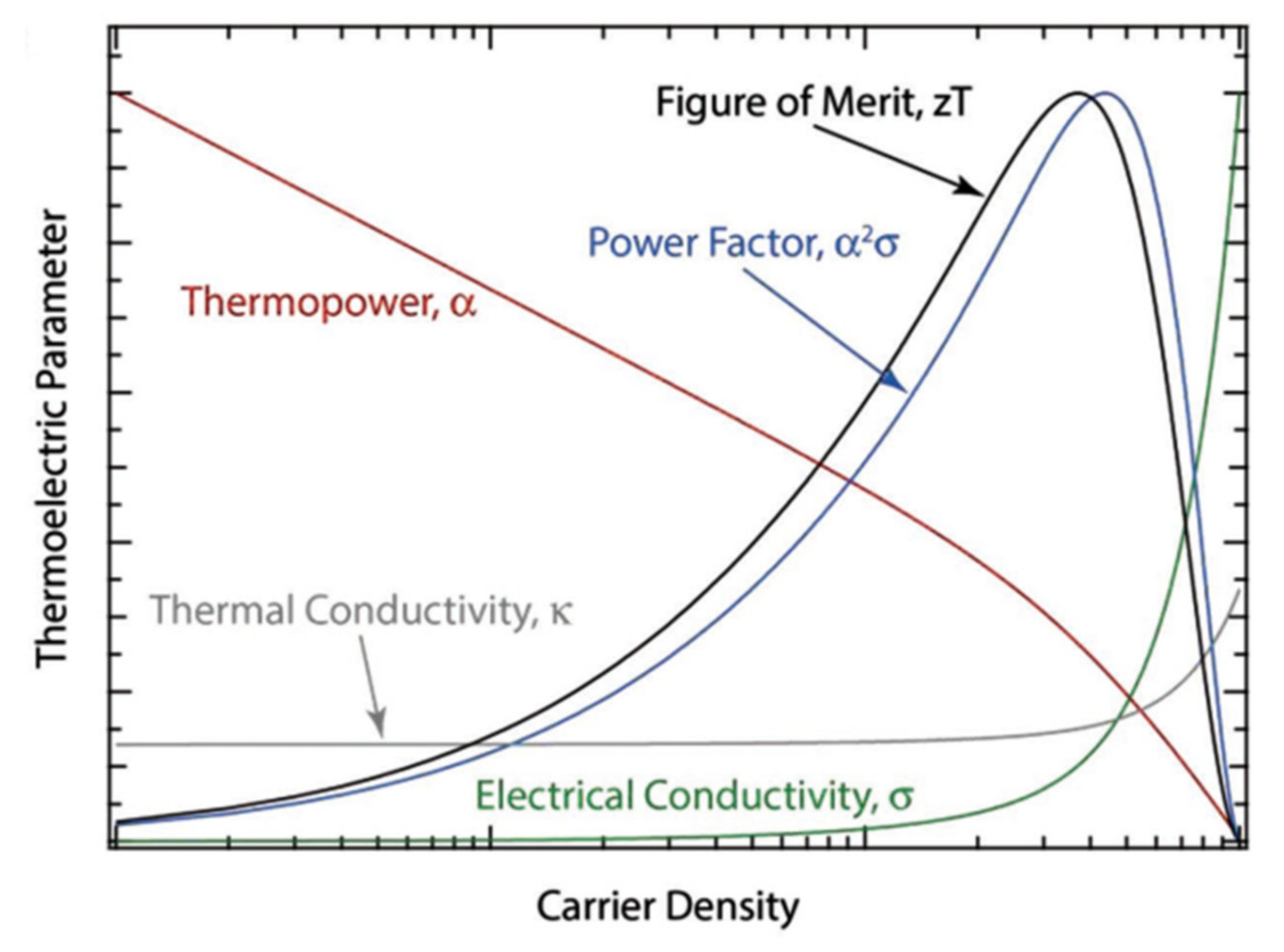
3.2.2. Thermoelectrics
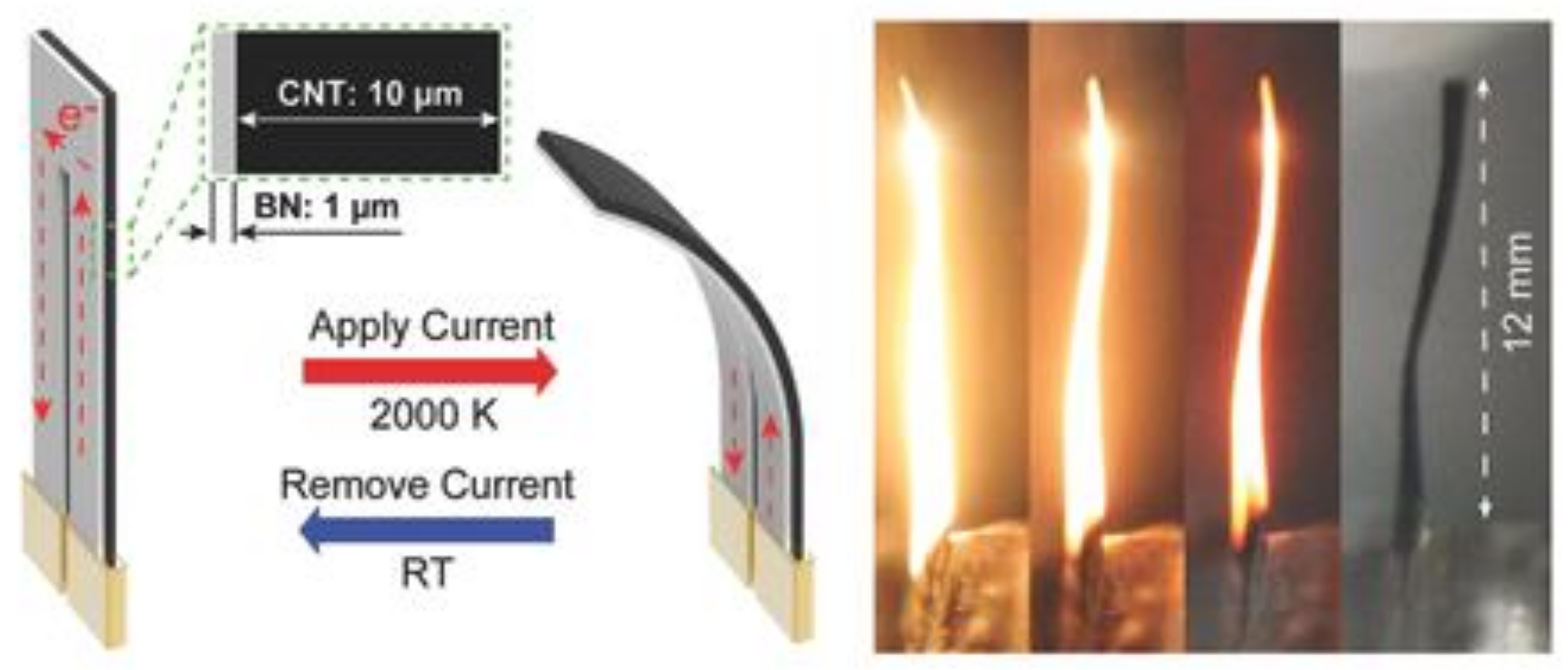
3.2.3. Electrothermics
3.3. Radiant Energy
3.3.1. Solar Energy
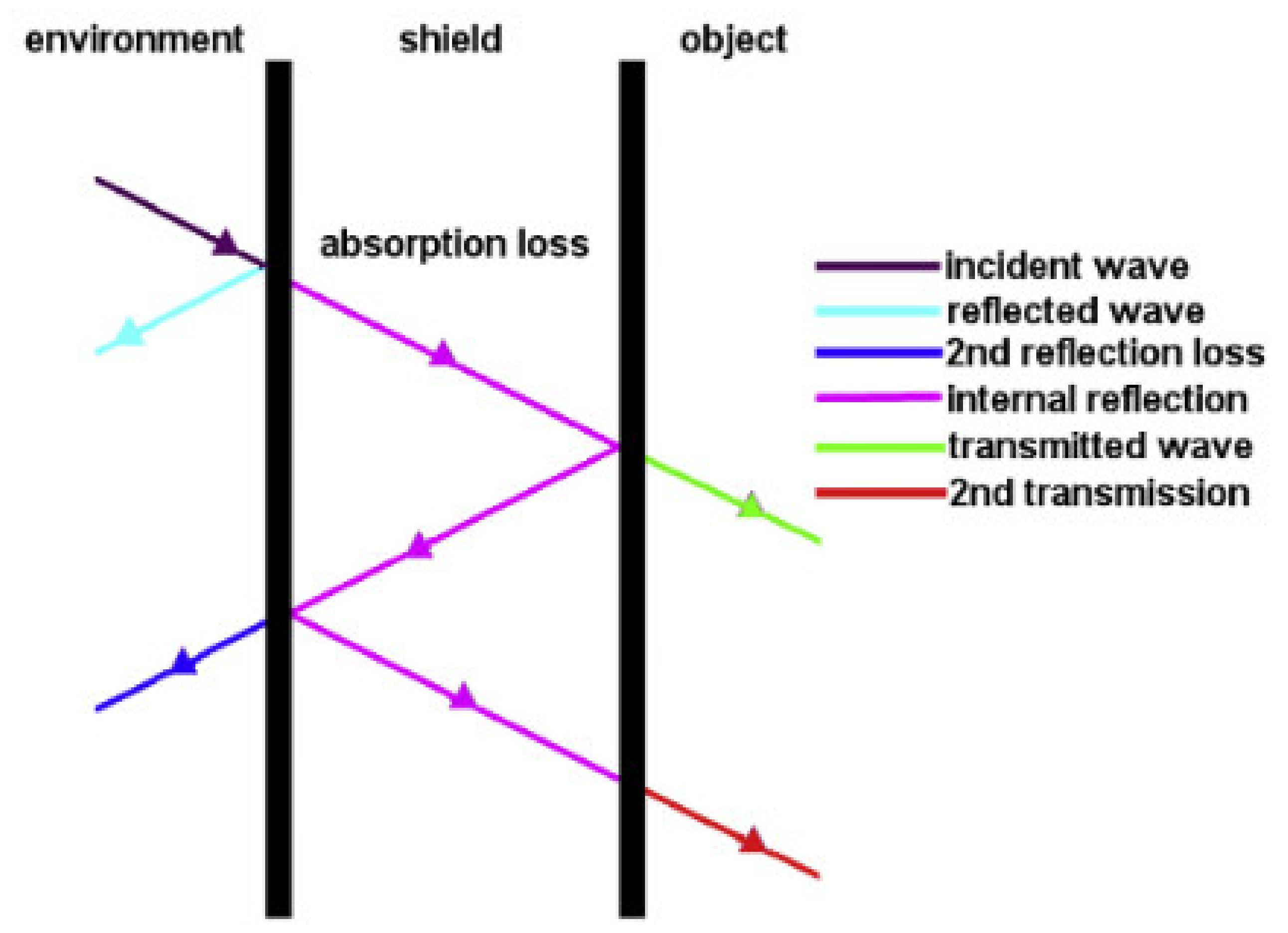
3.3.2. EMI Shielding
3.3.3. Photocatalysis
3.4. Mechanical Energy
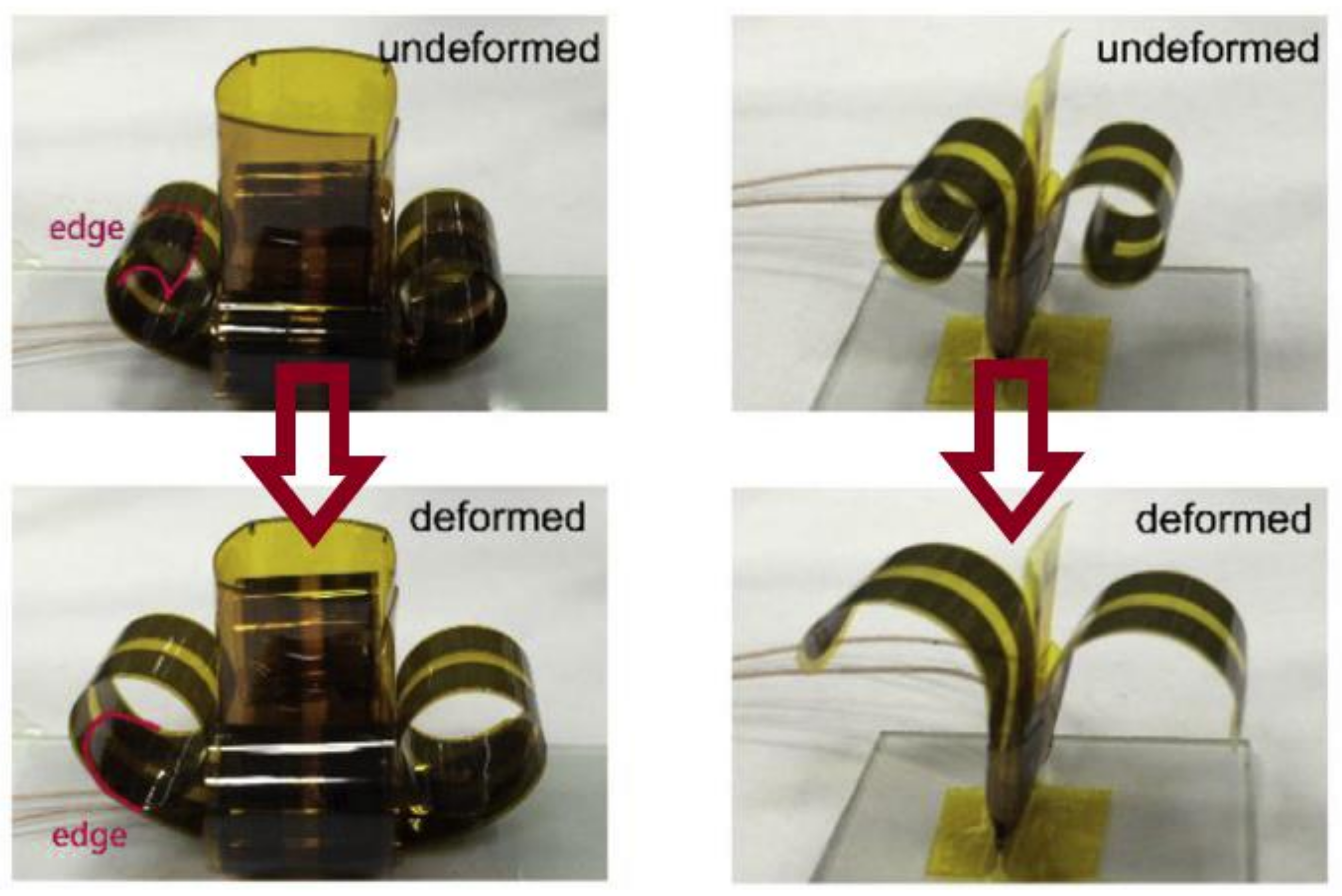
3.4.1. Piezoelectrics
3.4.2. Actuators
4. Conclusions and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ritchie, H.; Roser, M. Energy. Our World in Data. 2014. Available online: https://ourworldindata.org/energy (accessed on 29 March 2021).
- Pakdel, A.; Zhi, C.; Bando, Y.; Golberg, D. Low-Dimensional Boron Nitride Nanomaterials. Mater. Today 2012, 15, 256–265. [Google Scholar] [CrossRef]
- Kufer, D.; Konstantatos, G. Photo-FETs: Phototransistors Enabled by 2D and 0D Nanomaterials. ACS Photonics 2016, 3, 2197–2210. [Google Scholar] [CrossRef]
- Fang, L.; Feng, J.J.; Shi, X.; Si, T.; Song, Y.; Jia, H.; Li, Y.; Li, H.-W.; Zhang, Q. Turning Bulk Materials into 0D, 1D and 2D Metallic Nanomaterials by Selective Aqueous Corrosion. Chem. Commun. 2019, 55, 10476–10479. [Google Scholar] [CrossRef]
- Rajendran, R.; Shrestha, L.K.; Minami, K.; Subramanian, M.; Jayavel, R.; Ariga, K. Dimensionally Integrated Nanoarchitectonics for a Novel Composite from 0D, 1D, and 2D Nanomaterials: RGO/CNT/CeO2 Ternary Nanocomposites with Electrochemical Performance. J. Mater. Chem. A 2014, 2, 18480–18487. [Google Scholar] [CrossRef]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Brady, G.J.; Way, A.J.; Safron, N.S.; Evensen, H.T.; Gopalan, P.; Arnold, M.S. Quasi-Ballistic Carbon Nanotube Array Transistors with Current Density Exceeding Si and GaAs. Sci. Adv. 2016, 2, e1601240. [Google Scholar] [CrossRef] [PubMed]
- Kumanek, B.; Janas, D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Koziol, K.K. A Review of Production Methods of Carbon Nanotube and Graphene Thin Films for Electrothermal Applications. Nanoscale 2014, 6, 3037–3045. [Google Scholar] [CrossRef] [PubMed]
- Lekawa-Raus, A.; Patmore, J.; Kurzepa, L.; Bulmer, J.; Koziol, K. Electrical Properties of Carbon Nanotube Based Fibers and Their Future Use in Electrical Wiring. Adv. Funct. Mater. 2014, 24, 3661–3682. [Google Scholar] [CrossRef]
- Wang, K.; Luo, S.; Wu, Y.; He, X.; Zhao, F.; Wang, J.; Jiang, K.; Fan, S. Super-Aligned Carbon Nanotube Films as Current Collectors for Lightweight and Flexible Lithium Ion Batteries. Adv. Funct. Mater. 2013, 23, 846–853. [Google Scholar] [CrossRef]
- Li, H.; Lu, X.; Yuan, D.; Sun, J.; Erden, F.; Wang, F.; He, C. Lightweight Flexible Carbon Nanotube/Polyaniline Films with Outstanding EMI Shielding Properties. J. Mater. Chem. C 2017, 5, 8694–8698. [Google Scholar] [CrossRef]
- Kanoun, O.; Müller, C.; Benchirouf, A.; Sanli, A.; Dinh, T.N.; Al-Hamry, A.; Bu, L.; Gerlach, C.; Bouhamed, A. Flexible Carbon Nanotube Films for High Performance Strain Sensors. Sensors 2014, 14, 10042–10071. [Google Scholar] [CrossRef]
- Janas, D.; Vilatela, A.C.; Koziol, K.K.K. Performance of Carbon Nanotube Wires in Extreme Conditions. Carbon 2013, 62, 438–446. [Google Scholar] [CrossRef]
- Janas, D.; Cabrero-Vilatela, A.; Bulmer, J.; Kurzepa, L.; Koziol, K.K. Carbon Nanotube Wires for High-Temperature Performance. Carbon 2013, 64, 305–314. [Google Scholar] [CrossRef]
- Janas, D. From Bio to Nano: A Review of Sustainable Methods of Synthesis of Carbon Nanotubes. Sustainability 2020, 12, 4115. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon Nanotubes: A Potential Material for Energy Conversion and Storage. Prog. Energy Combust. Sci. 2018, 64, 219–253. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Wang, Y.; Zhang, Q. Roles of Carbon Nanotubes in Novel Energy Storage Devices. Carbon 2017, 122, 462–474. [Google Scholar] [CrossRef]
- Yu, D.; Goh, K.; Wang, H.; Wei, L.; Jiang, W.; Zhang, Q.; Dai, L.; Chen, Y. Scalable Synthesis of Hierarchically Structured Carbon Nanotube–Graphene Fibres for Capacitive Energy Storage. Nat. Nanotechnol. 2014, 9, 555–562. [Google Scholar] [CrossRef]
- Zhou, Y.; Azumi, R. Carbon Nanotube Based Transparent Conductive Films: Progress, Challenges, and Perspectives. Sci. Technol. Adv. Mater. 2016, 17, 493–516. [Google Scholar] [CrossRef]
- Yu, L.; Shearer, C.; Shapter, J. Recent Development of Carbon Nanotube Transparent Conductive Films. Chem. Rev. 2016, 116, 13413–13453. [Google Scholar] [CrossRef] [PubMed]
- Rossell, M.D.; Kuebel, C.; Ilari, G.; Rechberger, F.; Heiligtag, F.J.; Niederberger, M.; Koziej, D.; Erni, R. Impact of Sonication Pretreatment on Carbon Nanotubes: A Transmission Electron Microscopy Study. Carbon 2013, 61, 404–411. [Google Scholar] [CrossRef]
