Abstract
A high level of moisture in the insulation system of power transformers is often the cause of their failure. This can be prevented by drying a transformer in its place of installation. In the article the application of molecular sieves (MS) in the drying process of the transformer insulation system was analyzed. The water sorption isotherms of 3A MS in mineral oil, natural ester, and synthetic ester at 35 °C were determined, which was not described in the literature before. An evaluation of the influence of temperature on the drying dynamics of electro-insulating liquids using MS was also carried out. The drying dynamics were tested at three temperatures, i.e., 35 °C, 50 °C, and 65 °C, which allowed to analyze the effect of temperature on a short-term or continuous drying process. The tests showed that 3A MS’s ability to adsorb water varied depending on the type of electro-insulating liquid. The determined water sorption isotherms, described by means of Langmuir adsorption model, make it possible to calculate the amount of MS needed for drying transformers with different liquid insulation. The research and analyses show the MS’s great potential for the drying of transformer insulation systems.
1. Introduction
The insulation system is one of the most important elements of a power transformer, and the insulation system’s condition is one of the factors that influences the power transformer’s overall reliability. The insulation systems of grid and distribution transformers are usually made of cellulose materials that are impregnated with mineral oil. This is a proven solution that has been used in power engineering on a mass scale for nearly 100 years [1]. Recently, alternatives to mineral oil, e.g., synthetic and natural esters, have become increasingly popular. Growing interest in these liquids results mainly stems from their high flash point and fire point, and from their biodegradability, while they simultaneously maintain good dielectric parameters that are similar to mineral oil [2,3]. Another parameter that significantly differentiates these liquids from mineral oil is water solubility, which for both natural and synthetic ester at 20 °C is equal to 858 ppm and 1758 ppm, respectively. For mineral oil, this value is equal to only about 47 ppm [4].
After many years of a given transformer’s operation, regardless of the choice of materials that were used to construct its insulation system, network operators face problems connected with the system’s moisture, and water content is the basic parameter used to determine the condition of the transformer’s insulation.
After the power transformer production process, the water content in its solid insulation usually ranges from 0.5% to 1%. Unfortunately, during long-term operation of the device, exceeding even 50 years [5,6,7], the water content in its insulation system constantly increases [8]. The increase in the water content is mainly caused by the cellulose oxidation process and by moisture ingress into the tank, particularly in transformers with a free-breathing conservator [9]. In this case situation the moistening process will be faster for units with esters than with mineral oil.
Transformers with cellulose insulation with a moisture content above 2.0% are considered wet, and their operation carries the risk of failure [10]. In order to prevent this failure, it is necessary to take remedial actions such as reducing the transformer’s load or applying short-term drying of its insulation system.
On-site and short-term drying of a transformer insulation system is a difficult and expensive procedure, and in some cases it is impossible to conduct solely due to technical reasons. The inability to effectively dry distribution transformers is most often due to their tank’s low mechanical strength. This inhibits the use of an appropriate vacuum while drying the insulation, which then largely determines this process’ efficiency [11].
It should be noted that a problematic issue associated with moisture in an insulation system already appears before a 2% water content in the cellulose is exceeded. Even below this value the water will accelerate the cellulose aging process [12,13]. Aging should be understood here as the cellulose depolymerization process. As a result of the process of hydrolysis, oxygen bridges are broken between anhydrous-β-glucose monomers (C6H10O5) [14], the consequence of which is a gradual decrease in the degree of polymerization (DP), which for new cellulose usually ranges from 1100 to 1300. Below a degree of polymerization of 700, the tensile strength of cellulosic materials will begin to rapidly decrease. Below DP equal to 350, the mechanical strength of cellulose is poor, which in turn raises the risk of failure in the event of short-circuit currents in the windings [13].
The information presented above shows how important it is to maintain the insulation moisture level low throughout the transformer’s lifetime. The most important benefits of this approach are:
- Slowing down the cellulose depolymerization process, and thus extending the life of the transformer;
- Reducing the risk of transformer failure related to, among others, the occurrence of partial discharges [15,16] or bubble evolution [17,18];
- No restrictions on transformer operation;
- Eliminating of the risk of failure due to short-term drying requiring a vacuum.
Maintaining a low level of moisture in the transformer insulation system throughout the transformer’s lifetime is possible via circulating drying of the electro-insulating liquid by means of highly hygroscopic materials. Such materials should have a steep water sorption isotherm, i.e., for small relative humidity of a liquid the material should be able to adsorb large amounts of water. Materials that are very well suited for this purpose are molecular sieves (MS).
