Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste
Abstract
1. Introduction
- the presence of microorganisms with diverse physiology that are capable of fermenting a wide range of substrates;
- the reduced contamination risk and ability to use non-sterile substrates;
- higher hydrogen yields due to the concerted action of a diversified community of H2-synthesizing microorganisms.
2. Materials and Methods
2.1. The Production of Granular Microbial Preparation
2.2. Model Substrates for Assessing the Dark Fermentation Efficiency
2.3. The Fermentation Process
2.4. Assessment of the Fermentation Parameters
2.5. The Calculation of the Efficiency of Model Food Waste Digestion
- Waste degradation time (T, days)—defined as the amount of time passed between the start and the end of the fermentation (i.e., the termination of the gas synthesis);
- Hydrogen yield—calculated as the amount of H2 (L) synthesized per 1 kg (measured in total solids (TS)) or 1 L (measured in TOC) of waste;
- Waste degradation coefficient (Kd)—the degree of waste digestion. For SFW, this parameter was calculated as the ratio of initial and final weight of the waste based on TS (Kd = m1: m2, where m1 is the initial weight of dry waste; m2 is the weight of dry detritus). To determine the initial weight, the waste was dried to completion at 105 °C and weighed. To determine the weight of detritus, the non-fermented residues after fermentation were washed in distilled water, in the solution of weak organic acid, and then again in distilled water. The washed residues were dried at 105 °C and weighed. For LFW, the Kd was calculated as the ratio of the initial and final concentrations of TOC in the culture medium.
2.6. Theory/Calculation
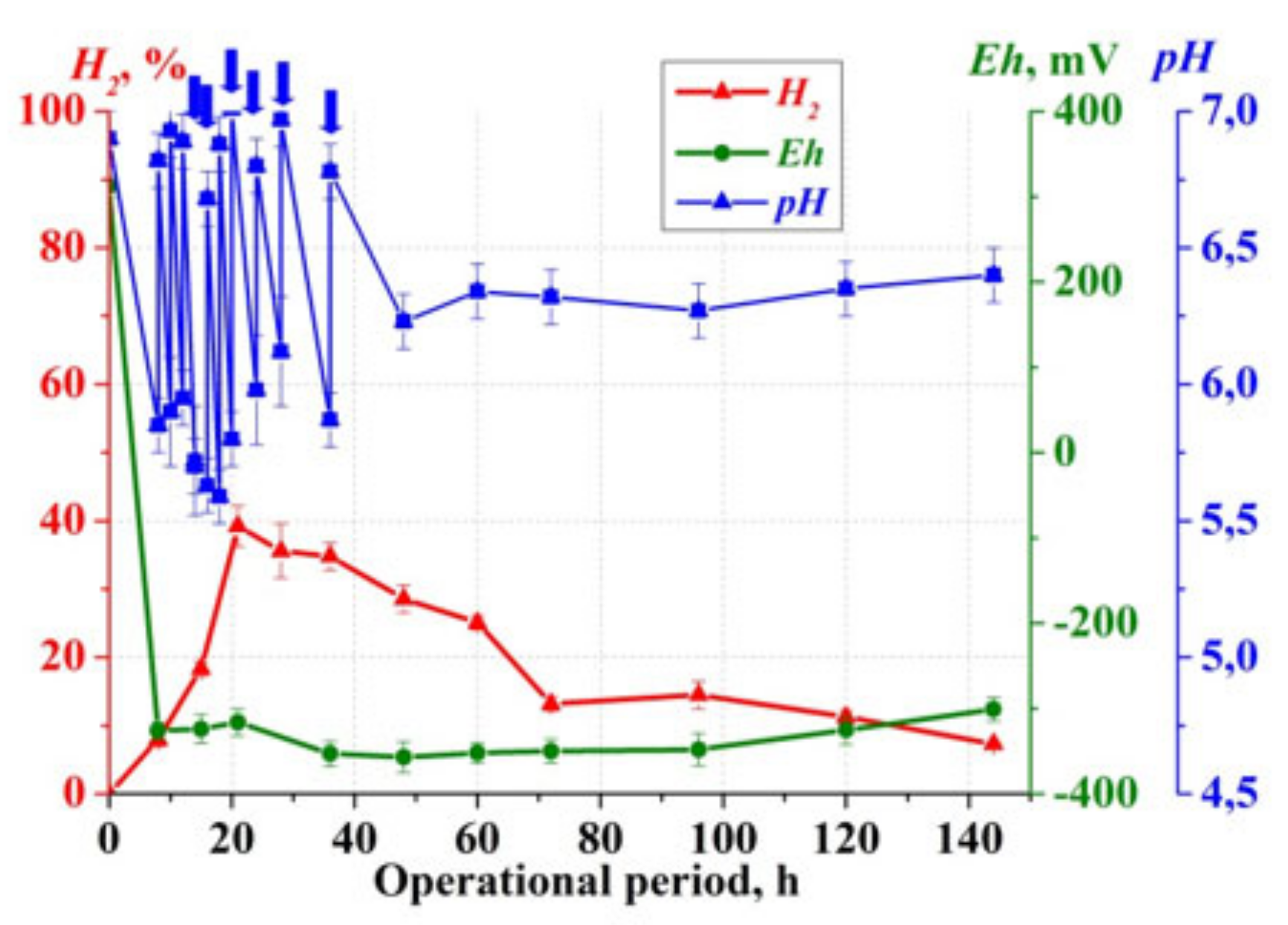
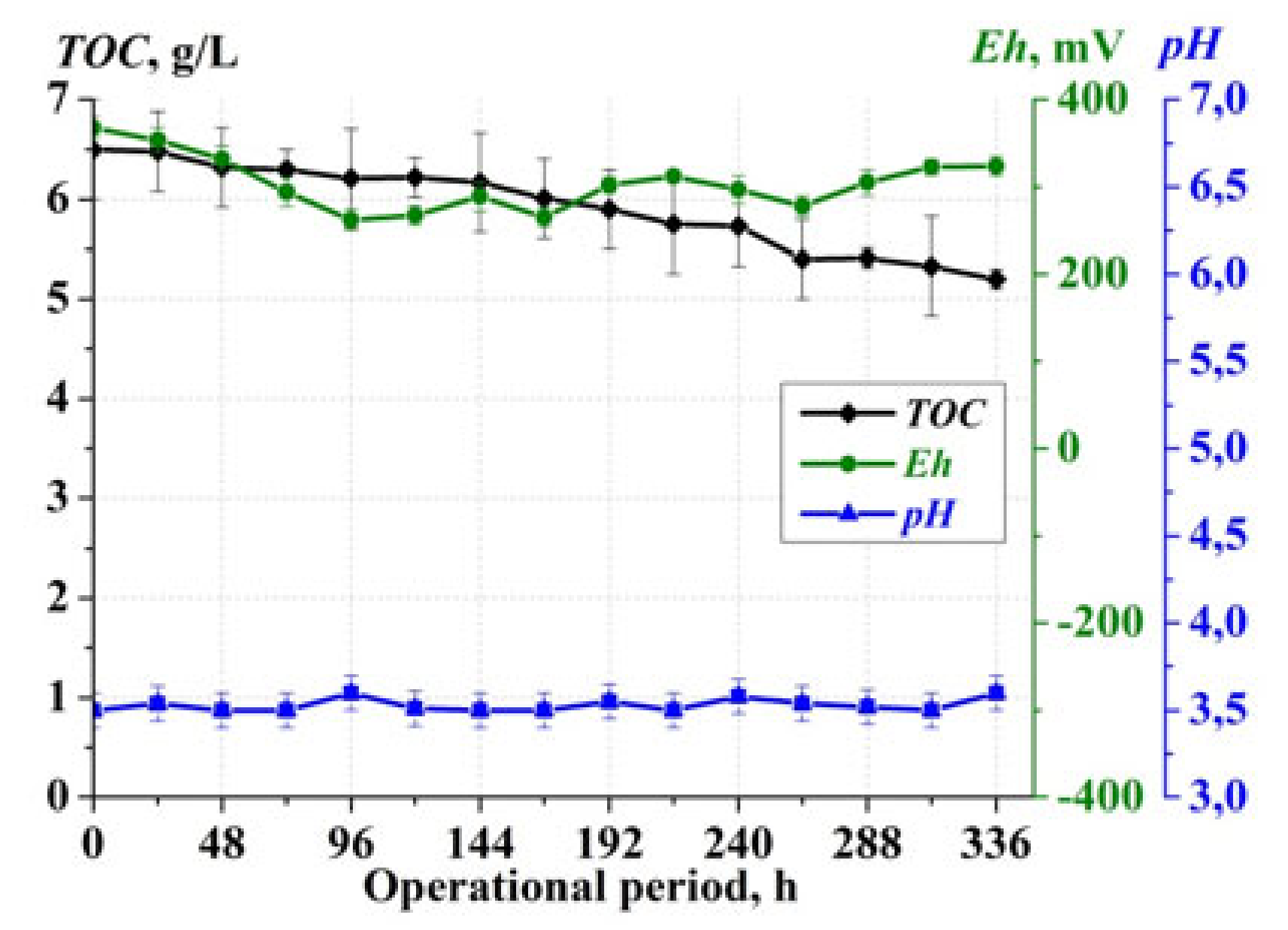
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christopher, K.; Dimitrios, R. A Review on Exergy Comparison of Hydrogen Production Methods from Renewable Energy Sources. Energy Environ. Sci. 2012, 5, 6640–6651. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Saratale, G.D.; Chen, S.-D.; Lo, Y.-C.; Saratale, R.G.; Chang, J.-S. Outlook of Biohydrogen Production from Lignocellulosic Feedstock Using Dark Fermentation—a Review. J. Sci. Ind. Res. 2008, 67, 962–979. [Google Scholar]
- Kovacova, M.; Kliestik, T.; Valaskova, K.; Durana, P.; Juhaszova, Z. Systematic Review of Variables Applied in Bankruptcy Prediction Models of Visegrad Group Countries. Oeconomia Copernic. 2019, 10, 743–772. [Google Scholar] [CrossRef]
