Abstract
Furfural is only derived from lignocellulosic biomass and is an important chemical used in the plastics, agrochemical, and pharmaceutical industries. The existing industrial furfural production process, involving reaction and purification steps, suffers from a low yield and intensive energy use. Hence, major improvements are needed to sustainably upgrade the furfural production process. In this study, the conventional furfural process based on a continuous stirred tank reactor and distillation columns was designed and optimized from an actual aqueous xylose solution via a biomass pretreatment step. Subsequently, a reactive distillation (RD) and extraction/distillation (ED) configuration was proposed for the reaction and purification steps, respectively, to improve the process efficiency. RD can remove furfural instantly from the reactive liquid phase and can separate heavy components from the raw furfural stream, while the ED configuration with toluene and butyl chloride used as extracting solvents can effectively separate furfural from a dilute aqueous stream. The results showed that the hybrid RD-ED process using a butyl chloride solvent saves up to 51.8% and 57.4% of the total investment costs and total annual costs, respectively, compared to the conventional process. Furthermore, environmental impacts were evaluated and compared for all structural alternatives.
1. Introduction
The world economy is currently overdependent on mineral resources, leading to negative environmental impacts such as global climate change, resource depletion, and global warming. Therefore, methods for the development of renewable resources in the production of chemicals and fuels have been intensively explored. Lignocellulosic biomass, which is the most promising candidate to replace fossil fuels, is a readily renewable resource for industry. The conversion of biomass into chemicals conceptually promises sustainable, inherently safe, eco-friendly production.
Furfural, which has been identified as one of the top 30 potential chemicals derived from biomass by the National Renewable Energy Laboratory (NREL), has been widely applied in industrial solvents, fungicides, fuel additives, resins, drugs, and plastics, among others [1]. Furfural is currently the only starting material for producing almost all furan compounds, and it plays a central role in the plastics, agrochemical, and pharmaceutical industries. The only route to producing furfural is from hemicellulose-rich agricultural wastes such as corn cobs and oat hulls [2]. This “green” chemical is considered the key renewable feedstock for producing biochemicals that can compete with oil-based chemicals [1,3].
The furfural industry has a long history that started when the Quaker Oats company established their first production plant in 1921, utilizing oat hulls and old equipment from a defunct cereal plant [4]. In this process, oat hulls are first mixed with sulfuric acid as the catalyst before entering the batch reactor. High-pressure steam is then injected to heat and hydrolyze the biomass feedstock with simultaneous steam stripping of the furfural dehydration products. Owing to low reactivity and poor mass transfer, the process suffers from low yields of approximately 50% and intensive energy use [4]. A significant improvement in furfural technology was achieved in 1960 when Quaker Oats developed a continuous industrial plant in Florida. The residence time was reduced to one hour instead of five hours in the previous batch process, while the furfural yield increased slightly (55%) [4]. Currently, a large proportion of the annual global production of furfural (300,000 tons) originates from China, using small-scale fixed-bed processes with a furfural yield of approximately 50% [5]. Meanwhile, the world’s largest furfural plant with a capacity of 35000 tons per year, located in the Dominican Republic, still employs a batch process [5]. Surprisingly, furfural technologies have shown little improvement thus far, as most existing furfural industrial processes are based on the original Quaker Oats process [4]. Therefore, a significant technological advancement is needed to upgrade furfural production to compete with oil-based products.
In recent years, several advanced furfural processes on a laboratory or pilot scale have been reported, such as Escher Wyss, Rosenlew, Supratherm, Stake, Supray Yield, and Voest Alpine [4]. The Escher Wyss process, which uses a fluid bed reactor, reduces the mean residence time to 45 min at an operating temperature of 170 °C. However, this process was abandoned due to a considerably low yield and severe corrosion. The Stake process, based on a “feeder gun” system, hydrolyzes biomass at 230 °C, resulting in a significant decrease in residence time (6.3 min). A furfural yield of 66% was obtained without adding acid catalyst [4]. The Bosh project in Durban, South Africa, reported a concept called Suprayield that targeted a furfural yield of 100% [4]. Theoretically, this “analytical furfural process” could achieve a 100% yield by keeping the reaction medium in a state of boiling at approximately 240 °C, which allowed any furfural products in the liquid phase to be instantly removed by the vapor phase. Recently, De Jong and Marcotulliio proposed a promising conceptual furfural production process using straw as the feedstock and utilizing a continuous multi-turbine column reactor [5]. The main benefit of this process was that the furfural product was removed immediately from the liquid phase, avoiding loss reactions that occur in the liquid phase. As a result, a high furfural yield of 86% and a short residence time of 24.6 min were achieved in the simulation study.
Reactive distillation (RD), a well-known technique among separation processes, can be advantageously applied to overcome the difficulties and limitations encountered in several chemical processes. In fact, combining a reaction and a separation step in a distillation column can serve as a simpler process and can afford higher yields by overcoming equilibrium limitations and poor selectivity of the desired products [6]. Hence, the interest in RD as a promising technology not only to reduce investment costs but also to improve product selectivity and conversion has increased substantially in recent years [7].
