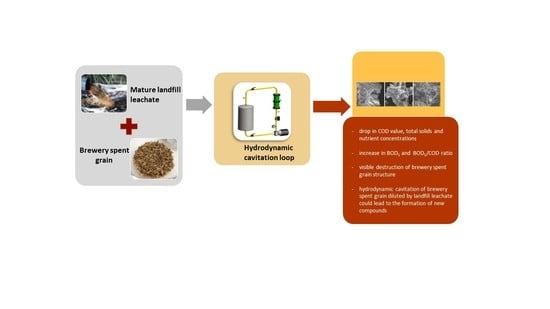
Mature Landfill Leachate as a Medium for Hydrodynamic Cavitation of Brewery Spent Grain
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
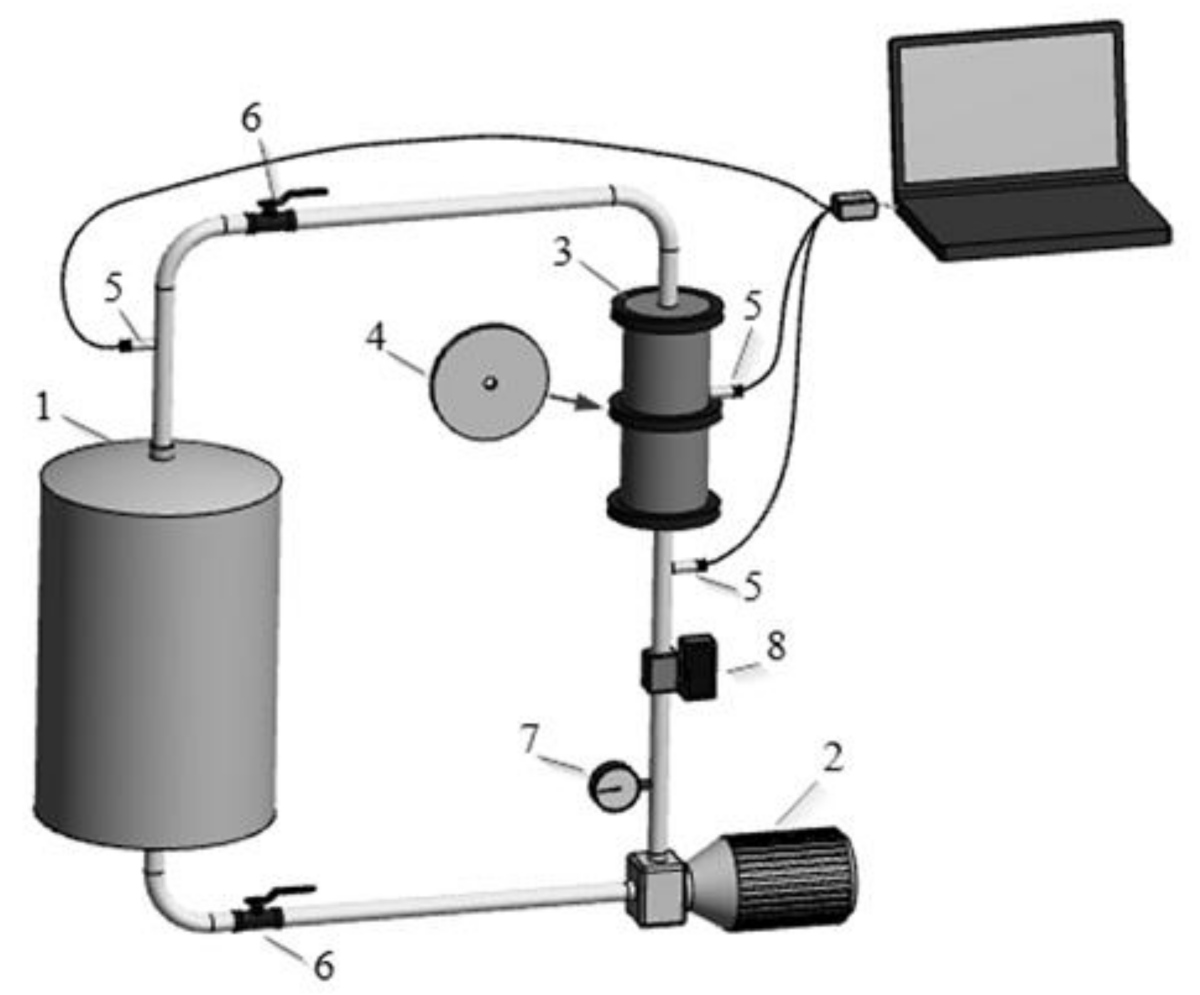
2.2. Experimental Installation and Methodology
2.3. Composition Analysis
2.3.1. Substrate Characteristics Analysis
2.3.2. SEM Analysis
2.3.3. FTIR-PAS Analysis
3. Results and Discussion
3.1. Characteristics of MLL, BSG and Their Mixture
3.2. Effects of HD on the Biodegradability of BSG and MLL Mixture
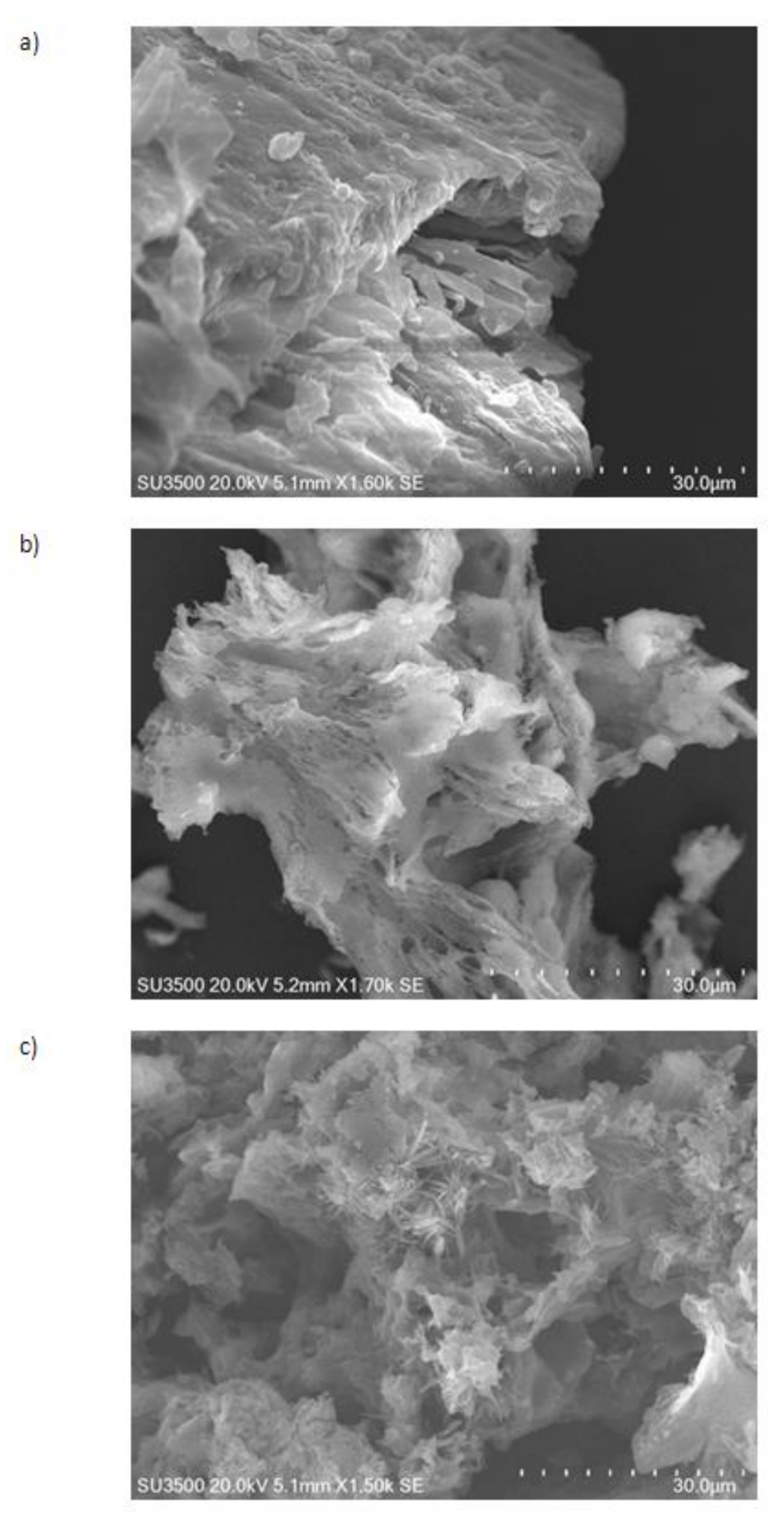
3.3. Morphological Structure of Raw and Cavitated Material
3.4. FTIR-PAS Analysis
3.5. Energy Efficiency of Hydrodynamic Cavitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fillaudeau, L.; Blanpain-Avet, P.; Daufin, G. Water, wastewater and waste management in brewing industries. J. Clean. Prod. 2006, 14, 463–471. [Google Scholar] [CrossRef]
- Rommi, K.; Niemi, P.; Kemppainen, K.; Kruus, K. Impact of thermochemical pre-treatment and carbohydrate and protein hydrolyzing enzyme treatment on fractionation of protein and lignin from brewer’s spent grain. J. Cereal Sci. 2018, 79, 168–173. [Google Scholar] [CrossRef]
- Xiros, C.; Christakopoulos, P. Biotechnological Potential of Brewers Spent Grain and its Recent Applications. Waste Biomass Valorization 2012, 3, 213–232. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, S.; Abu-Ghannam, N.; Jaiswal, A.K. A comparative analysis of pretreatment strategies on the properties and hydrolysis of brewers’ spent grain. Bioresour. Technol. 2018, 248, 272–279. [Google Scholar] [CrossRef]
- Mussatto, S.; Dragone, G.; Roberto, I. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Lu, Z.X.; Walker, K.Z.; Muir, J.G.; Mascara, T.; O’Dea, K. Arabinoxylan fiber, a byproduct of wheat flour processing, reduces the postprandial glucose response in normoglycemic subjects. Am. J. Clin. Nutr. 2000, 71, 1123–1128. [Google Scholar] [CrossRef] [PubMed]
- Stojceska, V.; Ainsworth, P. The effect of different enzymes on the quality of high-fibre enriched brewer’s spent grain breads. Food Chem. 2008, 110, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Stojceska, V.; Ainsworth, P.; Plunkett, A.; Ibanogˇlu, S. The recycling of brewer’s processing by-product into ready-to-eat snacks using extrusion technology. J. Cereal Sci. 2008, 47, 469–479. [Google Scholar] [CrossRef]
- Ktenioudaki, A.; Crofton, E.C.; Scannell, A.G.; Hannon, J.A.; Kilcawley, K.N.; Gallagher, E. Sensory properties and aromatic composition of baked snacks containing brewer’s spent grain. J. Cereal Sci. 2013, 57, 384–390. [Google Scholar] [CrossRef]
