Empirical Analysis of High Voltage Battery Pack Cells for Electric Racing Vehicles †
Abstract
1. Introduction
- 400 V DC maximum to match the motor controller’s voltage limit
- 7 kWh battery capacity
- 80 kW power output
- 200 A of maximum discharge current
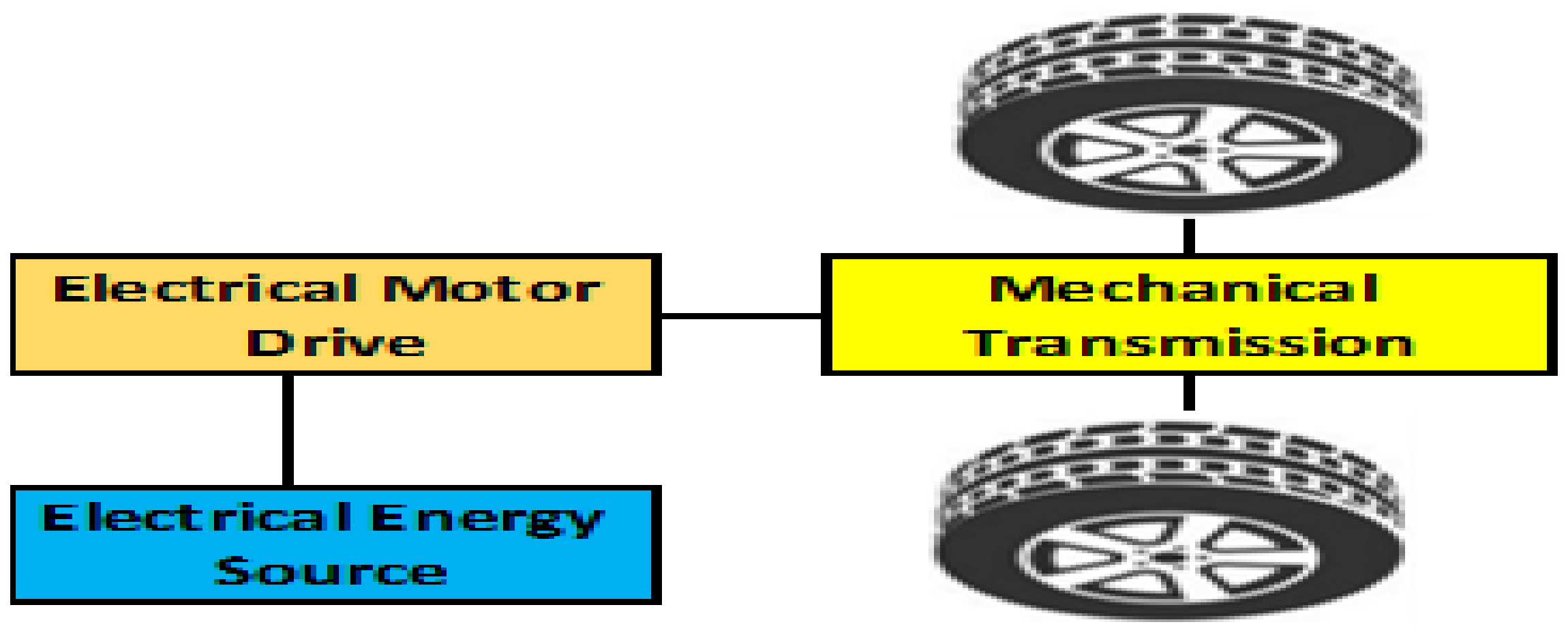
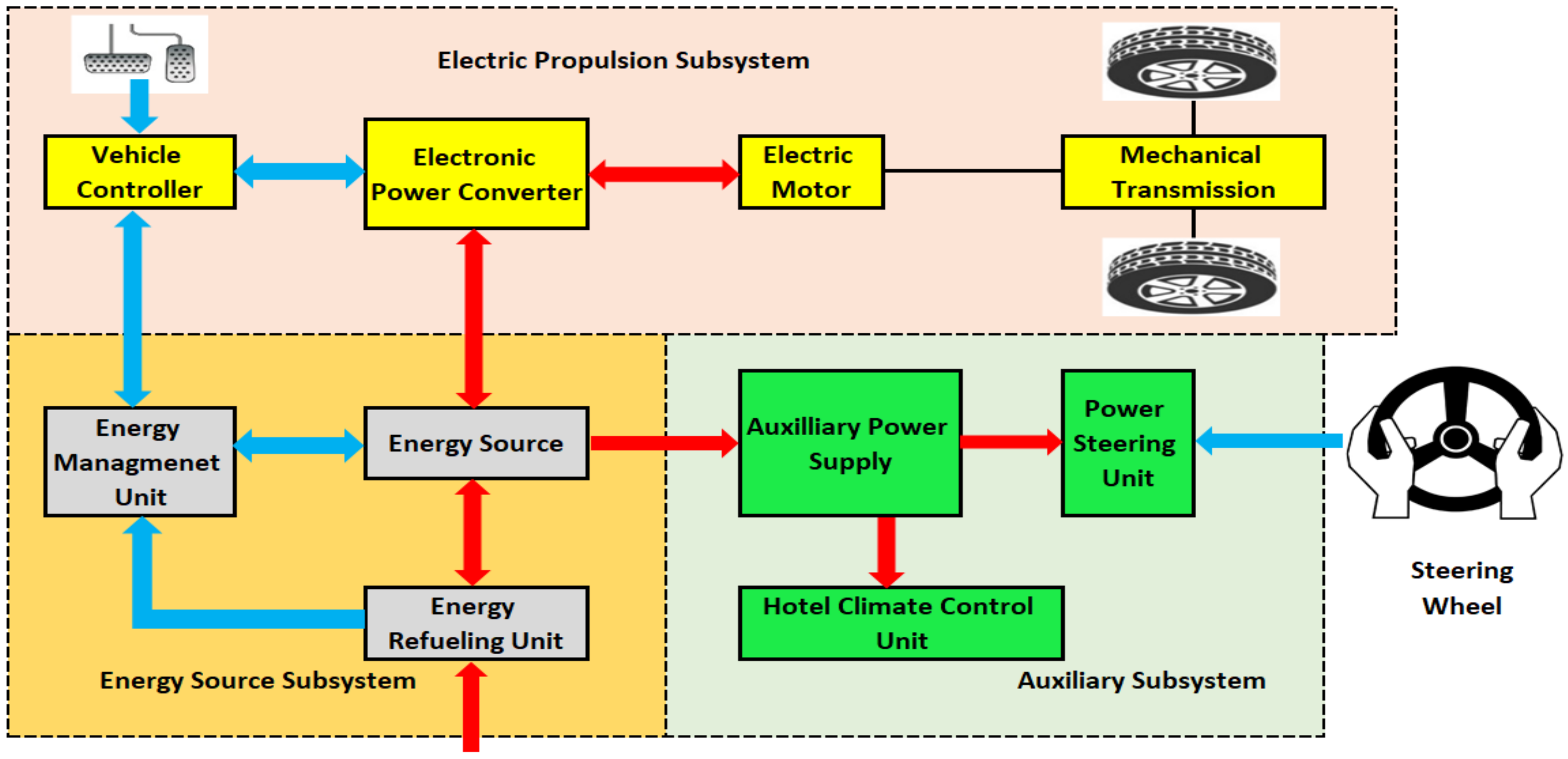
2. Battery Energy Storage System (BESS)
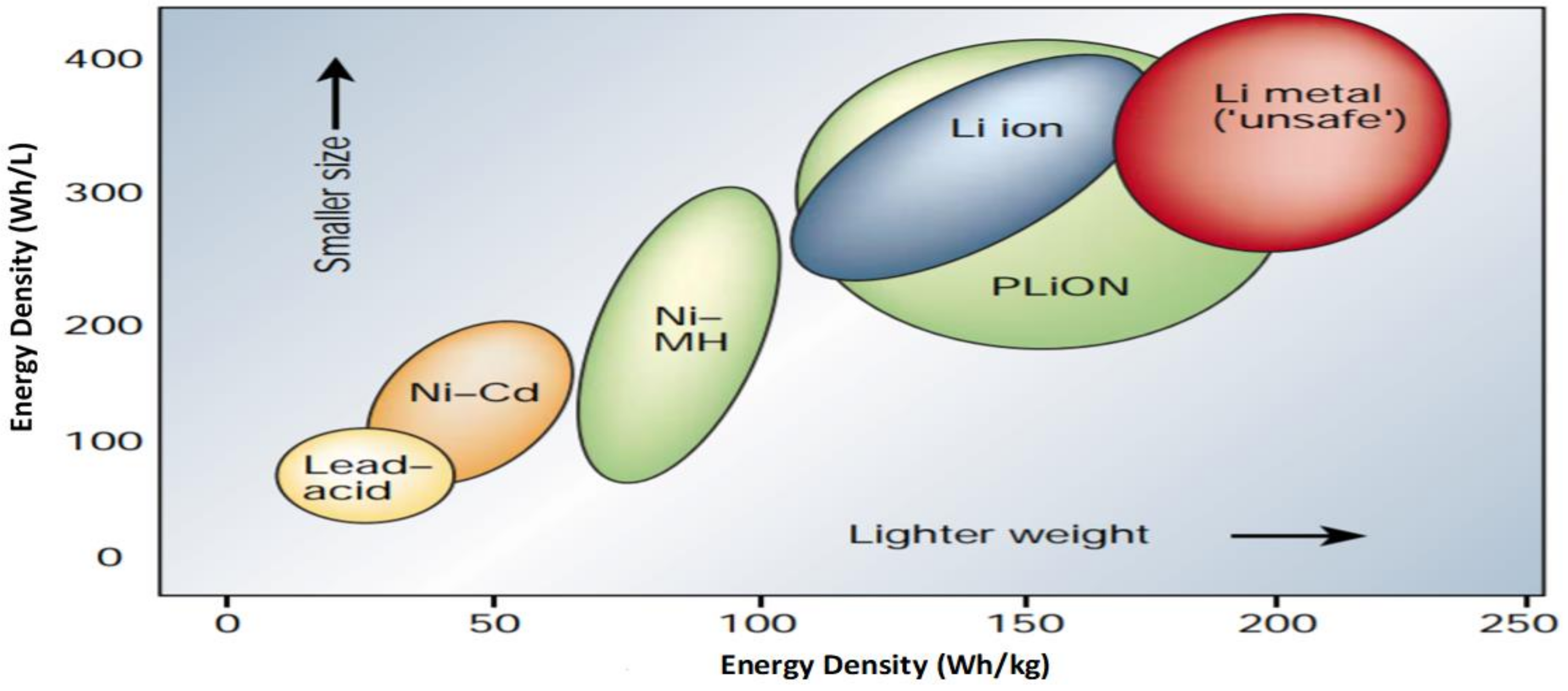