MS which can be used to dry a transformer insulation system are crystalline aluminosilicates called zeolites. Their basic characteristic is the presence of a crystal structure with a homogeneous pore system. The presence of an extensive specific surface area in zeolites that is only available to particles with a diameter smaller than the critical diameter of the pores causes the formation of a molecular sieve effect. The expanded specific surface area enables the adsorption of a large amount of water, which then determines the possibility of using this material for effective drying of the transformer insulation system [19,20,21].
There are several solutions on the market that use MS for circulating drying of the transformer insulation system. The devices’ principle is based on forced circulation of mineral oil between the transformer tank and a container filled with MS in which the liquid dielectric is dried. Dried mineral oil returns to the tank. As a result, since the system strives to maintain a state of moisture equilibrium, water migrates from the solid insulation to the liquid insulation, which causes a slow but gradual drying of the cellulose. Installing such a system on a new transformer allows to maintain a low water content in the cellulose insulation. Unfortunately, the manufacturers of drying systems do not provide detailed data on their solutions. For example, there is no information such as the type of MS used or the method of calculating the amount of MS needed to carry out the drying process.
A relatively small number of papers on the use of MS for circulating drying of a transformer insulation system can be found in the literature. The authors of paper [22] analyzed the impact of MS type (3A and 4A) on the dynamics of mineral oil drying depending on the initial water content of the dried liquid, its temperature, and the weight ratio of MS to mineral oil. The paper states that the choice of 3A MS for mineral oil drying is a more reasonable option than 4A MS due to the process’ better dynamics at higher temperature values and the more selective operation of this type of sieve. This selectivity results from the MS’s pore size, which is equal to ca. 3Å, which only allows for adsorption of particles smaller than the size of these pores. These particles include water, hydrogen and, to a very limited extent, carbon monoxide and acetylene. The adsorption of gas molecules plays a significant role when dissolved gas is used in oil analysis to diagnose the transformers. The authors of paper [22] also showed the significant effect of temperature on drying dynamics, namely, high temperature improves the dynamics of the drying process but, according to the authors, it also increases the probability of CO2 and C2H2 adsorption.
The authors of paper [23] presented the results of the drying of transformers by means of 4A MS. They found that MS are particularly well suited to maintaining a low moisture level of transformer cellulose insulation. The paper also states that the adsorption capacity of MS decreases along with an increasing oil temperature from 18–20% at 20 °C to 3–4% at 100 °C. This aspect seems to be very important in the context of determining the optimal temperature of how MS operate.
The above shows that high temperature improves the dynamics of the drying process but it can significantly reduce this process’ efficiency. For this reason, the authors conducted the research on the influence of temperature on the dynamics of various insulating liquids drying. The tests were carried out for the temperature ranging from 35 °C to 65 °C, which corresponds to the temperature of the insulating liquid in a transformer.
Paper [19] presents the results of an experiment involving the use of MS to dry mineral oil. The obtained drying effect of mineral oil was smaller than the result estimated from sorption isotherms for a 3A sieve determined in the air. On the basis of these studies it can be concluded that it is impossible to properly calculate the MS mass needed to dry the transformer insulation or to maintain its low moisture level based on sorption isotherms determined in the air.
On the basis of their research and the literature as presented above, the authors of this paper defined the following research objectives:
- Determine the water sorption isotherms of 3A MS in mineral oil, natural ester and synthetic ester—mainly in the context of calculating the correct amount of MS for short-term and continuous drying of the transformer insulation system;
- Evaluate the influence of temperature on the drying dynamics of insulating liquids—mainly in the context of selecting the proper operating temperature of MS.
Water sorption isotherms of 3A MS in mineral oil, natural ester, and synthetic ester are necessary to determine the amount of molecular sieve needed to dry transformer insulation. This knowledge (not available in literature) allows to simplify the drying system and apply a new approach in its operation. Existing systems use expensive water content meters. Thanks to them, it is possible to capture the moment when the molecular sieve is very wet and further insulation drying is impossible. Opting out of these meters will lower the price of drying system. However, in order to correctly estimate the amount of sieve, it is necessary to conduct tests that will provide detailed information on the material characteristics of molecular sieves and the course of the drying process—in particular its dynamics (also in terms of moisture balance between solid insulation and insulating liquid).