- Nguyen, T.-A.D.; Han, S.J.; Kim, J.P.; Kim, M.S.; Sim, S.J. Hydrogen Production of the Hyperthermophilic Eubacterium, Thermotoga Neapolitana under N2 Sparging Condition. Bioresour. Technol. 2010, 101, S38–S41. [Google Scholar] [CrossRef]
- Yun, Y.-M.; Lee, M.-K.; Im, S.-W.; Marone, A.; Trably, E.; Shin, S.-R.; Kim, M.-G.; Cho, S.-K.; Kim, D.-H. Biohydrogen Production from Food Waste: Current Status, Limitations, and Future Perspectives. Bioresour. Technol. 2018, 248, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, C.; Giuliano, A.; Bolzonella, D.; Pavan, P.; Cecchi, F. Bio-Hythane Production from Food Waste by Dark Fermentation Coupled with Anaerobic Digestion Process: A Long-Term Pilot Scale Experience. Int. J. Hydrogen Energy 2012, 37, 11549–11555. [Google Scholar] [CrossRef]
- Paritosh, K.; Kushwaha, S.K.; Yadav, M.; Pareek, N.; Chawade, A.; Vivekanand, V. Food Waste to Energy: An Overview of Sustainable Approaches for Food Waste Management and Nutrient Recycling. Biomed Res. Int. 2017, 2017, 1–19. [Google Scholar] [CrossRef]
- Han, W.; Hu, Y.; Li, S.; Li, F.; Tang, J. Biohydrogen Production in the Suspended and Attached Microbial Growth Systems from Waste Pastry Hydrolysate. Bioresour. Technol. 2016, 218, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Uçkun Kiran, E.; Trzcinski, A.P.; Ng, W.J.; Liu, Y. Bioconversion of Food Waste to Energy: A Review. Fuel 2014, 134, 389–399. [Google Scholar] [CrossRef]
- Guerrero, L.A.; Maas, G.; Hogland, W. Solid Waste Management Challenges for Cities in Developing Countries. Waste Manag. 2013, 33, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, I.K.; Kargi, F. Bio-Hydrogen Production from Waste Materials. Enzym. Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Ting, C.H.; Lee, D.J. Production of Hydrogen and Methane from Wastewater Sludge Using Anaerobic Fermentation. Int. J. Hydrogen Energy 2007, 32, 677–682. [Google Scholar] [CrossRef]
- Yasin, N.H.M.; Mumtaz, T.; Hassan, M.A.; Abd Rahman, N. Food Waste and Food Processing Waste for Biohydrogen Production: A Review. J. Environ. Manag. 2013, 130, 375–385. [Google Scholar] [CrossRef]
- Vatsala, T.M.; Raj, S.M.; Manimaran, A. A Pilot-Scale Study of Biohydrogen Production from Distillery Effluent Using Defined Bacterial Co-Culture. Int. J. Hydrogen Energy 2008, 33, 5404–5415. [Google Scholar] [CrossRef]
