1. Introduction
It was only a few years ago that most refrigeration appliances used refrigerants containing chlorine; these had a destructive effect on the ozone layer. The use of chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), which actually possess very good thermodynamic properties, is now prohibited. Apart from the destructive impact on the ozone layer, refrigerants also affect the natural environment by contributing to the greenhouse effect. Bearing in mind the changes taking place in the environment, the European Parliament approved Regulation No. 517/2014 [
1] on fluorinated greenhouse gases, which significantly limits the possibility of using the refrigerants currently available on the market, especially those with a high Global Warming Potential (GWP). The purpose of legal regulations is to limit global climate change in accordance with the Paris Agreements and to prevent the adverse effects of this change. In response, greater use of natural refrigerants and hydrocarbon derivatives is necessary, as they have a lower environmental impact. The extensive use of carbon dioxide or hydrocarbons, as well as hydrofluoroolefins (HFOs) and hydrofluorocarbons (HFCs) with a GWP not exceeding 150, is a key challenge in transforming the refrigerant market.
HFO substances quickly decompose in the lower atmosphere due to the double carbon bond in the molecule; this guarantees very low GWP, but also results in flammability [
2]. The most popular refrigerant in this group is R1234yf, which is used as a replacement for R134a in automotive air-conditioning systems. The second leading representative of the new generation of refrigerants is R1234ze(E), which has zero Ozone Depletion Potential (ODP) and a low GWP. It has been proposed as a replacement for R32, R410A, and R134a. However, R1234ze(E) is not an ideal substitute for the phased-out refrigerants, as it has a lower specific cooling capacity [
3], a lower coefficient of performance (COP) [
4], and a lower heat transfer coefficient [
5,
6]. According to EN 378-1 [
7], both HFO refrigerants have a very low level of GWP, at only 4 and 7, respectively. In addition to compressor cycles, HFOs are also considered for operation in other types of refrigeration equipment, such as ejector devices [
8,
9] or combined systems [
10,
11].
Combining HFO refrigerants with HFCs has become popular as a way to improve their properties, especially low cooling capacity, due to low latent heat of vaporization and specific refrigerating effect. In scientific research, it has often been combined with R32, a high-pressure refrigerant with satisfactory thermodynamic properties, high latent heat, and a relatively low GWP of 675 [
12]. The R32–R1234ze(E) mixture was tested in the proportions of 0.5/0.5 and 0.2/0.8 to determine the heat transfer coefficient obtained in a horizontal tube [
13] and the isobaric heat capacity (a mole fraction of R32 from 0.226 to 0.946) [
2]. Another proposed combination of HFO/HFC refrigerants is the R32–R1234yf mixture, at different weight proportions, tested to determine a truncated virial equation of state [
14].
Akasaka [
15] presented models of thermodynamic properties for the mixtures R32–R1234ze(E) and R32/R1234yf, the uncertainties of which are 1% for the bubble point pressure and 0.25% for the liquid density. The author states that while the models show slightly greater uncertainties than the typical Helmholtz energy state equations for pure fluids, they are applicable to preliminary analysis of refrigeration equipment and heat pumps. R1234ze(E), like R1234yf, was also combined with R134a to determine the vapor–liquid balance of the mixtures [
2] and to test the possibility of replacing pure R134a with new substances in home refrigerators.
Aprea et al. [
16] proposed a mixture of R1234yf–R134a with a 10% HFC weight share, defined by the GWP limit of 150. This mixture achieved a 17% lower life-cycle climate performance (LCCP) index than pure R134a.
Other experimental results have shown that for R134a–R1234ze(E) mixture, despite the larger required refrigerant charge, a shorter daily operating time was obtained while maintaining the set temperature level, which resulted in a reduction of energy consumption by 14% compared to R134a [
17]. Another combination is the R152a–R1234ze(E) mixture, for which the equilibrium (vapor + liquid) was tested; this is one of the most important parameters used to calculate and optimize cooling cycle efficiency, as well as the organic Rankine cycle and other chemical processes [
18].
Ternary mixtures are another way to combine the new generation of refrigerants with HFCs. Various mixtures containing HFO are currently commercially available. These include, among others:
R447A, which has a favorable GWP of 572, and, in terms of heat exchange, can be treated as a potential alternative to R410A (GWP = 2088) [
19];
R457A and R459B, with a low GWP (less than 150), which can provide a smaller refrigerant charge and improve energy efficiency in relation to R404A [
20];
It is not only synthetic refrigerants that are combined together. Natural substances, such as carbon dioxide and hydrocarbons, are also used to create refrigeration blends. Kondou et al. [
21] examined the heat transfer coefficient during evaporation and condensation of the ternary mixture R32–R1234yf–R744 with different weight shares (0.29/0.62/0.09, 0.43/0.53/0.04 and 0.06/0.34/0.6) and compared them with the binary R32–R1234ze(E) mixture (with weight shares of 0.4/0.6 and 0.73/0.27). Saengsikhiao et al. [
22] considered using R463A as a replacement for R404A. It has been shown that R463A may operate at a higher ambient temperature, achieving higher COP in a low-temperature application, and lower GWP compared to R404A.
A further example of combining HFCs with HFOs and natural substances is R134a–R1234yf–R600a. Isobutane has good latent heat and excellent thermodynamic performance but is highly flammable and explosive. R1234yf is also slightly flammable, so non-flammable R134a has been added to reduce this flammability. The vapor–liquid equilibrium data were collected for the mixture [
23]. Research has also been carried out on implementing a model for forecasting vapor + liquid balance data in a binary mixture of CO2, HFC, or HC, with two low GWP refrigerants (R1234yf and R1234ze (E)) [
24]. Another flammable natural refrigerant that has attracted interest in recent years is dimethyl ether (DME or RE170), which is widely used in the chemical industry, medicine, etc., and is therefore considered an alternative refrigerant. It has a low boiling point, high latent heat, and is non-toxic, slightly corrosive, and more environmentally friendly, because its ODP and GWP are 0 and 1, respectively. The study investigated the flammability of the RE170–R1234yf–R134a mixture (with weight fractions of 0.1/0.8/0.1) [
25].
