Tribo-Electrostatic Separation Analysis of a Beneficial Solution in the Recycling of Mixed Poly(Ethylene Terephthalate) and High-Density Polyethylene
Abstract
1. Introduction
1.1. Electrostatic Separation Method
1.2. Triboelectrification Process
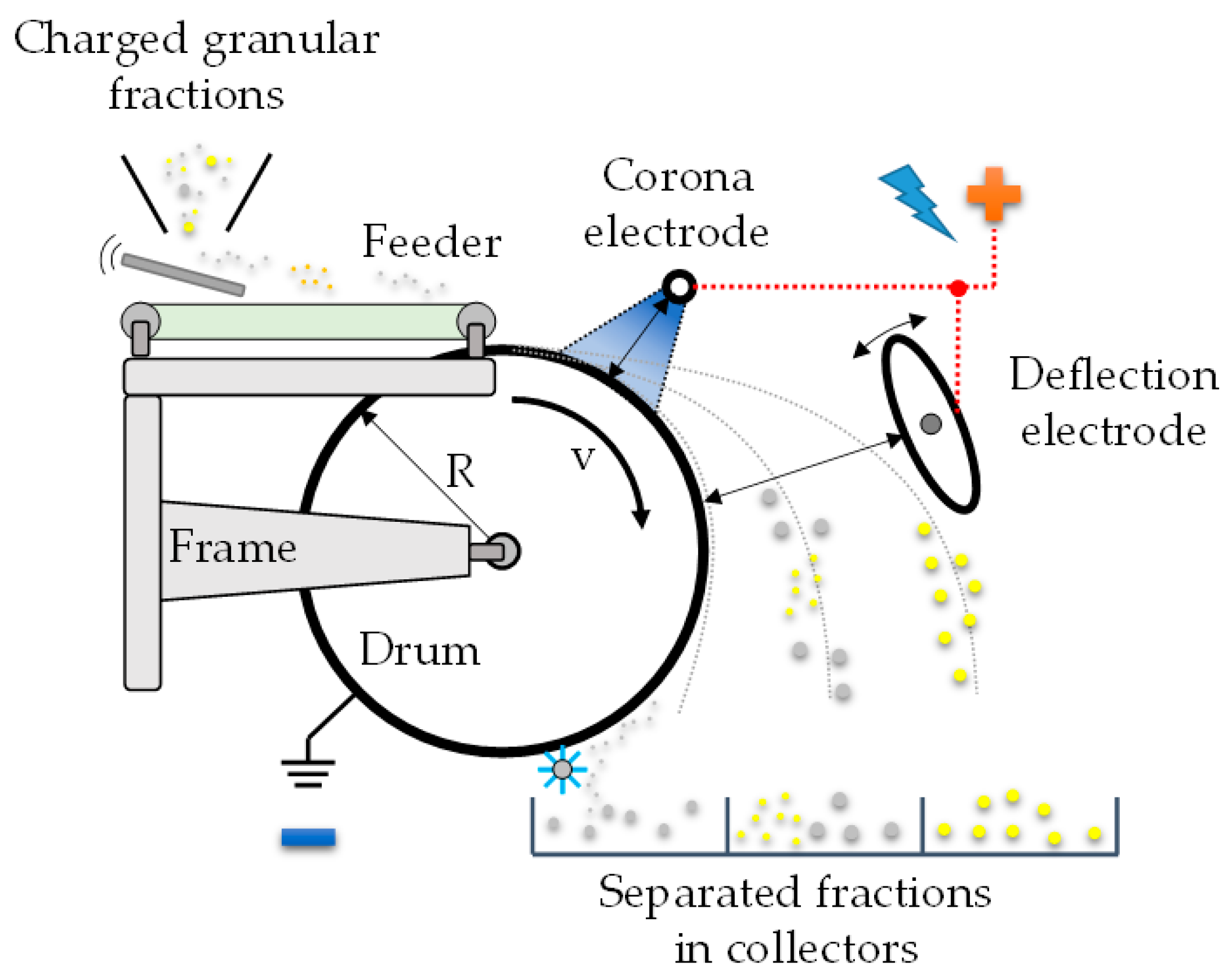
1.3. Types of Electrostatic Separators
2. Materials and Methods
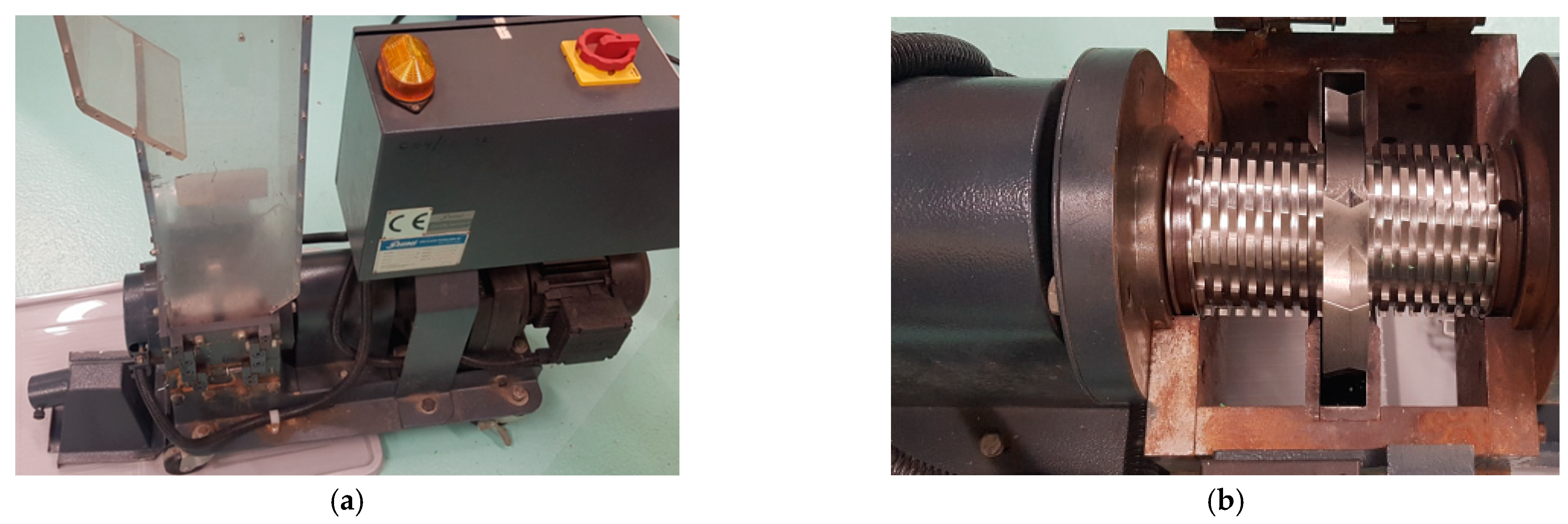
2.1. Materials and Samples Preparation
2.2. Electrification of Polymer Fractions
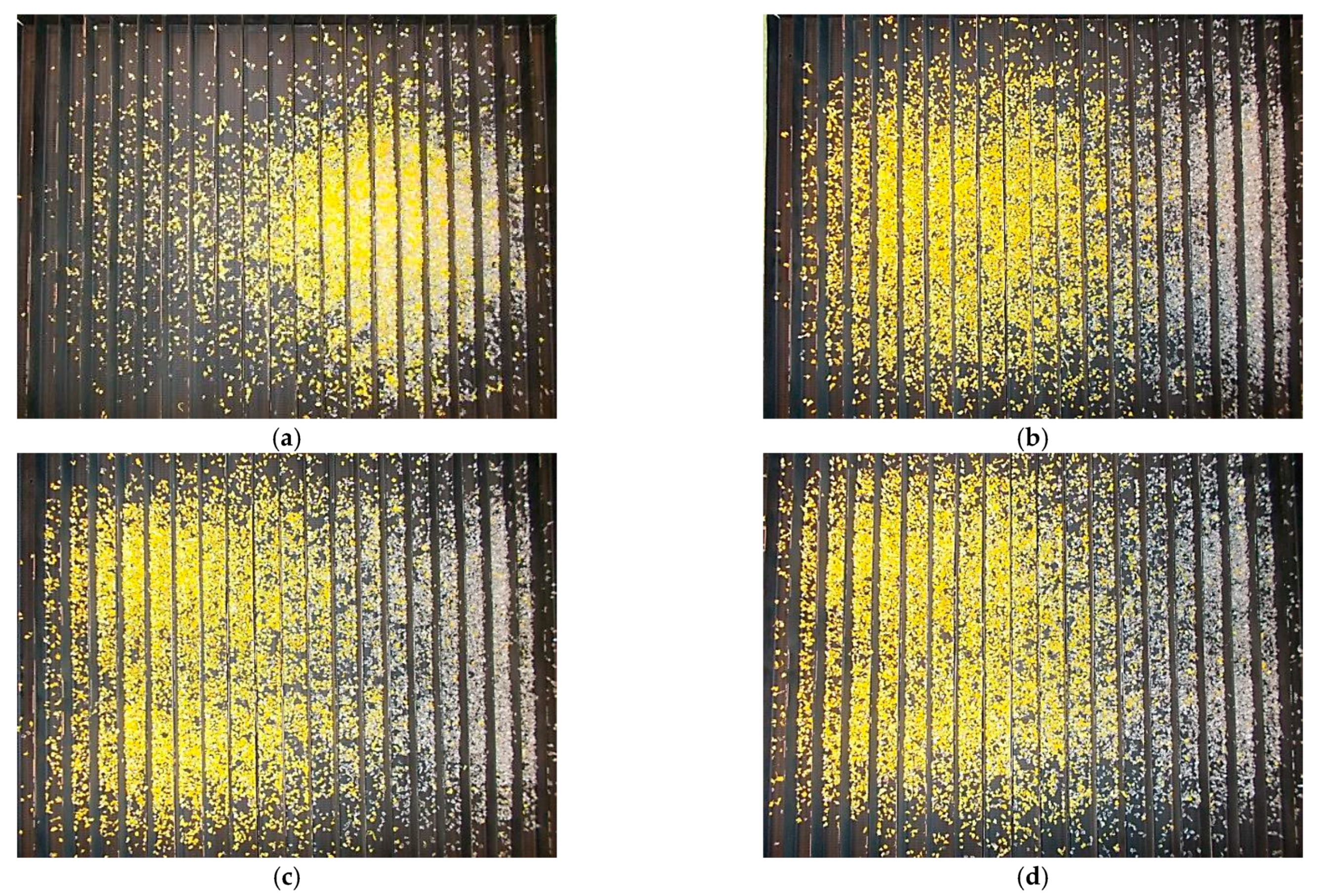
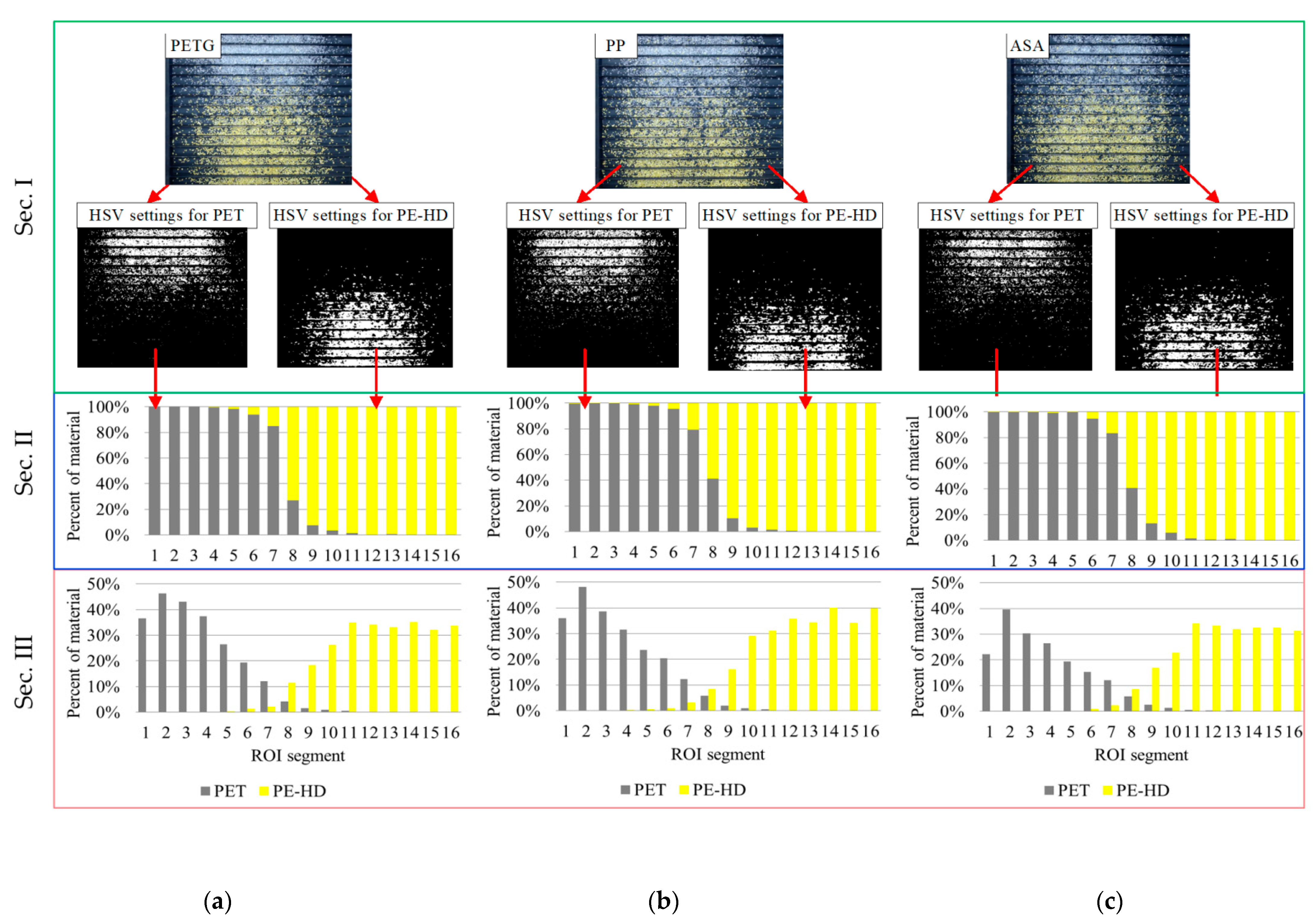
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaleski, P.; Chawla, Y. Circular Economy in Poland: Profitability Analysis for Two Methods of Waste Processing in Small Municipalities. Energies 2020, 13, 5166. [Google Scholar] [CrossRef]
- Duda, A.; Fenicki, A.; Molski, P.; Szostak, E.; Duda, P. Design and Operation of a Modern Polish Plant for Plastic Waste Recycling through the Degradative Depolymerization Process. A Case Study. Energies 2020, 13, 6620. [Google Scholar] [CrossRef]
- Rolewicz-Kalińska, A.; Lelicińska-Serafin, K.; Manczarski, P. The Circular Economy and Organic Fraction of Municipal Solid Waste Recycling Strategies. Energies 2020, 13, 4366. [Google Scholar] [CrossRef]
