The Development of the Temperature Disturbance Zone in the Surrounding of a Salt Cavern Caused by the Leaching Process for Safety Hydrogen Storage
Abstract
1. Introduction
2. Material and Methods
2.1. Characteristics of Salt Cavern Leaching Process
- endothermic process of salt dissolving,
- heat transfer between the cavern filling brine and rock salt massif,
- heat transfer within the cavern filling brine, and
- heat transfer in the rock salt massif.
2.2. Numerical Model of the Cavern Leaching Process and Rock Salt Massif Cooling
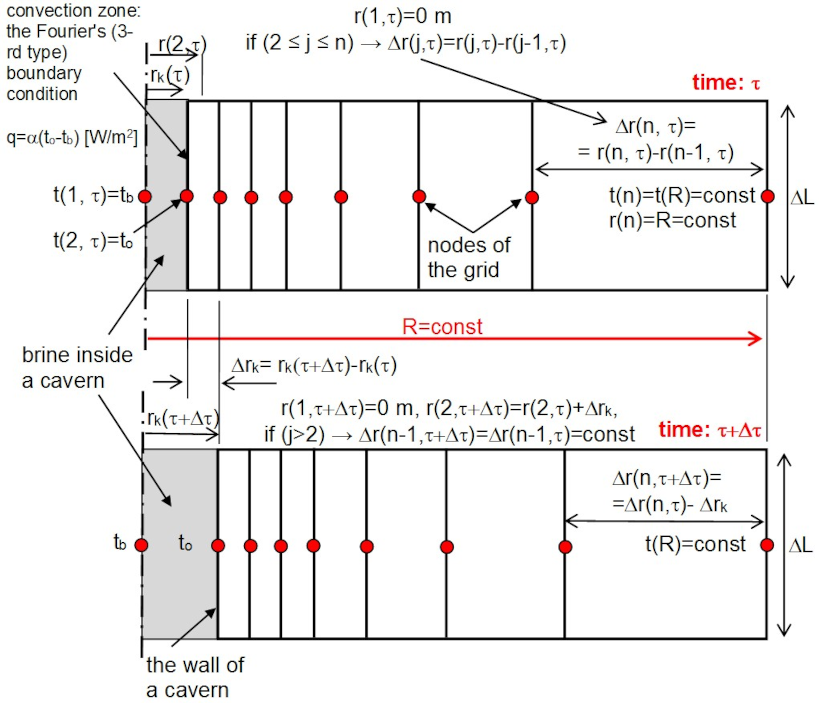
2.3. Full Model Description
- The cavern radius change over time
- Thermal conduction in the vicinity of the cavern
- Discretization criteria and boundary conditions in the vicinity of salt cavern
- Brine concentration in the cavern
- Brine and water properties
- specific heat variability, depending on its concentration and temperature and determined on the basis of equations given by Jamieson et al. [27], and
- density variability, depending on the concentration of solutes, pressure, and temperature and determined on the basis of equations given by McCain [28]
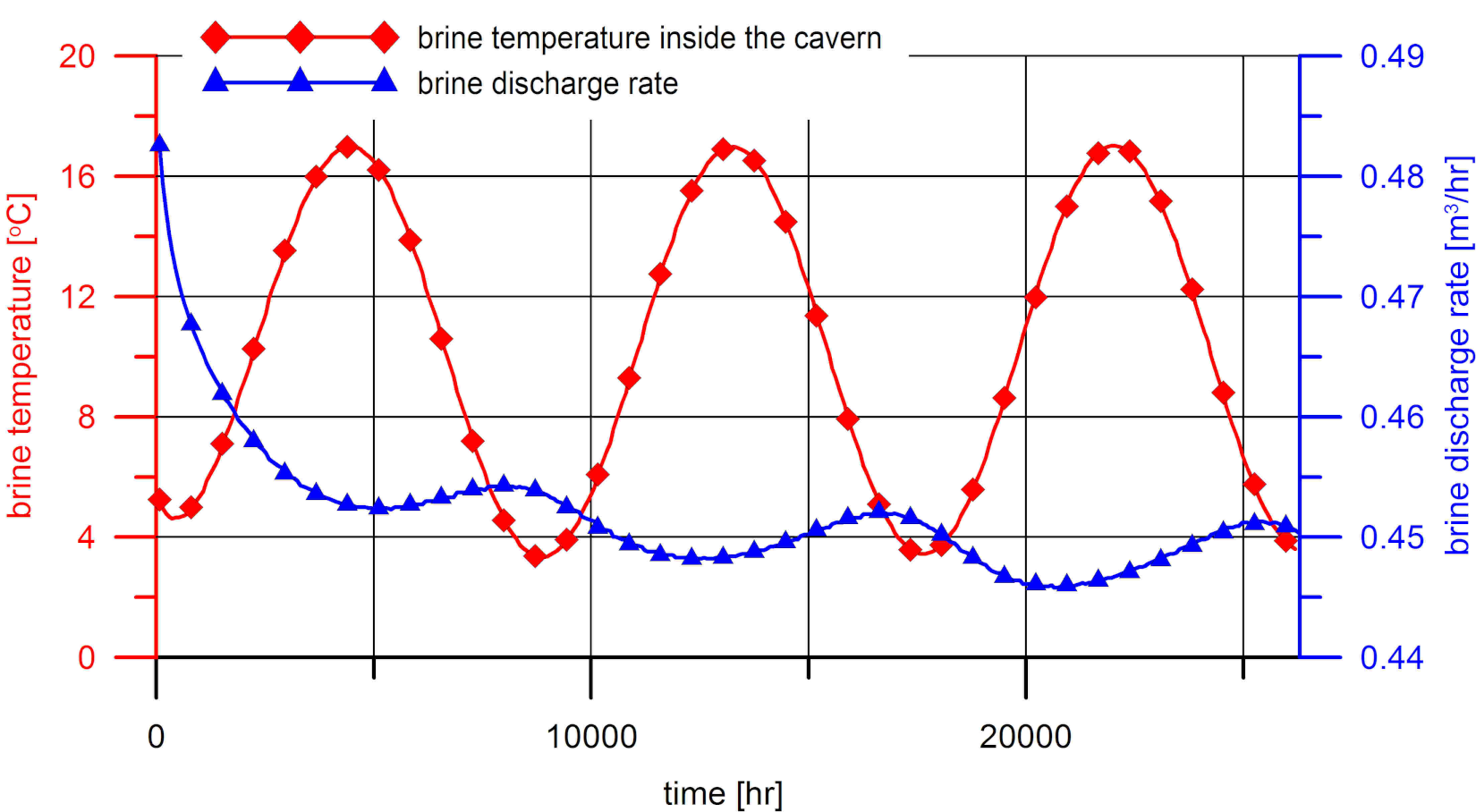
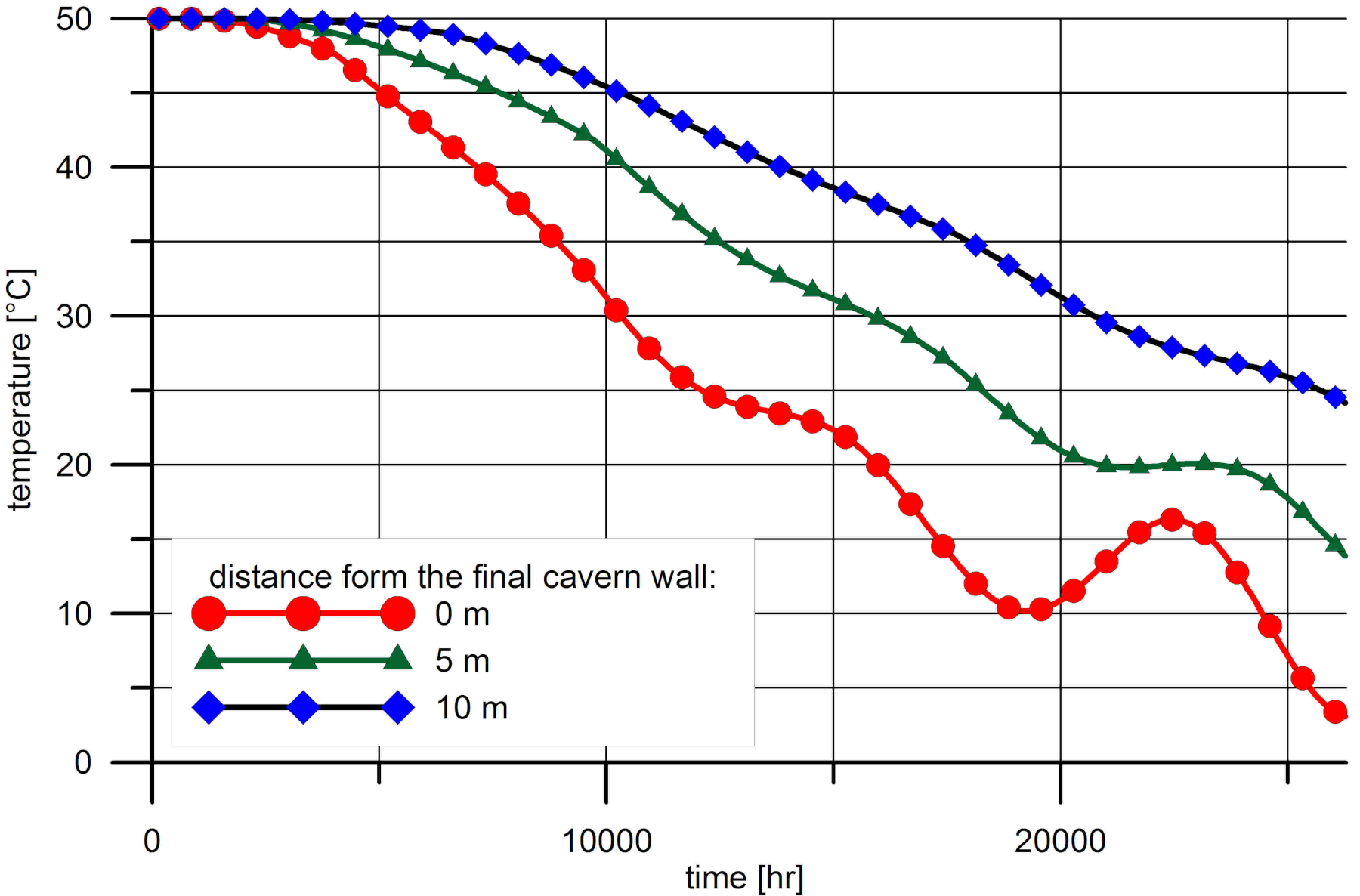
3. Results of Numerical Calculations
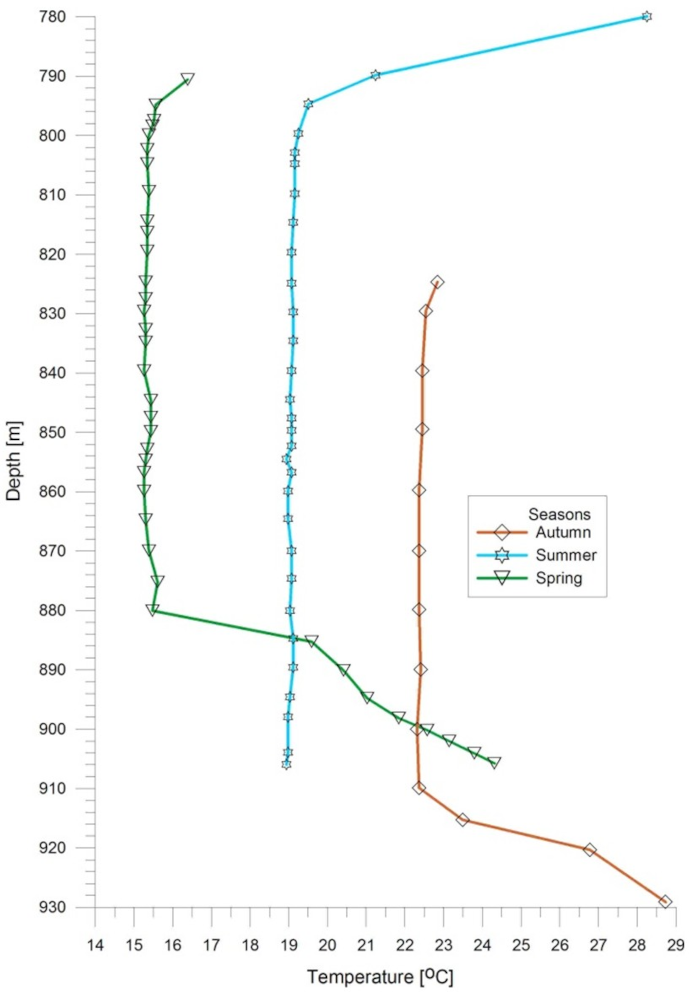
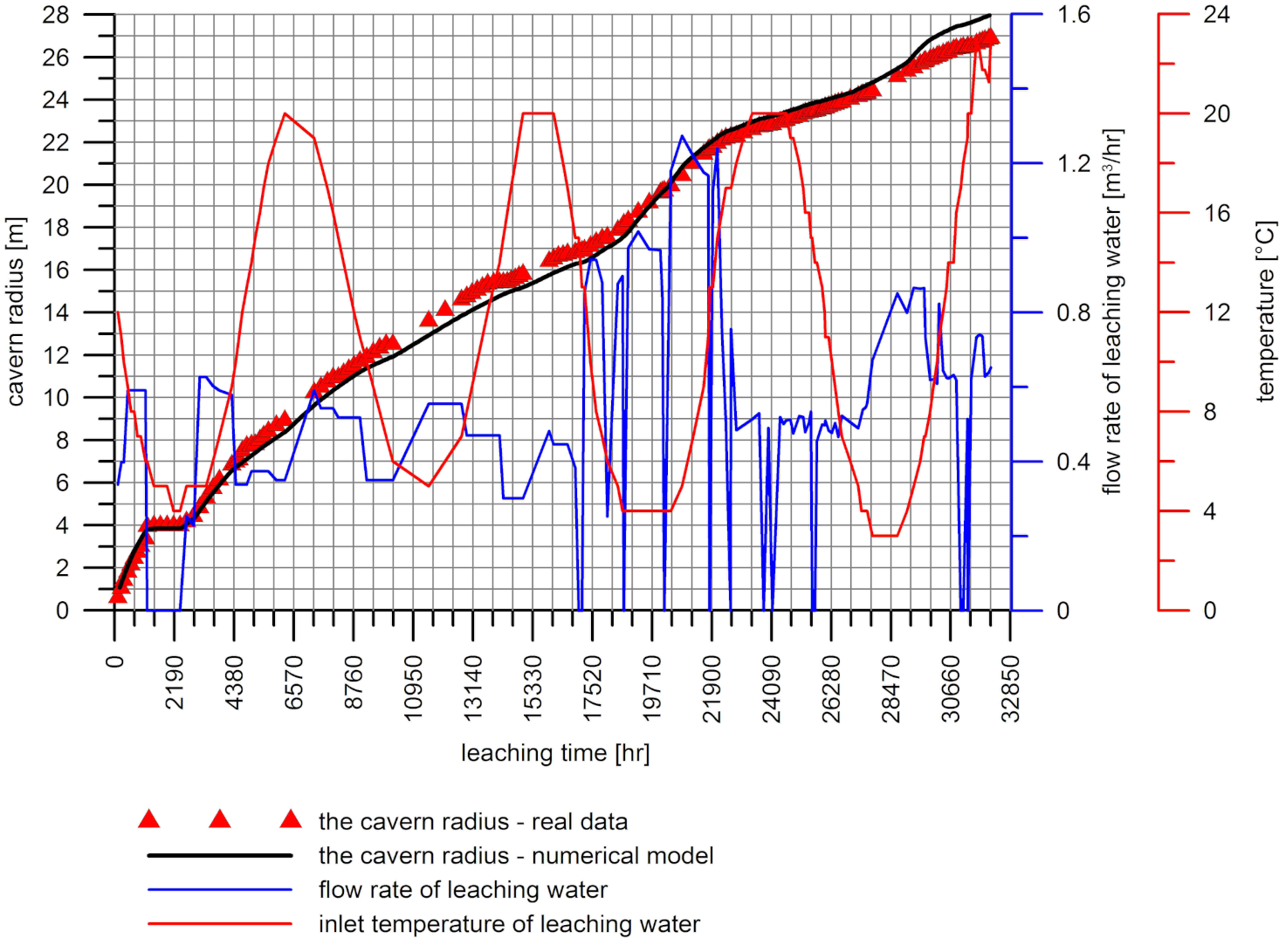
4. Comparison of Calculation Results with Measurement Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| convective heat transfer coefficient [] | |