There are many possible refrigerant combinations; HFO is usually combined with well-known substances from the HFC group due to their excellent environmental parameters. As shown above, it is mainly R1234yf and R1234ze(E) that are selected for analysis. Others are not as popular for a variety of reasons, including a normal boiling points above 0 °C or even non-zero ODP. In this study, along with R1234ze(E) and R1234yf, it was decided to use one of the rarer hydrofluoroolefins as a substrate for the newly defined mixture, namely R1243zf (a GWP of approx. 1, an ODP of 0).
Finding the perfect refrigerant, which has favorable thermodynamic properties and can work efficiently in the refrigeration cycle, while also being cheap, easily accessible, and safe for the environment, is a very difficult task, if only because of the numerous legal and technical changes taking place in the refrigeration sector. There are many possibilities for combining and mixing pure refrigerants together to achieve the desired properties and to have a negligible effect on the atmosphere. In this work, four ternary mixtures were proposed and theoretically tested for their cooling capacities in theoretical cooling circuits to determine the optimal composition for the new blends. Significant effort has gone into developing and testing different refrigerant blends. However, there are still many unresolved issues and opportunities that need to be investigated. In light of the state of the art in this field, combining and mixing pure refrigerants together to achieve the desired properties while having a negligible effect on the atmosphere is still an open problem and a meaningful endeavor. This combining and mixing took several aspects into account, such as the use of the rarer hydrofluoroolefins (instead of commonly used refrigerants) as a substrate for the newly defined mixture, the triangular design, which involves the use of