- Graf, A.; Zakharko, Y.; Schießl, S.P.; Backes, C.; Pfohl, M.; Flavel, B.S.; Zaumseil, J. Large Scale, Selective Dispersion of Long Single-Walled Carbon Nanotubes with High Photoluminescence Quantum Yield by Shear Force Mixing. Carbon 2016, 105, 593–599. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical Oxidation of Multiwalled Carbon Nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Singer, G.; Siedlaczek, P.; Sinn, G.; Rennhofer, H.; Mičušík, M.; Omastová, M.; Unterlass, M.M.; Wendrinsky, J.; Milotti, V.; Fedi, F.; et al. Acid Free Oxidation and Simple Dispersion Method of MWCNT for High-Performance CFRP. Nanomaterials 2018, 8, 912. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, L. Dispersion of Multiwall Carbon Nanotubes by Sodium Dodecyl Sulfate for Preparation of Modified Electrodes toward Detecting Hydrogen Peroxide. Mater. Lett. 2007, 61, 3571–3574. [Google Scholar] [CrossRef]
- Yang, K.; Yi, Z.; Jing, Q.; Yue, R.; Jiang, W.; Lin, D. Sonication-Assisted Dispersion of Carbon Nanotubes in Aqueous Solutions of the Anionic Surfactant SDBS: The Role of Sonication Energy. Chin. Sci. Bull. 2013, 58, 2082–2090. [Google Scholar] [CrossRef]
- Keinänen, P.; Siljander, S.; Koivula, M.; Sethi, J.; Sarlin, E.; Vuorinen, J.; Kanerva, M. Optimized Dispersion Quality of Aqueous Carbon Nanotube Colloids as a Function of Sonochemical Yield and Surfactant/CNT Ratio. Heliyon 2018, 4, e00787. [Google Scholar] [CrossRef]
- Parveen, S.; Rana, S.; Fangueiro, R.; Paiva, M.C. Characterizing Dispersion and Long Term Stability of Concentrated Carbon Nanotube Aqueous Suspensions for Fabricating Ductile Cementitious Composites. Powder Technol. 2017, 307, 1–9. [Google Scholar] [CrossRef]
- Mirri, F.; Ma, A.W.K.; Hsu, T.T.; Behabtu, N.; Eichmann, S.L.; Young, C.C.; Tsentalovich, D.E.; Pasquali, M. High-Performance Carbon Nanotube Transparent Conductive Films by Scalable Dip Coating. ACS Nano 2012, 6, 9737–9744. [Google Scholar] [CrossRef]
- Song, Y.I.; Kim, G.Y.; Choi, H.K.; Jeong, H.J.; Kim, K.K.; Yang, C.-M.; Lim, S.C.; An, K.H.; Jung, K.T.; Lee, Y.H. Fabrication of Carbon Nanotube Field Emitters Using a Dip-Coating Method. Chem. Vap. Depos. 2006, 12, 375–379. [Google Scholar] [CrossRef]
- Kim, P.; Kang, T.J. Large-Area Fluidic Assembly of Single-Walled Carbon Nanotubes through Dip-Coating and Directional Evaporation. Micro Nano Syst. Lett. 2017, 5, 18. [Google Scholar] [CrossRef]
- Kang, T.J.; Yoon, J.-W.; Kim, D.-I.; Kum, S.S.; Huh, Y.-H.; Hahn, J.-H.; Moon, S.H.; Lee, H.-Y.; Kim, Y.H. Sandwich-Type Laminated Nanocomposites Developed by Selective Dip-Coating of Carbon Nanotubes. Adv. Mater. 2007, 19, 427–432. [Google Scholar] [CrossRef]
- Spotnitz, M.E.; Ryan, D.; Stone, H.A. Dip Coating for the Alignment of Carbon Nanotubes on Curved Surfaces. J. Mater. Chem. 2004, 14, 1299–1302. [Google Scholar] [CrossRef]
- Pyo, S.; Jo, E.; Kwon, D.; Kim, W.; Chang, W.; Kim, J. Fabrication of Carbon Nanotube-Coated Fabric for Highly Sensitive Pressure Sensor. In Proceedings of the 2017 19th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Kaohsiung, Taiwan, 18–22 June 2017; pp. 962–965. [Google Scholar]
- Tchoul, M.N.; Ford, W.T.; Ha, M.L.P.; Chavez-Sumarriva, I.; Grady, B.P.; Lolli, G.; Resasco, D.E.; Arepalli, S. Composites of Single-Walled Carbon Nanotubes and Polystyrene: Preparation and Electrical Conductivity. Chem. Mater. 2008, 20, 3120–3126. [Google Scholar] [CrossRef]
- Song, Y.I.; Yang, C.-M.; Kim, D.Y.; Kanoh, H.; Kaneko, K. Flexible Transparent Conducting Single-Wall Carbon Nanotube Film with Network Bridging Method. J. Colloid Interface Sci. 2008, 318, 365–371. [Google Scholar] [CrossRef] [PubMed]
- WU, B.; BAI, L. Effect of Non-Ionic Surfactants on the Dispersion of Multiwalled Carbon Nanotubes at High Loading in Ethanol. Acta Phys. Chim. Sin. 2009, 25, 1065–1069. [Google Scholar]
- Meyer, F.; Minoia, A.; Raquez, J.M.; Spasova, M.; Lazzaroni, R.; Dubois, P. Poly(Amino-Methacrylate) as Versatile Agent for Carbon Nanotube Dispersion: An Experimental, Theoretical and Application Study. J. Mater. Chem. 2010, 20, 6873–6880. [Google Scholar] [CrossRef]
- Liu, L.; Han, J.; Xu, L.; Zhou, J.; Zhao, C.; Ding, S.; Shi, H.; Xiao, M.; Ding, L.; Ma, Z.; et al. Aligned, High-Density Semiconducting Carbon Nanotube Arrays for High-Performance Electronics. Science 2020, 368, 850–856. [Google Scholar] [CrossRef]
- Kerdcharoen, T.; Wongchoosuk, C. 11—Carbon nanotube and metal oxide hybrid materials for gas sensing. In Semiconductor Gas Sensors; Jaaniso, R., Tan, O.K., Eds.; Woodhead Publishing Series in Electronic and Optical Materials; Woodhead Publishing: Cambridge, UK; Sawston, UK, 2013; pp. 386–407. ISBN 978-0-85709-236-6. [Google Scholar]
- Park, J.-M.; Wang, Z.-J.; Kwon, D.-J.; Gu, G.-Y.; Lawrence DeVries, K. Electrical Properties of Transparent CNT and ITO Coatings on PET Substrate Including Nano-Structural Aspects. Solid-State Electron. 2013, 79, 147–151. [Google Scholar] [CrossRef]