- Li, Q.; Chai, L.; Wang, Q.; Yang, Z.; Yan, H.; Wang, Y. Fast esterification of spent grain for enhanced heavy metal ions adsorption. Bioresour. Technol. 2010, 101, 3796–3799. [Google Scholar] [CrossRef]
- Li, Q.; Chai, L.; Qin, W. Cadmium (II) adsorption on esterified spent grain: Equilibrium modeling and possible mechanisms. Chem. Eng. J. 2012, 197, 173–180. [Google Scholar] [CrossRef]
- Chai, L.; Li, Q.; Zhu, Y.; Zhang, Z.; Wang, Q.; Wang, Y.; Yang, Z. Synthesis of thiol-functionalized spent grain as a novel adsorbent for divalent metal ions. Bioresour. Technol. 2010, 101, 6269–6272. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xiong, C. Adsorptive removal of As(III) ions from water using spent grain modified by polyacrylamide. J. Environ. Sci. 2016, 45, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Manyuchi, M.; Frank, R.; Mbohwa, C.; Muzenda, E. Potential to Use Sorghum Brewers Spent Grains as a Boiler Fuel. BioResources. 2017, 12, 7228–7724. [Google Scholar]
- Akunna, J. Anaerobic treatment of brewery wastes. In Brewing Microbiology; Woodhead Publishing: Cambridge, England, 2015; pp. 407–424. [Google Scholar]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: Challenges and opportunities. Bioresour. Technol. 2016, 199, 92–102. [Google Scholar] [CrossRef]
- Zieliński, M.; Dębowski, M.; Kisielewska, M.; Nowicka, A.; Rokicka, M.; Szwarc, K. Cavitation-based pretreatment strategies to enhance biogas production in a small-scale agricultural biogas plant. Energy Sustain. Dev. 2019, 49, 21–26. [Google Scholar] [CrossRef]
- Dular, M.; Griessler-Bulc, T.; Gutierrez-Aguirre, I.; Heath, E.; Kosjek, T.; Klemenčič, A.K.; Oder, M.; Petkovšek, M.; Rački, N.; Ravnikar, M.; et al. Use of hydrodynamic cavitation in (waste)water treatment. Ultrason. Sonochem. 2016, 29, 577–588. [Google Scholar] [CrossRef]
- Szulżyk-Cieplak, J. Removal of Hardly Bio-Degradabale Organic Compounds from Wastewater By Means of Reagentless Methods. J. Ecol. Eng. 2017, 18, 63–71. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation—A review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Thanekar, P.; Gogate, P. Application of Hydrodynamic Cavitation Reactors for Treatment of Wastewater Containing Organic Pollutants: Intensification Using Hybrid Approaches. Fluids 2018, 3, 98. [Google Scholar] [CrossRef]
- Jawale, R.H.; Gogate, P.R. Novel approaches based on hydrodynamic cavitation for treatment of wastewater containing potassium thiocyanate. Ultrason. Sonochem. 2019, 52, 214–223. [Google Scholar] [CrossRef] [PubMed]