three refrigerant components, and the thermodynamic and operational parameters. Such an approach is hardly visible in the literature, which confirms the novelty of the presented research. Its realization required the application of new approaches that employed advanced analysis of the four ternary mixtures, along with their experimental verification. This allowed for the advanced testing of their cooling capacities in theoretical cooling circuits to determine the optimal composition for the new blends. It should also be highlighted that the weight fraction of the presented blends was determined (and not fixed, as a frequent goal of scientific articles) to find the optimal mixture for potentially replacing the existing refrigerants. Consequently, this article contributes to the state of the art in the new HFC/HFO blend selection by developing appropriate methods that can be feasibly implemented.
2. Materials and Methods
2.1. Methodology and Prerequisites for the Mixture Components
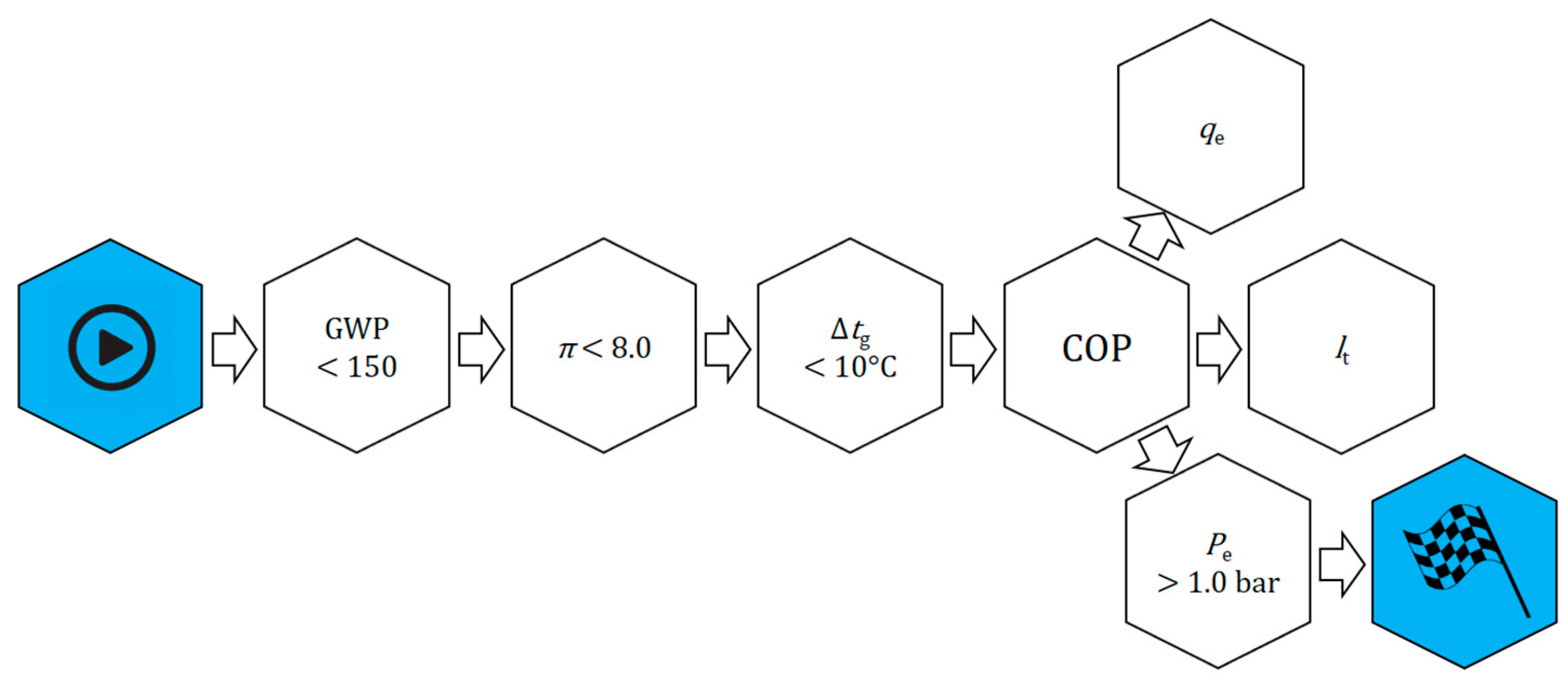
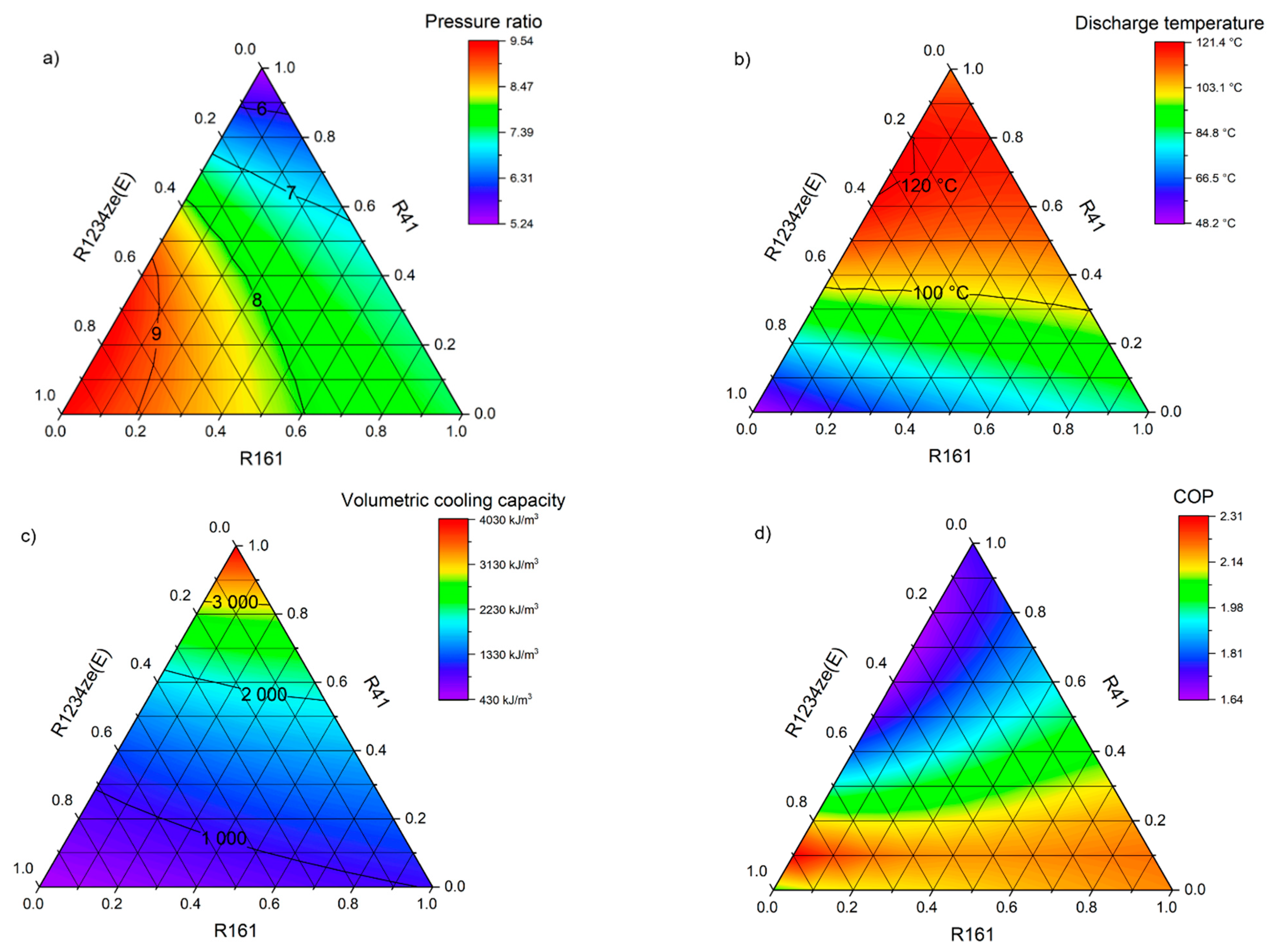
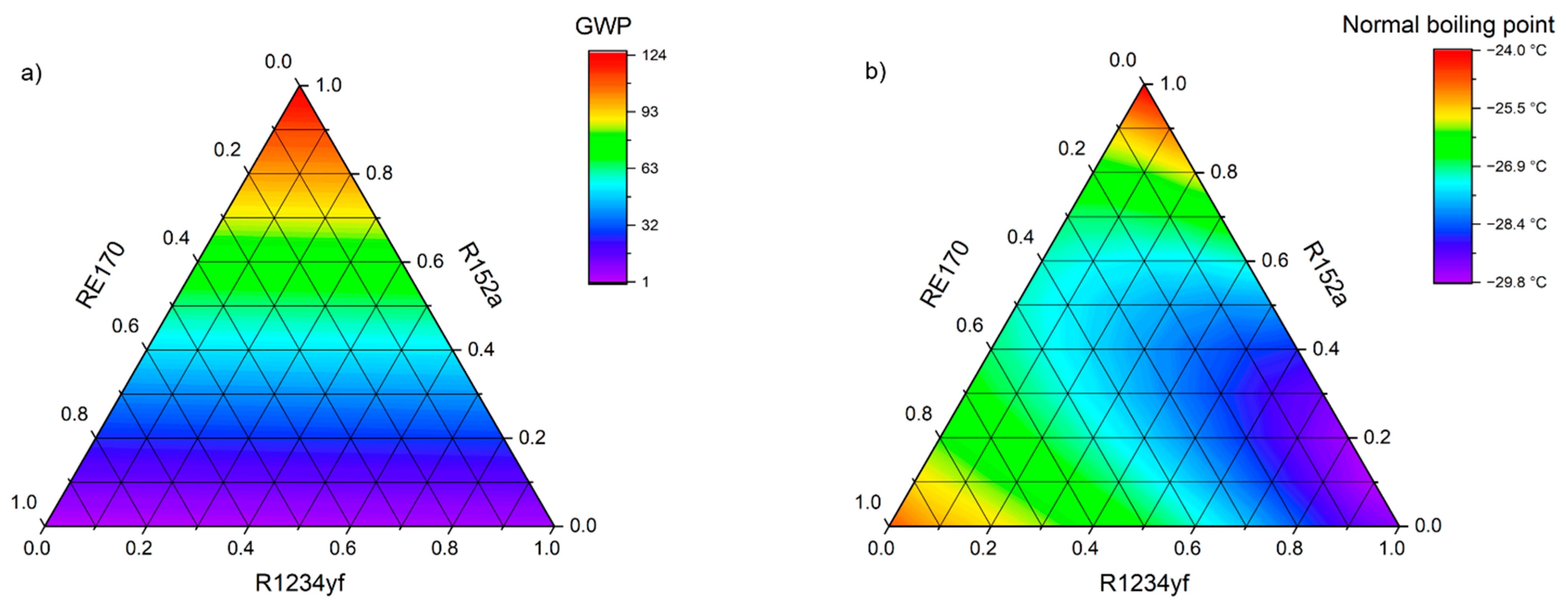
The selection of the optimal zeotropic or azeotropic mixture components was based on a triangular-basis plan, which involves the use of three refrigerant components. The sum of the weight shares of the selected substances must always be equal to 1 (100%), and the single share must be zero or positive. Thus, each blend will be defined by three independent variables. The shares of individual components (pure refrigerants) in the mixture change from 0 to 1 (from 0% to 100%) in steps of 0.1 (10%). The basic properties and operating parameters of each mixture, depending on their composition, are presented on ternary charts. In the corners of the graph are the pure substances selected as mixture components. The edges of the triangle depict binary mixtures, while the ternary mixtures are inside the triangle.