- Cha, J.E.; Kim, S.Y.; Lee, S.H. Effect of Continuous Multi-Walled Carbon Nanotubes on Thermal and Mechanical Properties of Flexible Composite Film. Nanomaterials 2016, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Wetzold, N.; Elsner, H.; Kroll, L.; Hübler, A.C. Carbon Nanotube Areas—Printed on Textile and Paper Substrates. Nanomater. Nanotechnol. 2011, 1, 3. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Cho, J.; Roether, J.A.; Thomas, B.J.C.; Jane Minay, E.; Shaffer, M.S.P. Electrophoretic Deposition of Carbon Nanotubes. Carbon 2006, 44, 3149–3160. [Google Scholar] [CrossRef]
- Liu, K.; Sun, Y.; Liu, P.; Wang, J.; Li, Q.; Fan, S.; Jiang, K. Periodically Striped Films Produced from Super-Aligned Carbon Nanotube Arrays. Nanotechnology 2009, 20, 335705. [Google Scholar] [CrossRef] [PubMed]
- Janas, D.; Koziol, K.K. Carbon Nanotube Fibers and Films: Synthesis, Applications and Perspectives of the Direct-Spinning Method. Nanoscale 2016, 8, 19475–19490. [Google Scholar] [CrossRef]
- Kymakis, E.; Stylianakis, M.M.; Spyropoulos, G.D.; Stratakis, E.; Koudoumas, E.; Fotakis, C. Spin Coated Carbon Nanotubes as the Hole Transport Layer in Organic Photovoltaics. Sol. Energy Mater. Sol. Cells 2012, 96, 298–301. [Google Scholar] [CrossRef]
- Jo, J.W.; Jung, J.W.; Lee, J.U.; Jo, W.H. Fabrication of Highly Conductive and Transparent Thin Films from Single-Walled Carbon Nanotubes Using a New Non-Ionic Surfactant via Spin Coating. ACS Nano 2010, 4, 5382–5388. [Google Scholar] [CrossRef] [PubMed]
- Holubowitch, N.E.; Landon, J.; Lippert, C.A.; Craddock, J.D.; Weisenberger, M.C.; Liu, K. Spray-Coated Multiwalled Carbon Nanotube Composite Electrodes for Thermal Energy Scavenging Electrochemical Cells. ACS Appl. Mater. Interfaces 2016, 8, 22159–22167. [Google Scholar] [CrossRef]
- Tuukkanen, S.; Välimäki, M.; Lehtimäki, S.; Vuorinen, T.; Lupo, D. Behaviour of One-Step Spray-Coated Carbon Nanotube Supercapacitor in Ambient Light Harvester Circuit with Printed Organic Solar Cell and Electrochromic Display. Sci. Rep. 2016, 6, 22967. [Google Scholar] [CrossRef]
- Park, C.; Kim, S.W.; Lee, Y.-S.; Lee, S.H.; Song, K.H.; Park, L.S. Spray Coating of Carbon Nanotube on Polyethylene Terephthalate Film for Touch Panel Application. J. Nanosci. Nanotechnol. 2012, 12, 5351–5355. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.; Lee, K.; Choi, E.; Kim, A.; Lee, S.-B. Spray-Coated Carbon Nanotube Thin-Film Transistors with Striped Transport Channels. Nanotechnology 2012, 23, 505203. [Google Scholar] [CrossRef]
- Tutak, W.; Chhowalla, M.; Sesti, F. The Chemical and Physical Characteristics of Single-Walled Carbon Nanotube Film Impact on Osteoblastic Cell Response. Nanotechnology 2010, 21, 315102. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.K.; Ortiz, R.P.; Alaboson, J.M.P.; Emery, J.D.; Bedzyk, M.J.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Fundamental Performance Limits of Carbon Nanotube Thin-Film Transistors Achieved Using Hybrid Molecular Dielectrics. ACS Nano 2012, 6, 7480–7488. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, D.; Peng, H.-X. A Pressurized Filtration Technique for Fabricating Carbon Nanotube Buckypaper: Structure, Mechanical and Conductive Properties. Microporous Mesoporous Mater. 2014, 184, 127–133. [Google Scholar] [CrossRef]
- Rojas, J.A.; Ardila-Rodríguez, L.A.; Diniz, M.F.; Gonçalves, M.; Ribeiro, B.; Rezende, M.C. Highly Porous Multiwalled Carbon Nanotube Buckypaper Using Electrospun Polyacrylonitrile Nanofiber as a Sacrificial Material. Heliyon 2019, 5, e01386. [Google Scholar] [CrossRef]
- Xu, G.-H.; Zhang, Q.; Huang, J.-Q.; Zhao, M.-Q.; Zhou, W.-P.; Wei, F. A Two-Step Shearing Strategy to Disperse Long Carbon Nanotubes from Vertically Aligned Multiwalled Carbon Nanotube Arrays for Transparent Conductive Films. Langmuir 2010, 26, 2798–2804. [Google Scholar] [CrossRef]
- He, X.; Gao, W.; Xie, L.; Li, B.; Zhang, Q.; Lei, S.; Robinson, J.M.; Hároz, E.H.; Doorn, S.K.; Wang, W.; et al. Wafer-Scale Monodomain Films of Spontaneously Aligned Single-Walled Carbon Nanotubes. Nat. Nanotechnol. 2016, 11, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Andrews, J.B.; Kumar, A.; Franklin, A.D. Improving Contact Interfaces in Fully Printed Carbon Nanotube Thin-Film Transistors. ACS Nano 2016, 10, 5221–5229. [Google Scholar] [CrossRef]
- Lakshmanan, S.; Kanwal, A.; Liu, S.; Patlolla, A.; Iqbal, Z.; Mitra, S.; Thomas, G.A.; Fagan, J.A.; Farrow, R.C. Improved Electrophoretic Deposition of Vertical Single Wall Carbon Nanotubes with Nanoscopic Electrostatic Lenses. Micromachines 2020, 11, 324. [Google Scholar] [CrossRef]
- Thomas, B.J.C.; Shaffer, M.S.P.; Freeman, S.; Koopman, M.; Chawla, K.K.; Boccaccini, A.R. Electrophoretic Deposition of Carbon Nanotubes on Metallic Surfaces. Available online: https://www.scientific.net/KEM.314.141 (accessed on 4 February 2021).
