Analysis of the Ignition Behavior Based on Similarity Factor Method
Abstract
1. Introduction
2. Definition of the Ignition Delay in a CVB
3. Similarity Factor Method
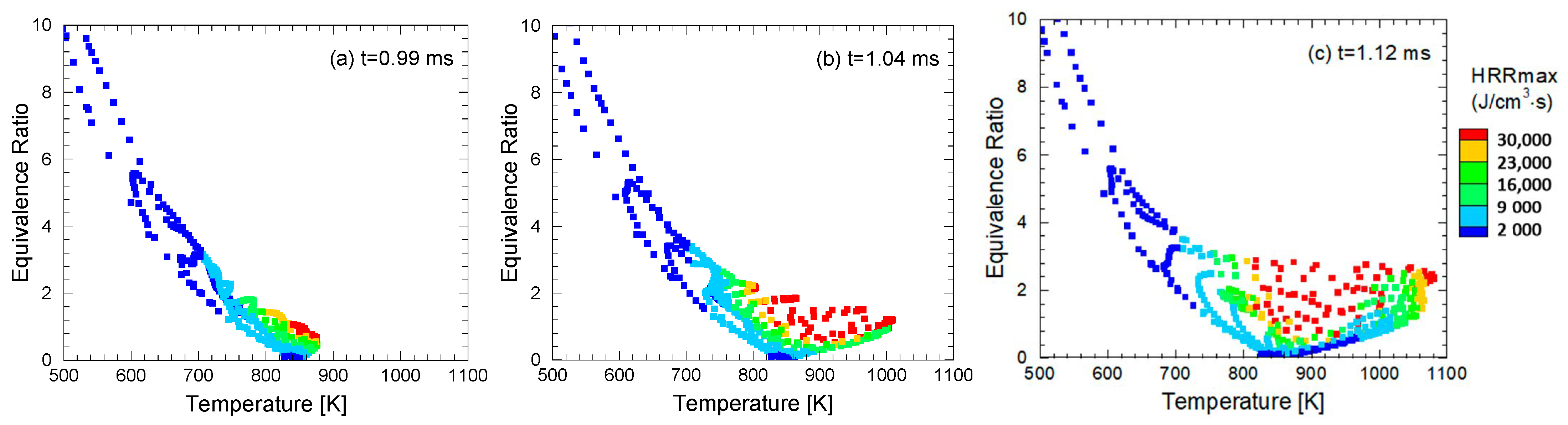
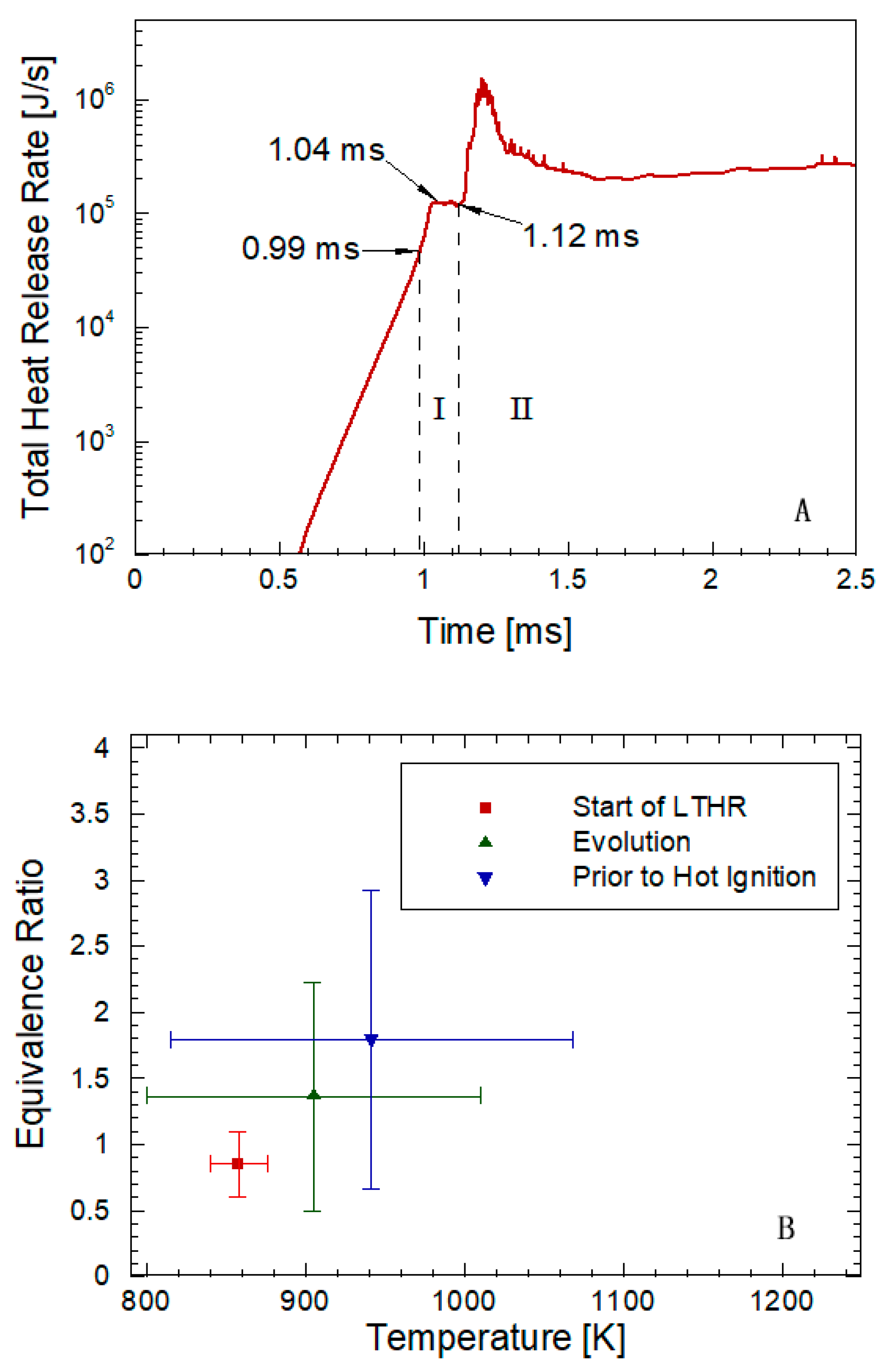
4. Expanded Application Research of the Similarity Factor Method
4.1. Validation by Different Mechanisms