The basic criteria for selecting the constituent substances were the environmental parameters of a zero ODP and a low GWP. A condition was also set regarding the normal boiling point, the value of which had to be at least −25 °C to avoid under-pressure conditions. Given the final evaporation temperature levels obtained, all the proposed mixtures were assigned to the implementation of air-conditioning or freezing cycles and compared to the corresponding reference refrigerants. An additional criterion was the small distribution of individual components in already existing and commercially available mixtures. It was decided that the newly created working fluid should contain at least one alternative refrigerant (natural or from the HFO group), the rest being HFCs. The criteria were ranked according to their weight and checked in accordance with the diagram presented in
Figure 1. The main initial consideration when creating mixtures and selecting their components was to limit the GWP value to 750 for air-conditioning systems (or 150 if possible) and 150 for low-temperature refrigeration devices. Obtaining low temperatures for the assumed operating conditions of the condenser may be associated with obtaining correspondingly higher pressure ratios in the system (compression ratio). In this case, the operation of the system may be disadvantageous due to a number of phenomena, such as low volumetric efficiency of the compressor and decrease in refrigerant mass flow due to an increase in specific vapor volume, increase in the compressor power consumption, high discharge temperature, and deterioration of lubricating properties of oils. In order to avoid the above-mentioned problems, it was assumed that the maximum value of compression ratio of the analyzed mixtures should not exceed 8.0.
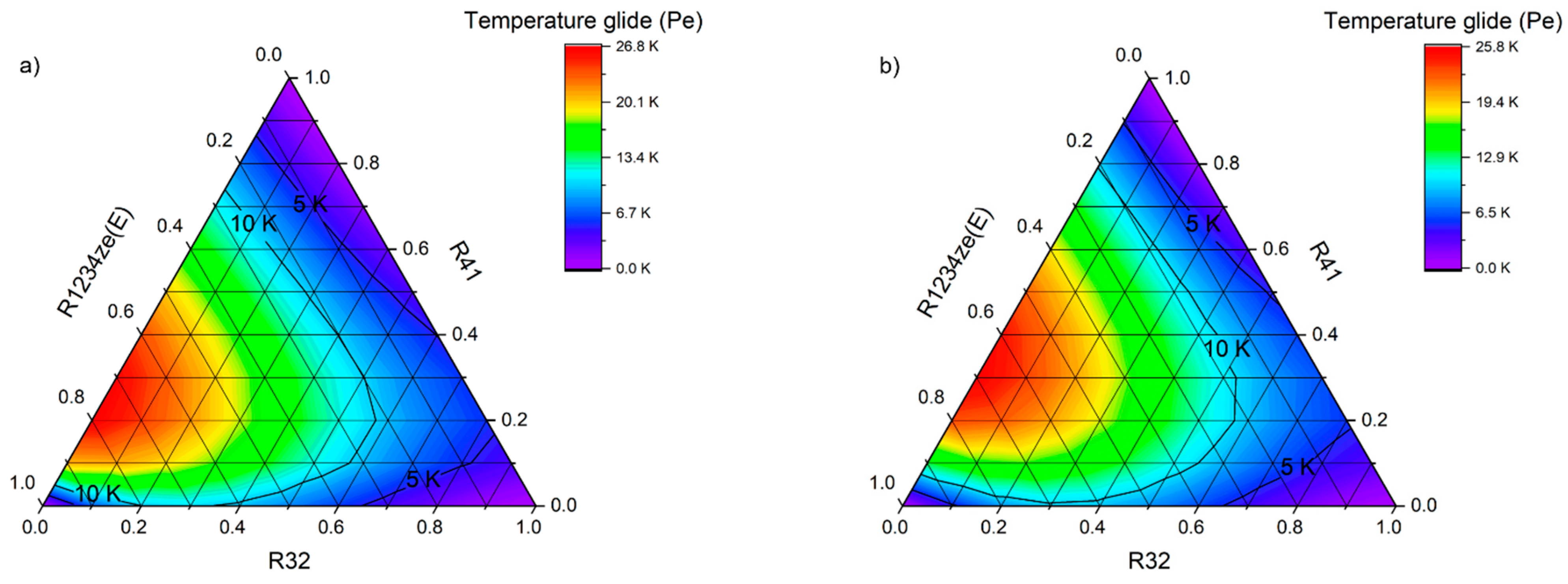
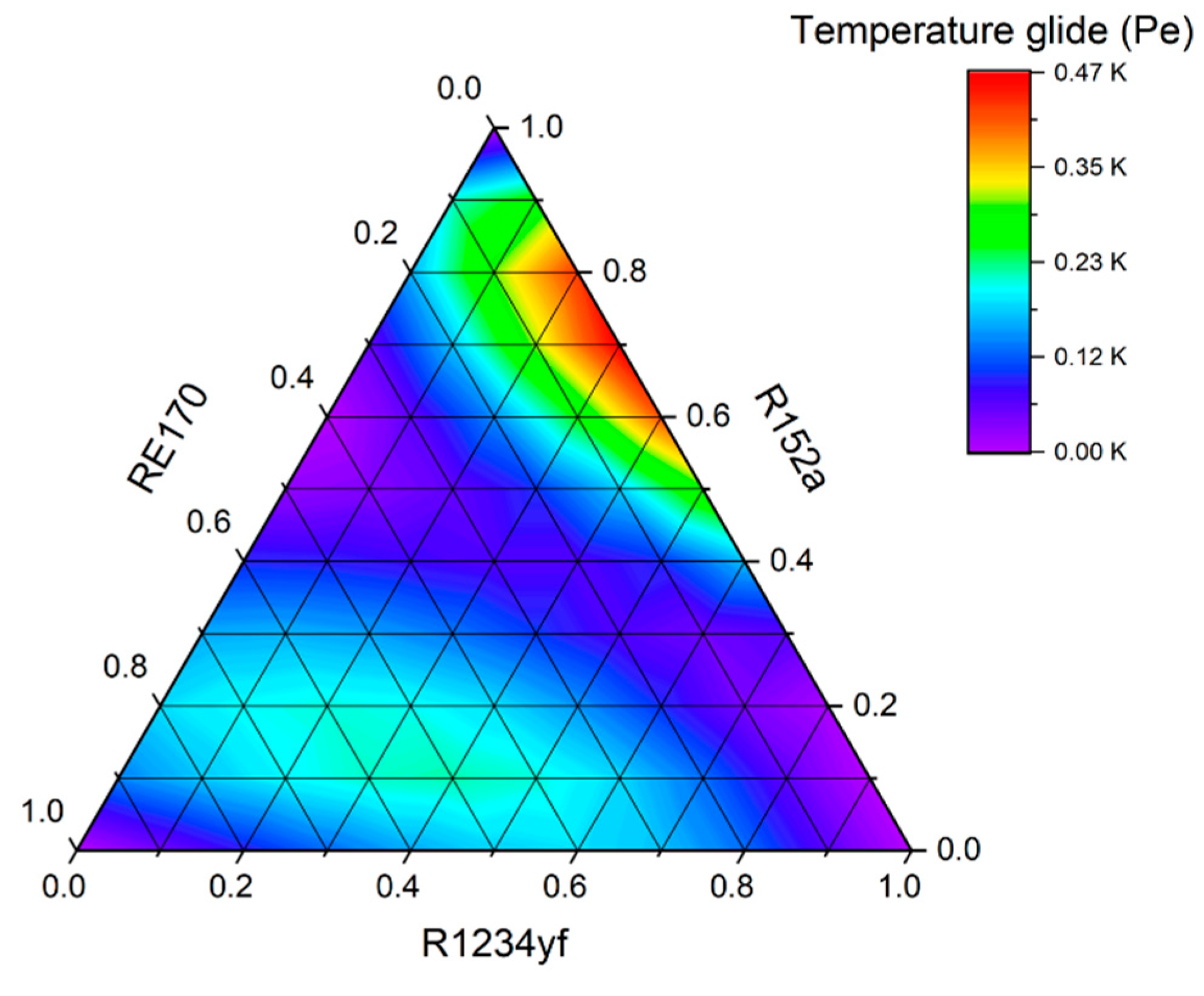
An equally important issue in the operation of real cooling cycles is the temperature glide, which may cause evaporator malfunctions. Components with extremely different vapour pressures can cause frosting to the initial sections of the evaporator due to the evaporation of low-boiling components. On the other hand, components with a high boiling point may not completely evaporate, which can lead to fractionation of the refrigerant inside the system and change its operating parameters. In case of extreme temperature glides, it is necessary to increase the vapour superheat set point to prevent the compressor from sucking in liquid refrigerant. In this analysis, the temperature glide of the mixtures was limited to 10 K. After meeting the initial criteria, the working parameters of the mixtures were analyzed.