2. Determining of the Water Sorption Isotherms of 3A Molecular Sieves in Mineral Oil, Natural Ester and Synthetic Ester
2.1. Materials Preparation and Measurement Procedure
In order to determine the water sorption isotherms, samples were prepared of insulating liquids with a high relative saturation of water (RS) that was equal to ca. 70% at a temperature of 35 °C. This corresponded to the absolute water content in mineral oil, natural ester, and synthetic ester that was, respectively, equal to 61 ppm, 787 ppm, and 1553 ppm. The assumed water content in the liquids was obtained by mixing both dry and moist liquids in appropriate proportions. The prepared liquids were poured into 500 mL bottles. After 24 h, the initial water content was measured in each sample using the Karl Fischer coulometric method (KFT) in accordance with the standard [24]. Subsequently, a 3A molecular sieve dried at 250 °C was added to the bottles. In order to obtain different points on the water sorption isotherm, the weight of the dry MS was different in each bottle. The weight was estimated based on the water sorption isotherms of the 3A MS determined in air [19]. It was assumed that 1 g of molecular sieve is able to adsorb 0.1674 g of water. Eight bottles with different masses of the molecular sieve were prepared for each of the three insulating liquids, which thus allowed to obtain eight measuring points for each liquid, i.e., for mineral oil, natural ester, and synthetic ester.
Tightly sealed vessels with the insulating liquids and a molecular sieve were placed in a laboratory heating chamber to ensure a constant temperature of 35 °C. The movement of the liquids inside the bottles was forced by means of magnetic stirrers. After six weeks, the water content of the liquids was again determined using the KFT method. The time necessary to establish the equilibrium between water adsorbed by the molecular sieves and water dissolved in the insulating liquids was estimated based on the results of an experiment described in paper [25]. The mass of water adsorbed by the MS was determined on the basis of the difference between the initial and the final water content in the liquid. It was then referred to the mass of dry molecular sieves.
2.2. Results
As a result of the experiment, points on the water sorption isotherms of the 3A molecular sieve in mineral oil, natural ester, and synthetic ester at 35 °C were determined. The results are shown in Table 1.

Table 1.
Water content in the 3A molecular sieve for various values of relative saturation of water in mineral oil, synthetic ester, and natural ester, T = 35 °C.
The obtained results were described using the Langmuir water sorption isotherm as represented by the equation [20]:
where
- ar—real adsorption [mol/g],
- am—adsorption capacity [mol/g],
- K—adsorption equilibrium constant [–], and
- c—molar concentration of adsorbate [mol/dm3].
The Langmuir water sorption isotherm is a type I isotherm. It is based on the following assumptions [26]:
- Adsorption is localized, i.e., it occurs at active sites, where one of the adsorbate molecules can be adsorbed at each of these sites and cannot move;
- There are no interactions between adsorbate particles;
- An increase in the number of adsorbed molecules takes place until all of the active centers are filled, then the balance between adsorption and desorption is established;
- The adsorbed substance forms a monomolecular layer on the surface of the adsorbent.
Since the 3A molecular sieve is characterized by a very large increase in adsorption at low relative humidity values and approximately constant adsorption at high relative humidity values, the Langmuir water sorption isotherm properly describes well the obtained results.
The relationship between actual adsorption a and water content in the molecular sieve WCS expressed in % is represented by the equation:
where
- M—molar mass of water [18 g/mol].
The molar concentration of the adsorbate is described by the equation:
where
- mw—mass of water [g],
- dl—insulating liquid density [g/dm3],
- ml—mass of insulating liquid.
In Equation (3) the following formula can be substituted:
where
- RS—relative saturation of liquid [%],
- S—water saturation limits [ppm].
The Langmuir isotherm Equation (1) can be written in a linear form (y = Ax + B):
which makes it possible to calculate the coefficients of Equation (5) using the relationships:
and
Figure 1 presents the Langmuir water sorption isotherms determined based on the measurement results. In addition, Figure 1 presents the water sorption isotherm of a 3A molecular sieve in air (based on [19]) at 35 °C.
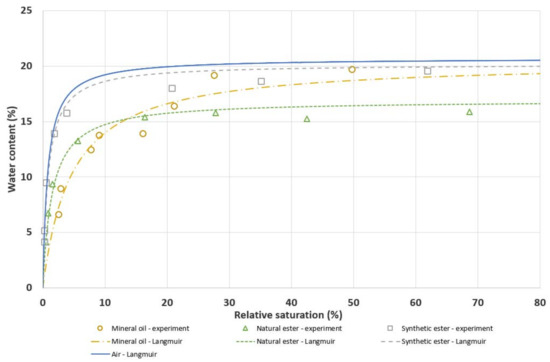
Figure 1.