- Choi, W.B.; Jin, Y.W.; Kim, H.Y.; Lee, S.J.; Yun, M.J.; Kang, J.H.; Choi, Y.S.; Park, N.S.; Lee, N.S.; Kim, J.M. Electrophoresis Deposition of Carbon Nanotubes for Triode-Type Field Emission Display. Appl. Phys. Lett. 2001, 78, 1547–1549. [Google Scholar] [CrossRef]
- Qingliang, S.; Hejun, L.; Fengling, Z.; Qiang, S.; Qiangang, F. Electrophoretic Deposition of Carbon Nanotubes for Improved Ablation Resistance of Carbon/Carbon Composites. Corros. Sci. 2018, 132, 204–213. [Google Scholar] [CrossRef]
- Pöhls, J.-H.; Johnson, M.B.; White, M.A.; Malik, R.; Ruff, B.; Jayasinghe, C.; Schulz, M.J.; Shanov, V. Physical Properties of Carbon Nanotube Sheets Drawn from Nanotube Arrays. Carbon 2012, 50, 4175–4183. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, K.; Feng, C.; Liu, P.; Zhang, L.; Kong, J.; Zhang, T.; Li, Q.; Fan, S. Spinning and Processing Continuous Yarns from 4-Inch Wafer Scale Super-Aligned Carbon Nanotube Arrays. Adv. Mater. 2006, 18, 1505–1510. [Google Scholar] [CrossRef]
- Li, Y.-L.; Kinloch, I.A.; Windle, A.H. Direct Spinning of Carbon Nanotube Fibers from Chemical Vapor Deposition Synthesis. Science 2004, 304, 276–278. [Google Scholar] [CrossRef]
- Janas, D.; Milowska, K.Z.; Bristowe, P.D.; Koziol, K.K. Improving the Electrical Properties of Carbon Nanotubes with Interhalogen Compounds. Nanoscale 2017, 9, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Lepak-Kuc, S.; Milowska, K.Z.; Boncel, S.; Szybowicz, M.; Dychalska, A.; Jozwik, I.; Koziol, K.K.; Jakubowska, M.; Lekawa-Raus, A. Highly Conductive Doped Hybrid Carbon Nanotube–Graphene Wires. ACS Appl. Mater. Interfaces 2019, 11, 33207–33220. [Google Scholar] [CrossRef]
- Tran, T.Q.; Fan, Z.; Liu, P.; Myint, S.M.; Duong, H.M. Super-Strong and Highly Conductive Carbon Nanotube Ribbons from Post-Treatment Methods. Carbon 2016, 99, 407–415. [Google Scholar] [CrossRef]
- Clancy, A.J.; White, E.R.; Tay, H.H.; Yau, H.C.; Shaffer, M.S.P. Systematic Comparison of Conventional and Reductive Single-Walled Carbon Nanotube Purifications. Carbon 2016, 108, 423–432. [Google Scholar] [CrossRef]
- Hansson, J.; Nylander, A.; Flygare, M.; Svensson, K.; Ye, L.; Nilsson, T.; Fu, Y.; Liu, J. Effects of High Temperature Treatment of Carbon Nanotube Arrays on Graphite: Increased Crystallinity, Anchoring and Inter-Tube Bonding. Nanotechnology 2020, 31, 455708. [Google Scholar] [CrossRef] [PubMed]
- Mattia, D.; Rossi, M.P.; Kim, B.M.; Korneva, G.; Bau, H.H.; Gogotsi, Y. Effect of Graphitization on the Wettability and Electrical Conductivity of CVD-Carbon Nanotubes and Films. J. Phys. Chem. B 2006, 110, 9850–9855. [Google Scholar] [CrossRef] [PubMed]
- Janas, D. Towards Monochiral Carbon Nanotubes: A Review of Progress in the Sorting of Single-Walled Carbon Nanotubes. Mater. Chem. Front. 2018, 2, 36–63. [Google Scholar] [CrossRef]
- Fagan, J.A. Aqueous Two-Polymer Phase Extraction of Single-Wall Carbon Nanotubes Using Surfactants. Nanoscale Adv. 2019, 1, 3307–3324. [Google Scholar] [CrossRef]
- Janas, D.; Koziol, K.K. Rapid Electrothermal Response of High-Temperature Carbon Nanotube Film Heaters. Carbon 2013, 59, 457–463. [Google Scholar] [CrossRef]
- Hou, G.; Wang, G.; Deng, Y.; Zhang, J.; Nshimiyimana, J.P.; Chi, X.; Hu, X.; Chu, W.; Dong, H.; Zhang, Z.; et al. Effective Enhancement of the Mechanical Properties of Macroscopic Single-Walled Carbon Nanotube Fibers by Pressure Treatment. RSC Adv. 2016, 6, 97012–97017. [Google Scholar] [CrossRef]
- Wang, J.N.; Luo, X.G.; Wu, T.; Chen, Y. High-Strength Carbon Nanotube Fibre-like Ribbon with High Ductility and High Electrical Conductivity. Nat. Commun. 2014, 5, 3848. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Tan, Y.F.; Hu, D.C.M.; Jewell, D.; Duong, H.M. Multi-Property Enhancement of Aligned Carbon Nanotube Thin Films from Floating Catalyst Method. Mater. Des. 2016, 108, 754–760. [Google Scholar] [CrossRef]
- Han, B.; Xue, X.; Xu, Y.; Zhao, Z.; Guo, E.; Liu, C.; Luo, L.; Hou, H. Preparation of Carbon Nanotube Film with High Alignment and Elevated Density. Carbon 2017, 122, 496–503. [Google Scholar] [CrossRef]
- Zhou, W.; Vavro, J.; Nemes, N.M.; Fischer, J.E.; Borondics, F.; Kamaras, K.; Tanner, D. Charge Transfer and Fermi Level Shift in P-Doped Single-Walled Carbon Nanotubes. Phys. Rev. B 2005, 71, 205423. [Google Scholar] [CrossRef]
- Kamarás, K.; Pekker, Á.; Botka, B.; Hu, H.; Niyogi, S.; Itkis, M.E.; Haddon, R.C. The Effect of Nitric Acid Doping on the Optical Properties of Carbon Nanotube Films. Phys. Status Solidi (B) 2010, 247, 2754–2757. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, J.; Vajtai, R.; Ajayan, P.M.; Barrera, E.V. Iodine Doped Carbon Nanotube Cables Exceeding Specific Electrical Conductivity of Metals. Sci. Rep. 2011, 1, 83. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.S.; Kim, H.J.; Fischer, J.E.; Thess, A.; Smalley, R.E. Conductivity Enhancement in Single-Walled Carbon Nanotube Bundles Doped with K and Br. Nature 1997, 388, 255–257. [Google Scholar] [CrossRef]
- Schmid, M.; Goze-Bac, C.; Krämer, S.; Roth, S.; Mehring, M.; Mathis, C.; Petit, P. Metallic Properties of Li-Intercalated Carbon Nanotubes Investigated by NMR. Phys. Rev. B 2006, 74, 073416. [Google Scholar] [CrossRef]
- Klinke, C.; Chen, J.; Afzali, A.; Avouris, P. Charge Transfer Induced Polarity Switching in Carbon Nanotube Transistors. Nano Lett. 2005, 5, 555–558. [Google Scholar] [CrossRef] [PubMed]
- Ayala, P.; Arenal, R.; Rümmeli, M.; Rubio, A.; Pichler, T. The Doping of Carbon Nanotubes with Nitrogen and Their Potential Applications. Carbon 2010, 48, 575–586. [Google Scholar] [CrossRef]
- Janas, D.; Herman, A.P.; Boncel, S.; Koziol, K.K.K. Iodine Monochloride as a Powerful Enhancer of Electrical Conductivity of Carbon Nanotube Wires. Carbon 2014, 73, 225–233. [Google Scholar] [CrossRef]
- Wang, X.; Behabtu, N.; Young, C.C.; Tsentalovich, D.E.; Pasquali, M.; Kono, J. High-Ampacity Power Cables of Tightly-Packed and Aligned Carbon Nanotubes. Adv. Funct. Mater. 2014, 24, 3241–3249. [Google Scholar] [CrossRef]
- Mokry, G.; Pozuelo, J.; Vilatela, J.J.; Sanz, J.; Baselga, J. High Ampacity Carbon Nanotube Materials. Nanomaterials 2019, 9, 383. [Google Scholar] [CrossRef]
- Subramaniam, C.; Yamada, T.; Kobashi, K.; Sekiguchi, A.; Futaba, D.N.; Yumura, M.; Hata, K. One Hundred Fold Increase in Current Carrying Capacity in a Carbon Nanotube–Copper Composite. Nat. Commun. 2013, 4, 2202. [Google Scholar] [CrossRef]
- Hong, S.; Myung, S. A Flexible Approach to Mobility. Nat. Nanotechnol. 2007, 2, 207–208. [Google Scholar] [CrossRef]
- Park, J.G.; Li, S.; Liang, R.; Fan, X.; Zhang, C.; Wang, B. The High Current-Carrying Capacity of Various Carbon Nanotube-Based Buckypapers. Nanotechnology 2008, 19, 185710. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, A.G. The Controversial Carbon Solid–Liquid–Vapour Triple Point. Nature 1978, 276, 695–696. [Google Scholar] [CrossRef]
- Cahill, J.A.; Kirshenbaum, A.D. The Density of liquid copper from its melting point (1356°k.) to 2500°k. and an estimate of its critical constants1,2. J. Phys. Chem. 1962, 66, 1080–1082. [Google Scholar] [CrossRef]
- Li, C.; Liu, M.; Ding, H.; He, L.; Wang, E.; Wang, B.; Fan, S.; Liu, K. A Lightly Fe-Doped (NiS2/MoS2)/Carbon Nanotube Hybrid Electrocatalyst Film with Laser-Drilled Micropores for Stabilized Overall Water Splitting and PH-Universal Hydrogen Evolution Reaction. J. Mater. Chem. A 2020, 8, 17527–17536. [Google Scholar] [CrossRef]
- Reuillard, B.; Warnan, J.; Leung, J.J.; Wakerley, D.W.; Reisner, E. A Poly(Cobaloxime)/Carbon Nanotube Electrode: Freestanding Buckypaper with Polymer-Enhanced H2-Evolution Performance. Angew. Chem. Int. Ed. 2016, 55, 3952–3957. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.M.; Jensen, K.; Waje, M.; Li, W.; Larsen, P.; Pauley, K.; Chen, Z.; Ramesh, P.; Itkis, M.E.; Yan, Y.; et al. High Performance Hydrogen Fuel Cells with Ultralow Pt Loading Carbon Nanotube Thin Film Catalysts. J. Phys. Chem. C 2007, 111, 17901–17904. [Google Scholar] [CrossRef]
- Sai Siddhardha, R.S.; Lakshminarayanan, V.; Ramamurthy, S.S. Spot-Free Catalysis Using Gold Carbon Nanotube & Gold Graphene Composites for Hydrogen Evolution Reaction. J. Power Sources 2015, 288, 441–450. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Jiang, Y.; Zhou, Y.; Jia, L.; Wang, C. Reduced Graphene Oxide-Polyimide/Carbon Nanotube Film Decorated with NiSe Nanoparticles for Electrocatalytic Hydrogen Evolution Reactions. Electrochim. Acta 2017, 243, 291–298. [Google Scholar] [CrossRef]
- Cui, W.; Liu, Q.; Cheng, N.; Asiri, A.M.; Sun, X. Activated Carbon Nanotubes: A Highly-Active Metal-Free Electrocatalyst for Hydrogen Evolution Reaction. Chem. Commun. 2014, 50, 9340–9342. [Google Scholar] [CrossRef]
