State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production
Abstract
1. Introduction
1.1. Biofuels
1.2. Transesterification of Triglycerides
1.3. Catalysts
2. Alkaline Earth Metal Catalysts
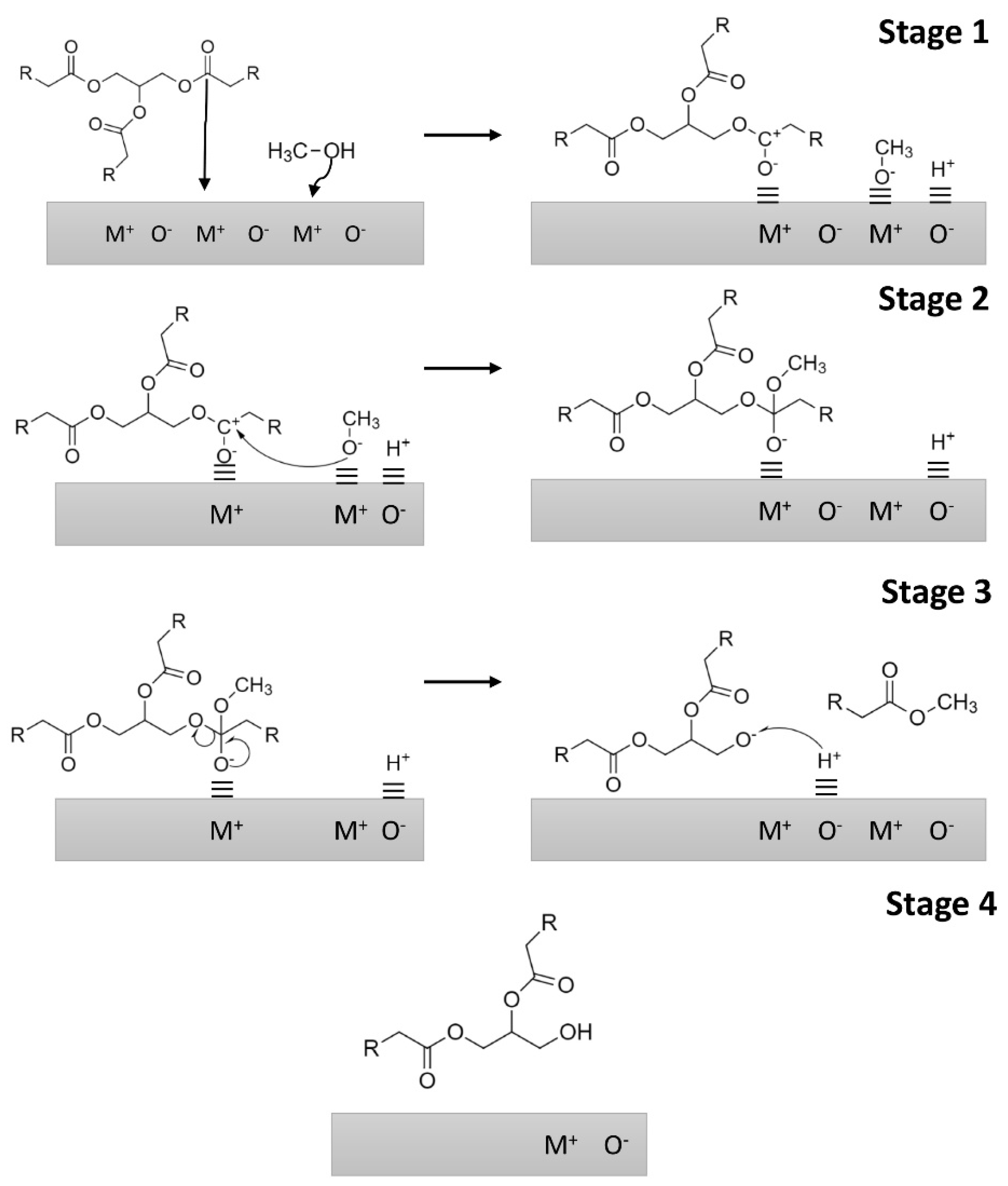
2.1. MgO
2.2. CaO
2.3. BaO and SrO
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Al Rashdan, M.; Al Zubi, M.; Al Okour, M. Effect of Using New Technology Vehicles on the World’s Environment and Petroleum Resources. J. Ecol. Eng. 2019, 20, 16–24. [Google Scholar] [CrossRef]
- Oil Prices. Available online: www.oilprice.com (accessed on 19 April 2020).