Four mixture types have been presented and tested in this paper:
R32–R41–R1234ze(E)—a mixture combining R32 that is currently gaining popularity, the R41, which has a low normal boiling point and a very low GWP, and R1234ze(E), the second most-studied HFO refrigerant;
R32–R161–R1234ze(E)—a mixture similar to the previous one. However, the second component has been changed to R161, which has a slightly higher boiling point at 1 bar pressure but an almost eight-times lower GWP;
R1234yf–R152a–RE170—this mixture combines the most popular refrigerant from the HFO group—R1234yf, with R152a often used in HFC/HFO mixtures, and a natural substance RE170, which has been increasingly used in refrigeration mixtures, since the early 2000s;
R1243zf–R152a–RE170—a mixture related to the previous one. However, the HFO group has been replaced by another, R1243zf, which thermodynamic properties are the most similar to widely used R134a, and which has a higher heat of vaporization than R1234yf.
All have been included in the European Patent (EP3309233A1) [
26], which relates to compositions for use in refrigeration and air-conditioning. However, we would like to highlight that this does not negate the possibility of studying them from the scientific standpoint, with no intention of using them commercially. Basic information on the pure components used is presented in
Table 1.
2.2. Theoretical Refrigeration Cycle—Assumptions
Two theoretical single-stage refrigeration cycles were determined for comparing the mixtures. In the first cycle, the evaporating temperature (
te) was set at 0 °C, with the condensing temperature (
tc) equal to 30 °C. This cycle corresponds to the work done by air-conditioning systems. The evaporating temperature in the second cycle was set to −30 °C, with the condensing temperature unchanged. This allowed to examine the behavior of mixtures in low-temperature systems. In both cases, it was assumed that the compression isentropic efficiency was
η = 0.7. For the purposes of theoretical analysis, it was assumed that the liquid subcooling in the condenser and the vapour superheating in the evaporator were equal to zero, as presented in
Figure 2. Pressure drops in the heat exchangers and in the pipeline flow were also omitted.