- Herceg, S.; Pinto Bautista, S.; Weiß, K.-A. Influence of Waste Management on the Environmental Footprint of Electricity Produced by Photovoltaic Systems. Energies 2020, 13, 2146. [Google Scholar] [CrossRef]
- Czarnecka-Komorowska, D.; Wiszumirska, K.; Garbacz, T. FILMS LDPE/LLDPE MADE FROM POST—CONSUMER PLASTICS: PROCESSING, STRUCTURE, MECHANICAL PROPERTIES. Adv. Sci. Technol. Res. J. 2018, 12, 134–142. [Google Scholar] [CrossRef]
- Saxena, M.; Morchhale, R.K.; Asokan, P.; Prasad, B.K. Plant Fiber—Industrial Waste Reinforced Polymer Composites as a Potential Wood Substitute Material. J. Compos. Mater. 2008, 42, 367–384. [Google Scholar] [CrossRef]
- Sommerhuber, P.F.; Welling, J.; Krause, A. Substitution Potentials of Recycled HDPE and Wood Particles from Post-Consumer Packaging Waste in Wood–Plastic Composites. Waste Manag. 2015, 46, 76–85. [Google Scholar] [CrossRef]
- Markowska, O.; Markowski, T.; Sobczyk, M. Analysis of the Mechanical Properties of Polymer Composites for the Production of Machine Parts Used as Substitutes for Elements Obtained from Metals. Polimery 2020, 65. [Google Scholar] [CrossRef]
- Dascalescu, L.; Zeghloul, T.; Iuga, A. Chapter 4—Electrostatic Separation of Metals and Plastics From Waste Electrical and Electronic Equipment. In WEEE Recycling; Chagnes, A., Cote, G., Ekberg, C., Nilsson, M., Retegan, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 75–106. ISBN 978-0-12-803363-0. [Google Scholar] [CrossRef]