Water sorption isotherms of the 3A molecular sieve in mineral oil, natural ester, synthetic ester, and air, T = 35 °C.
On the basis of Figure 1, it can be concluded that the molecular sieve’s ability to adsorb water differs depending on the type of liquid. At low values of relative saturation of water in the insulating liquid (RS < 5%), the tested molecular sieve exhibits the largest capacity for water sorption in synthetic ester; whereas the lowest water sorption capacity is observed in mineral oil. The water sorption isotherm of the 3A molecular sieve in synthetic ester at 35 °C is very similar to that in the air [19]. The high ability of the molecular sieve to dry liquids with a low relative water content is a key feature that determines the possibility of using it to dry a transformer insulation system. The obtained results were used to estimate the mass of the molecular sieve needed for short-term drying of transformer insulation or for maintaining a low level of moisture in it. This issue is discussed in Section 4.
3. Assessment of the Effect of Temperature on the Dynamics of Drying Insulating Liquids with a 3A Molecular Sieve
3.1. Materials Preparation and Measurement Procedure
In order to examine the effect of temperature on the dynamics of drying insulating liquids with a 3A molecular sieve, a total of 9 bottles filled with mineral oil, natural ester, or synthetic ester with a weight ML (Table 2) were prepared. The bottles were placed in three laboratory heating chambers at 35 °C, 50 °C, and 65 °C. Molecular sieves were added to the bottles in an amount allowing for adsorption of the water dissolved in each sample of the liquid. The initial water content WCL0 was determined by means of the KFT method. The weight of the molecular sieve MMS was estimated based on the water sorption isotherm of the 3A molecular sieve as presented in [19]. Table 2 presents a summary of the prepared samples.

Table 2.
Summary of the prepared samples.
At intervals allowing to capture the liquid drying dynamics, the water content in the samples was measured using the KFT method. At the initial stage of the study, the time intervals between consecutive measurements were several hours apart, whereas at the final stage the time intervals were as many as seven days apart between measurements.
3.2. Results
Figure 2, Figure 3 and Figure 4 present the results of measuring the water content in mineral oil, natural ester, and synthetic ester, respectively, during their drying with a 3A molecular sieve. The drying process was carried out at 35 °C, 50 °C, and 65 °C.
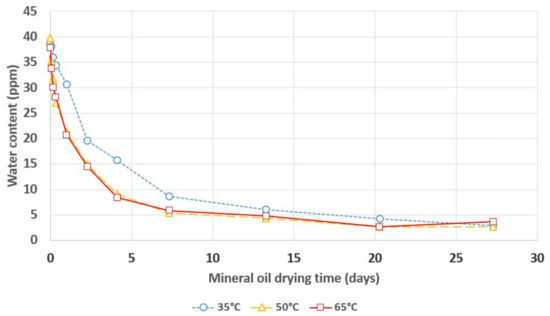
Figure 2.
Water content in mineral oil during its drying with a 3A molecular sieve.
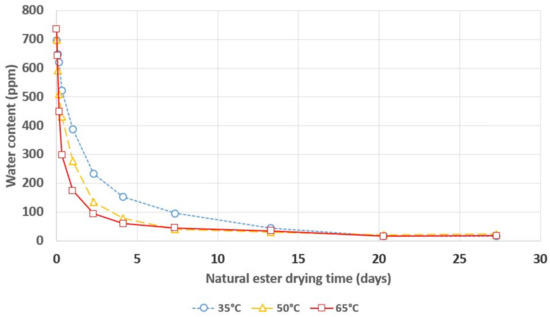
Figure 3.
Water content in natural ester during its drying with a 3A molecular sieve.
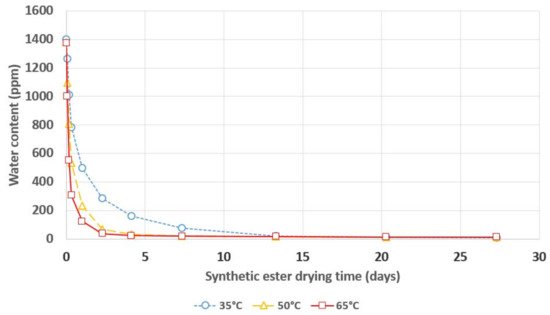
Figure 4.
Water content in synthetic ester during its drying with a 3A molecular sieve.