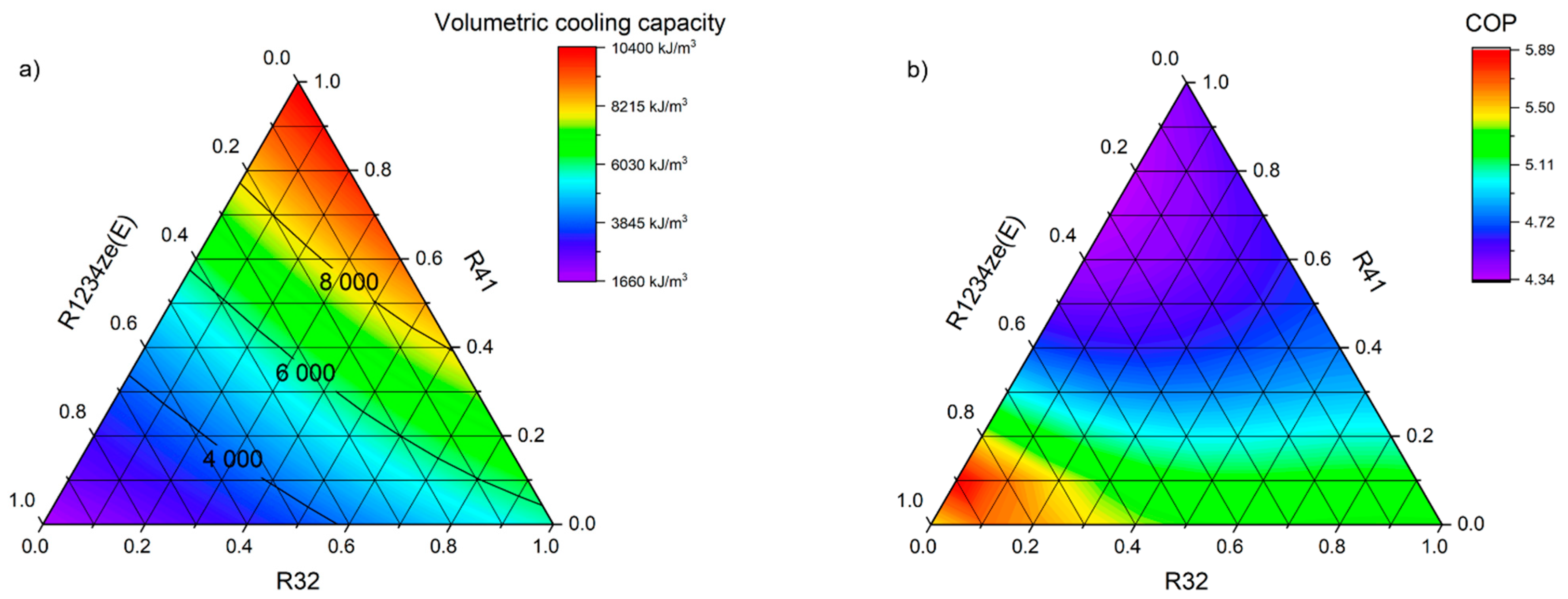
The theoretical analysis of comparative cycles allowed us to determine the basic operating parameters of the proposed mixtures. With the help of enthalpy at the characteristic operating points, the specific cooling capacity (qe), the specific work of the cycle (lt), the volumetric cooling capacity (defined as qe/vsuction), and the COP of each of the proposed mixtures were defined in terms of the assumed variability in the weight shares of the individual components. On the basis of the evaporation and condensation pressures obtained, the compression ratio and the temperature glide were determined. The specific refrigeration system parameters were determined for both the high-temperature and the low-temperature cycles. In addition to the operating parameters, the basic properties were also determined, such as the GWP, the critical point temperature and pressure, the normal boiling point (1 bar), and the molar mass.
The theoretical analysis of the newly defined zeotropic or azeotropic mixtures was carried out using the REFPROP 10.0 program [
27], from which the thermodynamic and transport properties of the fluids relevant for the study and their mixtures were taken, such as the critical temperature and pressure, the normal boiling point, and the molar mass. The thermodynamic properties of the mixtures were determined by employing a model that applies mixing rules to the Helmholtz energy of the mixture components, along with a departure function to account for the departure from ideal mixing. The same “XR0” mixing rule was used in REFPROP for all analyzed mixtures. It should be emphasized that in order to determine the properties of mixtures, it is necessary to have properties for binary subsystems, and among the mixtures under consideration, not all subsystems were tested experimentally. Therefore, it should be borne in mind that despite the generally good accuracy of determining thermal-flow properties, some of the presented values may differ from the actual ones, which requires confirmation in the field of further experimental studies.
The newly defined zeotropic or azeotropic mixtures will be compared (at work) to the refrigerants being withdrawn from cooling equipment as a result of the European Parliament regulation. It is important to find the parameters that will be favored by the proposed compositions. Taking into account the scope of application, the benchmark refrigerants for low temperature circuits will be R404A and R507A, and for high temperature circuits, R410A, R134a, R32, and R429A. The R429A mixture was used as a benchmark due to its similar composition to the two newly defined blends tested. This refrigerant consists of RE170, R152a, and R600a in the corresponding weight proportions of 60%, 10%, and 30%.