- Use of Recycled Plastics in Eco-Efficient Concrete—1st Edition. Available online: https://www.elsevier.com/books/use-of-recycled-plastics-in-eco-efficient-concrete/pacheco-torgal/978-0-08-102676-2 (accessed on 22 February 2021).
- Kim, B.-U.; Park, C.-H. Electrostatic Separation of Copper and Glass Particles in Pretreated Automobile Shredder Residue. Metals 2018, 8, 879. [Google Scholar] [CrossRef]
- Catinean, A.; Dascalescu, L.; Lungu, M.; Dumitran, L.M.; Samuila, A. Improving the Recovery of Copper from Electric Cable Waste Derived from Automotive Industry by Corona-Electrostatic Separation. Part. Sci. Technol. 2020, 1–8. [Google Scholar] [CrossRef]
- Kim, B.; Han, O.; Jeon, H.; Baek, S.; Park, C. Trajectory Analysis of Copper and Glass Particles in Electrostatic Separation for the Recycling of ASR. Metals 2017, 7, 434. [Google Scholar] [CrossRef]
- Benabderrahmane, A.; Medles, K.; Zeghloul, T.; Renoux, P.; Dascalescu, L.; Parenty, A. Triboelectric Charging and Electrostatic Separation of Granular Polymers Containing Brominated Flame Retardants. IEEE Trans. Ind. Appl. 2021, 57, 915–922. [Google Scholar] [CrossRef]
- Kelly, E.G.; Spottiswood, D.J. The Theory of Electrostatic Separations: A Review Part I. Fundamentals. Miner. Eng. 1989, 2, 33–46. [Google Scholar] [CrossRef]