3. Electrical Vehicle Battery Technology
| Company | Country | Vehicle Model | Battery Technology |
|---|---|---|---|
| GM | USA | Chevy-Volt | Li-ion |
| Saturn Vue Hybrid | NiMH | ||
| Ford | USA | Escape, Fusion, MKZ HEV | NiMH |
| Escape PHEV | Li-ion | ||
| Toyota | Japan | Prius, Lexus | NiMH |
| Honda | Japan | Civic, Insight | NiMH |
| Hyundai | South Korea | Sonata | Lithium polymer |
| Chrysler | USA | Chrysler 200C EV | Li-ion |
| BMW | Germany | X6 | NiMH |
| Mini E (2012) | Li-ion | ||
| BYD | China | E6 | Li-ion |
| Daimler Benz | Germany | ML450, S400 | NiMH |
| Smart EV (2010) | Li-ion | ||
| Mitsubishi | Japan | iMiEV (2010) | Li-ion |
| Nissan | Japan | Altima | NiMH |
| Leaf EV (2010) | Li-ion | ||
| Tesla | USA | Roadster (2009) | Li-ion |
| Think | Norway | Think EV | Li-ion, Sodium/metal chloride |
Selection of Cells
4. Comparative Analysis, Results and Discussion
4.1. Comparative Analysis of Li-ion Battery Cells from Different Manufacturers
- The temperature of the cell exceeded the maximum specified in the cell Material Safety Data Sheet (MSDS).
- The maximum current indicated in the MSDS was surpassed.
- The cell was operating below the lower voltage limit given in the MSDS.
4.2. Test 1: Continuous 15 A Discharge with No Active Cooling