| leaching coefficient determined in laboratory conditions for 20 , | |
| time step [s] | |
| enthalpy of NaCl dissolution in water [J/kg] | |
| height of the analyzed zone [m] | |
| mass of the NaCl dissolved in individual time step [kg] | |
| the cavern radius increase rate over time [m] | |
| volumetric stream of leaching water [m3/s] | |
| heat transfer coefficient [W/(m2 K)] | |
| leaching rate of the cavern wall with inclination by the solution with concentration and temperature and leaching properties at the depth h, [mm/h] | |
| density [kg/m3] | |
| density of the brine filling the cavern [kg3/s] | |
| leaching water density [kg3/s] | |
| density of NaCl | |
| the current time [s] | |
| c | specific heat [J/(kg )] |
| specific heat of the brine filling the cavern [J/(kg )] | |
| brine concentration in salt cavern expressed as the solute mass per unit volume of the solution [kg/m3] | |
| brine concentration in the cavern at the end of the current time step expressed as the mass of dissolved NaCl per volume unit [kg/m3] | |
| specific heat of leaching water per unit weight [J/(kg )] | |
| brine concentration in temperature expressed as the solute mass per unit volume of the solution [kg/m3] |
| cavern wall surface [m2] | |
| the rate of leaching by fresh water with temperature and cavern wall inclination and leaching properties at the depth h. In case of vertical cavern wall the value of function is equal to side leaching coefficient , and in case of the cavern roof it is equal to roof leaching coefficient [mm/h] | |