Figure 2 shows that the lowest dynamics of the water adsorption process by MS took place at 35 °C. Increasing the temperature to 50 °C resulted in a significant increase in the rate of water removal from the oil, particularly during the first days of the drying process. An increase in the drying temperature to 65 °C did not cause a further increase in the drying rate of the mineral oil. There was no effect of the drying temperature on the final water content in all of the analyzed mineral oil samples, and only the time needed to reach it changed.
In the case of drying both natural and synthetic ester, an even greater influence of the temperature on the dynamics of the drying process was observed than in the case of mineral oil. The increase in temperature of the electro-insulating liquid caused a significant increase in the rate of water removal from the esters. An increase in the drying dynamics was observed when the temperature increased from 35 °C to 50 °C and when the temperature increased from 50 °C to 65 °C.
The graphs of the water content in the insulating liquids during their drying process do not provide a complete picture of this process’ course. The water sorption isotherms presented in Section 2 clearly show that at low RS values there is a strong relationship between the MS’s adsorption capacity and the relative saturation of water in the insulating liquid. The purpose of drying is to reduce the water content in the insulating liquid to a very low level of RS. For a given water content expressed in ppm, the RS value changes along with temperature changes.
Taking into account the above, Figure 5, Figure 6 and Figure 7 show the changes in relative saturation of the insulating liquids during the drying process. The relative saturation of the insulating liquids was calculated based on the results of the water content in the liquids during their drying. These results were referred to the water saturation limit in mineral oil, natural ester and synthetic ester. The limit values of water saturation used for the calculations are presented in Table 3. These values were calculated on the basis of data contained in paper [4].
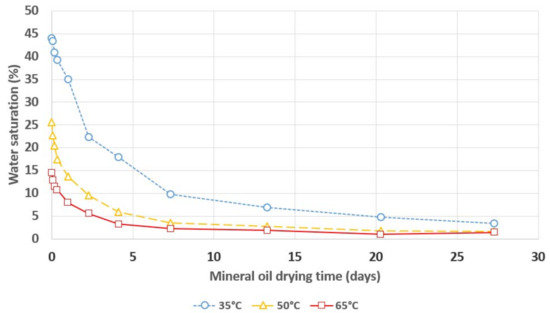
Figure 5.
Relative saturation of water in mineral oil during its drying with a 3A molecular sieve.
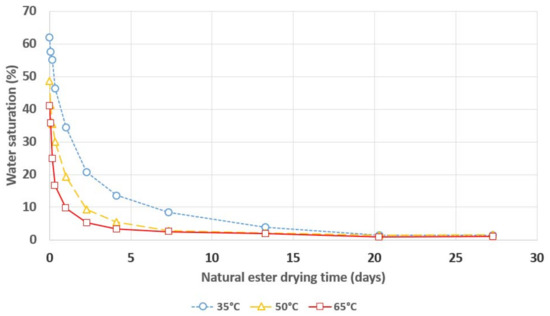
Figure 6.
Relative saturation of water in natural ester during its drying with a 3A molecular sieve.
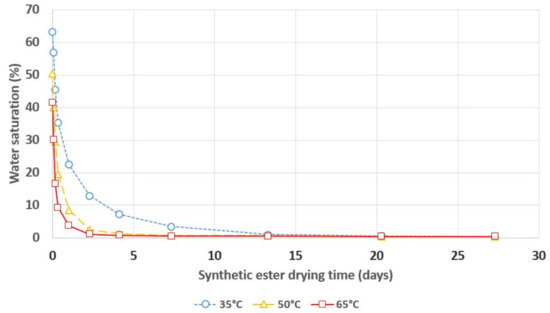
Figure 7.
Relative saturation of water in synthetic ester during its drying with a 3A molecular sieve.

Table 3.
Limits for the solubility of water in mineral oil, natural ester, and synthetic ester at 35 °C, 50 °C, and 65 °C [4].
The dependence of the insulating liquids’ relative saturation on their drying time confirms the 3A molecular sieve’s excellent ability to remove moisture from mineral oil, natural ester and synthetic ester. The clear influence of temperature on the drying process’ dynamics can be observed, i.e., increasing the temperature results in faster removal of moisture from the insulating liquids via the 3A molecular sieve. The ratio of the drying rate’s increase to the temperature’s increase is much higher if the temperature increases from 35 °C to 50 °C than if it increases from 50 °C to 65 °C. A temperature of 50 °C allows for the samples to be dried to a level of relative liquid saturation that is lower than 5% in a timeframe that does not exceed one week, regardless of the insulating liquid that is used. According to the standard IEC 60422, transformers whose relative oil saturation with water is lower than 5% are considered to be dry.