Mandalika and Runge proposed an acid-catalyzed batch RD process to produce furfural from biomass hydrolysates [8]. The furfural product was continuously removed as a dilute aqueous vapor stream from the top of the RD column. Consequently, high yields of furfural (greater than 85%) were consistently achieved with both biomass samples and a xylose solution in the presence of sulfuric acid. However, catalyst regeneration in batch processes may require complex and costly separation steps.
Metka et al. developed a continuous RD process for furfural production using solid catalysts and sulfolane as the solvent [6]. In this process, furfural was instantly removed from the liquid phase by steam-stripping of the heterogeneous azeotrope of furfural and water. As a result, furfural yields of 75% were achieved at 175 °C. Further optimization was needed to solve several technical issues, such as catalyst deactivation and solvent regeneration, to ensure that the process is feasible on a large scale. Recently, Jong and Marcotullio developed a continuous RD-assisted heat pump for the production of furfural from pentose [9,10]. The authors reported that a furfural yield of 87.5% was achieved, while the RD-assisted heat pump reduced energy consumption by 84% compared to the conventional process. However, this process used simplified feed comprising only pentose and water. Furthermore, the process conditions were only partially disclosed due to confidentiality issues. Notably, most previous studies focused on improving the reaction steps of furfural production on a small scale; very few studies have focused on the furfural purification step, which is also energy intensive. Nhien et al. proposed a hybrid extraction and distillation process using butyl chloride (BC) as the extracting solvent to improve the purification process [11]. As a result, the proposed hybrid process reduced the total annual cost (TAC) by 19.2% compared to the conventional distillation process.
Even though several studies focused on furfural production from xylose, very few studies considered an actual xylose solution that consists of many components such as dextrose, xylose, extractives, furfural, and levulinic acid (LA). Different compositions of raw materials result in varying degrees of profitability for the entire process. Furthermore, there is inadequate detailed design data for both the reaction and purification steps on a large scale. To solve these issues, in this study, a novel hybrid RD with extraction-distillation (RD-ED) process was proposed for furfural production from an actual xylose solution. First, a conventional reaction-distillation (R-D) process consisting of a continuous stirred tank reactor (CSTR) and distillation columns for the reaction and purification sections, respectively, was designed and optimized. Subsequently, RD was proposed to replace the conventional reactor, while an ED configuration with toluene and BC as extracting solvents was proposed for the purification process. All processes were rigorously simulated using the commercial simulator, Aspen HYSYS version 10. For a fair comparison, all structural alternatives were assessed for both economic and environmental impacts. Consequently, promising design and operating conditions for the furfural production process were explored.
2. Methods
2.1. Design and Simulation
In this study, a furfural plant was designed with a capacity of 90 kilotons furfural per year. The production capacity was designed based on the NREL plant design, which produces ethanol from corn stover [12]. The NREL biorefinery processes 2205 dry ton corn stover per day with the annual production of 120.4 kilotons of 99.5 wt% ethanol. Our idea was to develop a furfural production process, which utilizes the aqueous xylose stream in the NREL process. In particular, after the pretreatment step, the cellulose/lignin slurry was sent to the ethanol production part in the NREL process whereas the hemicellulose solution (aqueous xylose stream) was introduced to the present furfural production process. Note that the xylose solution can be contaminated to a varying degree due to the way it is harvested. However, it is out of the scope of the present research. Figure 1 shows the schematic flowsheet of the furfural production from lignocellulosic biomass. First, a biomass feedstock such as corn stover underwent acidic hydrolysis with sulfuric acid as the catalyst in the pretreatment step, where hemicellulose was converted into its sugar monomers that mainly comprises the five-carbon sugar, xylose, and the six-carbon sugar glucose (dextrose). The aqueous xylose stream was then separated from the cellulose/lignin slurry using a filter and transferred for the furfural production process. Then, the xylose solution was introduced to a CSTR reactor, where xylose was dehydrated to furfural in the presence of sulfuric acid. The vapor stream containing approximately 4 wt% furfural was withdrawn from the top of the reactor and delivered to the purification step to achieve commercial-grade purity of furfural.
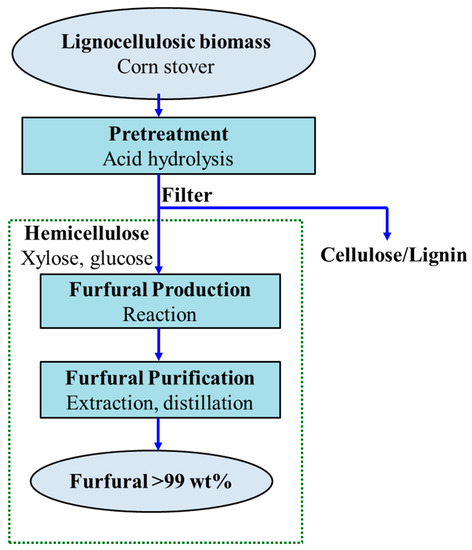
Figure 1.
