Facile Synthesis of Lanthanum Strontium Cobalt Ferrite (LSCF) Nanopowders Employing an Ion-Exchange Promoted Sol-Gel Process
Abstract
1. Introduction
2. Materials and Methods
3. Results
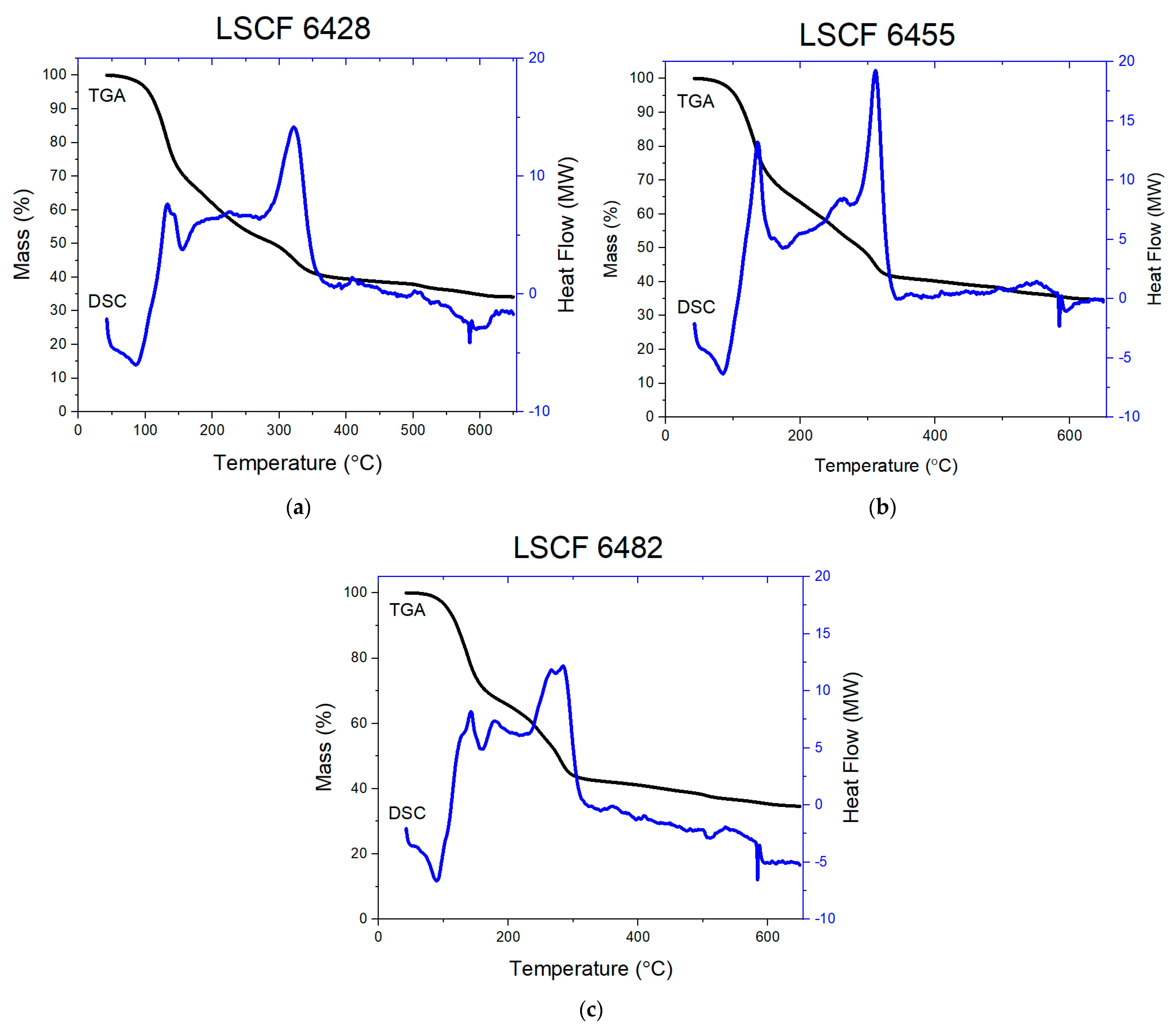
3.1. Thermal Analysis
- At low temperature around 100 °C, the initial dehydration of beads occurred liberating any bound water molecules. The samples first absorbed heat from the surroundings to have enough kinetic energy to release bound water molecules. This caused endothermic peaks in the DSC profile matching to a small weight loss in the TGA profile.