- Madeira, J.G.F.; Delgado, A.R.S.; Boloy, R.A.M.; Coutinho, E.R.; Loures, C.C.A. Exergetic and Economic Evaluation of Incorporation of Hydrogen Production in a Cassava Wastewater Plant. Appl. Therm. Eng. 2017, 123, 1072–1078. [Google Scholar] [CrossRef]
- van Niel, E.W.J.; Budde, M.A.W.; de Haas, G.G.; van der Wal, F.J.; Claassen, P.A.M.; Stams, A.J.M. Distinctive Properties of High Hydrogen Producing Extreme Thermophiles, Caldicellulosiruptor Saccharolyticus and Thermotoga Elfii. Int. J. Hydrogen Energy 2002, 27, 1391–1398. [Google Scholar] [CrossRef]
- Maroušek, J.; Strunecký, O.; Kolář, L.; Vochozka, M.; Kopecký, M.; Maroušková, A.; Batt, J.; Poliak, M.; Šoch, M.; Bartoš, P.; et al. Advances in Nutrient Management Make It Possible to Accelerate Biogas Production and Thus Improve the Economy of Food Waste Processing. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 1–10. [Google Scholar] [CrossRef]
- Han, W.; Fang, J.; Liu, Z.; Tang, J. Techno-Economic Evaluation of a Combined Bioprocess for Fermentative Hydrogen Production from Food Waste. Bioresour. Technol. 2016, 202, 107–112. [Google Scholar] [CrossRef]
- Ezeji, T.; Qureshi, N.; Blaschek, H.P. Butanol Production from Agricultural Residues: Impact of Degradation Products on Clostridium Beijerinckii Growth and Butanol Fermentation. Biotechnol. Bioeng. 2007, 97, 1460–1469. [Google Scholar] [CrossRef]
- Chen, W.-H. Biological Hydrogen Production by Anaerobic Fermentation. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2006. [Google Scholar] [CrossRef][Green Version]
- Wu, C.-W.; Whang, L.-M.; Cheng, H.-H.; Chan, K.-C. Fermentative Biohydrogen Production from Lactate and Acetate. Bioresour. Technol. 2012, 113, 30–36. [Google Scholar] [CrossRef]
- Ren, N.; Li, J.; Li, B.; Wang, Y.; Liu, S. Biohydrogen Production from Molasses by Anaerobic Fermentation with a Pilot-Scale Bioreactor System. Int. J. Hydrogen Energy 2006, 31, 2147–2157. [Google Scholar] [CrossRef]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R. A Review of Dark Fermentative Hydrogen Production from Biodegradable Municipal Waste Fractions. Waste Manag. 2013, 33, 1345–1361. [Google Scholar] [CrossRef]
- Kongjan, P.; Min, B.; Angelidaki, I. Biohydrogen Production from Xylose at Extreme Thermophilic Temperatures (70 °C) by Mixed Culture Fermentation. Water Res. 2009, 43, 1414–1424. [Google Scholar] [CrossRef]