4.3. Test 2: 30 A Discharge Current Tests with No Active Cooling
4.4. Test 3: 15 A Discharges with Active Air Cooling
4.5. Test 4: 30 A Discharge Current Test with Active Air Cooling
4.6. Key Findings and Summary
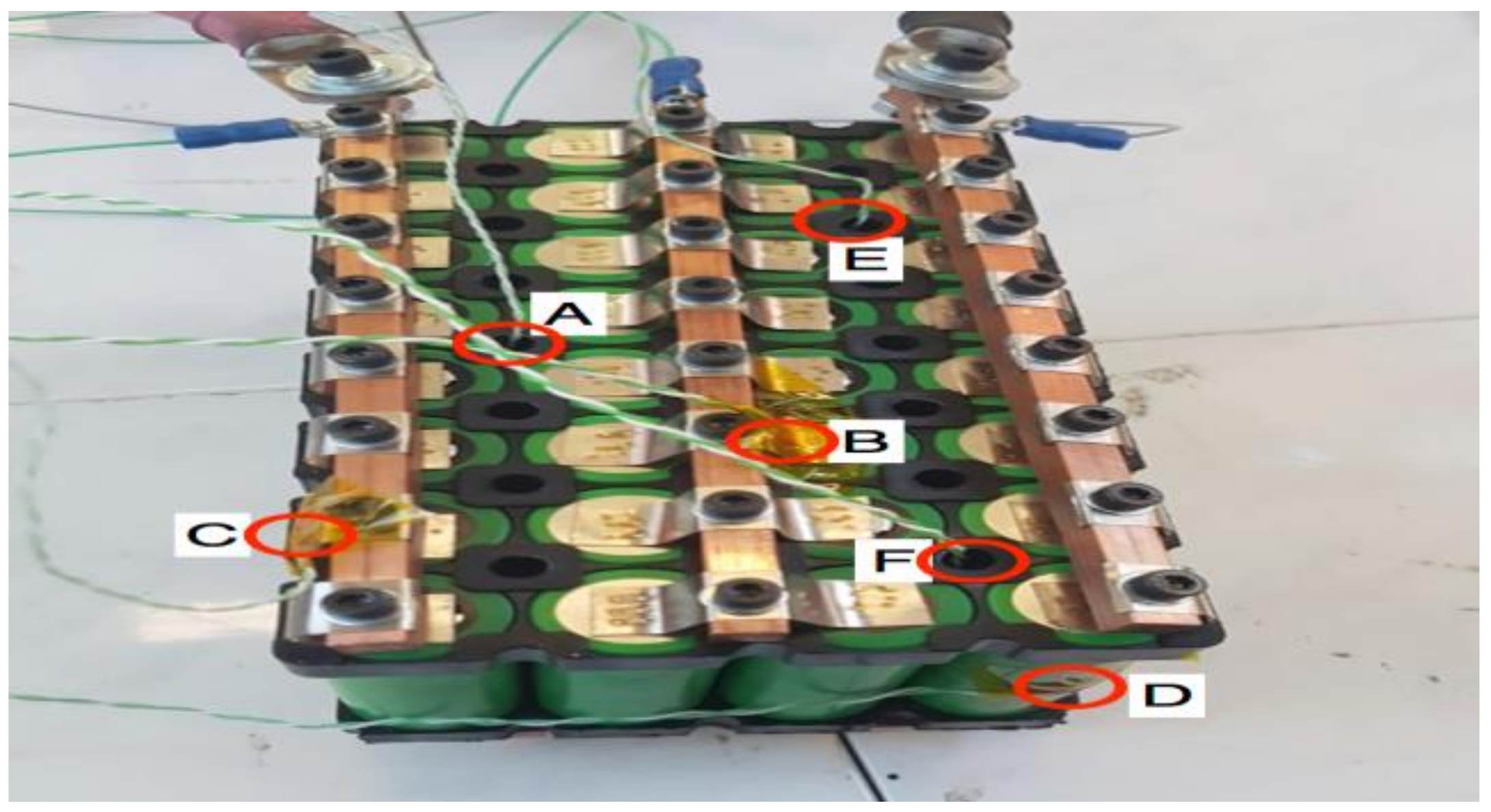
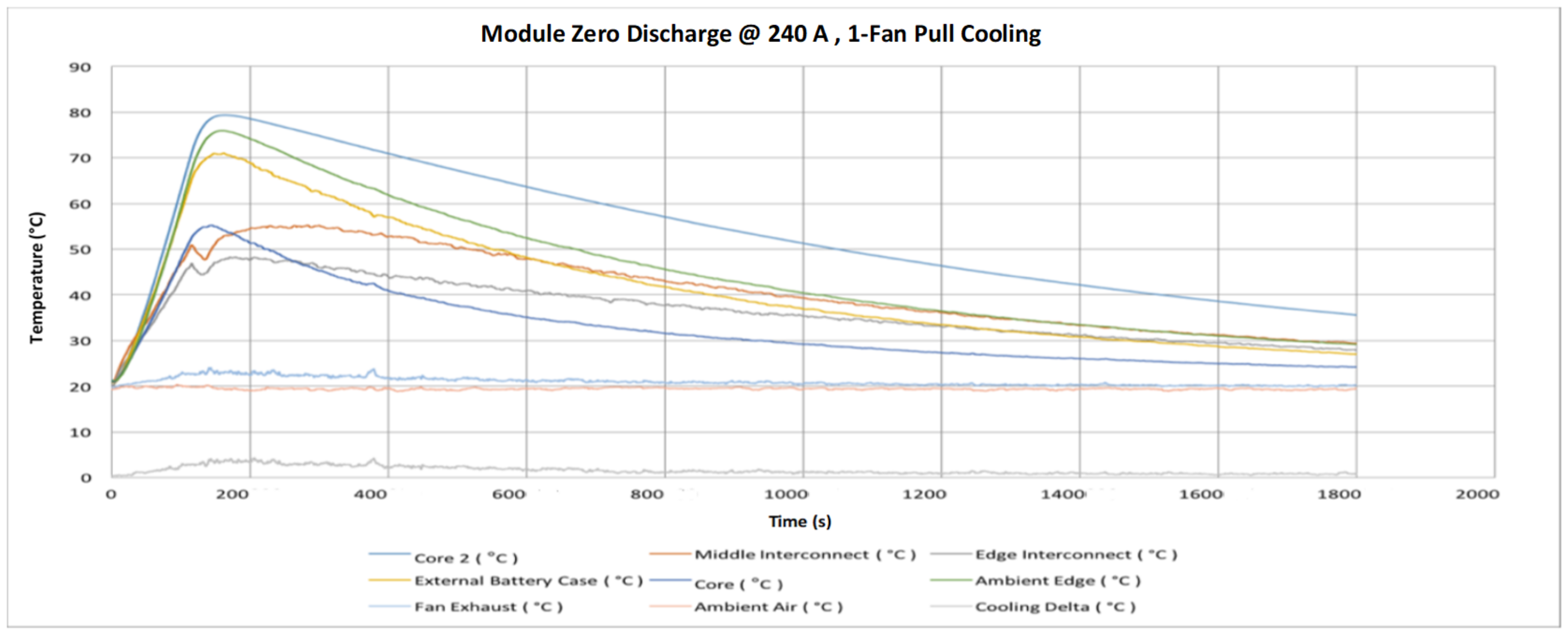
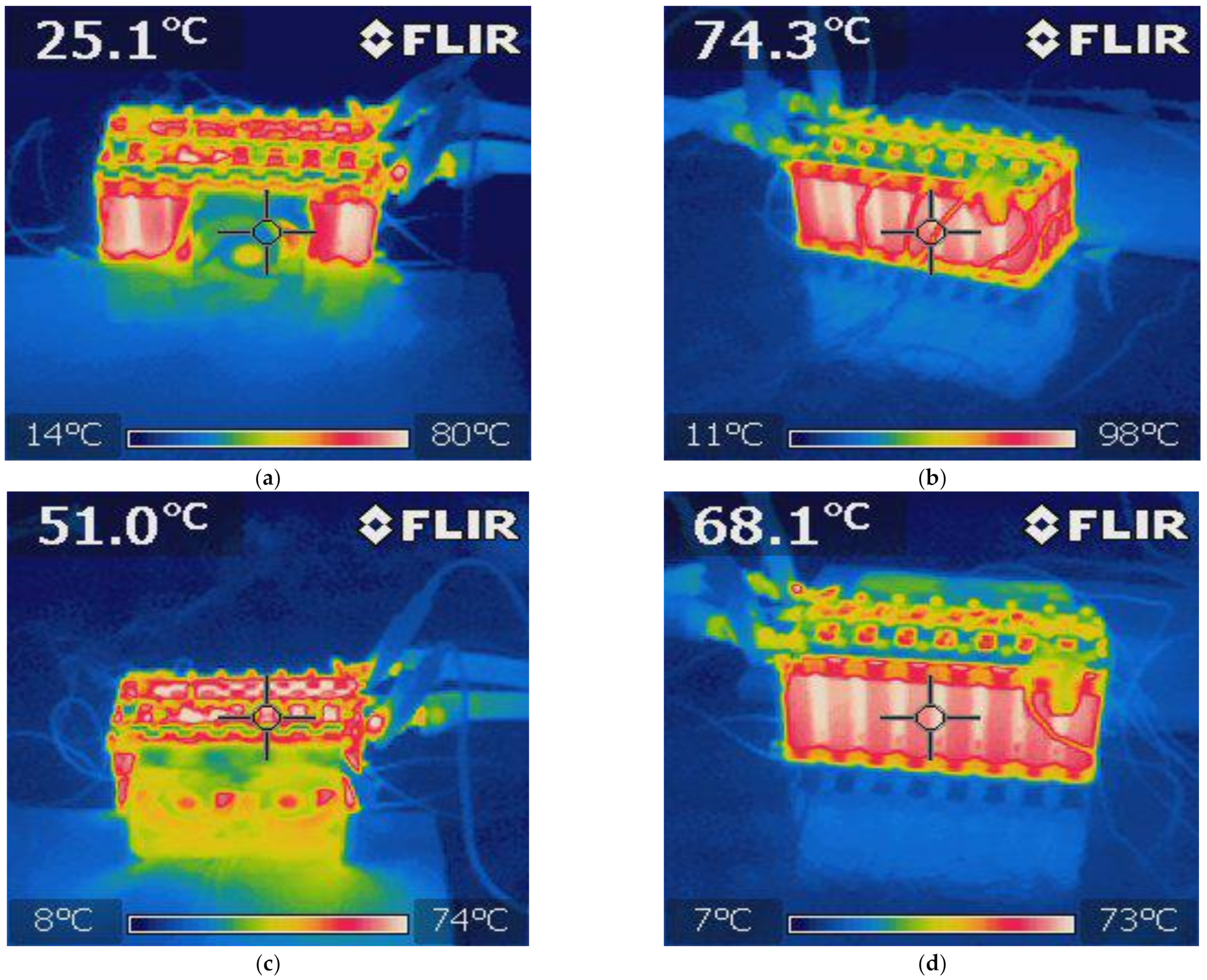
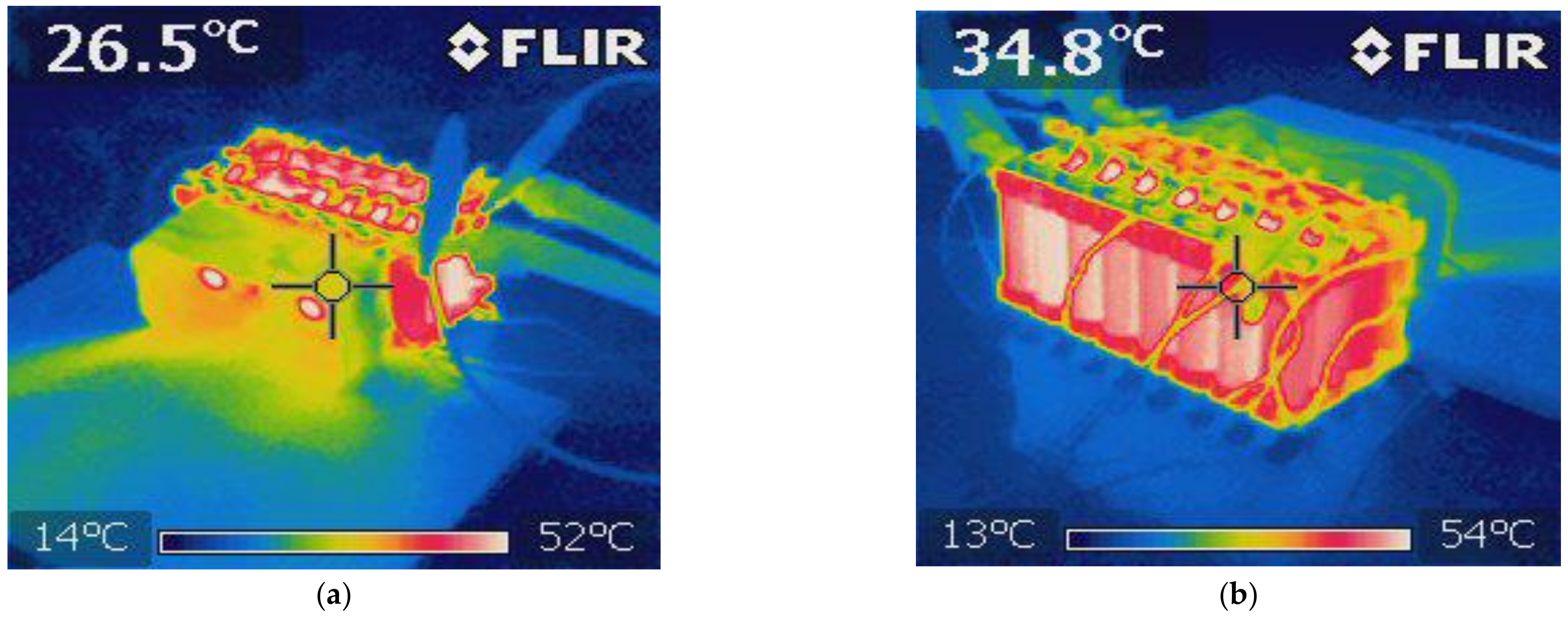
5. Battery Module Test
6. Conclusions