- Silveira, A.V.M.; Santana, M.P.; Tanabe, E.H.; Bertuol, D.A. Recovery of Valuable Materials from Spent Lithium Ion Batteries Using Electrostatic Separation. Int. J. Miner. Process. 2017, 169, 91–98. [Google Scholar] [CrossRef]
- Dias, P.; Schmidt, L.; Gomes, L.B.; Bettanin, A.; Veit, H.; Bernardes, A.M. Recycling Waste Crystalline Silicon Photovoltaic Modules by Electrostatic Separation. J. Sustain. Metall. 2018, 4, 176–186. [Google Scholar] [CrossRef]
- Felsing, S.; Kochleus, C.; Buchinger, S.; Brennholt, N.; Stock, F.; Reifferscheid, G. A New Approach in Separating Microplastics from Environmental Samples Based on Their Electrostatic Behavior. Environ. Pollut. 2018, 234, 20–28. [Google Scholar] [CrossRef]
- Richard, G.; Nadjem, A.; Medles, K.; Kachi, M.; Zeghloul, T.; Dascalescu, L. Tribocharging of PP Granules after Ex- Posure to a Dielectric Barrier Discharge (DBD). Effect of Exposure Duration, Voltage Amplitude and Frequency. Proc. Electrost. Soc. Am. Annu. Meet. ESA 2017, 7, 8–11. [Google Scholar]
- Iuga, A.; Samuila, A.; Morar, R.; Bilici, M.; Dascalescu, L. Tribocharging Techniques for the Electrostatic Separation of Granular Plastics from Waste Electric and Electronic Equipment. Part. Sci. Technol. 2016, 34, 45–54. [Google Scholar] [CrossRef]