- Maršálek, B.; Zezulka, Š.; Maršálková, E.; Pochylý, F.; Rudolf, P. Synergistic effects of trace concentrations of hydrogen peroxide used in a novel hydrodynamic cavitation device allows for selective removal of cyanobacteria. Chem. Eng. J. 2020, 382, 122383. [Google Scholar] [CrossRef]
- Patil, P.N.; Gogate, P.R.; Csoka, L.; Dregelyi-Kiss, A.; Horvath, M. Intensification of biogas production using pretreatment based on hydrodynamic cavitation. Ultrason. Sonochem. 2016, 30, 79–86. [Google Scholar] [CrossRef]
- Garuti, M.; Langone, M.; Fabbri, C.; Piccinini, S. Monitoring of full-scale hydrodynamic cavitation pretreatment in agricultural biogas plant. Bioresour. Technol. 2018, 247, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Han, J.-I. Simultaneous treatment (cell disruption and lipid extraction) of wet microalgae using hydrodynamic cavitation for enhancing the lipid yield. Bioresour. Technol. 2015, 186, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Mevada, J.; Devi, S.; Pandit, A. Large scale microbial cell disruption using hydrodynamic cavitation: Energy saving options. Biochem. Eng. J. 2019, 143, 151–160. [Google Scholar] [CrossRef]
- Hilares, R.T.; Dionízio, R.; Prado, C.; Ahmed, M.; Da Silva, S.; Santos, J.; Medeiros, R. Pretreatment of sugarcane bagasse using hydrodynamic cavitation technology: Semi-continuous and continuous process. Bioresour. Technol. 2019, 290, 121777. [Google Scholar] [CrossRef] [PubMed]
- Shankar, V.; Lundberg, A.; Frenander, K.; Ingelsten, S.; Landström, L.; Pamidi, T.; Johansson, Ö. Flow Induced Venturi Cavitation to Improve Energy Efficiency in Pulp Production. J. Fluid Flow Heat Mass Transf. 2018, 5, 10–17. [Google Scholar] [CrossRef]
- Madison, M.J.; Coward-Kelly, G.; Liang, C.; Karim, M.N.; Falls, M.; Holtzapple, M.T. Mechanical pretreatment of biomass—Part I: Acoustic and hydrodynamic cavitation. Biomass Bioenergy 2017, 98, 135–141. [Google Scholar] [CrossRef]
- Yi, C.; Lu, Q.; Wang, Y.; Wang, Y.; Yang, B. Degradation of organic wastewater by hydrodynamic cavitation combined with acoustic cavitation. Ultrason. Sonochem. 2018, 43, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Niemi, P.; Faulds, C.B.; Sibakov, J.; Holopainen, U.; Poutanen, K.; Buchert, J. Effect of a milling pre-treatment on the enzymatic hydrolysis of carbohydrates in brewer’s spent grain. Bioresour. Technol. 2012, 116, 155–160. [Google Scholar] [CrossRef]
- Montusiewicz, A.; Pasieczna-Patkowska, S.; Lebiocka, M.; Szaja, A.; Szymańska-Chargot, M. Hydrodynamic cavitation of brewery spent grain diluted by wastewater. Chem. Eng. J. 2017, 313, 946–956. [Google Scholar] [CrossRef]