4. Discussion
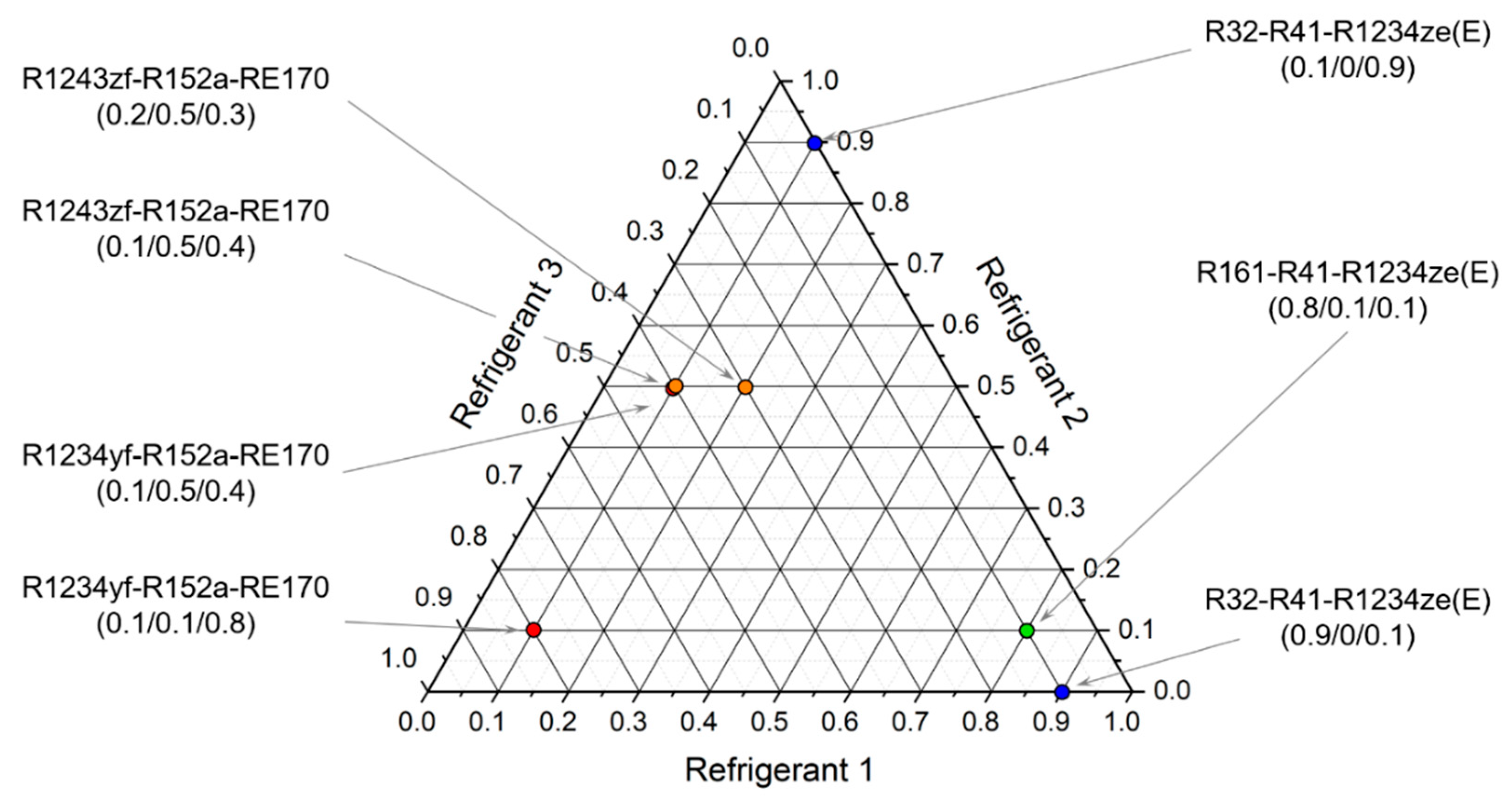
Summarizing the results presented in this study, seven optimal mixture compositions were selected, of which six are ternary mixtures and two are binary:
R32–R41–R1234ze(E) 0.9/0/0.1 air-conditioning cycle
R32–R41–R1234ze(E) 0.1/0.9/0 low-temperature cycle
R161–R41–R1234ze(E) 0.8/0.1/0.1 both cycles
R1234yf–R152a–RE170 0.1/0.1/0.8 air-conditioning cycle
R1234yf–R152a–RE170 0.1/0.5/0.4 air-conditioning cycle
R1243zf–R152a–RE170 0.2/0.5/0.3 air-conditioning cycle
R1243zf–R152a–RE170 0.1/0.5/0.4 air-conditioning cycle
Their distribution on the experiment plan is shown in
Figure 17.
There were many criteria for proving the applicability of a given refrigerant. The main ones were the temperature glide values achieved at
pe and within the whole range of working pressures. Based on the temperature differences obtained for the evaporation process, for which the limit value was set as 10 K, a significant proportion of the points for the first two mixtures were rejected. The temperature drop in the evaporation process for the assumed cooling cycle and variable evaporation temperatures for selected mixtures are presented in
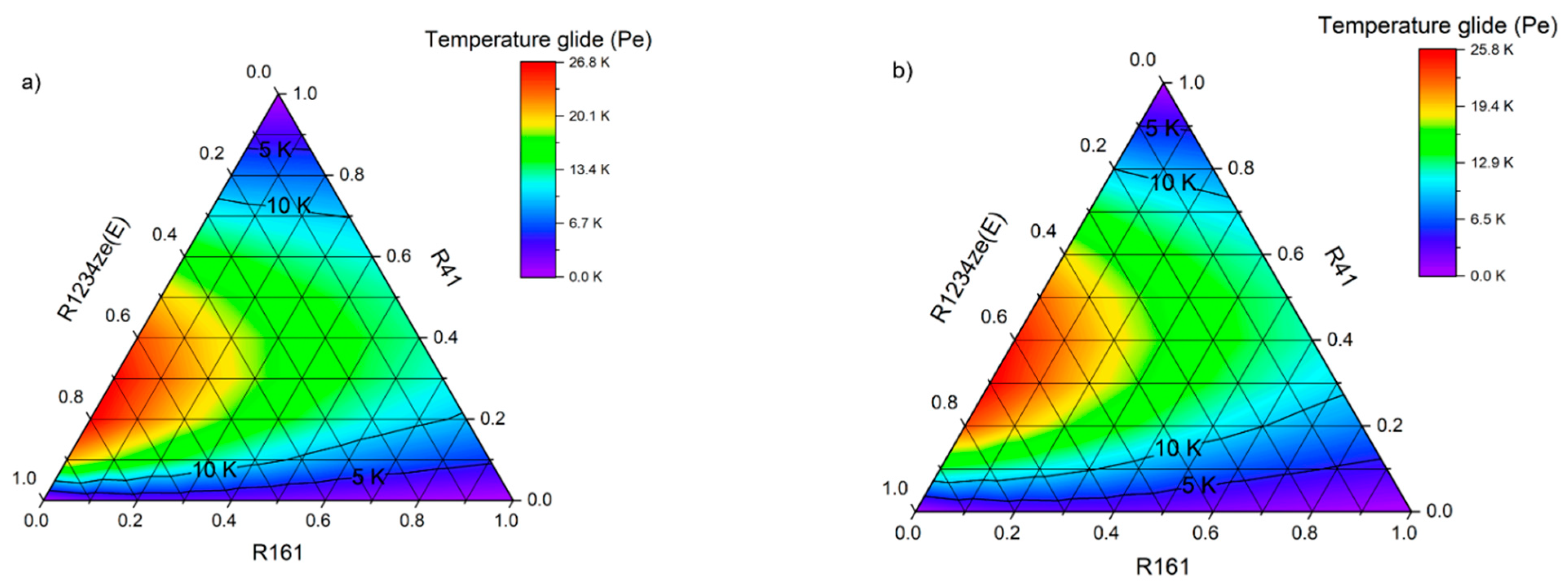
Figure 18. The figure shows that only for the mixture R161–R41–R1234ze(E), significant changes in the evaporation temperature are obtained, and therefore this mixture must be classified as zeotropic (ZEO). At the same time, it is clearly visible that with the increase in the evaporation temperature, the temperature glide increases significantly.