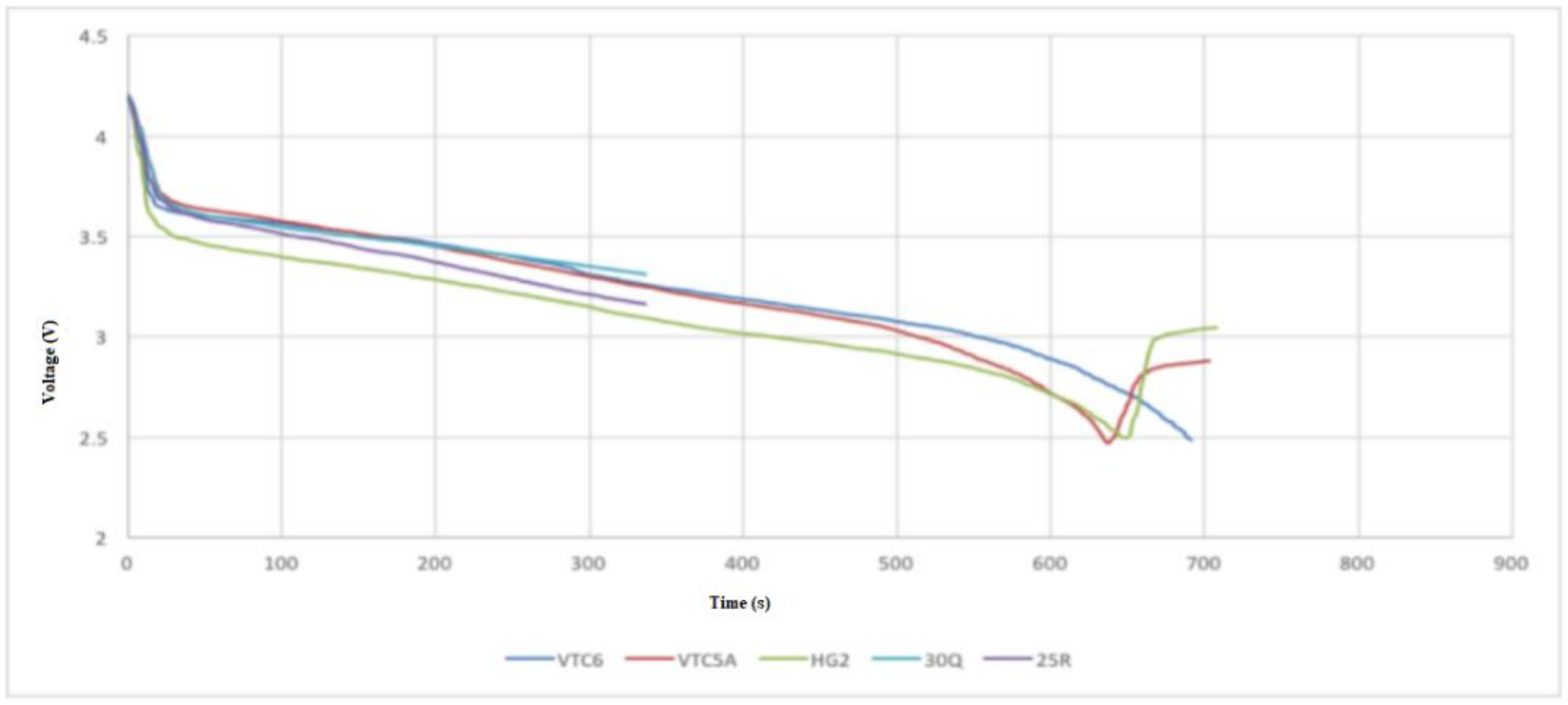
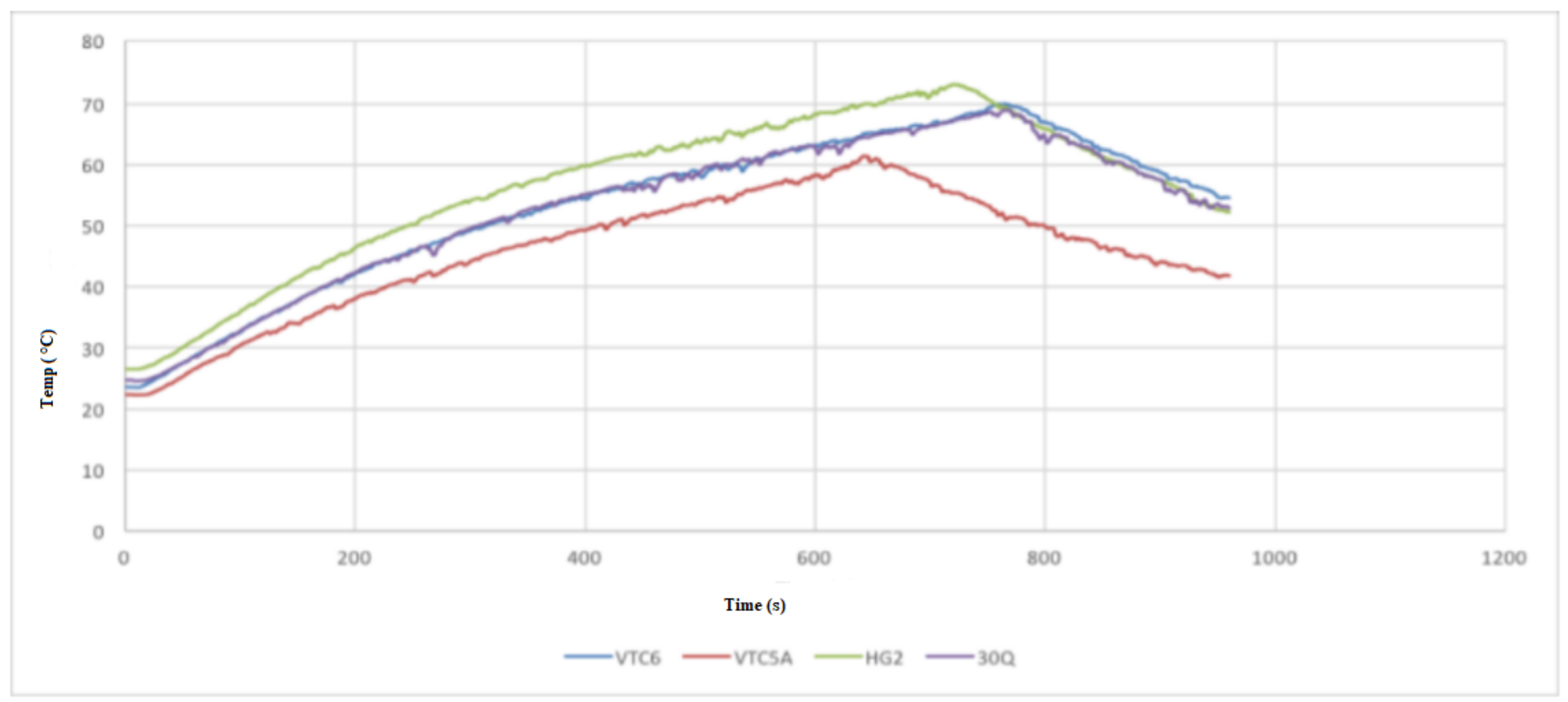
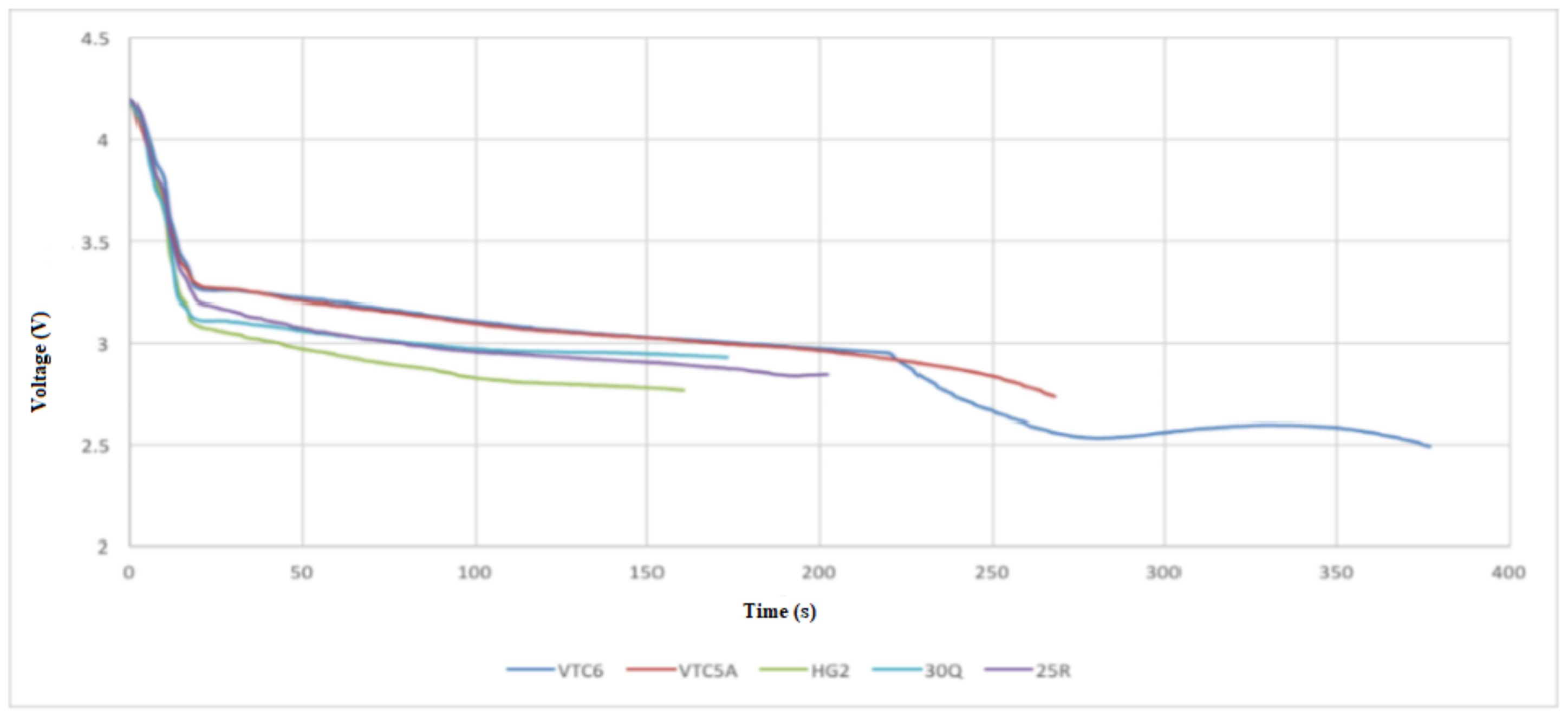
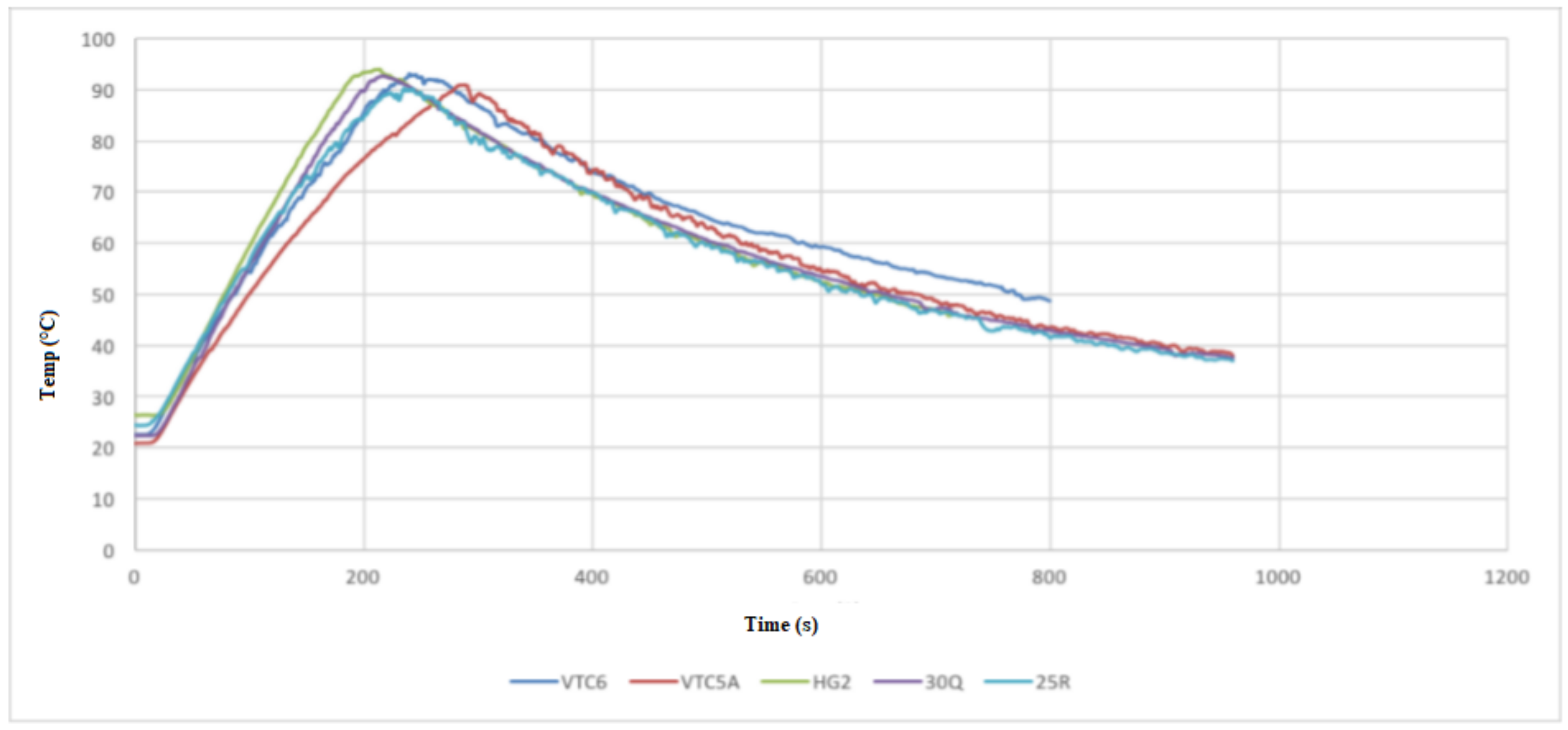
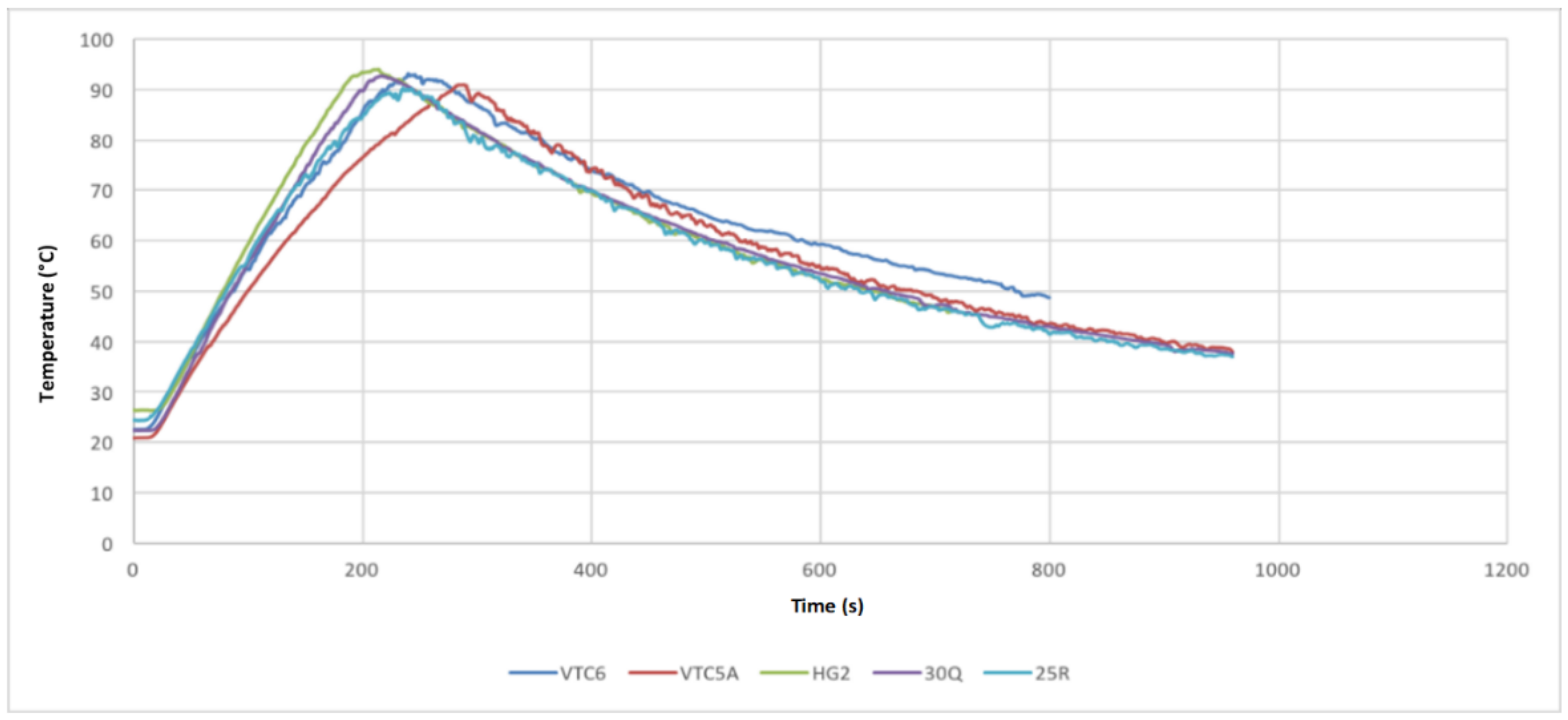
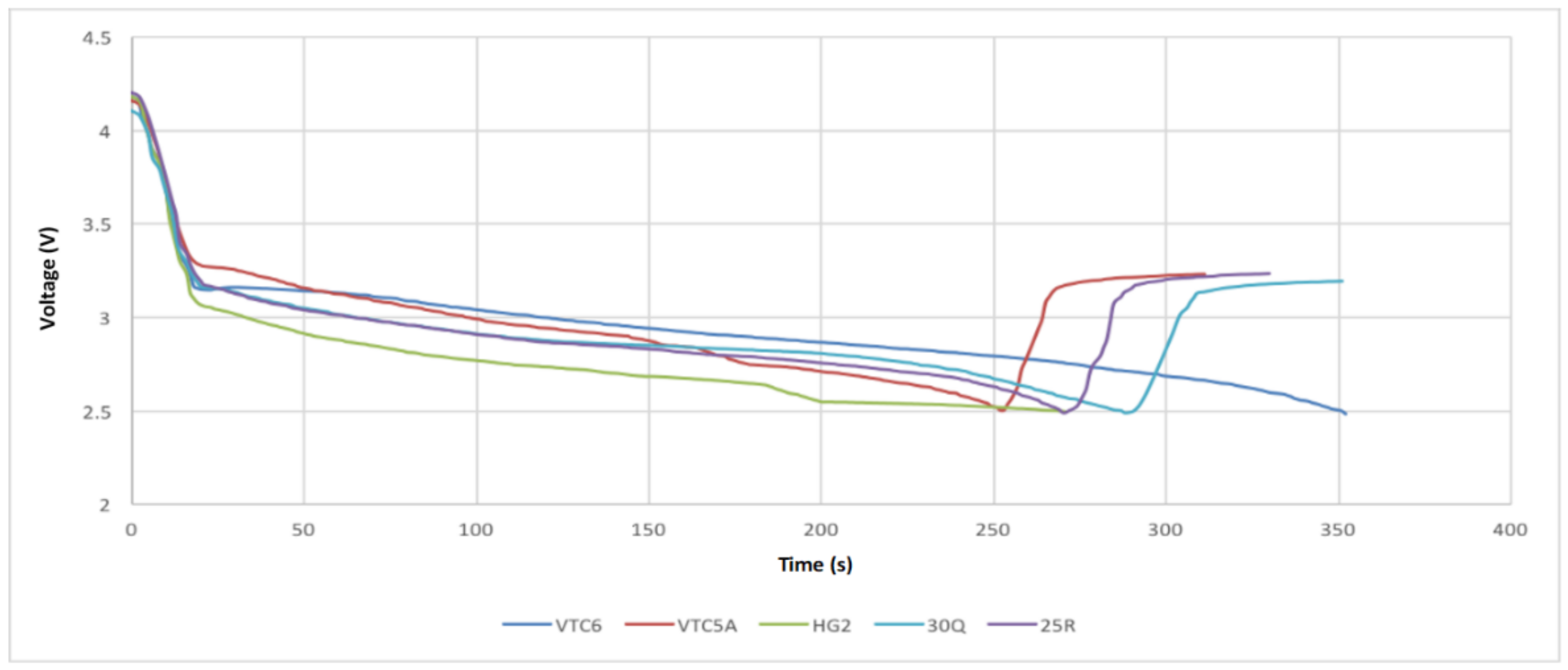
- Test 1 was performed at continuous 15 A discharge with no active cooling applied. This test demonstrated that the 30Q, VTC6, and VTC5A gave better thermal performance than the HG2.