Schematic flowsheet of the furfural production process from lignocellulosic biomass.
All processes in this study were rigorously simulated using Aspen HYSYS V.10. The physical property data of all components were taken from the Aspen database and the National Renewable Energy Laboratory (NREL) report [12]. The non-random two-liquid (NRTL) thermodynamic package was used to calculate the liquid activity coefficients, while the Hayden–O’Connell (HOC) equation of state was used to calculate the thermodynamic properties of the vapor phase. In particular, the HOC equation can reliably predict mixtures containing carboxylic acids, which may be formed during the solvation of polar compounds and dimerization in the vapor phase [13]. In addition, UNIFAC (UNIQUAC Functional-group Activity Coefficients) was used to estimate all missing binary parameters in the vapor–liquid equilibrium (VLE) data as well as all binary parameters in the liquid–liquid equilibrium data for the extraction process.
For the design and optimization of the distillation column, several simulations were first run to determine the initial structure. Next, the total number of trays and feed location were adjusted to minimize the TAC while maintaining the target product purity and recovery. The minimized TAC provided an optimal tradeoff between capital and operating costs and determined the optimal structural design and operating conditions. The detailed optimization procedure for a distillation column was reported in our previous study [3].
2.2. Reactions and Kinetics
In the present furfural production process, the xylose solution feed was introduced to the reactor where all reactions occurred. As shown in Table 1, the feed contained 87.6 wt% water, 5.8 wt% xylose, 1.2 wt% glucose (dextrose), and 0.8 wt% sulfuric acid as well as other components. In the presence of sulfuric acid, xylose was dehydrated to furfural, while dextrose was dehydrated to hydroxymethylfurfural (HMF). In this study, all reactions were modeled in Aspen HYSYS by implementing kinetic data obtained from the work of Carrosco and Roy and Bhandari et al. [14,15].

Table 1.
Stream table of the reaction-distillation process.
Xylose → Furfural + H2O
where R is the ideal gas constant and T (Kelvin) is the absolute temperature.
k1 = 3.67 × 109 exp(−1.01 × 109/RT)
Note that HMF reacts very quickly to formic acid (FA) and LA. Consequently, the reaction products of glucose decomposition were modeled as LA and FA [16]:
Glucose → HMF → LA + FA + Water
k2 = (2.43 × 1014)exp(−1.373 × 105/RT)
2.3. Economic Evaluation
For a fair comparison of the economic impact, the total investment cost (TIC), total operating costs (TOC), and TAC of all process alternatives were estimated using correlations from the Smith textbook [17]. For a preliminary design, an order-of-magnitude estimate with an error level of 25–40% is sufficient [18]. Therefore, for all the equipment cost calculations, Guthrie’s modular method was applied [18,19]. The tray sizing function in Aspen HYSYS was used to estimate the column diameters, tray spacing, and column heights. All reboilers, condensers, heat exchangers, column vessels, and tray stacks were considered in the TIC. A Chemical Engineering Index of 607.5 (corresponding to 2019) was used to update the TIC estimates. The utility prices listed in Table A1 in Appendix A were used for the TOC calculations [19]. A plant lifetime of 10 years and a fixed interest rate (8%) were assumed for the TAC estimates.
2.4. Environmental Assessment
In addition to economic evaluations, total annual carbon dioxide (CO2) emissions (TCE) of all the processes was calculated for a fair comparison of the environmental impact. For steam reboilers, Gadalla’s modular method was applied to calculate the CO2 emissions [20]:
where NHV is the net heating value of the fuel and C% is the carbon content. For natural gas, the NHV is 48,900 kJ/kg and C% is 0.41 kg/kg. The molar mass ratio of CO2 to C was a = 3.67. In addition, Qfuel denotes the amount of fuel used and is calculated as follows:
where Qproc is the heat duty requirement of the system while λproc (kJ/kg) and hproc (kJ/kg) are the latent heat and enthalpy of steam, respectively. The flame, stack, and ambient temperatures were TFTB (1800 °C), Tstack (160 °C), and T0 (25 °C), respectively.
3. Results and Discussion
In this study, the furfural plant was designed with a capacity of 90 kilotons of furfural per year, which amounts to approximately 20% of the global market volume in 2019 [21]. The following sections first detail the design of the furfural R-D process and its optimization. Subsequently, several structural alternatives, such as a reaction-extraction/distillation (R-ED) process, a reactive distillation-distillation (RD-D) process, and a RD-ED process, were proposed to improve the conventional process. All processes were assessed in terms of both economic and environmental impacts. Finally, the most promising design was selected for the furfural production process.
3.1. Furfural R-D Process
Figure 2 shows a schematic of the furfural R-D production process from the xylose feed solution via the biomass pretreatment step. The detailed feed conditions and component compositions are listed in Table 1 as stream 11 [12,22].