- Calin, L.; Caliap, L.; Neamtu, V.; Morar, R.; Iuga, A.; Samuila, A.; Dascalescu, L. Tribocharging of Granular Plastic Mixtures in View of Electrostatic Separation. IEEE Trans. Ind. Appl. 2008, 44, 1045–1051. [Google Scholar] [CrossRef]
- Landauer, J.; Foerst, P. Influence of Particle Charge and Size Distribution on Triboelectric Separation—New Evidence Revealed by In Situ Particle Size Measurements. Processes 2019, 7, 381. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, Y.; Zhong, Y.; Luo, M.; Du, Y.; Wang, L.; Wang, H. Flotation Separation of Polyethylene Terephthalate from Waste Packaging Plastics through Ethylene Glycol Pretreatment Assisted by Sonication. Waste Manag. 2020, 105, 309–316. [Google Scholar] [CrossRef]
- Bonifazi, G.; Capobianco, G.; Serranti, S. A Hierarchical Classification Approach for Recognition of Low-Density (LDPE) and High-Density Polyethylene (HDPE) in Mixed Plastic Waste Based on Short-Wave Infrared (SWIR) Hyperspectral Imaging. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2018, 198, 115–122. [Google Scholar] [CrossRef]
- Park, C.-H.; Jeon, H.-S.; Yu, H.-S.; Han, O.-H.; Park, J.-K. Application of Electrostatic Separation to the Recycling of Plastic Wastes: Separation of PVC, PET, and ABS. Environ. Sci. Technol. 2008, 42, 249–255. [Google Scholar] [CrossRef]