4.2. Injection Pressure Verification
4.3. Verification of Nozzle Diameter
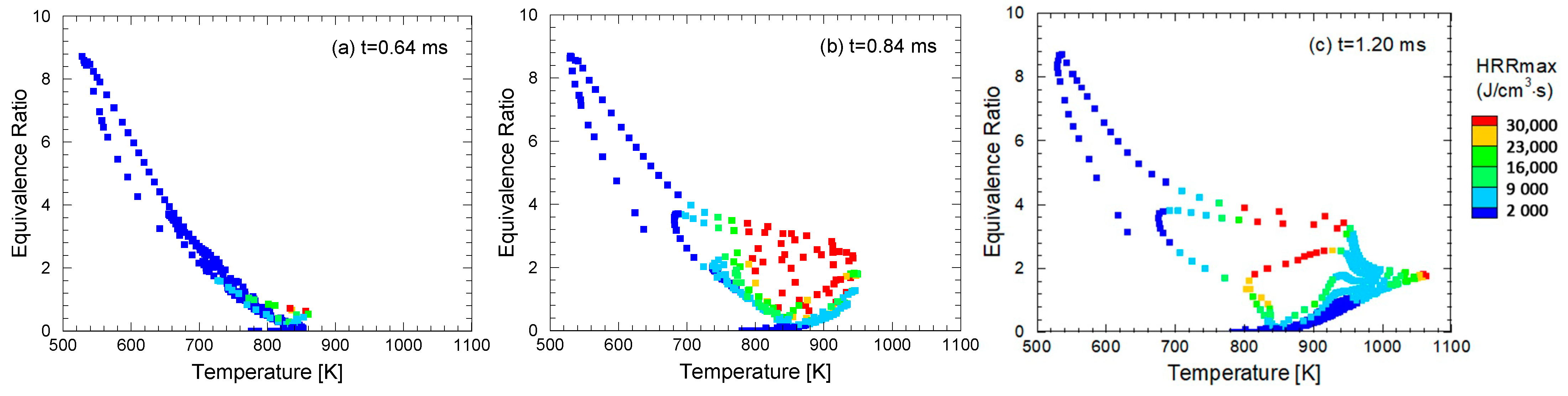
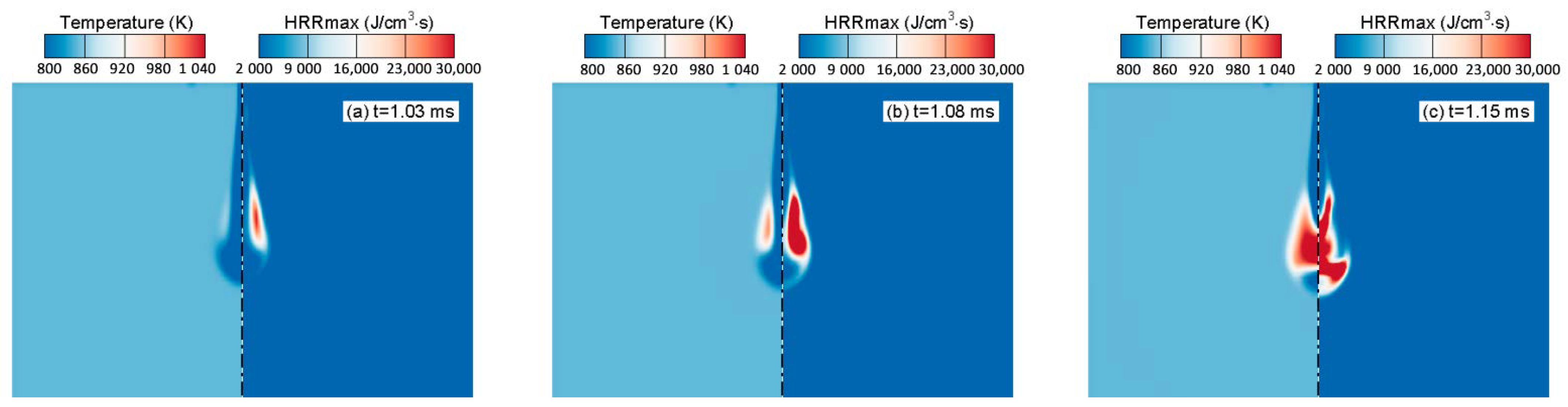
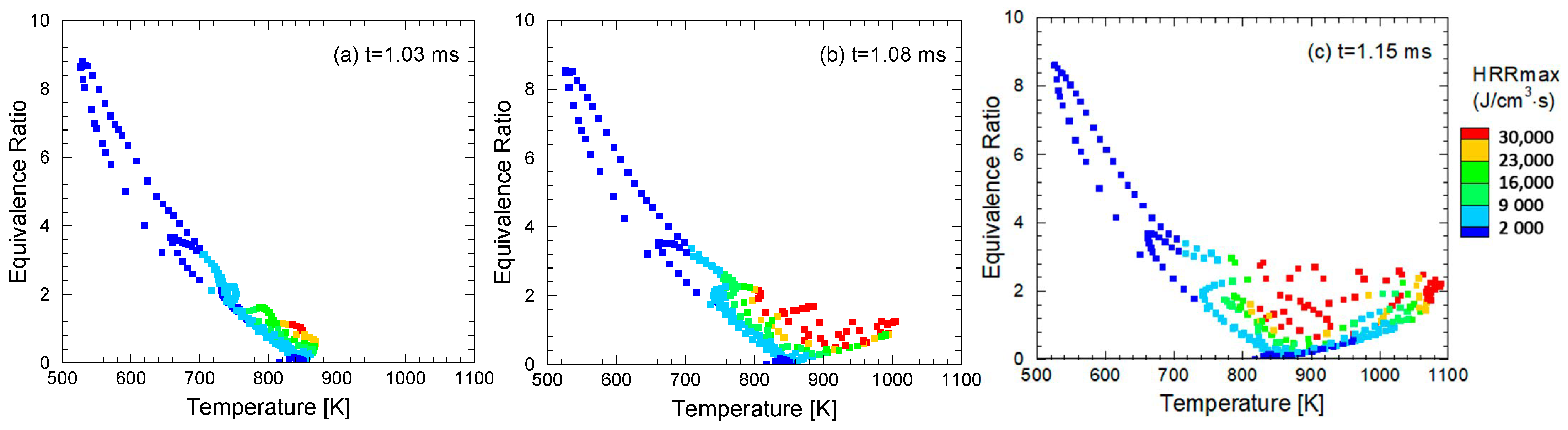
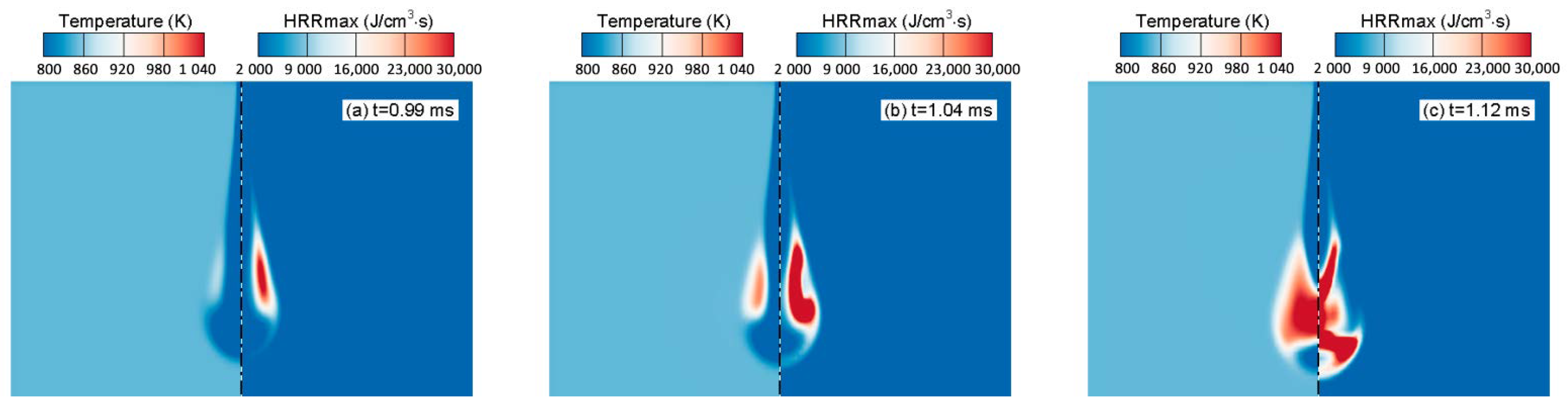
4.4. Comparison of Similarity Factors
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Monsalve-Serrano, J.; Belgiorno, G.; Di Blasio, G.; Guzmán-Mendoza, M. 1D simulation and experimental analysis on the effects of the injection parameters in methane–diesel dual-fuel combustion. Energies 2020, 13, 3734. [Google Scholar] [CrossRef]
- Mueller, C.J.; Cannella, W.J.; Bays, J.T.; Bruno, T.J.; DeFabio, K.; Dettman, H.D.; Gieleciak, R.M.; Huber, M.L.; Kweon, C.B.; McConnell, S.S.; et al. Diesel surrogate fuels for engine testing and chemical-kinetic modeling: Compositions and properties. Energy Fuels 2016, 30, 1445–1461. [Google Scholar] [CrossRef]
- ECN. Available online: http://www.sandia.gov/ecn/cvdata/dsearch/frameset.php (accessed on 8 October 2016).