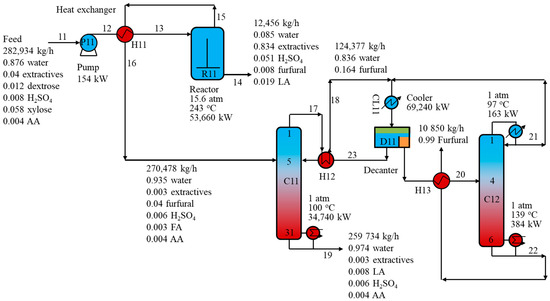
Figure 2.
Schematic of the furfural reaction-distillation process.
First, the feed stream was pressurized from 1 atm to 15.6 atm before exchanging heat with the reactor (R11) output stream (stream 15) to achieve a temperature of 203 °C. The heated feed was then introduced to the top of reactor R11. Reactor R1 was modeled as a CSTR reactor with the kinetic reactions described in Section 2.2. Figure 3 shows the relationship between the component flow rates and reaction temperature in reactor R11. Obviously, once the temperature reached 243 °C, the decomposition of xylose and dextrose occurred rapidly. Therefore, the operating temperature of the reactor was designed at 243 °C, which is in agreement with the report of the Suprayield process of the Bosh project in Durban [4]. Moreover, the operating pressure of 15.6 atm was taken from Marcotulio’s work [9] and sulfuric acid of 0.8 wt% acted as a catalyst. In particular, xylose and glucose were dehydrated to furfural and HMF, respectively. However, HMF is highly unstable and reacts quickly to form LA and FA.
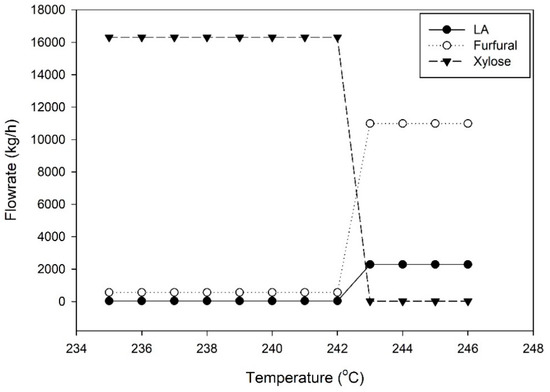
Figure 3.
Component flowrates versus reaction temperature in the furfural reactor.
The reactor top vapor (stream 15) consisting of 4 wt% furfural and 93.5 wt% water exchanged heat with stream 12 before being introduced to the distillation column C11, which is commonly called an azeotropic column. Note that, apart from furfural and water, stream 15 also contained numerous other components such as 955 kg/h extractives, 1694 kg/h sulfuric acid, 2061 kg/h LA, 897 kg/h FA, and 1180 kg/h acetic acid (AA). This may result in a more complex purification process to achieve the purity target of furfural. The function of column C11 was to separate the heterogeneous azeotrope of furfural-water to the top of the column and other heavy components, such as extractives, LA, and sulfuric acid, to the bottom of the column. This heavy fraction was sent to the wastewater treatment section for reuse in the biomass pretreatment step. Typically, the sulfate ions in the waste streams can be treated by over-liming and were removed from the process as gypsum. In the wastewater treatment step, the waste streams were processed by anaerobic digestion and aerobic digestion to digest organic matter in the stream. Anaerobic digestion produces a biogas stream that can be used for a combustor, whereas aerobic digestion produces a relatively clean water stream that can be reused in the process. The C1 top vapor stream (stream 17) containing 16.3 wt% furfural was cooled by the decanter output (stream 23) and was condensed and further cooled by a cooler to 40 °C before being sent to decanter D1. The furfural-water azeotrope was split naturally by decanter D1 into two liquid phases. The light aqueous phase exchanged heat with the C11 top stream before being returned by reflux to C1. Meanwhile, the heavy phase rich in furfural was heated by the furfural product stream from the bottom of C12 before being introduced to C12. Finally, furfural at the desired purity of 99.0 wt% was collected from the bottom of C12, while the azeotrope from the top of C12 was fed to decanter D11 before recycling back to C11. A total furfural recovery of 98.7 wt% was achieved in the purification process. For a fair comparison, the furfural purity and recovery were designed at 99.0 wt% and 98.7 wt%, respectively, for all structural alternatives of the purification process. All stream information is presented in detail in Table 1.
Table 2 lists the detailed designed parameters for all distillation columns in the R-D process. Valve trays, which have better turndown properties than sieve trays, were selected for all columns; thus, they are more flexible when the feed flowrate varies. Simple heat integration techniques such as feed preheating were applied to R11, C11, and C12 to maximize heat recovery. The results show that R11 and C11 consumed the most energy at 53,660 kW and 35,070 kW, respectively.

Table 2.
Design parameters of all distillation columns.
3.2. Improvement of the Conventional Furfural Production Process
3.2.1. Furfural R-ED Process
Owing to a large amount of water and numerous minor components in the C11 feed stream, C11 in the R-D process required considerable energy. For this type of feed, liquid–liquid equilibrium may be more suitable from an energy viewpoint. The effectiveness of toluene and BC solvents in furfural purification was reported for a simplified feed to a furfural reactor [11]. Therefore, toluene and BC were selected as extracting solvents for the furfural recovery process in this study.