4. Discussion
The experiments conducted in this study proved the very high potential of the 3A molecular sieve in drying mineral oil, natural ester, and synthetic ester. Since drying the insulating liquid in the transformer causes indirect drying of the transformer’s solid insulation, it is possible to estimate the amount of 3A molecular sieve that is necessary for complete drying of the insulation system. The water sorption isotherms shown in Figure 1 were used for this purpose.
Table 4 presents the total mass of the insulating materials in the 25 MVA transformer. An initial moisture content of cellulose insulation equal to 3.0% was used for the calculations. It was assumed that after the drying process the final water content in the cellulose insulation should be equal to 1.0%, which in a state of moisture equilibrium at 35 °C corresponds to 5% of the relative saturation of the insulating liquid. Taking into account the mass of the cellulosic materials of the analyzed transformer (1228.5 kg) and the mass of the oil (9900 kg), the mass of the water that should have been adsorbed by the molecular sieve was equal to 24.74 kg.

Table 4.
Total mass of the insulating materials in the 25 MVA transformer [27].
Based on the obtained water sorption isotherms for the 3A molecular sieve, it was assumed that for the relative saturation of mineral oil with water equal to 5%, the molecular sieve’s sorption capacity was equal to 12.06% at 35 °C (Figure 1). This means that 205.17 kg of MS was needed to adsorb 24.74 kg of water. In the case of drying the same transformer but with the electrical insulating system impregnated with either natural ester or synthetic ester, the amount of molecular sieve needed would be smaller due to the sieve’s greater capacity to adsorb water in these liquids. Assuming that the sorption capacity of 3A MS in both natural and synthetic esters was equal to 13.38% and 17.35%, respectively (for RS = 5%), the amount of MS needed to dry the insulation to a level of 1% was equal to 200.27 kg and 166.93 kg, respectively.
It is also possible to use a 3A molecular sieve for continuous drying of the transformer’s electrical insulation system in order to maintain its moisture at a constant low level, similar to the moisture content of a brand-new device. Drying the insulation in a permanently mounted circulating drying system removes water that appears in the transformer’s electro-insulating system during its lifetime. The increase in the transformer’s moisture depends on its construction and operating conditions. According to paper [9], the moisture content of solid insulation in a free-breathing transformer increases 0.2% per year. For a sealed transformer, this value is equal to 0.06% per year. The amount of 3A molecular sieve needed to counteract such an increase in moisture, for the 25 MVA transformer as described above, is summarized in Table 5. For comparison, calculations are presented for two construction variants, namely, free-breathing and sealed transformers.

Table 5.
Amount of 3A molecular sieve needed to adsorb the annual increase in moisture content of solid insulation in free-breathing and in sealed transformers.
In the case of a free-breathing transformer impregnated with mineral oil, 200 kg of the 3A molecular sieve should be suffice for nearly 10 years of continuous drying of the transformer. In the case of a sealed transformer impregnated with natural ester, such a quantity of molecular sieve would be sufficient for 47 years of protecting the electrical insulating system against moisture, and thus probably for the device’s entire lifetime.
Taking into consideration the results of the experiment testing the dynamics of drying insulating liquids depending on the temperature, the short-term drying of a transformer insulation system should be carried out at the highest temperature that will not cause cellulose degradation. As the drying temperature increases, the dynamics of water adsorption by the molecular sieve also increase. This conclusion is also confirmed by the results of research presented in paper [22]. The increase in insulation temperature causes an increase in the rate of water migration from cellulose to the insulating liquid. Taking the above into account, it can be concluded that a high temperature promotes the process of short-term drying of the transformer.
5. Conclusions
The study proved that there is a significant difference in the ability of 3A MS to adsorb water from mineral oil, synthetic ester, and natural ester. These differences are particularly important in the case of a low relative humidity of the insulating liquid below 5%. This relative humidity, i.e., below 5%, allows to maintain the water content in solid insulation at a level of ca. 1% for a temperature in the range of 30 °C to 70 °C, or for its effective short-term drying.
On the basis of the determined water sorption isotherms, described by means of Langmuir adsorption model, it is possible to calculate the amount of MS needed for short-term drying of the insulation system or for maintaining a low level of moisture throughout the transformer’s lifetime. This is the novelty of the work, which was not described in the literature before. This knowledge allows to simplify the drying system available in the market and apply a new approach in its operation.