- Lillo, P.M.; Pickett, L.M.; Persson, H.; Andersson, O.; Kook, S. Diesel spray ignition detection and spatial/temporal correction. SAE Int. J. Engines 2012, 5, 1330–1346. [Google Scholar] [CrossRef]
- Zhou, L.; Luo, K.H.; Qin, W.; Jia, M.; Shuai, S.J. Large eddy simulation of spray and combustion characteristics with realistic chemistry and high-order numerical scheme under diesel engine-like conditions. Energy Convers. Manag. 2015, 93, 377–387. [Google Scholar] [CrossRef]
- Payri, R.; Viera, J.P.; Pei, Y.; Som, S. Experimental and numerical study of lift-off length and ignition delay of a two-component diesel surrogate. Fuel 2015, 158, 957–967. [Google Scholar] [CrossRef]
- Fu, X.; Aggarwal, S.K. Fuel unsaturation effects on NOx and PAH formation in spray flames. Fuel 2015, 160, 1–15. [Google Scholar] [CrossRef]
- Pei, Y.; Davis, M.J.; Pickett, L.M.; Som, S. Engine Combustion Network (ECN), Global sensitivity analysis of Spray A for different combustion vessels. Combust. Flame 2015, 162, 2337–2347. [Google Scholar] [CrossRef]
- Ethan, E.W.; Louis-Marie, M.; Mark, P.B.M. Measurements of liquid length, vapor penetration, ignition delay, and flame lift-off length for the engine combustion network ‘spray B’ in a 2.34 L heavy-duty optical diesel engine. SAE Int. J. Engines 2016, 9, 910–931. [Google Scholar]
- Pickett, L.M.; Genzale, C.L.; Bruneaux, G.; Malbec, L.M.; Hermant, L.; Christiansen, C.; Schramm, J. Comparison of diesel spray combustion in different high-temperature, high-pressure facilities. SAE Int. J. Engines 2010, 3, 156–181. [Google Scholar] [CrossRef]
- Wang, H.; Sheen, D.A. Combustion kinetic model uncertainty quantification, propagation and minimization. Prog. Energy Combust. Sci. 2015, 47, 1–31. [Google Scholar] [CrossRef]
- Som, S.; Longman, D.E.; Luo, Z.; Plomer, M.; Lu, T.; Senecal, P.K.; Pomraning, E. Simulating flame lift-off characteristics of diesel and biodiesel fuels using detailed chemical-kinetic mechanisms and large eddy simulation turbulence model. J. Energy Resour. Technol. 2012, 134, 032204. [Google Scholar] [CrossRef]
- Deng, F.; Pan, Y.; Sun, W.; Yang, F.; Zhang, Y.; Huang, Z. Comparative study of the effects of nitrous oxide and oxygen on ethylene ignition. Energy Fuels 2017, 31, 14116–14128. [Google Scholar] [CrossRef]
- Capriolo, G.; Alekseev, V.A.; Konnov, A.A. An experimental and kinetic study of propanal oxidation. Combust. Flame 2018, 197, 11–21. [Google Scholar] [CrossRef]
- Zhou, C.W.; Li, Y.; O’connor, E.; Somers, K.P.; Thion, S.; Keesee, C.; Mathieu, O.; Petersen, E.L.; DeVerter, T.A.; Oehlschlaeger, M.A.; et al. A comprehensive experimental and modeling study of isobutene oxidation. Combust. Flame 2016, 167, 353–379. [Google Scholar] [CrossRef]
- Comandini, A.; Pengloan, G.; Abid, S.; Chaumeix, N. Experimental and modeling study of styrene oxidation in spherical reactor and shock tube. Combust. Flame 2016, 173, 425–440. [Google Scholar] [CrossRef]