- Poggi-Varaldo, H.M.; Munoz-Paez, K.M.; Escamilla-Alvarado, C.; Robledo-Narváez, P.N.; Ponce-Noyola, M.T.; Calva-Calva, G.; Ríos-Leal, E.; Galíndez-Mayer, J.; Estrada-Vázquez, C.; Ortega-Clemente, A.; et al. Biohydrogen, Biomethane and Bioelectricity as Crucial Components of Biorefinery of Organic Wastes: A Review. Waste Manag Res 2014, 32, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Sinha, P.; Pandey, A. An Evaluative Report and Challenges for Fermentative Biohydrogen Production. Int. J. Hydrogen Energy 2011, 36, 7460–7478. [Google Scholar] [CrossRef]
- Ntaikou, I.; Antonopoulou, G.; Lyberatos, G. Biohydrogen Production from Biomass and Wastes via Dark Fermentation: A Review. Waste Biomass Valor 2010, 1, 21–39. [Google Scholar] [CrossRef]
- Moreno-Andrade, I.; Buitrón, G. Evaluation of particle size and initial concentration of total solids on biohydrogen production from food waste. Fresenius Environ. Bull. 2015, 24, 8. [Google Scholar]
- Berezkin, V.G.; Drugov, Y.S. Gas Chromatography in Air Pollution Analysis; Elsevier: Amsterdam, The Netherlands, 1991; ISBN 978-0-08-085856-2. [Google Scholar]
- Suslova, O.; Govorukha, V.; Brovarskaya, O.; Matveeva, N.; Tashyreva, H.; Tashyrev, O. Method for Determining Organic Compound Concentration in Biological Systems by Permanganate Redox Titration. Int. J. Bioautom. 2014, 18, 45–52. [Google Scholar]
- Noike, T.; Mizuno, O. Hydrogen Fermentation of Organic Municipal Wastes. Water Sci. Technol. 2000, 42, 155–162. [Google Scholar] [CrossRef]
- Hovorukha, V.M.; Tashyrev, O.B.; Matvieieva, N.A.; Tashyreva, H.O.; Havryliuk, O.A.; Bielikova, O.I.; Sioma, I.B. Integrated Approach for Development of Environmental Biotechnologies for Treatment of Solid Organic Waste and Obtaining of Biohydrogen and Lignocellulosic Substrate. Environ. Res. Eng. Manag. 2019, 74, 31–42. [Google Scholar] [CrossRef]
- Ghimire, A.; Sposito, F.; Frunzo, L.; Trably, E.; Escudié, R.; Pirozzi, F.; Lens, P.N.L.; Esposito, G. Effects of Operational Parameters on Dark Fermentative Hydrogen Production from Biodegradable Complex Waste Biomass. Waste Manag. 2016, 50, 55–64. [Google Scholar] [CrossRef]
- Lay, J.-J.; Fan, K.-S.; Chang, J.; Ku, C.-H. Influence of Chemical Nature of Organic Wastes on Their Conversion to Hydrogen by Heat-Shock Digested Sludge. Int. J. Hydrogen Energy 2003, 28, 1361–1367. [Google Scholar] [CrossRef]
- Saxena, R.C.; Adhikari, D.K.; Goyal, H.B. Biomass-Based Energy Fuel through Biochemical Routes: A Review. Renew. Sustain. Energy Rev. 2009, 13, 167–178. [Google Scholar] [CrossRef]