The research results are important as regards the construction of an efficient system for the circular drying of insulating liquids. In order to ensure the best results for short-term drying, the liquid should be taken from the upper part of the transformer tank due to the higher temperature than from the bottom of the tank. In commercially available drying systems, mineral oil is taken from the bottom of the tank, which may reduce drying efficiency. In the case of continuous drying of the transformer insulation system in order to maintain a constant low moisture level, the liquid temperature and thus the place of its withdrawal from the tank have no significant effect.
Taking into account the high water solubility in natural and synthetic esters the problem of moisture in transformers with a free-breathing conservator will be serious. For this reason, the research on the application of MS for drying transformers insulated with esters are necessary.
The directions of further research are related to
- Assessing of the mechanical strength of MS in terms of the sieve’s erosion associated with the flow of insulating liquid;
- Evaluating of the selectivity of gases sorption by MS in the aspect of using gas chromatography for transformers diagnostics;
- Analyzing the impact of moisture migration dynamics between cellulose and electro-insulating liquid on drying process.
Author Contributions
Conceptualization, M.C. and P.P.; methodology, M.C. and P.P; formal analysis, M.C.; investigation, M.C. and P.P.; resources, M.C. and P.P.; data curation, M.C.; writing—original draft preparation, M.C. and P.P.; writing—review and editing, M.C. and P.P.; visualization, M.C.; supervision, P.P.; funding acquisition, P.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ministry of Science and Higher Education, grant number 0711/SBAD/4456.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Prevost, T.A.; Oommen, T.V. Cellulose insulation in oil-filled power transformers: Part I—History and development. IEEE Electr. Insul. Mag. 2006, 22, 28–35. [Google Scholar] [CrossRef]
- Athanassatou, H.; Duart, J.C.; Perrier, C.; Sitar, I.; Walker, J.; Claiborne, C.; Boche, T.; Cherry, D.; Darwin, A.; Gockenbach, E.; et al. Experiences in Service with New Insulating Liquids; Cigré Technical Brochure 436; International Council on Large Electric Systems (CIGRE): Paris, France, 2010. [Google Scholar]
- Dombek, G.; Nadolny, Z.; Marcinkowska, A. Effects of nanoparticles materials on heat transfer in electro-insulating liquids. Appl. Sci. 2018, 8, 2538. [Google Scholar] [CrossRef]
- Przybylek, P. Water saturation limit of insulating liquids and hygroscopicity of cellulose in aspect of moisture determination in oil-paper insulation. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 1886–1893. [Google Scholar] [CrossRef]
- Murugan, R.; Ramasamy, R. Understanding the power transformer component failures for health index-based maintenance planning in electric utilities. Eng. Fail. Anal. 2018, 96, 274–288. [Google Scholar] [CrossRef]
- Wang, M.; Vandermaar, A.J.; Srivastava, K.D. Review of condition assessment of power transformer in service. IEEE Electr. Insul. Mag. 2002, 18, 12–25. [Google Scholar] [CrossRef]
- Yousof, M.F.M.; Ekanayake, C.; Saha, T.K. Locating inter-disc faults in transformer winding using frequency response analysis. In Proceedings of the Australasian Universities Power Engineering Conference (AUPEC), Hobart, Australia, 29 September–3 October 2013; pp. 1–6. [Google Scholar] [CrossRef]
- Gielniak, J.; Graczkowski, A.; Moranda, H.; Przybylek, P.; Walczak, K.; Nadolny, Z.; Moscicka-Grzesiak, H.; Feser, K.; Gubanski, S.M. Moisture in cellulose insulation of power transformers—Statistics. IEEE Trans. Dielectr. Electr. Insul. 2013, 20, 982–987. [Google Scholar] [CrossRef]
- Sokolov, V.; Aubin, J.; Davydov, V.; Gasser, H.P.; Grin, P.; Koch, M.; Lundgaard, L.; Roizman, O.; Scala, M.; Tenbohlen, S.; et al. Moisture Equilibrium and Moisture Migration within Transformer Insulation Systems; Cigré Technical Brochure 349; International Council on Large Electric Systems (CIGRE): Paris, France, 2008. [Google Scholar]