| side leaching coefficient, typically 6–20 mm/h—determined in laboratory conditions | |
| vertical leaching coefficient, typically 14–30 mm/h—determined in laboratory conditions | |
| mass of the NaCl dissolved in leaching water in individual time step in solution injection to cavern in current time step [kg] | |
| mass of the NaCl dissolved in the brine withdrawn from the cavern [kg] | |
| mass of the NaCl dissolved in the brine filling the cavern at the start of the current time step [kg] | |
| r | radius [m] |
| t | temperature [] |
| brine temperature [] | |
| cavern wall temperature [] | |
| reference temperature in which coefficient was determined, | |
| brine temperature in time [] | |
| temperature of brine in salt cavern [] | |
| leaching water temperature [] | |
| cavern wall temperature [] | |
| volume of the brine filling the cavern (cavern volume) [m3] | |
| volume of the fresh water within the brine filling the cavern in the individual time step m |
References
- Bujakowski, W.; Tarkowski, R. Termiczna charakterystyka górotworu w rejonie wysadów solnych. Przegląd Górniczy 2001, 57, 15–20. [Google Scholar]
- Pająk, L.; Gonet, A.; Śliwa, T.; Knez, D. Analiza możliwości wykorzystania magazynów ciekłego propanu, lokowanych w strefie kawern wysadów solnych, do produkcji energii. Wiert. Naft. Gaz 2010, 27, 657–667. [Google Scholar]