- Singh, N.; Hui, D.; Singh, R.; Ahuja, I.P.S.; Feo, L.; Fraternali, F. Recycling of Plastic Solid Waste: A State of Art Review and Future Applications. Compos. Part B Eng. 2017, 115, 409–422. [Google Scholar] [CrossRef]
- Rybarczyk, D.; Jędryczka, C.; Regulski, R.; Sędziak, D.; Netter, K.; Czarnecka-Komorowska, D.; Barczewski, M.; Barański, M. Assessment of the Electrostatic Separation Effectiveness of Plastic Waste Using a Vision System. Sensors 2020, 20, 7201. [Google Scholar] [CrossRef]
- Younes, K.; Younes, M.; Meziane, R.; Samuila, A.; Dascalescu, L. Modified Tribo-Charging Device for the Electrostatic Separation of Plastics from Granular Industrial Wastes. Sep. Sci. Technol. 2017, 52, 1246–1256. [Google Scholar] [CrossRef]
- Zeghloul, T.; Mekhalef Benhafssa, A.; Richard, G.; Medles, K.; Dascalescu, L. Effect of Particle Size on the Tribo-Aero-Electrostatic Separation of Plastics. J. Electrost. 2017, 88, 24–28. [Google Scholar] [CrossRef]
- Skowron, M.; Syrek, P.; Surowiak, A. The Application of the Electrodynamic Separator in Minerals Beneficiation. IOP Conf. Ser. Mater. Sci. Eng. 2017, 200, 012017. [Google Scholar] [CrossRef]
- Lu, H.; Li, J.; Guo, J.; Xu, Z. Movement Behavior in Electrostatic Separation: Recycling of Metal Materials from Waste Printed Circuit Board. J. Mater. Process. Technol. 2008, 197, 101–108. [Google Scholar] [CrossRef]