- At higher temperature (100–300 °C), the decomposition peak was exothermic due to the on-going oxidative decomposition of the dried beads. A large loss in weight was observed in the TGA profile due to the breakage of G-G, G-M, and M-M weaker linkages in the alginate polysaccharide molecule causing a substantial liberation of bridging oxygen. At once, this stimulated the oxidation of La3+, Sr2+, Co2+, and Fe3+ in the alginate structure to form a mixture of the respective metastable metal oxides phases.
- The further decomposition occurred as the temperature increased (300–500 °C) and resulted in a small weight loss in the TGA profile, which was caused by the decomposition of metastable oxides to more stable oxides (La2O3, SrO, CoO, and Fe2O3) that manifested in a small exothermic peak.
- The further increase in temperature, around 500 °C, entirely oxidized the remaining β-D-manuronic acid (M) and α-L-guluronic acid (G), as well as the simultaneous formation of LSCF pseudoquaternary compound leading to the large exothermic peak in the DSC profile.
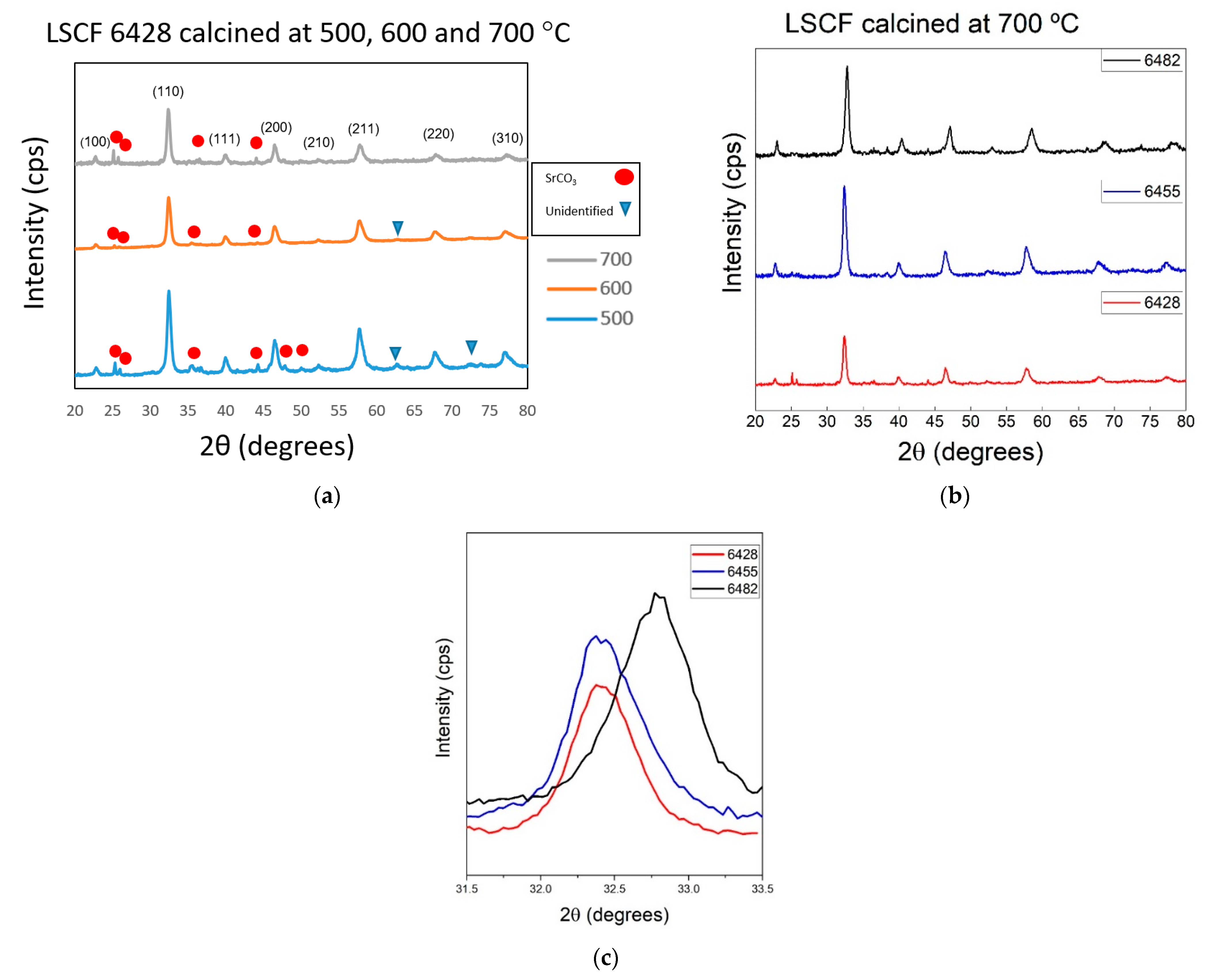
3.2. Material Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choudhury, A.; Chandra, H.; Arora, A. Application of solid oxide fuel cell technology for power generation—A review. Renew. Sust. Energ. Rev. 2013, 20, 430–442. [Google Scholar] [CrossRef]
- Conceiocao, L.D.; Silva, A.M.; Ribeiro, N.F.P.; Souza, M.M.V.M. Combustion synthesis of (LSCF) porous materials for application as cathode in IT-SOFC. Mater. Res. Bull. 2011, 46, 308–314. [Google Scholar] [CrossRef]