Toluene Solvent Process
Figure 4 depicts the R-ED process using a toluene solvent with key design parameters and stream information. The top vapor stream from the furfural reactor first exchanged heat with the solvent-rich stream before being cooled to 40 °C. The dilute furfural stream was then fed to extractor E21 from the top, while the toluene solvent was introduced from the E21 bottom. The E21 works with countercurrent liquid flows and produces an aqueous stream at the bottom and a toluene-rich stream at the top. In order to achieve a furfural recovery of 99.9 wt% via extractor E21, a feed-to-solvent ratio of 2.68 was designed. The raffinate containing water, sulfuric acid, LA, FA, AA, and extractives was sent to the wastewater treatment section. However, the toluene-rich stream still contained some heavy components such as sulfuric acid (156 kg/h) and LA (192 kg/h) so that a configuration of two distillation columns was needed to achieve the target furfural purity of 99.0 wt%. The extract was introduced to distillation column C21 to separated toluene from the top and furfural and other heavy components from the bottom. The produced solvent was cooled to 40 °C and mixed with the solvent makeup stream before being recycled back to the extractor. An additional column, C22, was required to separate furfural from heavy components to achieve the required purity of 99.0 wt%. The heavy fraction in the C22 bottom stream was then sent to the wastewater treatment section. In addition, simple heat integration was applied to improve the heat recovery of the entire process. In particular, the reactor output was used to preheat the reactor feed and the C21 feed, as shown in Figure 2. The results showed that, for the purification section, the ED configuration with toluene as the solvent saved up to 60.4% of the reboiler energy requirement compared to the distillation configuration in the R-D process. Overall, the R-ED process reduced the energy requirement by 23.9% compared with the R-D process.
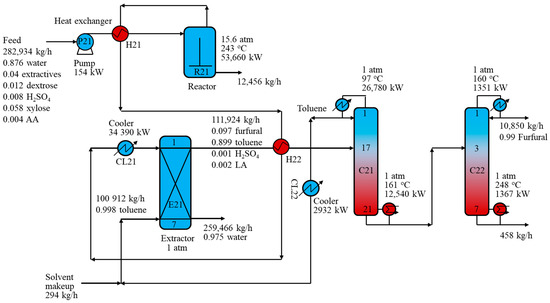
Figure 4.
Schematic of the furfural reaction-extraction/distillation process with toluene solvent.
BC Solvent Process
Figure 5 shows the key design and process parameters of the R-ED process using a BC solvent. Similar to the R-D process with a toluene solvent, the dilute furfural stream from the furfural reactor exchanged heat with the stream from the top of the extractor and was cooled to 40 °C before being fed to extractor E31. Extractor E31 generated a light phase comprising mainly BC and furfural and a heavy phase comprising mainly water and other components such as extractives, LA, AA, FA, and sulfuric acid. To obtain a furfural recovery of 99.9 wt% via extractor E31, a feed-to-solvent ratio of 7.84 was designed. Obviously, BC solvent was more favorable to extract furfural than toluene solvent, resulting in a decrease of 65.7% flowrate compared to the toluene flowrate. Furthermore, most of the heavy components, such as LA, sulfuric acid, and extractives were sent to the aqueous phase; hence, only one distillation column (instead of two distillation columns in the ED configuration with the toluene solvent) was required to achieve the required purity of 99.0 wt%. The solvent-rich stream from the top of E31 was then sent to distillation column (C31) to separate 99.0 wt% furfural from the bottom and toluene from the top. The heat of reactor output was utilized to preheat the reactor feed and the C31 feed streams to improve the heat recovery in the whole process. The results showed that the purification section using a BC solvent reduced the reboiler energy requirement by 95.9% compared to the purification section in the R-D process. Overall, the R-ED process with a BC solvent saved up to 37.9% of TOC and 35.9% of TIC compared to the R-D process.
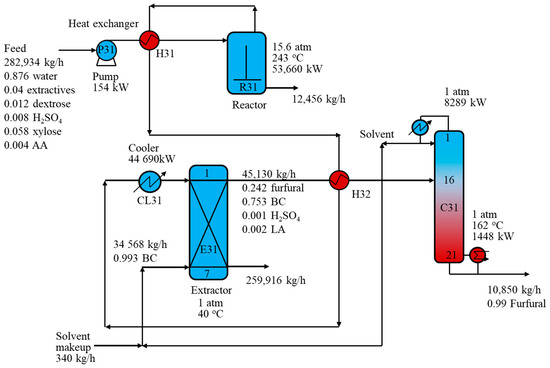
Figure 5.
Schematic of the furfural reaction-extraction/distillation process with butyl chloride solvent.