- Tomaszewska, B.; Pająk, L. Dynamics of clogging processes in injection wells used to pump highly mineralized thermal waters into the sandstone structures lying under the Polish Lowlands. Arch. Environ. Prot. 2012, 38, 105–117. [Google Scholar] [CrossRef]
- Tomaszewska, B. The prognosis of scaling phenomena in geothermal system using the geochemical modeling methods. Gospod. Surowcami Miner. Miner. Resour. Manag. 2008, 24, 399–407. [Google Scholar]
- AbuAisha, M.; Rouabhi, A.; Billiotte, J.; Hadj-Hassen, F. Non–isothermal two–phase hydrogen transport in rock salt during cycling in underground caverns. Int. J. Hydrogen Energy 2020, 46, 6632–6647. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Chen, J.; Jiang, D.; Fei, W.; Fan, J.; Li, Y. Feasibility evaluation of large-scale underground hydrogen storage in bedded salt rocks of China: A case study in Jiangsu province. Energy 2020, 198, 117348. [Google Scholar] [CrossRef]
- Sicsic, P.; Bérest, P. Thermal cracking following a blowout in a gas-storage cavern. Int. J. Rock Mech. Min. Sci. 2014, 71, 320–329. [Google Scholar] [CrossRef]
- Wang, T.; Ma, H.; Yang, C.; Shi, X.; Daemen, J. Gas seepage around bedded salt cavern gas storage. J. Nat. Gas Sci. Eng. 2015, 26, 61–71. [Google Scholar] [CrossRef]
- Labaune, P.; Rouabhi, A. Dilatancy and tensile criteria for salt cavern design in the context of cyclic loading for energy storage. J. Nat. Gas Sci. Eng. 2019, 62, 314–329. [Google Scholar] [CrossRef]
- Shkuratnik, V.; Kravchenko, O.; Filimonov, Y.L. Stresses and Temperature Affecting Acoustic Emission and Rheological Characteristics of Rock Salt. J. Min. Sci. 2019, 55, 531–537. [Google Scholar] [CrossRef]