- Li, H.; Yu, L.; Lu, X.; Ouyang, L.; Sun, S.; Huang, Z. Autoignition of ternary blends for gasoline surrogate at wide temperature ranges and at elevated pressure, Shock tube measurements and detailed kinetic modeling. Fuel 2016, 181, 916–925. [Google Scholar] [CrossRef]
- Zeng, M.; Yuan, W.; Li, W.; Zhang, Y.; Cao, C.; Li, T.; Zou, J. Comprehensive experimental and kinetic modeling study of n-tetradecane combustion. Energy Fuels 2017, 31, 12712–12720. [Google Scholar] [CrossRef]
- Dames, E.E.; Rosen, A.S.; Weber, B.W.; Gao, C.W.; Sung, C.J.; Green, W.H. A detailed combined experimental and theoretical study on dimethyl ether/propane blended oxidation. Combust. Flame 2016, 168, 310–330. [Google Scholar] [CrossRef]
- Cai, L.; Kruse, S.; Felsmann, D.; Thies, C.; Yalamanchi, K.K.; Pitsch, H. Experimental design for discrimination of chemical kinetic models for oxy-methane combustion. Energy Fuels 2017, 31, 5533–5542. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Liu, Y.; Li, Y.; Xie, M.; Yin, H. Application of a decoupling methodology for development of skeletal oxidation mechanisms for heavy n-alkanes from n-octane to n-hexadecane. Energy Fuels 2013, 27, 3467–3479. [Google Scholar] [CrossRef]
- Minwegen, H.; Burke, U.; Heufer, K.A. An experimental and theoretical comparison of C3–C5 linear ketones. Proc. Combust. Inst. 2017, 36, 561–568. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Pitz, W.J.; Herbinet, O.; Curran, H.J.; Silke, E.J. A comprehensive detailed chemical kinetic reaction mechanism for combustion of n-alkane hydrocarbons from n-octane to n-hexadecane. Combust. Flame 2009, 156, 181–199. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Han, Y.; Tan, M.; Xu, Y.; Tian, J.; Yan, J.; Chai, J.; Liu, J.; Yu, X. Experimental and kinetic modeling study of autoignition characteristics of n-heptane/ethanol by constant volume bomb and detail reaction mechanism. Energy Fuels 2017, 31, 13610–13626. [Google Scholar] [CrossRef]
- Li, R.; Liu, Z.; Han, Y.; Tan, M.; Xu, Y.; Tian, J.; Yan, J.; Chai, J.; Liu, J.; Yu, X. Understanding of the ignition behavior of n-heptane spray in constant volume combustion bombs focusing on chemical kinetics. Energy Fuels 2019, 33, 12830–12838. [Google Scholar]
- Fan, W.; Jia, M.; Chang, Y.; Li, Y. Similarity analysis of the chemical kinetic mechanism on the ignition delay in shock tubes and homogeneous charge compression ignition (HCCI) engines. In Proceedings of the International Powertrains, Fuels & Lubricants Meeting, Beijing, China, 8 October 2017. [Google Scholar]
- Kyoung, H.K.; Claus, B.; Dohoy, J. Fuel-sensitive ignition delay models for a local and global description of direct injection internal combustion engines. J. Eng. Gas Turbines Power 2015, 137, 111510. [Google Scholar]
- Edgar, B.L.; Dibble, R.W.; Naegeli, D.W. Autoignition of Dimethyl Ether and Dimethoxy Methane Sprays at High Pressures; SAE International: Warrendale, PA, USA, 1997. [Google Scholar]
- Ganesan, V. Internal combustion engines. Epfl 1958, 25, 299–308. [Google Scholar]