- Test 2 was conducted at 30 A discharge current when no active cooling applied. In this test, the VTC5A outperformed the other cells, having the longest discharge time prior to arriving at the thermal cutoff point. The 25R and VTC6 performed equally well in the thermal tests. None of the cells under test was able to fully discharge at 30 A without reaching thermal cutoff.
- Test 3 was carried out at 15 A discharge current when active air cooling applied. Under this test conditions, all the cells performed very well, with the VTC5AV and VTC6 performing better than the rest. This mainly due to their low internal resistance, which appears in the increased voltage under load during the consistent inclination gradient at discharge. Similar to Test 3, the VTC5A had the best performance with longest discharge time before the module reaches the thermal cutoff point. The HG2 had the weakest performance with the shortest discharge time.
- Test 4 was performed at 15 A discharge current when active air cooling applied. It is found when conducting this test that the VTC6 maintained the highest charge, taking more than 60 s longer to fully discharge because of its greater capacity. The VTC5A maintained a low voltage drop until the 50 s to 80 s mark, at which time the VTC6 kept a higher voltage than all other cells until the test finished.
- Based on the 4 test applied, the VTC5A cell gave the best performance among other brands.
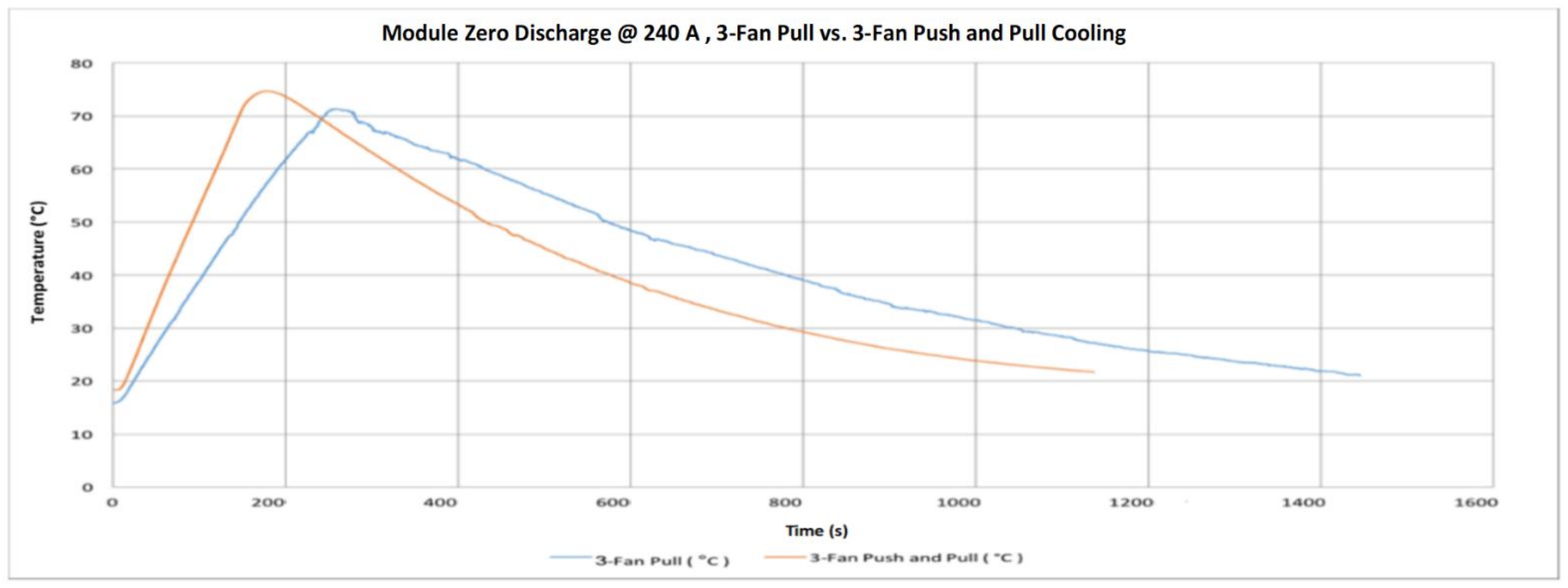
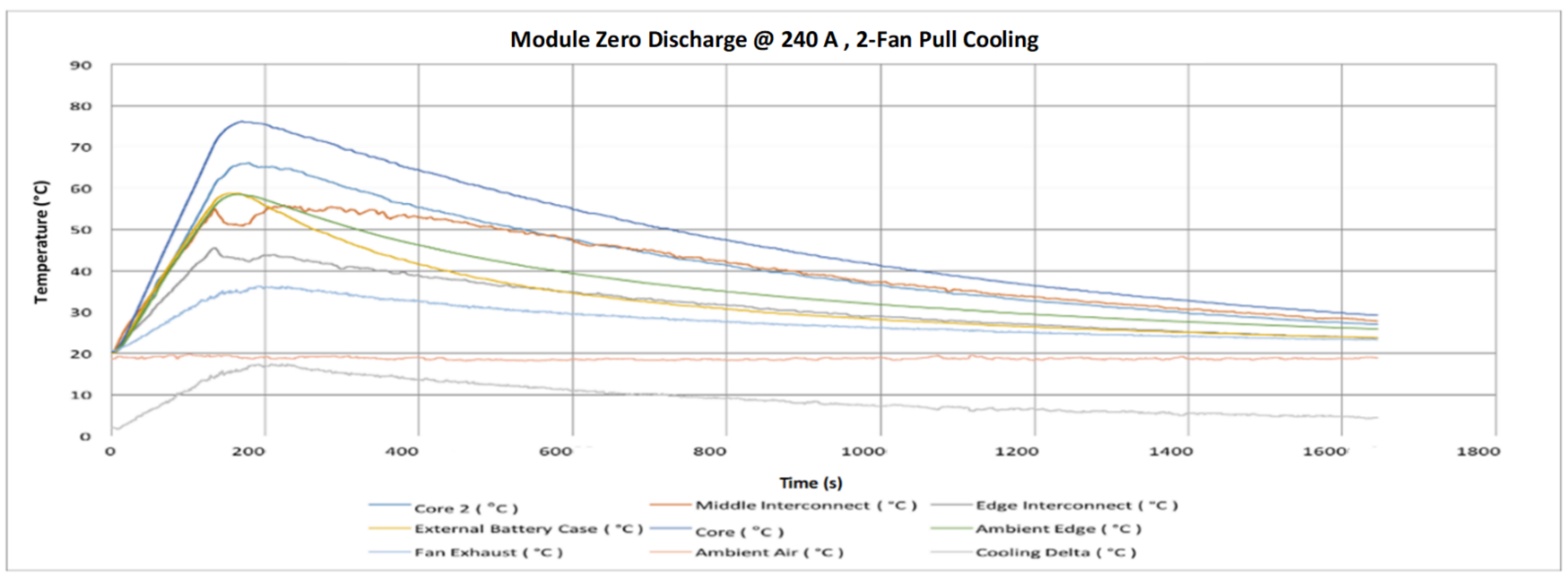
- Air cooling system found very efficient in significantly cooling the modules because of the large temperature contrast between the surrounding air and the module. The cooling system is required to ensure operating within allowable limits.