- Vargas, R.A.; Bonturim, E.; Andreoli, M.; Chiba, R.; Seo, E.S.M. Characterization of LSCF-based composite and LSCF as cathodes for intermediate temperature SOFCs. Mater. Sci. Forum 2012, 727–728, 657–662. [Google Scholar] [CrossRef]
- Badwal, S.P.S.; Giddey, S.; Munnings, C.; Kulkarni, A. Review of Progress in High Temperature Solid Oxide Fuel Cells. J. Aust. Ceram. Soc. 2014, 50, 23–37. [Google Scholar]
- Sun, Y.; He, S.; Saunders, M.; Chen, K.; Shao, Z.; Jiang, S.P. A comparative study of surface segregation and interface of La0·6Sr0·4Co0·2Fe0·8O3-δ electrode on GDC and YSZ electrolytes of solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 2606–2616. [Google Scholar] [CrossRef]
- Simner, S.P.; Anderson, M.D.; Engelhard, M.H.; Stevenson, J.W. Degradation mechanisms of La-Sr-Co-Fe-O3SOFC cathodes Electrochem. Solid State Lett. 2006, 9, A478–A481. [Google Scholar] [CrossRef]
- Wang, Z.; Kale, G.M.; Ghadiri, M. Synthesis and characterization of CexGd1−xO2−δ nanopowders employing an alginate mediated ion-exchange process. Chem. Eng. J. 2012, 198–199, 149–153. [Google Scholar] [CrossRef]
- Sardar, S.; Kale, G.; Ghadiri, M.; Cespedas, O. Structural Study of Holmium Zirconate Nanoparticles obtained through Carbon Neutral Sol-gel Process. Thermochim. Acta 2019, 676, 120–129. [Google Scholar] [CrossRef]
- Rahayu, S.; Forrester, J.S.; Kale, G.M.; Ghadiri, M. Promising solid electrolyte material for an IT-SOFC: Crystal structure of the cerium gadolinium holmium oxide Ce₀.₈Gd₀.₁Ho₀.₁O₁.₉ between 295 and 1023 K. Acta Cryst. Sect. C 2018, 74, 236–239. [Google Scholar] [CrossRef] [PubMed]