On the other hand, all the mixtures containing the R152a and RE170 fluids are characterized by a small temperature glide, the value of which does not exceed 0.5 K. The binary mixture R32–R1234ze(E) shows a temperature glide of almost exactly 1 K (limit for near-azeotropic mixtures).
Figure 18 also shows that among the selected mixtures, R32–R41 achieves much higher evaporating pressures.
Table 2 shows a comparison of the newly defined mixtures with the reference refrigerants dedicated to low-temperature installations. The parameters that are favorable to the new mixtures are the GWP and specific cooling capacity. Compared to currently used refrigerants, it was found that the most optimal is a R32–R41 binary mixture with a mass fraction of 0.9/0.1, for which the specific cooling capacity is 243 kJ/kg and is more than twice as high as for the R404A or R507A. The great advantage of the R32–R41 binary mixture is the increase in volumetric cooling capacity by over 2700 kJ/m
3. The disadvantages are the slightly higher temperature glide and high working pressures. The biggest problem seems to be the much higher discharge temperature exceeding 110 °C, which is typical for the currently implemented and used R404A substitutes. Conversely, R161–R41–R1234ze(E) obtains a lower volumetric cooling capacity, which is dictated by a much higher specific vapor volume, at the same time showing a much higher temperature glide, which can eliminate this refrigerant from systems requiring precise evaporator temperature. The use of this mixture, however, allows to significantly improve the COP; compared to R404A, the increase is as much as 15.8%. However, the use of these mixtures requires a profound change in the refrigerant market, as currently R41 is not widely available.
Table 3 shows the properties of the mixtures considered as refrigerants in air conditioning cycle. R410A, R134a, R32, and R429A were used as reference. An indicator that definitely favors the new refrigerants is the GWP, the value of which for R32-free mixtures does not exceed 63 and is less than half the permissible limit. Of the reference substances, only R429A has the same low GWP. The COP for the new mixtures are also favorable, since they all exceed a value of 5.34. When comparing the temperature glide, it can be observed that it is almost zero for the mixtures containing RE170, which is a slight advantage over R429A. These mixtures achieve a volumetric cooling capacity almost identical to that of R134a and approximately 6–12% higher than that of R429A. However, they are not in competition with R32 or mixtures containing it, so a higher amount of refrigerant in the system will be required. In terms of the obtained volumetric cooling capacity, the mixtures R32–R1234ze(E) and R161–R41–R1234ze (E) may be an interesting proposition. For both mixtures, the obtained values are much higher than for R134a, and in the case of a binary mixture R32–R1234ze(E) also at a similar level as for R32 and R410A. By analyzing all the variables, it can be summarized that the mixture R1234yf–R152a–RE170 with the weight shares of 0.1/0.5/0.4 seems to be the most promising for the implementation in air-conditioning cycles.
The conclusions drawn from the theoretical analysis should be confirmed by means of experimental studies of the various evaporation and condensation temperatures. To do this, mixtures with similar compositions should be tested, with a smaller jump in the weight shares of the individual components. However, this is future research work, as the scope of this work only included theoretical considerations regarding the new mixtures.
5. Conclusions
Referring to the applicable environmental parameter limits set on refrigerants, four new refrigerant mixtures have been proposed in this work. The optimal weight shares of the individual components were estimated by analyzing the GWP, thermodynamic, and operational parameters.
Theoretical tests were performed using the REFPROP 10.0 program, and the collected data allowed for a preliminary estimation to be made. On the basis of theoretical analyzes, it was shown that all the proposed compositions, except for the R161–R41–R1234ze(E) mixture, can be classified as near-azeotropes or even azeotropes, because their temperature glide in a wide range of evaporation pressure does not exceed 1K. At optimal compositions, the share of HFOs in all mixtures does not exceed 20%. After considering the advantages and disadvantages of the refrigerants proposed, it was determined that the most optimal composition in high-temperature (air conditioning) systems was the R1234yf–R152a–RE170 mixture with a weight share of 0.1/0.5/0.4. This is argued by its low GWP, equal to 63, the relatively high COP of 5.61, the relatively low normal boiling point of -27.27 °C, and a lower weight share of the most flammable components (flammability class 3) than in the case of R429A.
The analyzes also show that it is extremely difficult to find a blend with a negligible impact on the greenhouse effect and at the same time good thermodynamic properties, which could be used as a replacement for R404A or R507A in low-temperature systems. Both of the proposed mixtures have disadvantages compared to currently used refrigerants. Although the R32–R41 achieves high volumetric cooling capacity, it is also characterized by high evaporating and condensing pressures and high discharge temperatures, which will result in higher thermal and force loads of the compressor working elements and may lead to their shorter life span. On the other hand, the R161–R41–R1234ze(E) mixture, despite the high coefficient of performance, shows nearly ten times higher temperature glide than R404A, which may cause evaporator malfunctions. Components with extremely different vapor pressures can cause excessive frosting to the initial sections of the evaporator due to the evaporation of low-boiling components. On the other hand, components with a high boiling point may not completely evaporate, which can lead to fractionation of the refrigerant inside the system and change its operating parameters. In case of extreme temperature glides, it is necessary to increase the vapor superheat set point to prevent the compressor from sucking in liquid refrigerant, which obviously affects the efficiency of the system. Therefore, further research should be directed towards this application. It should be emphasized that the presented analyzes do not explore the issue of using these mixtures in cooling cycles completely. Above all, further studies of the flammability and safe use of the presented mixtures are required, as all the components used are flammable, and a significant part of them belong to the highest flammability class.