- Veit, H.M.; Diehl, T.R.; Salami, A.P.; Rodrigues, J.S.; Bernardes, A.M.; Tenório, J.A.S. Utilization of Magnetic and Electrostatic Separation in the Recycling of Printed Circuit Boards Scrap. Waste Manag. 2005, 25, 67–74. [Google Scholar] [CrossRef]
- Cieśla, A.; Kraszewski, W.; Skowron, M.; Surowiak, A.; Syrek, P. Application of electrodynamic drum separator to electronic wastes separation. Gospod. Surowcami Miner. 2016, 32, 155–174. [Google Scholar] [CrossRef]

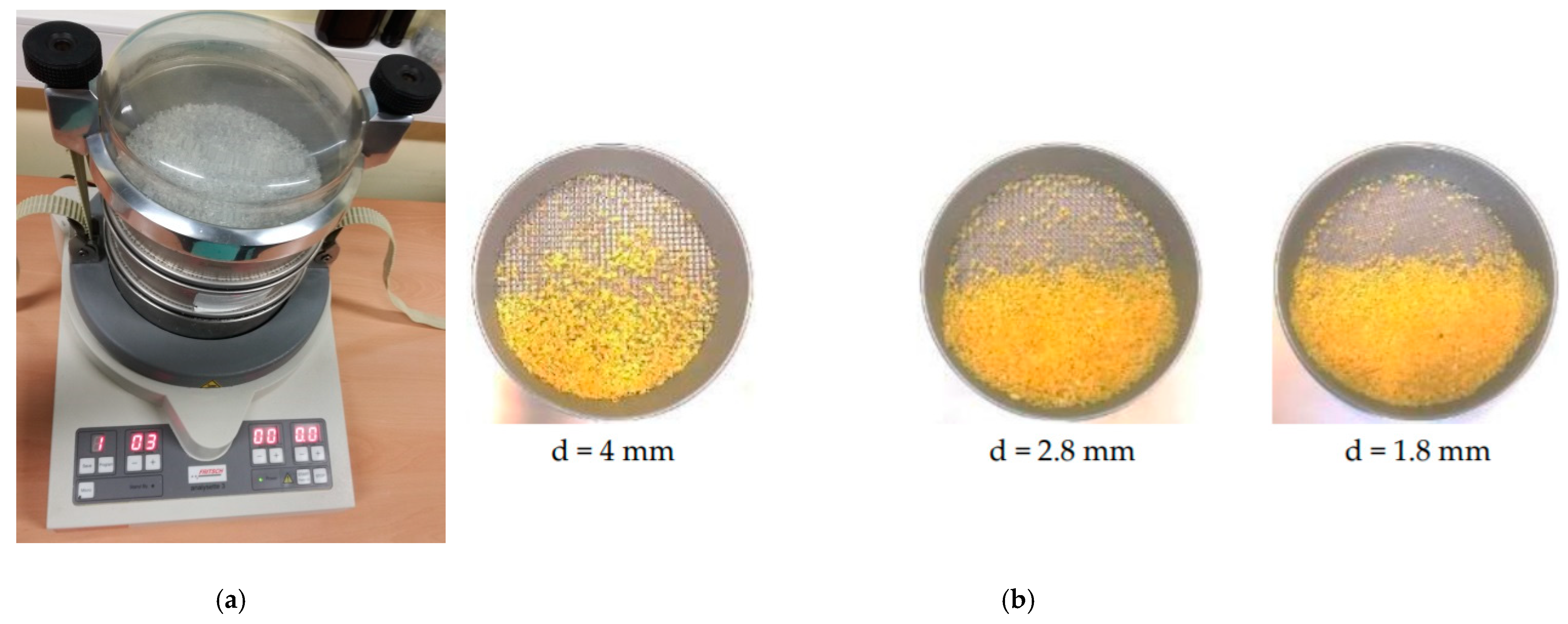
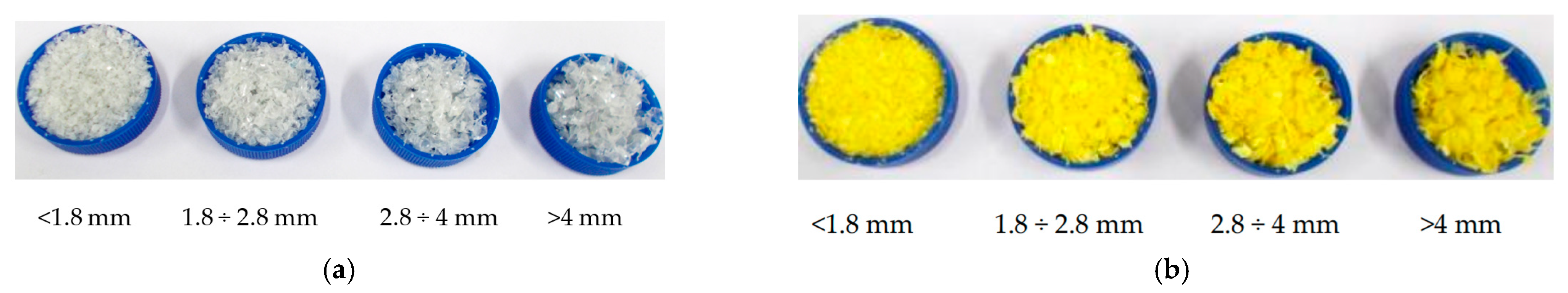
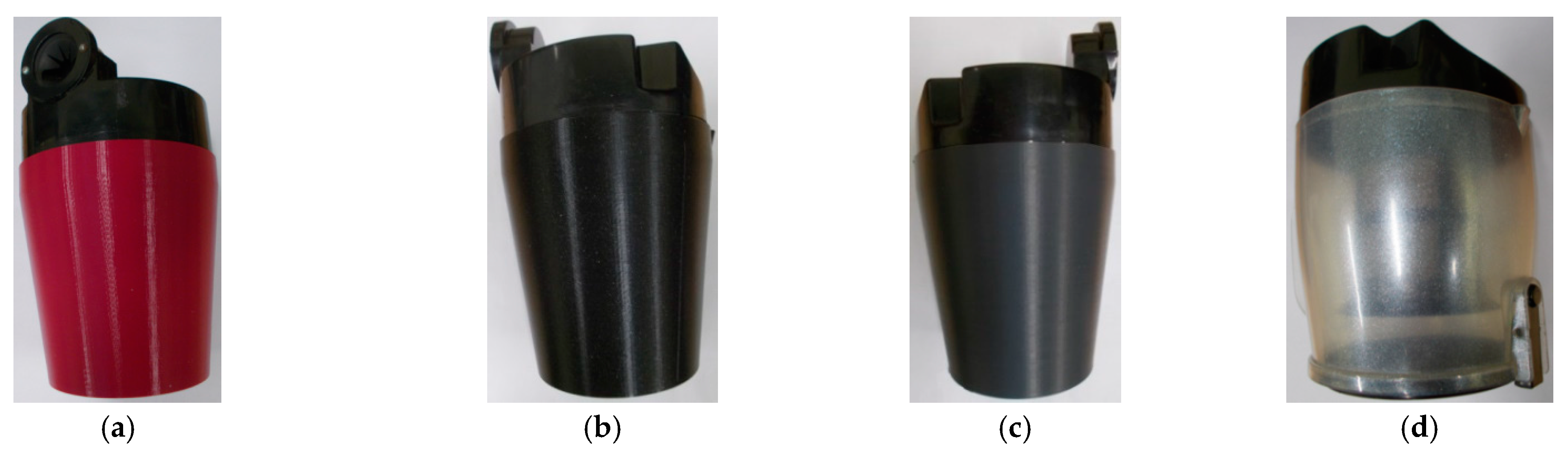
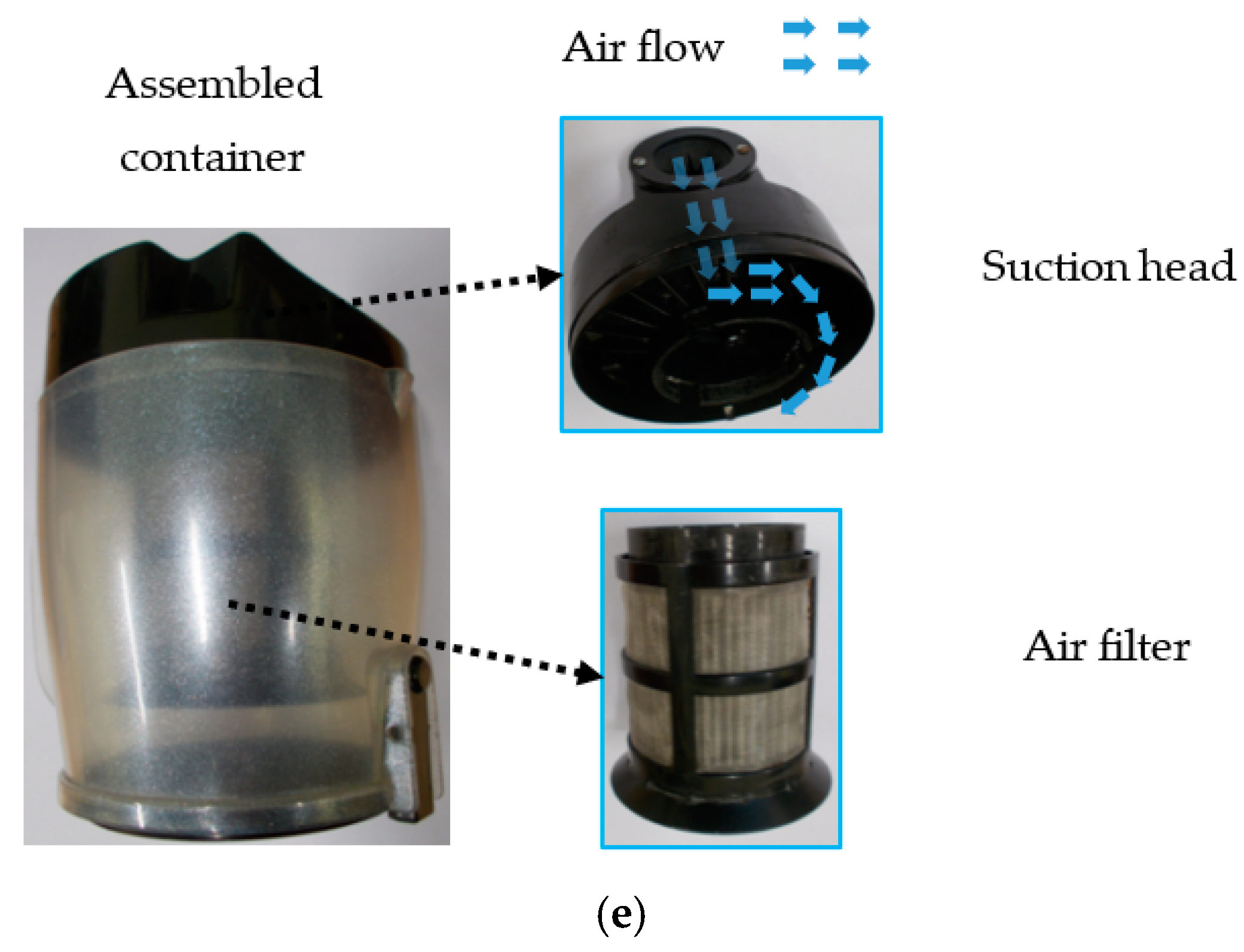
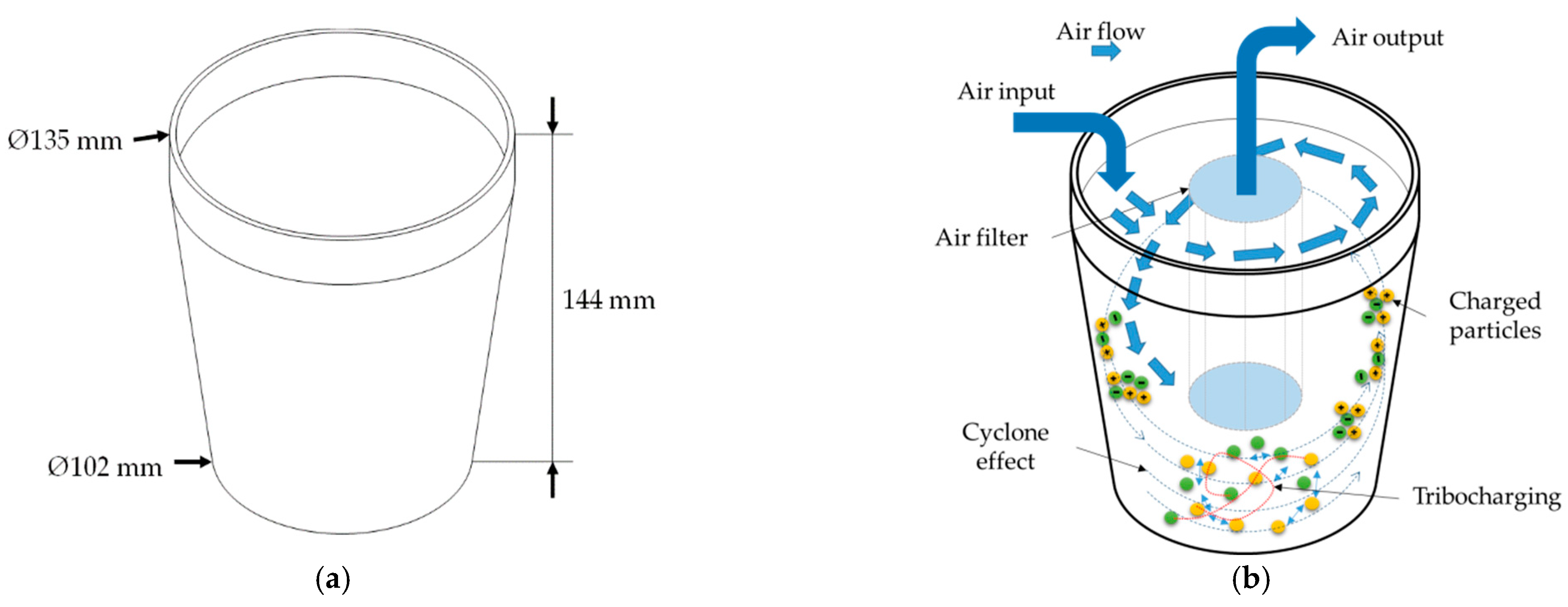

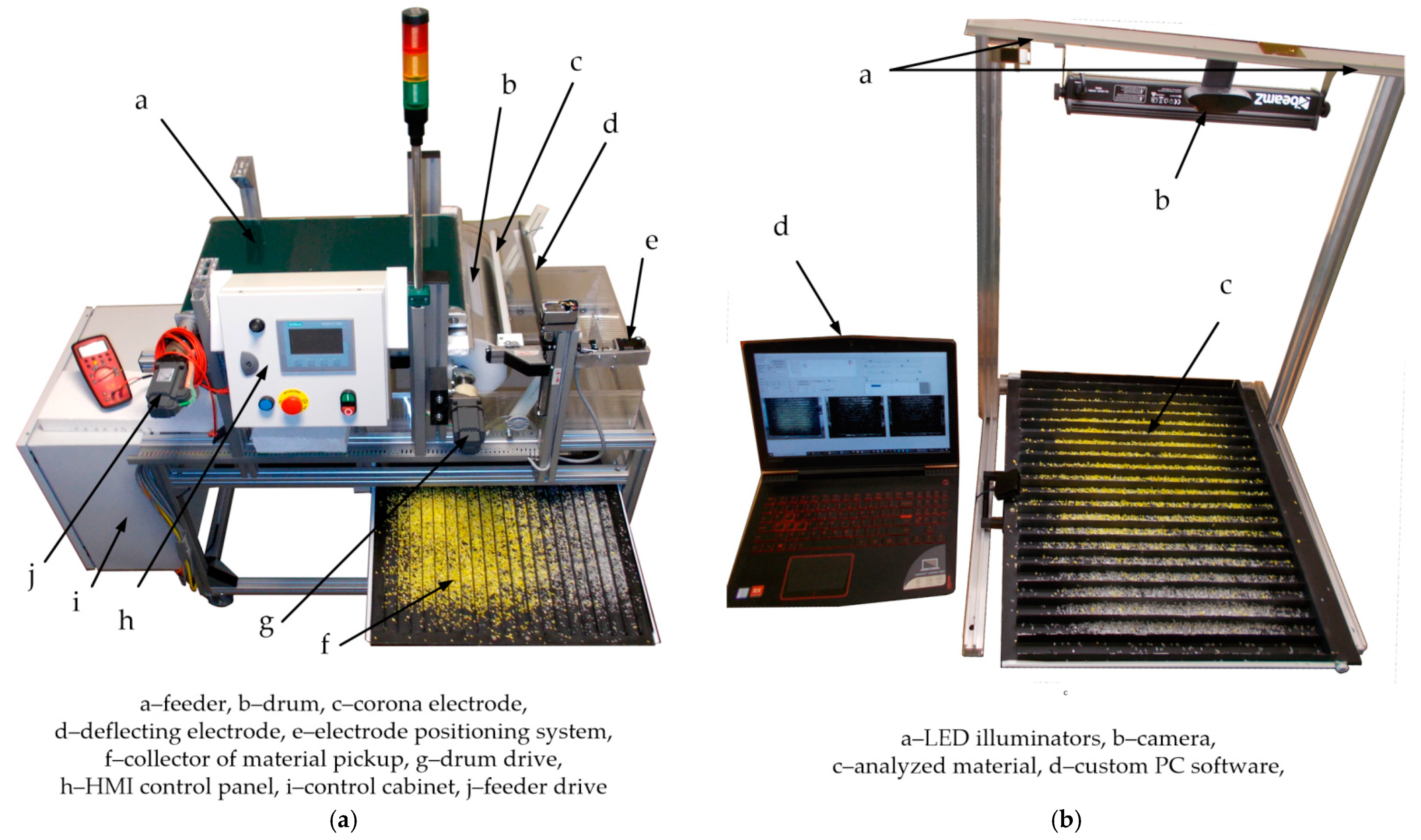
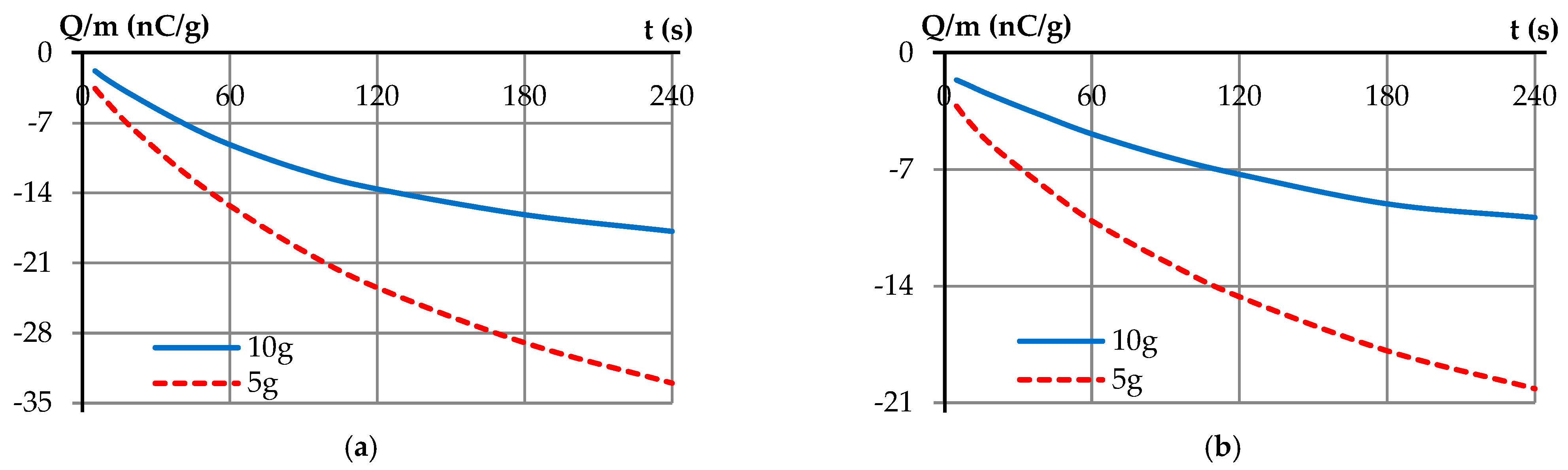
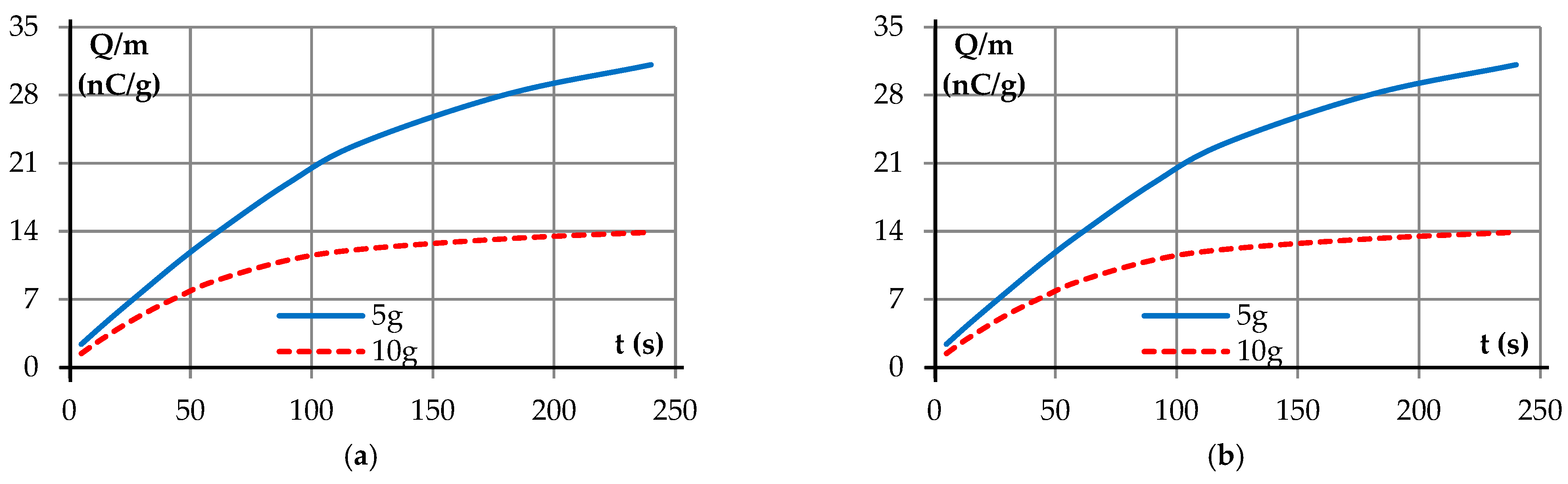
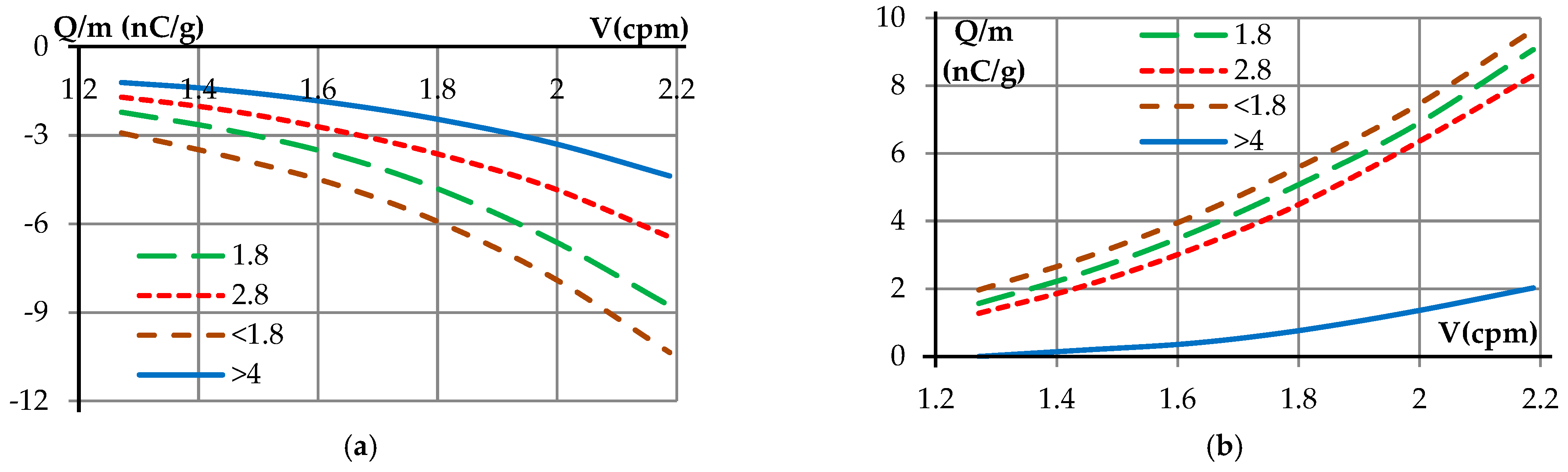
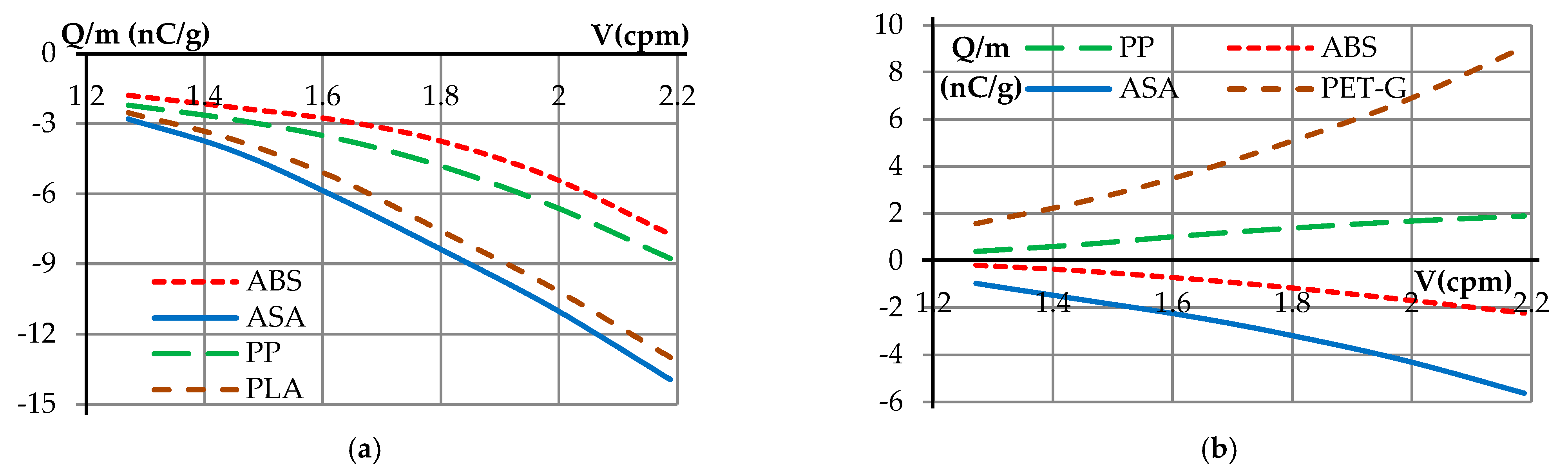
| Samples | <1.8 mm | 1.8–2.8 mm | 2.8–4 mm | >4 mm |
|---|---|---|---|---|
| PET | ~30% | ~40% | ~24% | ~6% |
| PE-HD | ~17% | ~37% | ~28% | ~18% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyskawinski, W.; Baranski, M.; Jedryczka, C.; Mikolajewicz, J.; Regulski, R.; Sedziak, D.; Netter, K.; Rybarczyk, D.; Czarnecka-Komorowska, D.; Barczewski, M. Tribo-Electrostatic Separation Analysis of a Beneficial Solution in the Recycling of Mixed Poly(Ethylene Terephthalate) and High-Density Polyethylene. Energies 2021, 14, 1755. https://doi.org/10.3390/en14061755