- Wang, L.; Pumera, M. Residual Metallic Impurities within Carbon Nanotubes Play a Dominant Role in Supposedly “Metal-Free” Oxygen Reduction Reactions. Chem. Commun. 2014, 50, 12662–12664. [Google Scholar] [CrossRef] [PubMed]
- Nikitin, A.; Ogasawara, H.; Mann, D.; Denecke, R.; Zhang, Z.; Dai, H.; Cho, K.; Nilsson, A. Hydrogenation of Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 2005, 95, 225507. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Voltammetry of Carbon Nanotubes and Graphenes: Excitement, Disappointment, and Reality. Chem. Rec. 2012, 12, 201–213. [Google Scholar] [CrossRef]
- Wang; Shan, H.; Hauge, R.H.; Pasquali, M.; Smalley, R.E. A Highly Selective, One-Pot Purification Method for Single-Walled Carbon Nanotubes. J. Phys. Chem. B 2007, 111, 1249–1252. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Guo, L.; Morris, D.; Kane, A.B.; Hurt, R.H. Targeted Removal of Bioavailable Metal as a Detoxification Strategy for Carbon Nanotubes. Carbon 2008, 46, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Pumera, M. Carbon Nanotubes Contain Residual Metal Catalyst Nanoparticles Even after Washing with Nitric Acid at Elevated Temperature Because These Metal Nanoparticles Are Sheathed by Several Graphene Sheets. Langmuir 2007, 23, 6453–6458. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron Encapsulated within Pod-like Carbon Nanotubes for Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Arenas, J.; Higgins, D.; Chen, Z.; Fowler, M.; Chen, Z. Mechanistic Analysis of Highly Active Nitrogen-Doped Carbon Nanotubes for the Oxygen Reduction Reaction. J. Power Sources 2012, 205, 215–221. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, C.; Dou, S.; Liu, D.; Wang, S. Oxidized Carbon Nanotubes as an Efficient Metal-Free Electrocatalyst for the Oxygen Reduction Reaction. RSC Adv. 2015, 5, 41901–41904. [Google Scholar] [CrossRef]
- Lee, W.J.; Maiti, U.N.; Lee, J.M.; Lim, J.; Han, T.H.; Kim, S.O. Nitrogen-Doped Carbon Nanotubes and Graphene Composite Structures for Energy and Catalytic Applications. Chem. Commun. 2014, 50, 6818–6830. [Google Scholar] [CrossRef] [PubMed]
- Yen, C.H.; Shimizu, K.; Lin, Y.-Y.; Bailey, F.; Cheng, I.F.; Wai, C.M. Chemical Fluid Deposition of Pt-Based Bimetallic Nanoparticles on Multiwalled Carbon Nanotubes for Direct Methanol Fuel Cell Application. Energy Fuels 2007, 21, 2268–2271. [Google Scholar] [CrossRef]
- Jeng, K.-T.; Chien, C.-C.; Hsu, N.-Y.; Yen, S.-C.; Chiou, S.-D.; Lin, S.-H.; Huang, W.-M. Performance of Direct Methanol Fuel Cell Using Carbon Nanotube-Supported Pt–Ru Anode Catalyst with Controlled Composition. J. Power Sources 2006, 160, 97–104. [Google Scholar] [CrossRef]
- Ramli, Z.A.C.; Kamarudin, S.K. Platinum-Based Catalysts on Various Carbon Supports and Conducting Polymers for Direct Methanol Fuel Cell Applications: A Review. Nanoscale Res. Lett. 2018, 13, 410. [Google Scholar] [CrossRef]
- Rohland, B.; Pietrzak, M.; Möller, S.; Bunescu, M.-C.; Wienecke, M.; Barfels, T. CNT-Based Cathode Material for DMFC. Fuller. Nanotub. Carbon Nanostruct. 2005, 13, 511–522. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Qi, G.; Cheng, J.; Wang, B. Vertically Aligned N-Doped Carbon Nanotubes Arrays as Efficient Binder-Free Catalysts for Flexible Li-CO2 Batteries. Energy Storage Mater. 2021, 35, 148–156. [Google Scholar] [CrossRef]
- Jiménez, C.; García, J.; Camarillo, R.; Martínez, F.; Rincón, J. Electrochemical CO2 Reduction to Fuels Using Pt/CNT Catalysts Synthesized in Supercritical Medium. Energy Fuels 2017, 31, 3038–3046. [Google Scholar] [CrossRef]
- Safdar Hossain, S.; Rahman, S.; Ahmed, S. Electrochemical Reduction of Carbon Dioxide over CNT-Supported Nanoscale Copper Electrocatalysts. J. Nanomater. 2014, 2014, 374318. [Google Scholar] [CrossRef]
- Hjorth, I.; Nord, M.; Rønning, M.; Yang, J.; Chen, D. Electrochemical Reduction of CO2 to Synthesis Gas on CNT Supported CuxZn1-x O Catalysts. Catal. Today 2020, 357, 311–321. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Z.; Zhang, X.; Li, L.; Li, Y.; Xu, H.; Li, X.; Yu, X.; Zhang, Z.; Liang, Y.; et al. Highly Selective and Active CO2 Reduction Electrocatalysts Based on Cobalt Phthalocyanine/Carbon Nanotube Hybrid Structures. Nat. Commun. 2017, 8, 14675. [Google Scholar] [CrossRef]
- Krukiewicz, K.; Janas, D.; Vallejo-Giraldo, C.; Biggs, M.J.P. Self-Supporting Carbon Nanotube Films as Flexible Neural Interfaces. Electrochim. Acta 2019, 295, 253–261. [Google Scholar] [CrossRef]
- Gerwig, R.; Fuchsberger, K.; Schroeppel, B.; Link, G.; Heusel, G.; Kraushaar, U.; Schuhmann, W.; Stett, A.; Stelzle, M. PEDOT–CNT Composite Microelectrodes for Recording and Electrostimulation Applications: Fabrication, Morphology, and Electrical Properties. Front. Neuroeng. 2012, 5, 8. [Google Scholar] [CrossRef]
- Mata, D.; Oliveira, F.J.; Neto, M.A.; Belmonte, M.; Bastos, A.C.; Lopes, M.A.; Gomes, P.S.; Fernandes, M.H.; Silva, R.F. Smart Electroconductive Bioactive Ceramics to Promote in Situ Electrostimulation of Bone. J. Mater. Chem. B 2015, 3, 1831–1845. [Google Scholar] [CrossRef]
- Eleftheriou, C.G.; Zimmermann, J.B.; Kjeldsen, H.D.; David-Pur, M.; Hanein, Y.; Sernagor, E. Carbon Nanotube Electrodes for Retinal Implants: A Study of Structural and Functional Integration over Time. Biomaterials 2017, 112, 108–121. [Google Scholar] [CrossRef]
- Samba, R.; Herrmann, T.; Zeck, G. PEDOT–CNT Coated Electrodes Stimulate Retinal Neurons at Low Voltage Amplitudes and Low Charge Densities. J. Neural Eng. 2015, 12, 016014. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Liu, Y.; Zhai, X.; Wang, F.; Ren, X.; Tao, F.; Li, T.; Wang, G.; Ren, F. Application of Carbon Nanotube-Based Materials as Interlayers in High-Performance Lithium-Sulfur Batteries: A Review. Front. Energy Res. 2020, 8. [Google Scholar] [CrossRef]
- Li, L.; Yang, H.; Zhou, D.; Zhou, Y. Progress in Application of CNTs in Lithium-Ion Batteries. Available online: https://www.hindawi.com/journals/jnm/2014/187891/ (accessed on 8 February 2021).
- Chew, S.Y.; Ng, S.H.; Wang, J.; Novák, P.; Krumeich, F.; Chou, S.L.; Chen, J.; Liu, H.K. Flexible Free-Standing Carbon Nanotube Films for Model Lithium-Ion Batteries. Carbon 2009, 47, 2976–2983. [Google Scholar] [CrossRef]
- Yoon, S.; Lee, S.; Kim, S.; Park, K.-W.; Cho, D.; Jeong, Y. Carbon Nanotube Film Anodes for Flexible Lithium Ion Batteries. J. Power Sources 2015, 279, 495–501. [Google Scholar] [CrossRef]
- Schulze, M.C.; Belson, R.M.; Kraynak, L.A.; Prieto, A.L. Electrodeposition of Sb/CNT Composite Films as Anodes for Li- and Na-Ion Batteries. Energy Storage Mater. 2020, 25, 572–584. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, B.B.Q. A Perspective: Carbon Nanotube Macro-Films for Energy Storage. Energy Environ. Sci. 2013, 6, 3183–3201. [Google Scholar] [CrossRef]
- Yu, D.; Dai, L. Self-Assembled Graphene/Carbon Nanotube Hybrid Films for Supercapacitors. J. Phys. Chem. Lett. 2010, 1, 467–470. [Google Scholar] [CrossRef]
- Ciszewski, M.; Koszorek, A.; Radko, T.; Szatkowski, P.; Janas, D. Review of the Selected Carbon-Based Materials for Symmetric Supercapacitor Application. J. Electron. Mater. 2019, 48, 717–744. [Google Scholar] [CrossRef]
- Díez, N.; Botas, C.; Mysyk, R.; Goikolea, E.; Rojo, T.; Carriazo, D. Highly Packed Graphene–CNT Films as Electrodes for Aqueous Supercapacitors with High Volumetric Performance. J. Mater. Chem. A 2018, 6, 3667–3673. [Google Scholar] [CrossRef]
- Lu, W.; Dai, L. Carbon Nanotube Supercapacitors. Carbon Nanotub. 2010. [Google Scholar] [CrossRef]
- Aval, L.F.; Ghoranneviss, M.; Pour, G.B. High-Performance Supercapacitors Based on the Carbon Nanotubes, Graphene and Graphite Nanoparticles Electrodes. Heliyon 2018, 4, e00862. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, Y.; Wu, H.; Jiang, K.; Li, Q.; Fan, S.; Li, J.; Wang, J. Ultrastretchable Carbon Nanotube Composite Electrodes for Flexible Lithium-Ion Batteries. Nanoscale 2018, 10, 19972–19978. [Google Scholar] [CrossRef] [PubMed]
- Frackowiak, E.; Béguin, F. Carbon Materials for the Electrochemical Storage of Energy in Capacitors. Carbon 2001, 39, 937–950. [Google Scholar] [CrossRef]
- Notarianni, M.; Liu, J.; Mirri, F.; Pasquali, M.; Motta, N. Graphene-Based Supercapacitor with Carbon Nanotube Film as Highly Efficient Current Collector. Nanotechnology 2014, 25, 435405. [Google Scholar] [CrossRef]
- Tiwari, P.; Janas, D.; Chandra, R. Ultrahigh Rate Supercapacitor Based on Self-Standing Carbon Nanotubes Supported Vertically Aligned MoS2 Sheets. MRS Adv. 2020, 5, 2495–2502. [Google Scholar] [CrossRef]