- Šarc, A.; Stepišnik-Perdih, T.; Petkovšek, M.; Dular, M. The issue of cavitation number value in studies of water treatment by hydrodynamic cavitation. Ultrason. Sonochem. 2017, 34, 51–59. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; APHA: Washington, DC, USA, 2012. [Google Scholar]
- Soest, P.J.V. Use of Detergents in the Analysis of Fibrous Feeds. II. A Rapid Method for the Determination of Fiber and Lignin. J. AOAC Int. 1963, 46, 829–835. [Google Scholar] [CrossRef]
- Chylińska, M.; Szymańska-Chargot, M.; Kruk, B.; Zdunek, A. Study on dietary fibre by Fourier transform-infrared spectroscopy and chemometric methods. Food Chem. 2016, 196, 114–122. [Google Scholar] [CrossRef]
- Tripathy, B.K.; Ramesh, G.; Debnath, A.; Kumar, M. Mature landfill leachate treatment using sonolytic-persulfate/hydrogen peroxide oxidation: Optimization of process parameters. Ultrason. Sonochem. 2019, 54, 210–219. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, A.; Jiang, G.; Li, Q. Transformation and degradation mechanism of landfill leachates in a combined process of SAARB and ozonation. Waste Manag. 2019, 85, 283–294. [Google Scholar] [CrossRef]
- Reinhart, D.R.; Townsend, T.G. Landfill Bioreactor Design and Operation; CRC Press: London, UK, 2018. [Google Scholar]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Cai, J.; Huai, X.; Liu, B.; Guo, Z. Application of Hydrodynamic Cavitation to Wastewater Treatment. Chem. Eng. Technol. 2016, 39, 1363–1376. [Google Scholar] [CrossRef]
- Simpson, A.; Ranade, V.V. Modelling of hydrodynamic cavitation with orifice: Influence of different orifice designs. Chem. Eng. Res. Des. 2018, 136, 698–711. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Ozonek, J.; Pasieczna-Patkowska, S. Application of hydrodynamic cavitation to improve the biodegradability of mature landfill leachate. Ultrason. Sonochem. 2015, 26, 378–387. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y. Degradation of alachlor in aqueous solution by using hydrodynamic cavitation. J. Hazard. Mater. 2009, 161, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Panjičko, M.; Zupančič, G.D.; Fanedl, L.; Logar, R.M.; Tišma, M.; Zelić, B. Biogas production from brewery spent grain as a mono-substrate in a two-stage process composed of solid-state anaerobic digestion and granular biomass reactors. J. Clean. Prod. 2017, 166, 519–529. [Google Scholar] [CrossRef]
- Ozonek, J. Application of Hydrodynamic Cavitation in Environmental Engineering; CRC Press: London, UK, 2012. [Google Scholar]