Lyskawinski W, Baranski M, Jedryczka C, Mikolajewicz J, Regulski R, Sedziak D, Netter K, Rybarczyk D, Czarnecka-Komorowska D, Barczewski M. Tribo-Electrostatic Separation Analysis of a Beneficial Solution in the Recycling of Mixed Poly(Ethylene Terephthalate) and High-Density Polyethylene. Energies. 2021; 14(6):1755. https://doi.org/10.3390/en14061755
Chicago/Turabian StyleLyskawinski, Wieslaw, Mariusz Baranski, Cezary Jedryczka, Jacek Mikolajewicz, Roman Regulski, Dariusz Sedziak, Krzysztof Netter, Dominik Rybarczyk, Dorota Czarnecka-Komorowska, and Mateusz Barczewski. 2021. "Tribo-Electrostatic Separation Analysis of a Beneficial Solution in the Recycling of Mixed Poly(Ethylene Terephthalate) and High-Density Polyethylene" Energies 14, no. 6: 1755. https://doi.org/10.3390/en14061755
APA StyleLyskawinski, W., Baranski, M., Jedryczka, C., Mikolajewicz, J., Regulski, R., Sedziak, D., Netter, K., Rybarczyk, D., Czarnecka-Komorowska, D., & Barczewski, M. (2021). Tribo-Electrostatic Separation Analysis of a Beneficial Solution in the Recycling of Mixed Poly(Ethylene Terephthalate) and High-Density Polyethylene. Energies, 14(6), 1755. https://doi.org/10.3390/en14061755