- Joseph, N.; Shafi, P.M.; Bose, A.C. Recent Advances in 2D-MoS2 and Its Composite Nanostructures for Supercapacitor Electrode Application. Energy Fuels 2020, 34, 6558–6597. [Google Scholar] [CrossRef]
- Das, C.M.; Kang, L.; Ouyang, Q.; Yong, K.-T. Advanced Low-Dimensional Carbon Materials for Flexible Devices. InfoMat 2020, 2, 698–714. [Google Scholar] [CrossRef]
- Pan, Z.; Liu, M.; Yang, J.; Qiu, Y.; Li, W.; Xu, Y.; Zhang, X.; Zhang, Y. High Electroactive Material Loading on a Carbon Nanotube@3D Graphene Aerogel for High-Performance Flexible All-Solid-State Asymmetric Supercapacitors. Adv. Funct. Mater. 2017, 27, 1701122. [Google Scholar] [CrossRef]
- Tiwari, P.; Janas, D.; Chandra, R. Self-Standing MoS2/CNT and MnO2/CNT One Dimensional Core Shell Heterostructures for Asymmetric Supercapacitor Application. Carbon 2021, 177, 291–303. [Google Scholar] [CrossRef]
- Hao, M.; Huang, Z.; Saviers, K.R.; Xiong, G.; Hodson, S.L.; Fisher, T.S. Characterization of Vertically Oriented Carbon Nanotube Arrays as High-Temperature Thermal Interface Materials. Int. J. Heat Mass Transf. 2017, 106, 1287–1293. [Google Scholar] [CrossRef]
- Qiu, L.; Scheider, K.; Radwan, S.A.; Larkin, L.S.; Saltonstall, C.B.; Feng, Y.; Zhang, X.; Norris, P.M. Thermal Transport Barrier in Carbon Nanotube Array Nano-Thermal Interface Materials. Carbon 2017, 120, 128–136. [Google Scholar] [CrossRef]
- Taphouse, J.H.; Bougher, T.L.; Singh, V.; Abadi, P.P.S.S.; Graham, S.; Cola, B.A. Carbon Nanotube Thermal Interfaces Enhanced with Sprayed on Nanoscale Polymer Coatings. Nanotechnology 2013, 24, 105401. [Google Scholar] [CrossRef]
- Qiu, L.; Guo, P.; Kong, Q.; Tan, C.W.; Liang, K.; Wei, J.; Tey, J.N.; Feng, Y.; Zhang, X.; Tay, B.K. Coating-Boosted Interfacial Thermal Transport for Carbon Nanotube Array Nano-Thermal Interface Materials. Carbon 2019, 145, 725–733. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Cassell, A.M.; Cruden, B.A. Implications of Catalyst Control for Carbon Nanotube Based Thermal Interface Materials. J. Appl. Phys. 2008, 104, 084310. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Suhir, E.; Wang, X. Thermal Properties of Carbon Nanotube Array Used for Integrated Circuit Cooling. J. Appl. Phys. 2006, 100, 074302. [Google Scholar] [CrossRef]
- Cola, B.A.; Amama, P.B.; Xu, X.; Fisher, T.S. Effects of Growth Temperature on Carbon Nanotube Array Thermal Interfaces. J. Heat Transf. 2008, 130. [Google Scholar] [CrossRef]
- Amama, P.B.; Cola, B.A.; Sands, T.D.; Xu, X.; Fisher, T.S. Dendrimer-Assisted Controlled Growth of Carbon Nanotubes for Enhanced Thermal Interface Conductance. Nanotechnology 2007, 18, 385303. [Google Scholar] [CrossRef]
- Cross, R.; Cola, B.A.; Fisher, T.; Xu, X.; Gall, K.; Graham, S. A Metallization and Bonding Approach for High Performance Carbon Nanotube Thermal Interface Materials. Nanotechnology 2010, 21, 445705. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Bodelot, L.; Lebental, B.; Lim, Y.D.; Shiau, L.L.; Gusarov, B.; Tan, C.W.; Liang, K.; Lu, C.; Tan, C.S.; et al. Novel Three-Dimensional Carbon Nanotube Networks as High Performance Thermal Interface Materials. Carbon 2018, 132, 359–369. [Google Scholar] [CrossRef]
- Jun, X.; Fisher, T.S. Enhanced Thermal Contact Conductance Using Carbon Nanotube Array Interfaces. IEEE Trans. Compon. Packag. Technol. 2006, 29, 261–267. [Google Scholar] [CrossRef]
- Qiu, L.; Wang, X.; Su, G.; Tang, D.; Zheng, X.; Zhu, J.; Wang, Z.; Norris, P.M.; Bradford, P.D.; Zhu, Y. Remarkably Enhanced Thermal Transport Based on a Flexible Horizontally-Aligned Carbon Nanotube Array Film. Sci. Rep. 2016, 6, 21014. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, M.; Di, J.; Xu, G.; Li, H.; Li, Q. Architecting Three-Dimensional Networks in Carbon Nanotube Buckypapers for Thermal Interface Materials. J. Phys. Chem. C 2012, 116, 3903–3909. [Google Scholar] [CrossRef]
- Blackburn, J.L.; Ferguson, A.J.; Cho, C.; Grunlan, J.C. Carbon-Nanotube-Based Thermoelectric Materials and Devices. Adv. Mater. 2018, 30, 1704386. [Google Scholar] [CrossRef] [PubMed]
- Hung, N.T.; Nugraha, A.R.T.; Hasdeo, E.H.; Dresselhaus, M.S.; Saito, R. Diameter Dependence of Thermoelectric Power of Semiconducting Carbon Nanotubes. Phys. Rev. B 2015, 92, 165426. [Google Scholar] [CrossRef]
- Piao, M.; Joo, M.-K.; Na, J.; Kim, Y.-J.; Mouis, M.; Ghibaudo, G.; Roth, S.; Kim, W.-Y.; Jang, H.-K.; Kennedy, G.P.; et al. Effect of Intertube Junctions on the Thermoelectric Power of Monodispersed Single Walled Carbon Nanotube Networks. J. Phys. Chem. C 2014, 118, 26454–26461. [Google Scholar] [CrossRef]
- Lian, F.; Llinas, J.P.; Li, Z.; Estrada, D.; Pop, E. Thermal Conductivity of Chirality-Sorted Carbon Nanotube Networks. Appl. Phys. Lett. 2016, 108, 103101. [Google Scholar] [CrossRef]
- Nonoguchi, Y.; Ohashi, K.; Kanazawa, R.; Ashiba, K.; Hata, K.; Nakagawa, T.; Adachi, C.; Tanase, T.; Kawai, T. Systematic Conversion of Single Walled Carbon Nanotubes into N-Type Thermoelectric Materials by Molecular Dopants. Sci. Rep. 2013, 3, 3344. [Google Scholar] [CrossRef]
- Nakai, Y.; Honda, K.; Yanagi, K.; Kataura, H.; Kato, T.; Yamamoto, T.; Maniwa, Y. Giant Seebeck Coefficient in Semiconducting Single-Wall Carbon Nanotube Film. Appl. Phys. Express 2014, 7, 025103. [Google Scholar] [CrossRef]
- Hone, J.; Ellwood, I.; Muno, M.; Mizel, A.; Cohen, M.L.; Zettl, A.; Rinzler, A.G.; Smalley, R.E. Thermoelectric Power of Single-Walled Carbon Nanotubes. Phys. Rev. Lett. 1998, 80, 1042–1045. [Google Scholar] [CrossRef]
- Jia, S.-L.; Geng, H.-Z.; Wang, L.; Tian, Y.; Xu, C.-X.; Shi, P.-P.; Gu, Z.-Z.; Yuan, X.-S.; Jing, L.-C.; Guo, Z.-Y.; et al. Carbon Nanotube-Based Flexible Electrothermal Film Heaters with a High Heating Rate. R. Soc. Open Sci. 2018, 5, 172072. [Google Scholar] [CrossRef]
- Park, J.; Jang, I.R.; Lee, K.; Kim, H.J. High Efficiency Crumpled Carbon Nanotube Heaters for Low Drift Hydrogen Sensing. Sensors 2019, 19, 3878. [Google Scholar] [CrossRef]
- Ning, W.; Wang, Z.; Liu, P.; Zhou, D.; Yang, S.; Wang, J.; Li, Q.; Fan, S.; Jiang, K. Multifunctional Super-Aligned Carbon Nanotube/Polyimide Composite Film Heaters and Actuators. Carbon 2018, 139, 1136–1143. [Google Scholar] [CrossRef]
- Jung, D.; Kim, D.; Lee, K.H.; Overzet, L.J.; Lee, G.S. Transparent Film Heaters Using Multi-Walled Carbon Nanotube Sheets. Sens. Actuators A Phys. 2013, 199, 176–180. [Google Scholar] [CrossRef]
- Fischer, T.; Rühling, J.; Wetzold, N.; Zillger, T.; Weissbach, T.; Göschel, T.; Würfel, M.; Hübler, A.; Kroll, L. Roll-to-Roll Printed Carbon Nanotubes on Textile Substrates as a Heating Layer in Fiber-Reinforced Epoxy Composites. J. Appl. Polym. Sci. 2018, 135, 45950. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Li, X.; Zhang, J.; Lou, H.; Shi, X.; Cheng, X.; Peng, H. A Smart, Stretchable Resistive Heater Textile. J. Mater. Chem. C 2016, 5, 41–46. [Google Scholar] [CrossRef]
- Luo, J.; Lu, H.; Zhang, Q.; Yao, Y.; Chen, M.; Li, Q. Flexible Carbon Nanotube/Polyurethane Electrothermal Films. Carbon 2016, 110, 343–349. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, Z.; Chen, W.; Lu, D.; Yang, L.; Yang, R.; Zhu, H.; Tang, Z.; Gui, X. Direct Patterning of Carbon Nanotube via Stamp Contact Printing Process for Stretchable and Sensitive Sensing Devices. Nano-Micro Lett. 2019, 11, 92. [Google Scholar] [CrossRef]
- Chang-Jian, S.-K.; Ho, J.-R. Laser Patterning of Carbon-Nanotubes Thin Films and Their Applications. Carbon Nanotub. Appl. Electron. Devices 2011. [Google Scholar] [CrossRef]
- Dong, L.; Youkey, S.; Bush, J.; Jiao, J.; Dubin, V.M.; Chebiam, R.V. Effects of Local Joule Heating on the Reduction of Contact Resistance between Carbon Nanotubes and Metal Electrodes. J. Appl. Phys. 2007, 101, 024320. [Google Scholar] [CrossRef]
- Costa, P.M.F.J.; Gautam, U.K.; Bando, Y.; Golberg, D. Direct Imaging of Joule Heating Dynamics and Temperature Profiling inside a Carbon Nanotube Interconnect. Nat. Commun. 2011, 2, 421. [Google Scholar] [CrossRef]
- Santini, C.A.; Vereecken, P.M.; Volodin, A.; Groeseneken, G.; Gendt, S.D.; Haesendonck, C.V. A Study of Joule Heating-Induced Breakdown of Carbon Nanotube Interconnects. Nanotechnology 2011, 22, 395202. [Google Scholar] [CrossRef]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown. Science 2001, 292, 706–709. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.G.; Hersam, M.; Arnold, M.; Martel, R.; Avouris, P. Current Saturation and Electrical Breakdown in Multiwalled Carbon Nanotubes. Phys. Rev. Lett. 2001, 86, 3128–3131. [Google Scholar] [CrossRef]