- Bérest, P.; Brouard, B.; Djakeun-Djizanne, H.; Hévin, G. Thermomechanical effects of a rapid depressurization in a gas cavern. Acta Geotech. 2014, 9, 181–186. [Google Scholar] [CrossRef]
- Sedaee, B.; Mohammadi, M.; Esfahanizadeh, L.; Fathi, Y. Comprehensive modeling and developing a software for salt cavern underground gas storage. J. Energy Storage 2019, 25, 100876. [Google Scholar] [CrossRef]
- Yu, W.; Huang, W.; Liu, H.; Wang, K.; Wen, K.; Gong, J.; Zhang, M. A systematic method for assessing the operating reliability of the underground gas storage in multiple salt caverns. J. Energy Storage 2020, 31, 101675. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Z.; Fan, J.; Jiang, D.; Li, Z.; Chen, J. Research on gas leakage and collapse in the cavern roof of underground natural gas storage in thinly bedded salt rocks. J. Energy Storage 2020, 31, 101669. [Google Scholar] [CrossRef]
- Cetinkaya, E. Underground, modelling peak-load exploitation of and storage, gas and gazu, modelowanie szczytowej eksploatacji podziemnych magazynów and tureckich, w kawernach solnych dla and warunków, i polskich. Arch. Min. Sci. 2007, 52, 21–47. [Google Scholar]
- Ślizowski, J.; Urbańczyk, K.; Serbin, K. Numeryczna analiza konwergencji pola komór magazynowych gazu w wysadzie solnym. Gospod. Surowcami Miner. 2010, 26, 85–93. [Google Scholar]
- Bérest, P. Heat transfer in salt caverns. Int. J. Rock Mech. Min. Sci. 2019, 120, 82–95. [Google Scholar] [CrossRef]