- Petkovic, S.; Adnadjevic, B.; Jovanovic, J. A novel advanced technology for removal of phenol from wastewaters in a Ventury reactor. Therm. Sci. 2019, 23, 1935–1942. [Google Scholar] [CrossRef]
- Zieliński, M.; Rusanowska, P.; Krzywik, A.; Dudek, M.; Nowicka, A.; Dębowski, M. Application of Hydrodynamic Cavitation for Improving Methane Fermentation of Sida hermaphrodita Silage. Energies 2019, 12, 526. [Google Scholar] [CrossRef]
- Hilares, R.T.; Dos Santos, J.C.; Ahmed, M.A.; Jeon, S.H.; Da Silva, S.S.; Han, J.-I. Hydrodynamic cavitation-assisted alkaline pretreatment as a new approach for sugarcane bagasse biorefineries. Bioresour. Technol. 2016, 214, 609–614. [Google Scholar] [CrossRef]
- Hilares, R.T.; De Almeida, G.F.; Ahmed, M.A.; Antunes, F.A.; Da Silva, S.S.; Han, J.-I.; Dos Santos, J.C. Hydrodynamic cavitation as an efficient pretreatment method for lignocellulosic biomass: A parametric study. Bioresour. Technol. 2017, 235, 301–308. [Google Scholar] [CrossRef]
- Thangavelu, K.; Desikan, R.; Taran, O.P.; Uthandi, S. Delignification of corncob via combined hydrodynamic cavitation and enzymatic pretreatment: Process optimization by response surface methodology. Biotechnol. Biofuels 2018, 11, 1–13. [Google Scholar] [CrossRef]
- Kim, I.; Lee, I.; Jeon, S.H.; Hwang, T.; Han, J.-I. Hydrodynamic cavitation as a novel pretreatment approach for bioethanol production from reed. Bioresour. Technol. 2015, 192, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzym. Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Reis, S.F.; Coelho, E.; Coimbra, M.A.; Abu-Ghannam, N. Influence of grain particle sizes on the structure of arabinoxylans from brewer’s spent grain. Carbohydr. Polym. 2015, 130, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies. Tables and Charts; John Wiley and Sons, Ltd.: Chichester, UK, 2001. [Google Scholar]
- Alu’Datt, M.H.; Gammoh, S.; Rababah, T.; Almomani, M.; Alhamad, M.N.; Ereifej, K.; Almajwal, A.; Tahat, A.; Hussein, N.M.; Nasser, S.A. Preparation, characterization, nanostructures and bio functional analysis of sonicated protein co-precipitates from brewers’ spent grain and soybean flour. Food Chem. 2018, 240, 784–798. [Google Scholar] [CrossRef] [PubMed]
- Mishra, K.P.; Gogate, P.R. Intensification of degradation of Rhodamine B using hydrodynamic cavitation in the presence of additives. Sep. Purif. Technol. 2010, 75, 385–391. [Google Scholar] [CrossRef]