- Otsuka, K.; Inoue, T.; Chiashi, S.; Maruyama, S. Selective Removal of Metallic Single-Walled Carbon Nanotubes in Full Length by Organic Film-Assisted Electrical Breakdown. Nanoscale 2014, 6, 8831–8835. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Hawkins, S.C.; Falzon, B.G. An Advanced Anti-Icing/de-Icing System Utilizing Highly Aligned Carbon Nanotube Webs. Carbon 2018, 136, 130–138. [Google Scholar] [CrossRef]
- Kataura, H.; Kumazawa, Y.; Maniwa, Y.; Umezu, I.; Suzuki, S.; Ohtsuka, Y.; Achiba, Y. Optical Properties of Single-Wall Carbon Nanotubes. Synth. Met. 1999, 103, 2555–2558. [Google Scholar] [CrossRef]
- Soetedjo, H.; Mora, M.F.; Garcia, C.D. Optical Properties of Single-Wall Carbon Nanotube Films Deposited on Si/SiO2 Wafers. Thin Solid Film. 2010, 518, 3954–3959. [Google Scholar] [CrossRef] [PubMed]
- Wieland, L.; Li, H.; Rust, C.; Chen, J.; Flavel, B.S. Carbon Nanotubes for Photovoltaics: From Lab to Industry. Adv. Energy Mater. 2021, 11, 2002880. [Google Scholar] [CrossRef]
- Contreras, M.A.; Barnes, T.; van de Lagemaat, J.; Rumbles, G.; Coutts, T.J.; Weeks, C.; Glatkowski, P.; Levitsky, I.; Peltola, J.; Britz, D.A. Replacement of Transparent Conductive Oxides by Single-Wall Carbon Nanotubes in Cu(In,Ga)Se2-Based Solar Cells. J. Phys. Chem. C 2007, 111, 14045–14048. [Google Scholar] [CrossRef]
- Aitola, K.; Domanski, K.; Correa-Baena, J.-P.; Sveinbjörnsson, K.; Saliba, M.; Abate, A.; Grätzel, M.; Kauppinen, E.; Johansson, E.M.J.; Tress, W.; et al. High Temperature-Stable Perovskite Solar Cell Based on Low-Cost Carbon Nanotube Hole Contact. Adv. Mater. 2017, 29, 1606398. [Google Scholar] [CrossRef] [PubMed]
- Habisreutinger, S.N.; Leijtens, T.; Eperon, G.E.; Stranks, S.D.; Nicholas, R.J.; Snaith, H.J. Carbon Nanotube/Polymer Composites as a Highly Stable Hole Collection Layer in Perovskite Solar Cells. Nano Lett. 2014, 14, 5561–5568. [Google Scholar] [CrossRef]
- Jeon, I.; Shawky, A.; Seo, S.; Qian, Y.; Anisimov, A.; Kauppinen, E.I.; Matsuo, Y.; Maruyama, S. Carbon Nanotubes to Outperform Metal Electrodes in Perovskite Solar Cells via Dopant Engineering and Hole-Selectivity Enhancement. J. Mater. Chem. A 2020, 8, 11141–11147. [Google Scholar] [CrossRef]
- Bati, A.S.R.; Yu, L.; Batmunkh, M.; Shapter, J.G. Recent Advances in Applications of Sorted Single-Walled Carbon Nanotubes. Adv. Funct. Mater. 2019, 29, 1902273. [Google Scholar] [CrossRef]
- Isborn, C.M.; Tang, C.; Martini, A.; Johnson, E.R.; Otero-de-la-Roza, A.; Tung, V.C. Carbon Nanotube Chirality Determines Efficiency of Electron Transfer to Fullerene in All-Carbon Photovoltaics. J. Phys. Chem. Lett. 2013, 4, 2914–2918. [Google Scholar] [CrossRef]
- Kolanowska, A.; Janas, D.; Herman, A.P.; Jędrysiak, R.G.; Giżewski, T.; Boncel, S. From Blackness to Invisibility—Carbon Nanotubes Role in the Attenuation of and Shielding from Radio Waves for Stealth Technology. Carbon 2018, 126, 31–52. [Google Scholar] [CrossRef]
- Al-Saleh, M.H.; Saadeh, W.H.; Sundararaj, U. EMI Shielding Effectiveness of Carbon Based Nanostructured Polymeric Materials: A Comparative Study. Carbon 2013, 60, 146–156. [Google Scholar] [CrossRef]
- Cui, K.; Wardle, B.L. Breakdown of Native Oxide Enables Multifunctional, Free-Form Carbon Nanotube–Metal Hierarchical Architectures. ACS Appl. Mater. Interfaces 2019, 11, 35212–35220. [Google Scholar] [CrossRef]
- Sankaran, S.; Deshmukh, K.; Ahamed, M.B.; Khadheer Pasha, S.K. Recent Advances in Electromagnetic Interference Shielding Properties of Metal and Carbon Filler Reinforced Flexible Polymer Composites: A Review. Compos. Part A Appl. Sci. Manuf. 2018, 114, 49–71. [Google Scholar] [CrossRef]
- Kim, H.M.; Kim, K.; Lee, S.J.; Joo, J.; Yoon, H.S.; Cho, S.J.; Lyu, S.C.; Lee, C.J. Charge Transport Properties of Composites of Multiwalled Carbon Nanotube with Metal Catalyst and Polymer: Application to Electromagnetic Interference Shielding. Curr. Appl. Phys. 2004, 4, 577–580. [Google Scholar] [CrossRef]
- Yuen, S.-M.; Ma, C.-C.M.; Chuang, C.-Y.; Yu, K.-C.; Wu, S.-Y.; Yang, C.-C.; Wei, M.-H. Effect of Processing Method on the Shielding Effectiveness of Electromagnetic Interference of MWCNT/PMMA Composites. Compos. Sci. Technol. 2008, 68, 963–968. [Google Scholar] [CrossRef]
- Chen, M.; Yin, X.; Li, M.; Chen, L.; Cheng, L.; Zhang, L. Electromagnetic Interference Shielding Properties of Silicon Nitride Ceramics Reinforced by in Situ Grown Carbon Nanotubes. Ceram. Int. 2015, 41, 2467–2475. [Google Scholar] [CrossRef]
- Gupta, T.K.; Singh, B.P.; Dhakate, S.R.; Singh, V.N.; Mathur, R.B. Improved Nanoindentation and Microwave Shielding Properties of Modified MWCNT Reinforced Polyurethane Composites. J. Mater. Chem. A 2013, 1, 9138–9149. [Google Scholar] [CrossRef]
- Singh, A.P.; Gupta, B.K.; Mishra, M.; Govind; Chandra, A.; Mathur, R.B.; Dhawan, S.K. Multiwalled Carbon Nanotube/Cement Composites with Exceptional Electromagnetic Interference Shielding Properties. Carbon 2013, 56, 86–96. [Google Scholar] [CrossRef]
- Al-Saleh, M.H. Influence of Conductive Network Structure on the EMI Shielding and Electrical Percolation of Carbon Nanotube/Polymer Nanocomposites. Synth. Met. 2015, 205, 78–84. [Google Scholar] [CrossRef]
- Verma, P.; Saini, P.; Malik, R.S.; Choudhary, V. Excellent Electromagnetic Interference Shielding and Mechanical Properties of High Loading Carbon-Nanotubes/Polymer Composites Designed Using Melt Recirculation Equipped Twin-Screw Extruder. Carbon 2015, 89, 308–317. [Google Scholar] [CrossRef]
- Xiang, C.; Pan, Y.; Guo, J. Electromagnetic Interference Shielding Effectiveness of Multiwalled Carbon Nanotube Reinforced Fused Silica Composites. Ceram. Int. 2007, 33, 1293–1297. [Google Scholar] [CrossRef]
- Zhang, C.-S.; Ni, Q.-Q.; Fu, S.-Y.; Kurashiki, K. Electromagnetic Interference Shielding Effect of Nanocomposites with Carbon Nanotube and Shape Memory Polymer. Compos. Sci. Technol. 2007, 67, 2973–2980. [Google Scholar] [CrossRef]
- Chao, Z.; Yu, Y.; Lei, F.; Hu, D. A Lightweight and Flexible CNT/Fe3O4 Composite with High Electromagnetic Interference Shielding Performance. Carbon Lett. 2020. [Google Scholar] [CrossRef]
- Zeng, S.; Li, X.; Li, M.; Zheng, J.; Shiju, E.; Yang, W.; Zhao, B.; Guo, X.; Zhang, R. Flexible PVDF/CNTs/Ni@CNTs Composite Films Possessing Excellent Electromagnetic Interference Shielding and Mechanical Properties under Heat Treatment. Carbon 2019, 155, 34–43. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Dong, C.; Chen, G.; Guan, H. Preparation and Electromagnetic Shielding Effectiveness of Cobalt Ferrite Nanoparticles/Carbon Nanotubes Composites. Nanomater. Nanotechnol. 2019, 9, 1847980419837821. [Google Scholar] [CrossRef]
- Feng, X.; Qin, X.; Liu, D.; Huang, Z.; Zhou, Y.; Lan, W.; Lu, F.; Qi, H. High Electromagnetic Interference Shielding Effectiveness of Carbon Nanotube–Cellulose Composite Films with Layered Structures. Macromol. Mater. Eng. 2018, 303, 1800377. [Google Scholar] [CrossRef]
- Chikyu, N.; Nakano, T.; Kletetschka, G.; Inoue, Y. Excellent Electromagnetic Interference Shielding Characteristics of a Unidirectionally Oriented Thin Multiwalled Carbon Nanotube/Polyethylene Film. Mater. Des. 2020, 195, 108918. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Wang, X.-Y.; Li, X.-M.; Liao, S.-Y.; Lin, Z.-Q.; Hu, Y.-G.; Zhao, T.; Zeng, X.-L.; Li, C.-H.; Yu, S.-H.; et al. Ultrathin Densified Carbon Nanotube Film with “Metal-like” Conductivity, Superior Mechanical Strength, and Ultrahigh Electromagnetic Interference Shielding Effectiveness. ACS Nano 2020, 14, 14134–14145. [Google Scholar] [CrossRef]
- Murakami, N.; Tango, Y.; Miyake, H.; Tajima, T.; Nishina, Y.; Kurashige, W.; Negishi, Y.; Takaguchi, Y. SWCNT Photocatalyst for Hydrogen Production from Water upon Photoexcitation of (8, 3) SWCNT at 680-Nm Light. Sci. Rep. 2017, 7, 43445. [Google Scholar] [CrossRef]
- Izawa, T.; Kalousek, V.; Miyamoto, D.; Murakami, N.; Miyake, H.; Tajima, T.; Kurashige, W.; Negishi, Y.; Ikeue, K.; Ohkubo, T.; et al. Carbon-Nanotube-Based Photocatalysts for Water Splitting in Cooperation with BiVO4 and [Co(Bpy)3]3+/2+. Chem. Lett. 2019, 48, 410–413. [Google Scholar] [CrossRef]
- Murakami, N.; Miyake, H.; Tajima, T.; Nishikawa, K.; Hirayama, R.; Takaguchi, Y. Enhanced Photosensitized Hydrogen Production by Encapsulation of Ferrocenyl Dyes into Single-Walled Carbon Nanotubes. J. Am. Chem. Soc. 2018, 140, 3821–3824. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Kang, X.; Zhang, S. CNT/g-C3N4 Photocatalysts with Enhanced Hydrogen Evolution Ability for Water Splitting Based on a Noncovalent Interaction. Int. J. Energy Res. 2018, 42, 1649–1656. [Google Scholar] [CrossRef]