- Serbin, K.; Ślizowski, J.; Urbańczyk, K.; Nagy, S. The influence of thermodynamic effects on gas storage cavern convergence. Int. J. Rock Mech. Min. Sci. 2015, 100, 166–171. [Google Scholar] [CrossRef]
- Ozarslan, A. Large-scale hydrogen energy storage in salt caverns. Int. J. Hydrogen Energy 2012, 37, 14265–14277. [Google Scholar] [CrossRef]
- Kunstman, A.; Poborska-Młynarska, K.; Urbańczyk, K. Solution Mining in Salt Deposits: Outline of rEcent Development Trends; AGH Uczelniane Wydawnictwa Naukowo-Dydaktyczne: Krakow, Poland, 2007. [Google Scholar]
- Kunstman, A.; Poborska-Młynarska, K.; Urbańczyk, K. Zarys Otworowego ugownictwa Solnego: Aktualne Kierunki Rozwoju; AGH Uczelniane Wydawnictwa Naukowo-Dydaktyczne: Krakow, Poland, 2002. [Google Scholar]
- Wiśniewski, S.; Wiśniewski, T.S. Wymiana Ciepła; Wydawnictwo Naukowe PWN, WNT: Warsaw, Poland, 2010. [Google Scholar]
- Robertson, E.C. Thermal Properties of Rocks. 1988. Available online: https://pubs.usgs.gov/of/1988/0441/report.pdf (accessed on 1 September 2020).
- Kunstman, A.; Urbanczyk, K. Computer Simulation of Temperature Distribution Around Well and Cavern During Leaching. In Proceedings of the SMRI Spring Meeting, New Orleans, LA, USA, 15–16 April 1995. [Google Scholar]
- Hobler, T. Ruch Ciepła i Wymienniki; Wydawnictwo Naukowe PWN: Warsaw, Poland, 1979. [Google Scholar]
- Weast, R.C. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1988; Volume 69. [Google Scholar]