- Finally, the VTC6 battery cell was selected, due to its thermal superiority, for the subsequent tests with focus on thermal temperatures of the module. Thermocouples and thermal imaging used as well to monitor the module temperatures at different cooling approaches.
- The key outcome of this further experiments indicates that a forced-air cooling system with a suitable battery pack can keep the temperature of the cell at a level that enables superior performance.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ehsani, M.; Gao, Y.; Gay, S.E.; Emadi, A. Modern Electric Hybrid Electric and Fuel Cell Vehicles; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Charadsuksawat, A.; Laoonual, Y.; Chollacoop, N. Comparative Study of Hybrid Electric Vehicle and Conventional Vehicle Under New European Driving Cycle and Bangkok Driving Cycle. In Proceedings of the 2018 IEEE Transportation Electrification Conference and Expo, Asia-Pacific (ITEC Asia-Pacific), Bangkok, Thailand, 6–9 June 2018; pp. 1–6. [Google Scholar]
- Markowitz, M. “Wells to Wheels: Electric Car Efficiency”, Energy Matters. Available online: https://matter2energy.wordpress.com/2013/02/22/wells-towheels-electric-car-efficiency/ (accessed on 26 March 2018).
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Zolot, M.D.; Kelly, K.; Keyser, M.; Mihalic, M.; Pesaran, A.; Hieronymus, A. Thermal evaluation of the Honda Insight battery pack. In Proceedings of the Intersociety Energy Conversion Engineering Conference SAE, Savannah, GA, USA, 29 July–2 August 2001; pp. 923–928. [Google Scholar]
- Kim, G.-H.; Pesaran, A. Battery Thermal Management Design Modeling. World Electr. Veh. J. 2007, 1, 126–133. [Google Scholar] [CrossRef]
- Wu, M.-S.; Liu, K.H.; Wang, Y.-Y.; Wan, C.-C. Heat dissipation design for lithium-ion batteries. J. Power Sources 2002, 109, 160–166. [Google Scholar] [CrossRef]
- Peng, X.; Cui, X.; Liao, X.; Garg, A. A Thermal Investigation and Optimization of an Air-Cooled Lithium-Ion Battery Pack. Energies 2020, 13, 2956. [Google Scholar] [CrossRef]
- Formula SAE, “Formula SAE Rules”, Formula SAE. Available online: https://www.fsaeonline.com/content/2017-18%20FSAE%20Rules%20PRELIMINARY.pdf (accessed on 30 June 2020).
- Kim, Y. Design and Management of Energy-Efficient Hybrid Electrical Energy Storage Systems; Springer International Publishing: Cham, Switzerland, 2014. [Google Scholar]
- Sehil, K. A Super-Capacitor-Based Energy Storage for Quick Variation in Stand-Alone PV Systems. Ph.D. Thesis, Brunel University, London, UK, 2018. [Google Scholar]
- Crompton, T. Battery Reference Book; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Sehil, K.; Darwish, M.; Marouchos, C.C.; Jeans, W.C. Comparative Analysis of High Voltage Battery Pack Cells for Electric Vehicle. In Proceedings of the 2020 55th International Universities Power Engineering Conference (UPEC), Torino, Italy, 1–4 September 2020; pp. 1–6. [Google Scholar]
- Schainker, R.B. Executive overview: Energy storage options for a sustainable energy future. In Proceedings of the IEEE Power Engineering Society General Meeting, Denver, CO, USA, 6–10 June 2004; pp. 2309–2314. [Google Scholar] [CrossRef]
- Kusko, A.; Dedad, J. Short-term, long-term, energy storage methods for standby electric power systems. In Proceedings of the Fourtieth IAS Annual Meeting, Conference Record of the 2005 Industry Applications Conference, Hong Kong, China, 2–6 October 2005; pp. 2672–2678. [Google Scholar] [CrossRef]
- Alamri, B.R.; Alamri, A.R. Technical review of energy storage technologies when integrated with intermittent renewable energy. In Proceedings of the 2009 International Conference on Sustainable Power Generation and Supply, Nanjing, China, 6–7 April 2009; pp. 1–5. [Google Scholar]
- Perrin, M.; Malbranche, P.; Mattera, F.; Simonin, L.; Sauer, D.U.; Lailler, P.; Joessen, A.; Willer, B.; Dahlen, M.; Ruddell, A.; et al. Evaluation and perspectives of storage technologies for PV electricity. In Proceedings of the 3rd World Conference on Photovoltaic Energy Conversion, Osaka, Japan, 11–18 May 2003; pp. 2194–2197. [Google Scholar]
- Glaize, C.; Geniès, S. Lithium Batteries and Other Electrochemical Storage Systems; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nat. Cell Biol. 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Sun, C. Advanced Battery Materials Hoboken; Wiley: New York, NY, USA, 2019. [Google Scholar]
- Young, K.; Wang, C.; Wang, L.Y.; Strunz, K. Electric Vehicle Battery Technologies. In Electric Vehicle Integration into Modern Power Networks; Springer: New York, NY, USA, 2013; pp. 15–56. [Google Scholar]







| Battey Type | Advantages | Disadvantages |
|---|---|---|
| Lithium Polymer (Li-po) | Robust Flexible Low profile Less likely to leak electrolyte | More expensive to manufacture Store less energy Shorter lifespan |
| Lithium Ion (Li-ion) | Generally higher energy density Better power performance No memory effect * Cheaper to manufacture | More prone to suffer from aging. More likely to combust if overcharged |
| Battery Pack Specification | |
|---|---|
| Max voltage (V) | 400 |
| Minimum discharge current (A) | 200 |
| Capacity (kWh) | 7 |
| Output power (kW) | 80 |
| Discharge (A) | ||||||
|---|---|---|---|---|---|---|
| Cell Name | mAh | Format | Module Required in Parallel | Pulse | Continuous | Peak Pack |
| Sony VTC6 | 3000 | Cylindrical | 6 | 30 | 15 | 180 |
| Sony VTC5A | 2500 | Cylindrical | 7 | 30 | 20 | 210 |
| LG DB 18650 HG2 | 3000 | Cylindrical | 6 | 20 | 20 | 120 |
| Samsung 18650 30Q | 3000 | Cylindrical | 6 | 15 | 15 | 90 |
| Samsung 18650 25R | 2500 | Cylindrical | 6 | 20 | 20 | 120 |
| Test | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| Start voltage (V) | 4.00 | 4.00 | 4.00 | 4.00 |
| End voltage (V) | 2.50 | 2.50 | 2.50 | 2.50 |
| Current (A) | 15 | 30 | 15 | 30 |
| Use of active cooling | No | No | Yes | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sehil, K.; Alamri, B.; Alqarni, M.; Sallama, A.; Darwish, M. Empirical Analysis of High Voltage Battery Pack Cells for Electric Racing Vehicles. Energies 2021, 14, 1556. https://doi.org/10.3390/en14061556
Sehil K, Alamri B, Alqarni M, Sallama A, Darwish M. Empirical Analysis of High Voltage Battery Pack Cells for Electric Racing Vehicles. Energies. 2021; 14(6):1556. https://doi.org/10.3390/en14061556
Chicago/Turabian StyleSehil, Khaled, Basem Alamri, Mohammed Alqarni, Abdulhafid Sallama, and Mohamed Darwish. 2021. "Empirical Analysis of High Voltage Battery Pack Cells for Electric Racing Vehicles" Energies 14, no. 6: 1556. https://doi.org/10.3390/en14061556
APA StyleSehil, K., Alamri, B., Alqarni, M., Sallama, A., & Darwish, M. (2021). Empirical Analysis of High Voltage Battery Pack Cells for Electric Racing Vehicles. Energies, 14(6), 1556. https://doi.org/10.3390/en14061556