- Tashyrev, O.; Govorukha, V.; Havryliuk, O. The effect of mixing modes on biohydrogen yield and spatial ph gradient at dark fermentation of solid food waste. Ecol. Eng. Environ. Prot. 2017, 53–62. [Google Scholar] [CrossRef]
- Norris, J.R.; Ribbons, D.W. Methods in Microbiology; Academic Press: Cambridge, MA, USA, 1970; ISBN 978-0-08-086027-5. [Google Scholar]
- Herrero, A.A.; Gomez, R.F.; Snedecor, B.; Tolman, C.J.; Roberts, M.F. Growth Inhibition of Clostridium Thermocellum by Carboxylic Acids: A Mechanism Based on Uncoupling by Weak Acids. Appl. Microbiol. Biotechnol. 1985, 22, 53–62. [Google Scholar] [CrossRef]
- Nakai, S.A.; Siebert, K.J. Validation of Bacterial Growth Inhibition Models Based on Molecular Properties of Organic Acids. Int. J. Food Microbiol. 2003, 86, 249–255. [Google Scholar] [CrossRef]
- Omar, H.; Rohani, S. Treatment of Landfill Waste, Leachate and Landfill Gas: A Review. Front. Chem. Sci. Eng. 2015, 9, 15–32. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, M.-S. Hydrogenases for Biological Hydrogen Production. Bioresour. Technol. 2011, 102, 8423–8431. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Patel, S.K.S.; Lee, J.-K.; Kalia, V.C. Extending the Limits of Bacillus for Novel Biotechnological Applications. Biotechnol. Adv. 2013, 31, 1543–1561. [Google Scholar] [CrossRef]
- Bartacek, J.; Zabranska, J.; Lens, P.N.L. Developments and Constraints in Fermentative Hydrogen Production. Biofuels Bioprod. Biorefining 2007, 1, 201–214. [Google Scholar] [CrossRef]
- Ito, T.; Nakashimada, Y.; Senba, K.; Matsui, T.; Nishio, N. Hydrogen and Ethanol Production from Glycerol-Containing Wastes Discharged after Biodiesel Manufacturing Process. J. Biosci. Bioeng. 2005, 100, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Yadav, K.K.; Kumar, V. A Review on Current Status of Municipal Solid Waste Management in India. J. Environ. Sci. 2015, 37, 206–217. [Google Scholar] [CrossRef]
- Wang, J.; Wan, W. Factors Influencing Fermentative Hydrogen Production: A Review. Int. J. Hydrogen Energy 2009, 34, 799–811. [Google Scholar] [CrossRef]
- Ghosh, D.; Hallenbeck, P.C. Fermentative Hydrogen Yields from Different Sugars by Batch Cultures of Metabolically Engineered Escherichia Coli DJT135. Int. J. Hydrogen Energy 2009, 34, 7979–7982. [Google Scholar] [CrossRef]
- Thong, S.; Prasertsan, P.; Karakashev, D.; Angelidaki, I. Thermophilic Fermentative Hydrogen Production by the Newly Isolated Thermoanaerobacterium Thermosaccharolyticum PSU-2. Int. J. Hydrogen Energy 2008, 33, 1204–1214. [Google Scholar] [CrossRef]