- Jamieson, D.; Tudhope, J.; Morris, R.; Cartwright, G. Physical properties of sea water solutions: Heat capacity. Desalination 1969, 7, 23–30. [Google Scholar] [CrossRef]
- McCain, W., Jr. Reservoir-Fluid Property Correlations-State of the Art (includes associated papers 23583 and 23594). SPE Reserv. Eng. 1991, 6, 266–272. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pająk, L.; Lankof, L.; Tomaszewska, B.; Wojnarowski, P.; Janiga, D. The Development of the Temperature Disturbance Zone in the Surrounding of a Salt Cavern Caused by the Leaching Process for Safety Hydrogen Storage. Energies 2021, 14, 803. https://doi.org/10.3390/en14040803
Pająk L, Lankof L, Tomaszewska B, Wojnarowski P, Janiga D. The Development of the Temperature Disturbance Zone in the Surrounding of a Salt Cavern Caused by the Leaching Process for Safety Hydrogen Storage. Energies. 2021; 14(4):803. https://doi.org/10.3390/en14040803
Chicago/Turabian StylePająk, Leszek, Leszek Lankof, Barbara Tomaszewska, Paweł Wojnarowski, and Damian Janiga. 2021. "The Development of the Temperature Disturbance Zone in the Surrounding of a Salt Cavern Caused by the Leaching Process for Safety Hydrogen Storage" Energies 14, no. 4: 803. https://doi.org/10.3390/en14040803
APA StylePająk, L., Lankof, L., Tomaszewska, B., Wojnarowski, P., & Janiga, D. (2021). The Development of the Temperature Disturbance Zone in the Surrounding of a Salt Cavern Caused by the Leaching Process for Safety Hydrogen Storage. Energies, 14(4), 803. https://doi.org/10.3390/en14040803