- Saharan, V.K.; Rizwani, M.A.; Malani, A.A.; Pandit, A.B. Effect of geometry of hydrodynamically cavitating device on degradation of orange-G. Ultrason. Sonochem. 2013, 20, 345–353. [Google Scholar] [CrossRef]

| Parameter | Unit | MLL before Cavitation | MLL after Cavitation |
|---|---|---|---|
| Total chemical oxygen demand (COD) | mg L−1 | 6140 ± 83 | 5470 ± 79 |
| Soluble chemical oxygen demand (SCOD) | mg L−1 | 4771 ± 37 | 4948 ± 32 |
| Dissolved organic carbon (DOC) | mg L−1 | 1486 ± 79 | 1555 ± 69 |
| Biochemical oxygen demand (BOD5) | mg L−1 | 249 ± 15 | 306 ± 16 |
| BOD5/COD | - | 0.041 ± 0.002 | 0.056 ± 0.002 |
| Total solids (TS) | g kg−1 | 13.5 ± 0.2 | 13.0 ± 0.1 |
| Volatile solids (VS) | g kg−1 | 3.7 ± 0.05 | 4.1 ± 0.03 |
| Alkalinity | mg L−1 | 13,052 ± 327 | 12,324 ± 308 |
| pH | - | 8.11 ± 0.05 | 8.60 ± 0.02 |
| Volatile fatty acids (VFA) | mg L−1 | 1178 ± 38 | 1163 ± 40 |
| Total nitrogen (TN) | mg L−1 | 3705 ± 128 | 3176 ± 106 |
| Ammonium nitrogen (NH4+-N) | mg L−1 | 1707 ± 389 | 1896 ± 302 |
| Total phosphorus (TP) | mg L−1 | 21.6 ± 13.7 | 21.1 ± 10.5 |
| Ortho-phosphate phosphorus (PO43−-P) | mg L−1 | 19.0 ± 2.8 | 18.4 ± 2.1 |
| Parameter | Unit | Average Value ± Standard Deviation |
|---|---|---|
| COD | g kg−1 | 307.9 ± 9.2 |
| TS | g kg−1 | 223.9 ± 4.3 |
| VS | g kg−1 | 217.2 ± 4.2 |
| Total carbon (TC) a | g kg−1 | 44.0 ± 2.2 |
| Inorganic carbon (IC) | % dry weight | nd. |
| Total Kjeldahl nitrogen (TKN) | % dry weight | 3.25 ± 0.07 |
| Cellulose (C) | % dry weight | 14.07 ± 0.49 |
| Hemicellulose (HC) | % dry weight | 41.07 ± 0.50 |
| Lignin (LG) | % dry weight | 5.13 ± 0.21 |
| Pectic polysaccharides, phenolic compounds, proteins, and monosaccharides analyzed totally (PPPM) | % dry weight | 39.73 ± 1.14 |
| Monosaccharides (MS) | g kg−1 TS | 47.54 ± 2.66 |
| Proteins (P) | % dry weight | 20.3 ± 0.42 |
| Phenolic compounds (PPh) | g kg−1 TS | 0.23 ± 0.03 |
| Parameter | Unit | Mixture of BSG and MLL before Cavitation | Mixture of BSG and MLL after Cavitation |
|---|---|---|---|
| COD | mg L−1 | 13,600 ± 420 | 12,000 ± 307 |
| SCOD | mg L−1 | 6058 ± 102 | 6139 ± 111 |
| DOC | mg L−1 | 1928 ± 309 | 2527 ± 343 |
| BOD5 | mg L−1 | 222 ± 17 | 321 ± 13 |
| BOD5/COD | - | 0.016 ± 0.002 | 0.027 ± 0.004 |
| TS | g kg−1 | 17.3 ± 0.3 | 16.7 ± 0.2 |
| VS | g kg−1 | 8.0 ± 0.07 | 8.2 ± 0.06 |
| Alkalinity | mg L−1 | 12,395 ± 345 | 13,950 ± 320 |
| pH | - | 8.26 ± 0.12 | 8.49 ± 0.07 |
| VFA | mg L−1 | 1811 ± 211 | 1853 ± 261 |
| TN | mg L−1 | 3573 ± 143 | 3100 ± 131 |
| NH4+-N | mg L−1 | 1677 ± 394 | 2730 ± 388 |
| TP | mg L−1 | 24.6 ± 15.3 | 24.5 ± 15.4 |
| PO43−-P | mg L−1 | 18.9 ± 2.9 | 19.7 ± 2.7 |