- Ye, A.; Fan, W.; Zhang, Q.; Deng, W.; Wang, Y. CdS–Graphene and CdS–CNT Nanocomposites as Visible-Light Photocatalysts for Hydrogen Evolution and Organic Dye Degradation. Catal. Sci. Technol. 2012, 2, 969–978. [Google Scholar] [CrossRef]
- Dai, K.; Zhang, X.; Fan, K.; Zeng, P.; Peng, T. Multiwalled Carbon Nanotube-TiO2 Nanocomposite for Visible-Light-Induced Photocatalytic Hydrogen Evolution. J. Nanomater. 2014, 2014, 694073. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J. Carbon-Based H2-Production Photocatalytic Materials. J. Photochem. Photobiol. C Photochem. Rev. 2016, 27, 72–99. [Google Scholar] [CrossRef]
- Nakashima, N. Nanocarbons for Energy Conversion: Supramolecular Approaches; Springer: Berlin/Heidelberg, Germany, 2019; ISBN 3-319-92917-8. [Google Scholar]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced Nanoarchitectures for Solar Photocatalytic Applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef]
- Kandy, M.M. Carbon-Based Photocatalysts for Enhanced Photocatalytic Reduction of CO2 to Solar Fuels. Sustain. Energy Fuels 2020, 4, 469–484. [Google Scholar] [CrossRef]
- Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. CO2 Photocatalytic Reduction with CNT/TiO2 Based Nanocomposites Prepared by High-Pressure Technology. J. Supercrit. Fluids 2020, 163, 104876. [Google Scholar] [CrossRef]
- Ong, W.-J.; Gui, M.M.; Chai, S.-P.; Mohamed, A.R. Direct Growth of Carbon Nanotubes on Ni/TiO2 as next Generation Catalysts for Photoreduction of CO2 to Methane by Water under Visible Light Irradiation. RSC Adv. 2013, 3, 4505–4509. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Jain, S.L.; Babalola, J.O.; Vorontsov, A.V.; Kumar, U. Insights into Reinforced Photocatalytic Activity of the CNT–TiO2 Nanocomposite for CO2 Reduction and Water Splitting. J. Phys. Chem. C 2019, 123, 367–378. [Google Scholar] [CrossRef]
- Lashgari, M.; Soodi, S.; Zeinalkhani, P. Photocatalytic Back-Conversion of CO2 into Oxygenate Fuels Using an Efficient ZnO/CuO/Carbon Nanotube Solar-Energy-Material: Artificial Photosynthesis. J. CO2 Util. 2017, 18, 89–97. [Google Scholar] [CrossRef]
- Fang, Z.; Li, S.; Gong, Y.; Liao, W.; Tian, S.; Shan, C.; He, C. Comparison of Catalytic Activity of Carbon-Based AgBr Nanocomposites for Conversion of CO2 under Visible Light. J. Saudi Chem. Soc. 2014, 18, 299–307. [Google Scholar] [CrossRef]
- Fu, Z.-C.; Xu, R.-C.; Moore, J.T.; Liang, F.; Nie, X.-C.; Mi, C.; Mo, J.; Xu, Y.; Xu, Q.-Q.; Yang, Z.; et al. Highly Efficient Photocatalytic System Constructed from CoP/Carbon Nanotubes or Graphene for Visible-Light-Driven CO2 Reduction. Chem. Eur. J. 2018, 24, 4273–4278. [Google Scholar] [CrossRef]
- Aoi, S.; Mase, K.; Ohkubo, K.; Fukuzumi, S. Photocatalytic Reduction of CO2 and H2O to CO and H2 with a Cobalt Chlorin Complex Adsorbed on Multi-Walled Carbon Nanotubes. Catal. Sci. Technol. 2016, 6, 4077–4080. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Qian, D.; Liu, W.K. Mechanical Properties of Carbon Nanotubes: Theoretical Predictions and Experimental Measurements. C. R. Phys. 2003, 4, 993–1008. [Google Scholar] [CrossRef]
- Salvetat, J.-P.; Bonard, J.-M.; Thomson, N.H.; Kulik, A.J.; Forró, L.; Benoit, W.; Zuppiroli, L. Mechanical Properties of Carbon Nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Sharma, S.K.; Gaur, H.; Kulkarni, M.; Patil, G.; Bhattacharya, B.; Sharma, A. PZT–PDMS Composite for Active Damping of Vibrations. Compos. Sci. Technol. 2013, 77, 42–51. [Google Scholar] [CrossRef]
- Rujijanagul, G.; Boonyakul, S.; Tunkasiri, T. Effect of the Particle Size of PZT on the Microstructure and the Piezoelectric Properties of 0-3 PZT/Polymer Composites. J. Mater. Sci. Lett. 2001, 20, 1943–1945. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, Y.; Li, H.; Ou, J. PZT/PVDF Composites Doped with Carbon Nanotubes. Sens. Actuators A Phys. 2013, 194, 228–231. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Z.-Y. Development of Polymer-Based 0–3 Composites with High Dielectric Constant. J. Adv. Dielect. 2011, 1, 389–406. [Google Scholar] [CrossRef]
- Wang, Z.; Keith Nelson, J.; Hillborg, H.; Zhao, S.; Schadler, L.S. Dielectric Constant and Breakdown Strength of Polymer Composites with High Aspect Ratio Fillers Studied by Finite Element Models. Compos. Sci. Technol. 2013, 76, 29–36. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, Y.J. High Performance Flexible Piezoelectric Pressure Sensor Based on CNTs-Doped 0–3 Ceramic-Epoxy Nanocomposites. Mater. Des. 2018, 151, 133–140. [Google Scholar] [CrossRef]
- Han, J.K.; Jeon, D.H.; Cho, S.Y.; Kang, S.W.; Lim, J.; Bu, S.D. Flexible Piezoelectric Generators by Using the Bending Motion Method of Direct-Grown-PZT Nanoparticles on Carbon Nanotubes. Nanomaterials 2017, 7, 308. [Google Scholar] [CrossRef]
- Banerjee, S.; Du, W.; Sundar, U.; Cook-Chennault, K.A. Piezoelectric and Dielectric Characterization of MWCNT-Based Nanocomposite Flexible Films. Available online: https://www.hindawi.com/journals/jnm/2018/6939621/ (accessed on 4 February 2021).
- Gau, C.; Ko, H.S.; Chen, H.T. Piezoresistive Characteristics of MWNT Nanocomposites and Fabrication as a Polymer Pressure Sensor. Nanotechnology 2009, 20, 185503. [Google Scholar] [CrossRef]
- Cao, C.L.; Hu, C.G.; Xiong, Y.F.; Han, X.Y.; Xi, Y.; Miao, J. Temperature Dependent Piezoresistive Effect of Multi-Walled Carbon Nanotube Films. Diam. Relat. Mater. 2007, 16, 388–392. [Google Scholar] [CrossRef]
- Chen, S.; Luo, J.; Wang, X.; Li, Q.; Zhou, L.; Liu, C.; Feng, C. Fabrication and Piezoresistive/Piezoelectric Sensing Characteristics of Carbon Nanotube/PVA/Nano-ZnO Flexible Composite. Sci. Rep. 2020, 10, 8895. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, X.; Zhu, Y.; Li, R.; Chen, H.; Gao, P.; Zhang, Y.; Li, T.; Liu, Y.; Li, Q. Hydrothermal Deposition of a Zinc Oxide Nanorod Array on a Carbon Nanotube Film as a Piezoelectric Generator. RSC Adv. 2014, 4, 43772–43777. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Costa, P.; Lanceros-Mendez, S. 3.9 Piezoelectric Energy Production. In Comprehensive Energy Systems; Dincer, I., Ed.; Elsevier: Oxford, UK, 2018; pp. 380–415. ISBN 978-0-12-814925-6. [Google Scholar]
- Hu, Y.; Kang, W.; Fang, Y.; Xie, L.; Qiu, L.; Jin, T. Piezoelectric Poly(Vinylidene Fluoride) (PVDF) Polymer-Based Sensor for Wrist Motion Signal Detection. Appl. Sci. 2018, 8, 836. [Google Scholar] [CrossRef]
- Wu, C.-M.; Chou, M.-H.; Zeng, W.-Y. Piezoelectric Response of Aligned Electrospun Polyvinylidene Fluoride/Carbon Nanotube Nanofibrous Membranes. Nanomaterials 2018, 8, 420. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fang, L.; Sun, B.; Li, X.; Kang, S.H. Review—Recent Progress in Flexible and Stretchable Piezoresistive Sensors and Their Applications. J. Electrochem. Soc. 2020, 167, 037561. [Google Scholar] [CrossRef]
- Kim, G.H.; Hong, S.M.; Seo, Y. Piezoelectric Properties of Poly(Vinylidene Fluoride) and Carbon Nanotube Blends: β-Phase Development. Phys. Chem. Chem. Phys. 2009, 11, 10506–10512. [Google Scholar] [CrossRef]
- Kabir, E.; Khatun, M.; Nasrin, L.; Raihan, M.J.; Rahman, M. Pure β-Phase Formation in Polyvinylidene Fluoride (PVDF)-Carbon Nanotube Composites. J. Phys. D Appl. Phys. 2017, 50, 163002. [Google Scholar] [CrossRef]
- Baughman, R.H.; Cui, C.; Zakhidov, A.A.; Iqbal, Z.; Barisci, J.N.; Spinks, G.M.; Wallace, G.G.; Mazzoldi, A.; Rossi, D.D.; Rinzler, A.G.; et al. Carbon Nanotube Actuators. Science 1999, 284, 1340–1344. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Yao, Y.; Luo, W.; Wan, J.; Dai, J.; Hitz, E.; Fu, K.K.; Hu, L. A Solution-Processed High-Temperature, Flexible, Thin-Film Actuator. Adv. Mater. 2016, 28, 8618–8624. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Sitti, M. Self-Sensing Paper Actuators Based on Graphite–Carbon Nanotube Hybrid Films. Adv. Sci. 2018, 5, 1800239. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xiao, P.; Ni, F.; Zhang, L.; Zhang, T.; Wang, S.; Zhou, W.; Lu, W.; Kuo, S.-W.; Chen, T. Biomimetic Underwater Self-Perceptive Actuating Soft System Based on Highly Compliant, Morphable and Conductive Sandwiched Thin Films. Nano Energy 2021, 81, 105617. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rdest, M.; Janas, D. Carbon Nanotube Films for Energy Applications. Energies 2021, 14, 1890. https://doi.org/10.3390/en14071890
Rdest M, Janas D. Carbon Nanotube Films for Energy Applications. Energies. 2021; 14(7):1890. https://doi.org/10.3390/en14071890
Chicago/Turabian StyleRdest, Monika, and Dawid Janas. 2021. "Carbon Nanotube Films for Energy Applications" Energies 14, no. 7: 1890. https://doi.org/10.3390/en14071890
APA StyleRdest, M., & Janas, D. (2021). Carbon Nanotube Films for Energy Applications. Energies, 14(7), 1890. https://doi.org/10.3390/en14071890