3.2.2. Furfural RD-D Process
The RD configuration has been effectively demonstrated at the laboratory scale in several studies, as mentioned in Introduction section. In this section, we simulated and propose a detailed design of the RD configuration at an industrial scale of the conventional process. Figure 6 presents a schematic of the RD-D process for furfural production. The feed was first pressurized to 23.5 atm and preheated by the stream from the top of RD41 before being fed to the RD column. The operating pressure was maintained at 23.5 atm to maintain the column temperature at approximately 226 °C. In the RD column, the furfural formation reaction and glucose decomposition occurred and were simulated using the kinetic data described in the Reactions and Kinetics section. The dilute furfural stream from the top of RD41 then exchanged heat with the RD41 feed and was introduced to the azeotropic distillation column C41. Similar to the purification section in the R-D process, the C41 product containing the heterogeneous azeotrope of furfural and water was cooled to 40 °C and fed to decanter D41. The liquid–liquid azeotrope was separated naturally in the decanter into two liquid phases. The light aqueous phase was preheated and recycled back to C41, while the heavy furfural phase was preheated and fed to the second distillation column C42. The bottom stream of C41, containing mainly water and AA, was sent to the wastewater treatment section before being reused in the biomass pretreatment process. In C42, furfural with a purity of 99.0 wt% was collected from the bottom while the furfural-water azeotrope was delivered to the top. This azeotropic stream was cooled before being recycled to decanter D41.
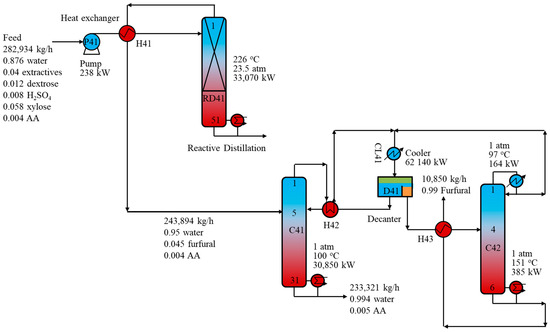
Figure 6.
Schematic of the reactive distillation-distillation process for furfural production.
In the RD column, as furfural was formed from xylose, it was instantly removed from the reacting liquid phase by the internal vapor. This largely prevented possible by-product reactions such as resinification and condensation. Notably, the furfural formation in the RD column occurred at a lower temperature (226 °C) than that in the conventional reactor (243 °C), resulting in a 38.4% reduction in the energy requirement compared to the conventional reactor.
Table 3 lists the component flow rates in the top vapor streams of the conventional reactor and the RD column. Both streams had similar furfural flowrates, but the top vapor stream from the RD column contained much less heavy components, such as sulfuric acid, LA, and extractives, than that stream from the conventional reactor. In total, the flowrate of the RD top stream is 9.8% less than that of the CSTR top stream. This may bring significant benefits to the purification process. The results showed that the azeotropic column C41 in the RD-D process had a 10.2% lower reboiler energy consumption than column C11 in the R-D process. Remarkably, the RD-D process had a 27.6% lower TOC than the RD-D process.

Table 3.
Component flowrates of the top vapors of the reactor and reactive distillation.
3.2.3. Furfural RD-ED Process
Toluene Solvent Process
Figure 7 depicts the hybrid RD-ED process using toluene as the extracting solvent for furfural production. In this process, the dilute furfural stream from the top of the RD column was cooled to 40 °C and fed to extractor E51 from the top, while the toluene solvent was introduced to the extractor from the bottom. A feed-to-solvent ratio of 2.78 was implemented to achieve a furfural recovery of 98.7 wt% via the purification process. Extractor E51’s function was to produce two liquid phases: an aqueous phase from the bottom and a toluene-rich phase from the top. The aqueous stream containing mostly water and some AA was sent to the wastewater treatment section, while the solvent phase containing toluene and furfural was heated by the dilute furfural stream and fed to distillation column C51. Subsequently, furfural at the required purity of 99.0 wt% was collected from C51, whereas toluene solvent from the C51 top was cooled before being recycled back to the extractor.
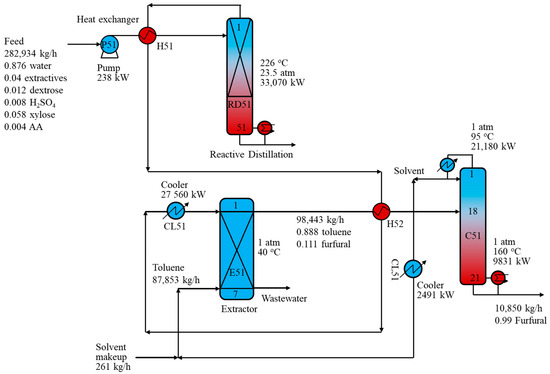
Figure 7.
Schematic of the reactive distillation-distillation process with toluene solvent.
Note that the furfural purification step in the RD-ED process with toluene solvent required only one distillation column to achieve a furfural purity of 99.0 wt%. This benefit was due to the RD column, in which most of the heavy components such as extractives, LA, and sulfuric acid were delivered to the bottom stream. As a result, the RD-ED process with toluene solvent saved up to 51.7% of the reboiler energy requirement compared to the conventional R-D process.