- Gupta, S.K.; Kumari, S.; Reddy, K.; Bux, F. Trends in Biohydrogen Production: Major Challenges and State-of-the-Art Developments. Environ. Technol. 2013, 34, 1653–1670. [Google Scholar] [CrossRef]
- Mizuno, O.; Dinsdale, R.; Hawkes, F.R.; Hawkes, D.L.; Noike, T. Enhancement of Hydrogen Production from Glucose by Nitrogen Gas Sparging. Bioresour. Technol. 2000, 73, 59–65. [Google Scholar] [CrossRef]
- Shi, X.-Y.; Yu, H.-Q. Continuous Production of Hydrogen from Mixed Volatile Fatty Acids with Rhodopseudomonas Capsulata. Int. J. Hydrogen Energy 2006, 31, 1641–1647. [Google Scholar] [CrossRef]
- Li, C.; Fang, H.H.P. Fermentative Hydrogen Production From Wastewater and Solid Wastes by Mixed Cultures. Crit. Rev. Environ. Sci. Technol. 2007, 37, 1–39. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.-H.; Li, L.; Sung, S. Biological Hydrogen Production: Effects of PH and Intermediate Products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Wang, A.-J.; Cao, G.-L.; Liu, W.-Z. Biohydrogen Production from Anaerobic Fermentation. In Biotechnology in China III: Biofuels and Bioenergy; Bai, F.-W., Liu, C.-G., Huang, H., Tsao, G.T., Eds.; Advances in Biochemical Engineering Biotechnology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 143–163. ISBN 978-3-642-28478-6. [Google Scholar]
- Ramos, C.; Buitrón, G.; Moreno-Andrade, I.; Chamy, R. Effect of the Initial Total Solids Concentration and Initial PH on the Bio-Hydrogen Production from Cafeteria Food Waste. Int. J. Hydrogen Energy 2012, 37, 13288–13295. [Google Scholar] [CrossRef]
- Fountoulakis, M.S.; Manios, T. Enhanced Methane and Hydrogen Production from Municipal Solid Waste and Agro-Industrial by-Products Co-Digested with Crude Glycerol. Bioresour. Technol. 2009, 100, 3043–3047. [Google Scholar] [CrossRef]
- Ma, J.; Ke, S.; Chen, Y. Mesophilic Biohydrogen Production from Food Waste. In Proceedings of the 2008 2nd International Conference on Bioinformatics and Biomedical Engineering, Shanghai, China, 16–18 May 2008; pp. 2841–2844. [Google Scholar]
- Das, D.; Veziroglu, T.N. Advances in Biological Hydrogen Production Processes. Int. J. Hydrogen Energy 2008, 33, 6046–6057. [Google Scholar] [CrossRef]
- Yokoi, H.; Saitsu, A.; Uchida, H.; Hirose, J.; Hayashi, S.; Takasaki, Y. Microbial Hydrogen Production from Sweet Potato Starch Residue. J. Biosci. Bioeng. 2001, 91, 58–63. [Google Scholar] [CrossRef]