- Ryan, T.W. Correlation of Physical and Chemical Ignition Delay to Cetane Number; SAE International: Warrendale, PA, USA, 1985. [Google Scholar]
- Vasu, S.S.; Davidson, D.F.; Hanson, R.K. Shock tube study of syngas ignition in rich CO2 mixtures and determination of the rate of H + O2 + CO2 → HO2 + CO2. Energy Fuels 2011, 25, 990–997. [Google Scholar] [CrossRef]
- Engine Combustion Network. ECN4 topic 5—Combustion submission guidelines. In Proceedings of the ECN 4th Workshop, Kyoto, Japan, 5–6 September 2015. [Google Scholar]
- Curran, H.J.; Gaffuri, P.; Pitz, W.J.; Westbrook, C.K. A comprehensive modeling study of n-heptane oxidation. Combust. Flame 1998, 114, 149–177. [Google Scholar] [CrossRef]
- Fraser, R.; Siebers, D.; Edwards, C. Autoignition of Methane and Natural Gas in a Simulated Diesel Environment; SAE International: Warrendale, PA, USA, 1991. [Google Scholar]
- Lapuerta, M.; Sanz-Argent, J.; Raine, R. Ignition characteristics of diesel fuel in a constant volume bomb under diesel-like conditions. Effect of the operation parameters. Energy Fuels 2014, 28, 5445–5454. [Google Scholar] [CrossRef]
- Burke, U.; Pitz, W.J.; Curran, H.J. Experimental and kinetic modeling study of the shock tube ignition of a large oxygenated fuel, Tri-propylene glycol mono-methyl ether. Combust. Flame 2015, 162, 2916–2927. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Haworth, D.C. Simulations of transient n-heptane and n-dodecane spray flames under engine-relevant conditions using a transported PDF method. Combust. Flame 2013, 160, 2083–2102. [Google Scholar] [CrossRef]
- Miraboutalebi, S.M.; Kazemi, P.; Bahrami, P. Fatty acid methyl ester (fame) composition used for estimation of biodiesel cetane number employing random forest and artificial neural networks, A new approach. Fuel 2016, 166, 143–151. [Google Scholar] [CrossRef]
- Wang, H.; Yao, M.; Reitz, R. Development of a reduced primary reference fuel mechanism for internal combustion engine combustion simulations. Energy Fuels 2013, 27, 7843–7853. [Google Scholar] [CrossRef]
- Chang, Y.; Jia, M.; Li, Y.; Xie, M. Application of the optimized decoupling methodology for the construction of a skeletal primary reference fuel (PRF) mechanism focusing on engine-relevant conditions. Front. Mech. Eng. 2015, 1, 2297–3079. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, W.; Yang, S.; Xu, K.; Zhu, M.; Xu, J. Analysis of the Ignition Behavior Based on Similarity Factor Method. Energies 2021, 14, 873. https://doi.org/10.3390/en14040873
Fan W, Yang S, Xu K, Zhu M, Xu J. Analysis of the Ignition Behavior Based on Similarity Factor Method. Energies. 2021; 14(4):873. https://doi.org/10.3390/en14040873
Chicago/Turabian StyleFan, Weiwei, Shengxiong Yang, Ke Xu, Mingdong Zhu, and Jie Xu. 2021. "Analysis of the Ignition Behavior Based on Similarity Factor Method" Energies 14, no. 4: 873. https://doi.org/10.3390/en14040873
APA StyleFan, W., Yang, S., Xu, K., Zhu, M., & Xu, J. (2021). Analysis of the Ignition Behavior Based on Similarity Factor Method. Energies, 14(4), 873. https://doi.org/10.3390/en14040873