- IEEE. IEEE Guide for Diagnostic Field Testing of Electric Power Apparatus—Part 1: Oil Filled Power Transformers, Regulators, and Reactors; IEEE Std. 62-1995; Institute of Electrical and Electronics Engineers (IEEE): Piscataway Township, NJ, USA, 1995. [Google Scholar]
- Przybylek, P.; Moranda, H.; Moscicka-Grzesiak, H.; Szczesniak, D. Application of synthetic ester for drying distribution transformer insulation—The influence of cellulose thickness on drying efficiency. Energies 2019, 12, 3874. [Google Scholar] [CrossRef]
- Shroff, D.H.; Stannett, A.W. A review of paper aging in power transformers. IEE Proc. C Gener. Transm. Distr. 1985, 132, 312–319. [Google Scholar] [CrossRef]
- Fofana, I.; Borsi, H.; Gockenbach, G.; Farzaneh, M. Ageing of Cellulose in Mineral-Oil Insulated Transformers; Cigré Technical Brochure 323; International Council on Large Electric Systems (CIGRE): Paris, France, 2007. [Google Scholar]
- IEC. Measurement of the Average Viscometric Degree of Polymerization of New and Aged Cellulosic Electrically Insulating Materials; IEC 60450; International Electrotechnical Commission (IEC): New York, NY, USA, 2004. [Google Scholar]
- Sikorski, W.; Walczak, K.; Przybylek, P. Moisture migration in an oil-paper insulation system in relation to online partial discharge monitoring of power transformers. Energies 2016, 9, 1082. [Google Scholar] [CrossRef]
- Dai, J.; Wang, Z.D.; Jarman, P. Moisture and aging effect on the creepage discharge characteristics at the oil/transformer-board interface under divergent field. In Proceedings of the Conference on Electrical Insulation and Dielectric Phenomena (CEIDP), Québec, QC, Canada, 26–29 October 2008; pp. 662–665. [Google Scholar] [CrossRef]
- Oommen, T.V.; Lindgren, S.R. Bubble evolution from transformer overload. In Proceedings of the Transmission and Distribution Conference Exposition, Atlanta, GA, USA, 2 November 2001; pp. 137–142. [Google Scholar] [CrossRef]
- Przybylek, P. Investigations of the temperature of bubble effect initiation oil-paper insulation. Prz. Elektrotech. 2010, 86, 166–169. [Google Scholar]
- Cybulski, M.; Przybylek, P. Water sorption isotherms for 3A and 13X molecular sieves in the aspect of their use for drying the transformer insulation system. Prz. Elektrotech. 2018, 86, 37–40. (In Polish) [Google Scholar] [CrossRef]
- Sarbak, Z. Nieorganiczne Materiały Nanoporowate; Wydawnictwo Naukowe Uniwersytetu im. Adama Mickiewicza: Poznan, Poland, 2010; pp. 103–138. [Google Scholar]
- Lukarska, M.H. Synthesis and Characterization of Composites Containing Organic Dyes Entrapped in the Molecular Sieves. Ph.D. Thesis, Adam Mickiewicz University, Poznan, Poland, 2015. [Google Scholar]
- Fofana, I.; Wasserberg, V.; Borsi, H.; Gockenbach, E.; Farzaneh, M. Drying of transformer insulation using zeolite. IEEE Electr. Insul. Mag. 2004, 20, 20–30. [Google Scholar] [CrossRef]
- Lelekakis, N.; Martin, D.; Guo, W.; Wijaya, J. A field study of two online dry-out methods for power transformers. IEEE Electr. Insul. Mag. 2012, 28, 32–39. [Google Scholar] [CrossRef]
- IEC. Insulating Liquids—Oil-Impregnated Paper and Pressboard—Determination of Water by Automatic Coulometric Karl Fischer Titration; IEC 60814; International Electrotechnical Commission (IEC): New York, NY, USA, 1997. [Google Scholar]
- Cybulski, M.; Przybylek, P. Applying of hygroscopic materials to dry transformer insulation. In Proceedings of the ITM Web of Conferences, Poznan, Poland, 23—24 April 2018; Volume 19, p. 01017. [Google Scholar] [CrossRef]
- Bushra, R.; Shahadat, M. Mechanism of adsorption on nanomaterials. In Advanced Environmental Analysis: Applications of Nanomaterials; Hussain, C.M., Kharisov, B., Eds.; The Royal Society of Chemistry: London, UK, 2017; Volume 1, pp. 90–111. [Google Scholar]
- Thomas, L. Drying of Power Transformer Insulation by Means of Synthetic Ester. Master’s Thesis, Poznan University of Technology, Poznan, Poland. (In Polish).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).