BC Solvent Process
Figure 8 depicts the hybrid RD-ED process using BC as the extracting solvent for furfural production. Similar to the RD-ED process with the toluene solvent, the dilute furfural stream from the RD column was cooled to 40 °C and introduced to the top of extractor E61. Meanwhile, the BC solvent was fed to extractor E61 from the bottom. The mass ratio of the dilute furfural stream to the BC solvent was set at 8.11 to maintain a furfural recovery of 98.7 wt% via the purification process. Extractor E61 generated a solvent-rich phase comprising BC and furfural from the top and an aqueous phase comprising water and other components such as AA and FA from the bottom. The aqueous stream was then sent to the wastewater treatment section before being reused in pretreatment. Meanwhile, the solvent-rich stream was sent to distillation column C61. The furfural product of 99.0 wt% furfural was recovered from the bottom of C61 while BC solvent was collected from the C61 top. The solvent stream was mixed with the solvent makeup and recycled back to the extractor E61. Remarkably, the RD-ED process with BC as the extracting solvent saved up 62% and 51.8% of the reboiler energy requirement and TIC, respectively, compared to the R-D process.
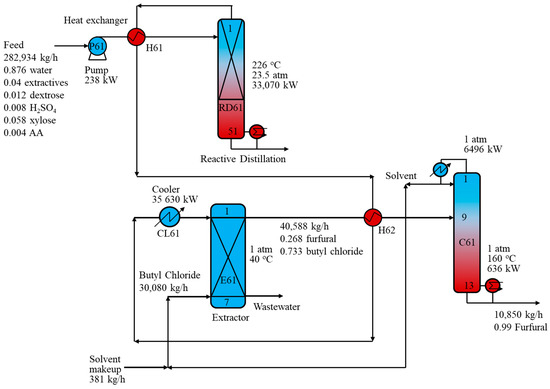
Figure 8.
Schematic of the reactive distillation-distillation process with a butyl chloride solvent.
Table 4 lists the key results of all process alternatives, including the energy requirements of the reboilers and condensers, TIC, TOC, TAC, and TCE. Remarkably, the hybrid RD-ED process with the BC solvent saved up to 51.8% and 57.4% TIC and TAC, respectively, compared to the R-D process. Figure 9 illustrates the costs and carbon emissions of all the processes. In terms of environmental impact, the hybrid RD-ED process with the BC solvent was the most favorable as it reduced TCE by 58.9% compared to the R-D process.

Table 4.
Comparison of different structural alternatives for furfural production process.
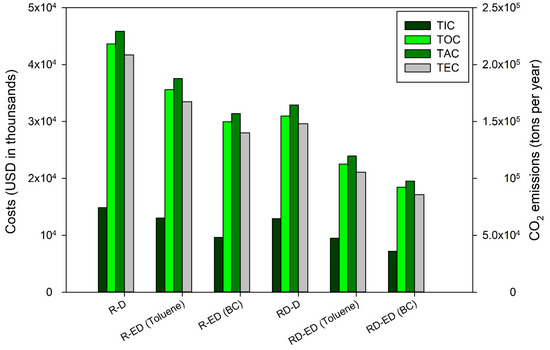
Figure 9.
Economic and environmental evaluations of all structural alternatives for furfural production process.
From the actual xylose solution, we proposed a cost-efficient design for the furfural production process. As a result, utilizing the hemicellulose fraction of lignocellulosic biomass can attain a higher certain degree of possible furfural in a biorefinery context.
4. Conclusions
In this study, a novel hybrid RD-ED process with BC as an effective extracting solvent was proposed for enhancing the process of producing furfural from an actual xylose solution. The RD configuration allowed furfural to be removed immediately from the reactive liquid phase by the internal vapor; thus, it required a lower reaction temperature compared to the conventional reactor with the same furfural yield. The results demonstrated that the RD process required 38.4% less TOC than the conventional reactor. Furthermore, most of the heavy components were delivered to the bottom of the RD column, resulting in significant benefits to the furfural purification process. In addition, the ED configuration with BC as the extracting solvent was more suitable for purification from a dilute aqueous stream, leading to a substantial improvement in energy efficiency compared to the purification process with only distillation columns. The novel hybrid RD-ED process with a BC solvent reduced TIC and TAC by 51.8% and 57.4%, respectively, compared to the conventional process. The proposed hybrid process was eco-friendly, emitting 56.9% less CO2 than the conventional process. The results of this study provide a strong basis for the design and improvement of more sustainable furfural production technologies.
Author Contributions
L.C.N. and N.V.D.L. contributed equally to this work by designing the study, by performing the process simulations, and by writing the article. M.L. conceived the core research concepts and advised academically. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Priority Research Centers Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Education (2014R1A6A1031189).