- Kim, H.Y. A Low Cost Production of Hydrogen from Carbonaceous Wastes. Int. J. Hydrogen Energy 2003, 28, 1179–1186. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Fan, Y.-T.; Xing, Y.; Pan, C.-M.; Zhang, G.-S.; Lay, J.-J. Enhanced Biohydrogen Production from Cornstalk Wastes with Acidification Pretreatment by Mixed Anaerobic Cultures. Biomass Bioenergy 2007, 31, 250–254. [Google Scholar] [CrossRef]
- Elbeshbishy, E.; Nakhla, G.; Hafez, H. Biochemical Methane Potential (BMP) of Food Waste and Primary Sludge: Influence of Inoculum Pre-Incubation and Inoculum Source. Bioresour. Technol. 2012, 110, 18–25. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen Fermentation of Food Waste without Inoculum Addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- Angenent, L.T.; Karim, K.; Al-Dahhan, M.H.; Wrenn, B.A.; Domíguez-Espinosa, R. Production of Bioenergy and Biochemicals from Industrial and Agricultural Wastewater. Trends Biotechnol. 2004, 22, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Tashyrev, O.; Hovorukha, V.; Havryliuk, O.; Sioma, I.; Matvieieva, N.; Tashyreva, H.; Yastremska, L.; Bielikova, O. Perspectives of the development of the industrial technologies of obtaining biohydrogen during multicomponent food waste microbial destruction. In Hydrogen in Alternative Energy and New Technologies; KIM: Kyiv, Ukraine, 2018; p. 294. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hovorukha, V.; Havryliuk, O.; Gladka, G.; Tashyrev, O.; Kalinichenko, A.; Sporek, M.; Dołhańczuk-Śródka, A. Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste. Energies 2021, 14, 1831. https://doi.org/10.3390/en14071831
Hovorukha V, Havryliuk O, Gladka G, Tashyrev O, Kalinichenko A, Sporek M, Dołhańczuk-Śródka A. Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste. Energies. 2021; 14(7):1831. https://doi.org/10.3390/en14071831
Chicago/Turabian StyleHovorukha, Vira, Olesia Havryliuk, Galina Gladka, Oleksandr Tashyrev, Antonina Kalinichenko, Monika Sporek, and Agnieszka Dołhańczuk-Śródka. 2021. "Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste" Energies 14, no. 7: 1831. https://doi.org/10.3390/en14071831
APA StyleHovorukha, V., Havryliuk, O., Gladka, G., Tashyrev, O., Kalinichenko, A., Sporek, M., & Dołhańczuk-Śródka, A. (2021). Hydrogen Dark Fermentation for Degradation of Solid and Liquid Food Waste. Energies, 14(7), 1831. https://doi.org/10.3390/en14071831