- Kosanke, K.L.; Kosanke, B.J.; Sturman, B.T.; Winokur, R.M. Encyclopedic Dictionary of Pyrotechnics; Journal of Pyrotechnics: Whitewater, WI, USA, 2012. [Google Scholar]
- Wang, Z.; Kale, G.M.; Ghadiri, M. Novel Ion-Exchange Process for the Preparation of Metal Oxide Nanopowders from Sodium Alginate. J. Am. Ceram. Soc. 2012, 95, 3124–3129. [Google Scholar] [CrossRef]
- Sardar, S.; Kale, G.M.; Cespedes, O.; Ghadiri, M. Environmentally sustainable facile synthesis of nanocrystalline Holmium Hafnate (Ho2Hf2O7): Promising new oxide-ion conducting solid electrolyte. Springer Nat. Appl. Sci. 2020, 2, 541–552. [Google Scholar] [CrossRef]
- Kale, G.M.; Jacob, K.T. Phase relations and thermodynamic properties of compounds in the pseudobinary system BaO-Y2O3. Solid State Ion. 1989, 43, 247–252. [Google Scholar] [CrossRef]
- Hardy, J.S.; Templeton, J.W.; Edwards, D.J.; Lu, Z.; Stevenson, J.W. 2012. Lattice Expansion of LSCF-6428 Cathodes Measured by In-Situ XRD during SOFC Operation. J. Power Sources 2012, 198, 76–82. [Google Scholar] [CrossRef]
- Cheng, J.; Zhang, M.; Jiang, Y.; Zou, L.; Gong, Y.; Chi, B.; Pu, J.; Jian, L. Perovskite La0.6Sr0.4Co0.2Fe0.8O3 as an effective electrocatalyst for non-aqueous lithium air batteries. Electrochim. Acta 2016, 191, 106–115. [Google Scholar] [CrossRef]
- Kuhn, J.N.; Ozkan, U.S. Effect of Co Content upon the Bulk Structure of Sr- and Co-doped LaFeO3. Catal. Lett. 2008, 121, 179–188. [Google Scholar] [CrossRef]
- Partovi, K.; Geppert, B.; Liang, F.; Rüscher, C.H.; Caro, J. Effect of the B-Site Composition on the Oxygen Permeability and the CO2 Stability of Pr0.6Sr0.4CoxFe1−xO3−δ (0.0 ≤ x ≤ 1.0) Membranes. Chem. Mater. 2015, 27, 2911–2919. [Google Scholar] [CrossRef]