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AA | acetic acid |
| BC | butyl chloride |
| CO2 | carbon dioxide |
| CSTR | continuous stirred tank reactor |
| ED | extraction/distillation |
| FA | formic acid |
| HMF | hydroxylmethylfurfural |
| LA | levulinic acid |
| NHV | net heating value |
| NREL | National Renewable Energy Laboratory |
| NRTL | non-random two-liquid |
| HOC | Hayden–O’Connell |
| RD | reactive distillation |
| R-D | reaction-distillation |
| RD-ED | reactive distillation-extraction/distillation |
| R-ED | reaction-extraction/distillation |
| TIC | total investment cost |
| TOC | total annual operating costs |
| TCE | total annual CO2 emissions |
| TAC | total annual costs |
| UNIFAC: UNIQUAC | Functional-group Activity Coefficients |
| VLE | vapor-liquid equilibrium |
Appendix A

Table A1.
Utility cost data [19].
Table A1.
Utility cost data [19].
| Utility | Price ($/GJ) |
|---|---|
| Cooling water | 0.35 |
| Low-pressure steam | 13.28 |
| Medium-pressure steam | 14.19 |
| High-pressure steam | 17.70 |
References
- Werpy, T.; Petersen, G. Top. Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2004. [Google Scholar]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2000; ISBN 9783527306732. [Google Scholar]
- Nhien, L.C.; Long, N.V.D.; Kim, S.; Lee, M. Design and optimization of intensified biorefinery process for furfural production through a systematic procedure. Biochem. Eng. J. 2016, 116, 166–175. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The Chemistry and Technology of Furfural and Its Many By-Products; Elsevier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- De Jong, W.; Marcotullio, G. Overview of Biorefineries based on Co-Production of Furfural, Existing Concepts and Novel Developments. Int. J. Chem. React. Eng. 2010, 8. [Google Scholar] [CrossRef]
- Metkar, P.S.; Till, E.J.; Corbin, D.R.; Pereira, C.J.; Hutchenson, K.W.; Sengupta, S.K. Reactive distillation process for the production of furfural using solid acid catalysts. Green Chem. 2015, 17, 1453–1466. [Google Scholar] [CrossRef]
- Malone, M.F.; Doherty, M.F. Reactive Distillation. Ind. Eng. Chem. Res. 2000, 39, 3953–3957. [Google Scholar] [CrossRef]
- Mandalika, A.; Runge, T. Enabling integrated biorefineries through high-yield conversion of fractionated pentosans into furfural. Green Chem. 2012, 14, 3175–3184. [Google Scholar] [CrossRef]
- Marcotullio, G. The Chemistry and Technology of Furfural Production in Modern Lignocellulose-Feedstock Biorefineries. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 2011. [Google Scholar]
- de Jong, W.; Marcotullio, G. Process for the Production of Furfural from Pentoses. U.S. Patent 12/944,403, 3 May 2012. [Google Scholar]
- Nhien, L.C.; Long, N.V.D.; Kim, S.; Lee, M. Techno-economic assessment of hybrid extraction and distillation processes for furfural production from lignocellulosic biomass. Biotechnol. Biofuels 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process. Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Laboratory: Golden, CO, USA, 2011. [Google Scholar]
- Aspentech. Aspen Physical Property System—Physical Property Methods V7.3; Aspen Technology, Inc.: Bedford, MA, USA, 2011. [Google Scholar]
- Carrasco, F.; Roy, C. Kinetic study of dilute-acid prehydrolysis of xylan-containing biomass. Wood Sci. Technol. 1992, 26, 189–208. [Google Scholar] [CrossRef]
- Bhandari, N.; Macdonald, D.G.; Bakhshi, N.N. Kinetic studies of corn stover saccharification using sulphuric acid. Biotechnol. Bioeng. 1984. [Google Scholar] [CrossRef] [PubMed]
- CHANG, C.; MA, X.; CEN, P. Kinetics of Levulinic Acid Formation from Glucose Decomposition at High Temperature. Chinese J. Chem. Eng. 2006, 14, 708–712. [Google Scholar] [CrossRef]
- Smith, R. Chemical Process. Design and Integration; Wiley: West Sussex, UK, 2016. [Google Scholar]
- Biegler, L.T.; Grossmann, I.E.; Westerberg, A.W. Systematic Methods of Chemical Process. Design; Prentice Hall Inc.: Uppler Saddle River, NJ, USA, 1997. [Google Scholar]
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.A.; Bhattacharyya, D. Analysis, Synthesis, and Design of Chemical Processes, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2016. [Google Scholar]
- Gadalla, M.A.; Olujic, Z.; Jansens, P.J.; Jobson, M.; Smith, R. Reducing CO2 emissions and energy consumption of heat-integrated distillation systems. Environ. Sci. Technol. 2005, 39, 6860–6870. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Furfural Market Analysis by Application (Furfuryl Alcohol, Solvent) and Segment Forecasts to 2020; Grand View Research: San Francisco, CA, USA, 2015. [Google Scholar]
- Strømsnes, L.M.; Moe, S. Process Modeling of a Biorefinery for Integrated Production of Ethanol and Furfural in HYSYS. Ph.D. Thesis, Norwegian University of Science and Technology, Tronholm, Norway, 2016. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).