| References | Aim of Work | Substrate | Process Parameters | Resluts |
|---|---|---|---|---|
| Zieliński et al. [51] | Biogas yield improvement | Sida hermaphrodita silage + cattle manure | Reactor type—rotor disintegrator, electric motor with a power of 4 KW at 2800 rpm | No change in the chemical composition of the lignocellulose biomass after cavitation was observed |
| Hilares et al. [52] | Lignin reduction improvement | Sugarcane bagasse | Reactor type—orifice, inlet pressure—3 bar, temperature—70 °C, catalyst—0.3 M NaOH | 51.52% of lignin removal |
| Hilares et al. [53] | Lignin reduction improvement | Sugarcane bagasse | Reactor type—orifice, inlet pressure—3 bar, temperature—64 °C, catalyst—0.48 M NaOH | 60.4% of lignin removal |
| Montusiewicz et al. [35] | Improvement of biodegradation index | BSG + primary treated municipal wastewater | Reactor type—orifice, inlet pressure—7 bar, temperature—20°C | BSG biodegradability index increased from 0.074 to 0.091 |
| Thangavelu et al. [54] | Lignin reduction improvement | Corncob | Reactor type—orifice, inlet pressure—0.5 bar, temperature—30 °C, catalyst—Laccase enzyme: 6.5 U g−1 of biomass | 47.44% of lignin removal |
| Kim et al. [55] | Lignin reduction improvement | Reed | Reactor type—orifice, inlet pressure—5 bar, temperature—77 °C, catalyst—3.0% NaOH | 35%–42% of lignin removal |
| Present study | Improvement of biodegradation index | BSG + MLL | Reactor type—orifice, inlet pressure—7 bar, temperature—20 °C | Improvement in both the BOD5 value and BOD5/COD ratio (i.e., 45% and 69%, respectively |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebiocka, M.; Montusiewicz, A.; Pasieczna-Patkowska, S.; Gułkowski, S. Mature Landfill Leachate as a Medium for Hydrodynamic Cavitation of Brewery Spent Grain. Energies 2021, 14, 1150. https://doi.org/10.3390/en14041150
Lebiocka M, Montusiewicz A, Pasieczna-Patkowska S, Gułkowski S. Mature Landfill Leachate as a Medium for Hydrodynamic Cavitation of Brewery Spent Grain. Energies. 2021; 14(4):1150. https://doi.org/10.3390/en14041150
Chicago/Turabian StyleLebiocka, Magdalena, Agnieszka Montusiewicz, Sylwia Pasieczna-Patkowska, and Sławomir Gułkowski. 2021. "Mature Landfill Leachate as a Medium for Hydrodynamic Cavitation of Brewery Spent Grain" Energies 14, no. 4: 1150. https://doi.org/10.3390/en14041150
APA StyleLebiocka, M., Montusiewicz, A., Pasieczna-Patkowska, S., & Gułkowski, S. (2021). Mature Landfill Leachate as a Medium for Hydrodynamic Cavitation of Brewery Spent Grain. Energies, 14(4), 1150. https://doi.org/10.3390/en14041150