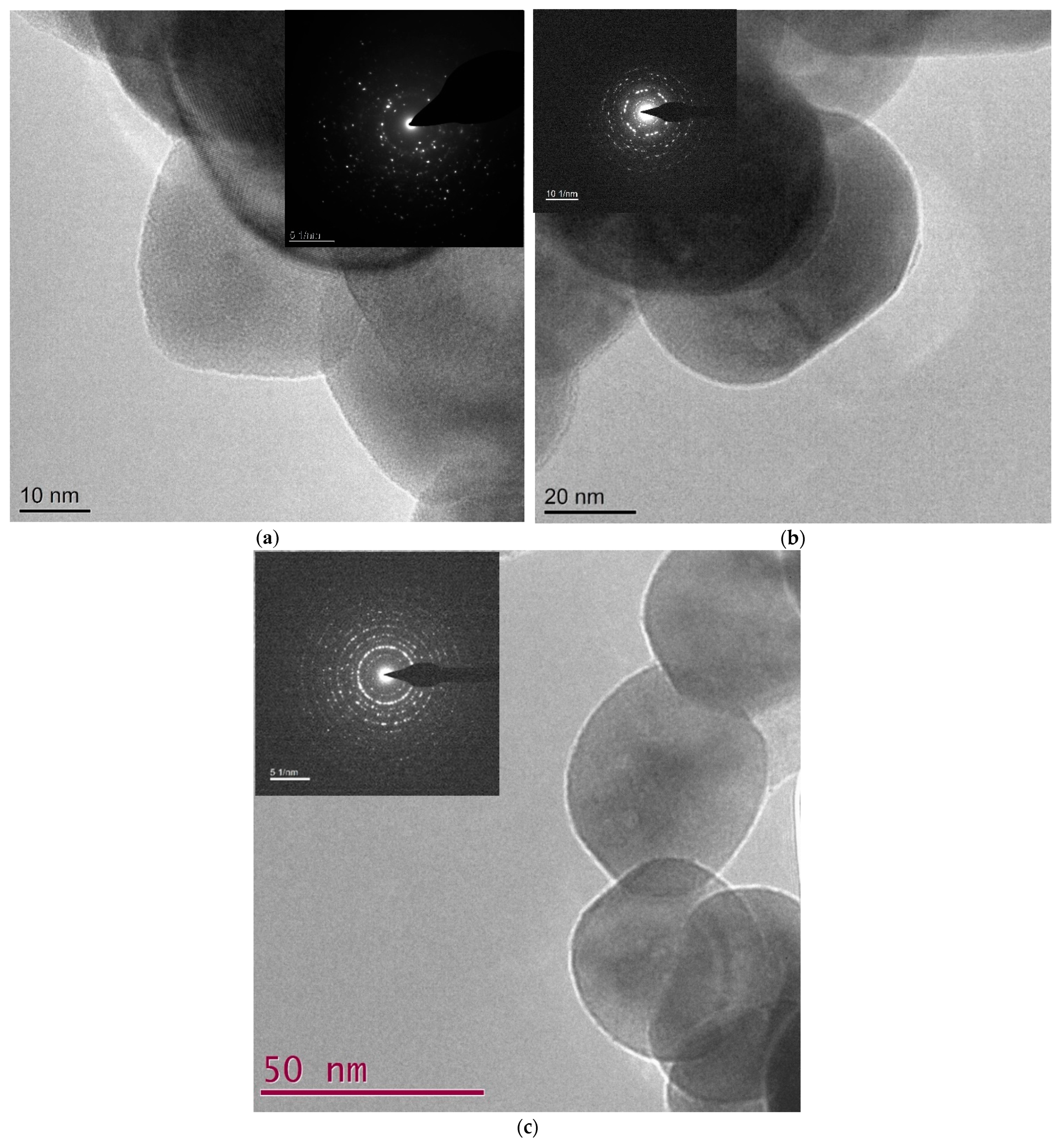

| LSCF | Miller Indices | XRD | TEM |
|---|---|---|---|
| h k l | (Å) | (Å) | |
| 6428 | 1 0 0 | 3.907 | 3.933 |
| 1 1 0 | 2.767 | 2.937 | |
| 1 1 1 | 2.257 | 2.377 | |
| 2 0 0 | 1.953 | 1.991 | |
| 6455 | 1 0 0 | 3.901 | 3.872 |
| 1 1 0 | 2.769 | 2.826 | |
| 1 1 1 | 2.253 | 2.362 | |
| 2 0 0 | 1.958 | 1.954 | |
| 6482 | 1 0 0 | 3.865 | 3.865 |
| 1 1 0 | 2.729 | 2.826 | |
| 1 1 1 | 2.232 | 2.259 | |
| 2 0 0 | 1.926 | 1.996 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahayu, S.; Fatah, A.A.; Kale, G.M. Facile Synthesis of Lanthanum Strontium Cobalt Ferrite (LSCF) Nanopowders Employing an Ion-Exchange Promoted Sol-Gel Process. Energies 2021, 14, 1800. https://doi.org/10.3390/en14071800
Rahayu S, Fatah AA, Kale GM. Facile Synthesis of Lanthanum Strontium Cobalt Ferrite (LSCF) Nanopowders Employing an Ion-Exchange Promoted Sol-Gel Process. Energies. 2021; 14(7):1800. https://doi.org/10.3390/en14071800
Chicago/Turabian StyleRahayu, Sri, Adi Ab Fatah, and Girish M. Kale. 2021. "Facile Synthesis of Lanthanum Strontium Cobalt Ferrite (LSCF) Nanopowders Employing an Ion-Exchange Promoted Sol-Gel Process" Energies 14, no. 7: 1800. https://doi.org/10.3390/en14071800
APA StyleRahayu, S., Fatah, A. A., & Kale, G. M. (2021). Facile Synthesis of Lanthanum Strontium Cobalt Ferrite (LSCF) Nanopowders Employing an Ion-Exchange Promoted Sol-Gel Process. Energies, 14(7), 1800. https://doi.org/10.3390/en14071800