- Tans, P.; Keeling, R. Trends in Atmospheric Carbon Dioxide. Available online: http://www.esrl.noaa.gov/gmd/ccgg/trends/mlo.html#mlo_full (accessed on 7 January 2012).
- Ballesteros, M.; Manzanares, P. Liquid Biofuels. In The Role of Bioenergy in the Bioeconomy; Elago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Amsterdam, The Netherlands, 2019; pp. 113–144. [Google Scholar]
- Oh, Y.-K.; Hwang, K.-R.; Kim, C.; Kim, J.R.; Lee, J.-S. Recent developments and key barriers to advanced biofuels: A short review. Bioresour. Technol. 2018, 257, 320–333. [Google Scholar] [CrossRef] [PubMed]
- IEA. Global Energy & CO2 Status Report. Available online: https://www.iea.org/geco/emissions/ (accessed on 19 April 2020).
- Höök, M.; Tang, X. Depletion of fossil fuels and anthropogenic climate change—A review. Energy Policy 2013, 52, 797–809. [Google Scholar] [CrossRef]
- Manaf, I.S.A.; Embong, N.H.; Khazaai, S.N.M.; Rahim, M.H.A.; Yusoff, M.M.; Lee, K.T.; Maniam, G.P. A review for key challenges of the development of biodiesel industry. Energy Convers. Manag. 2019, 185, 508–517. [Google Scholar] [CrossRef]
- Paris Agreement, U.N. The Paris Agreement. Available online: https://unfccc.int/process-and-meetings/the-paris-agreement/the-paris-agreement (accessed on 19 April 2019).
- Cespi, D.; Esposito, I.; Cucciniello, R.; Anastas, P.T. Beyond the beaker: Benign by design society. Curr. Res. Green Sustain. Chem. 2020, 3, 100028. [Google Scholar] [CrossRef]
- Sunde, K.; Brekke, A.; Solberg, B. Environmental impacts and costs of woody Biomass-to-Liquid (BTL) production and use—A review. For. Policy Econ. 2011, 13, 591–602. [Google Scholar] [CrossRef]
- Chanthawong, A.; Dhakal, S. Liquid Biofuels Development in Southeast Asian Countries: An Analysis of Market, Policies and Challenges. Waste Biomass-Valorization 2015, 7, 157–173. [Google Scholar] [CrossRef]
- Efthymiopoulos, I.; Hellier, P.; Ladommatos, N.; Kay, A.; Mills-Lamptey, B. Effect of Solvent Extraction Parameters on the Recovery of Oil From Spent Coffee Grounds for Biofuel Production. Waste Biomass-Valorization 2019, 10, 253–264. [Google Scholar] [CrossRef]
- Correa, D.F.; Beyer, H.L.; Possingham, H.P.; Thomas-Hall, S.R.; Schenk, P.M. Biodiversity impacts of bioenergy production: Microalgae vs. first generation biofuels. Renew. Sustain. Energy Rev. 2017, 74, 1131–1146. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- Hill, J.; Nelson, E.; Tilman, D.; Polasky, S.; Tiffany, D.G. Environmental, economic, and energetic costs and benefits of biodiesel and ethanol biofuels. Proc. Natl. Acad. Sci. USA 2006, 103, 11206–11210. [Google Scholar] [CrossRef]
- Van Gerpen, J. Biodiesel processing and production. Fuel Process. Technol. 2005, 86, 1097–1107. [Google Scholar] [CrossRef]
- Parada, M.P.; Osseweijer, P.; Duque, J.A.P. Sustainable biorefineries, an analysis of practices for incorporating sustainability in biorefinery design. Ind. Crop. Prod. 2017, 106, 105–123. [Google Scholar] [CrossRef]
- Abdullah, B.; Muhammad, S.A.F.S.; Shokravi, Z.; Ismail, S.; Kassim, K.A.; Mahmood, A.N.; Aziz, M.A. Fourth generation biofuel: A review on risks and mitigation strategies. Renew. Sustain. Energy Rev. 2019, 107, 37–50. [Google Scholar] [CrossRef]
- Amini, Z.; Ilham, Z.; Ong, H.C.; Mazaheri, H.; Chen, W.-H. State of the art and prospective of lipase-catalyzed transesterification reaction for biodiesel production. Energy Convers. Manag. 2017, 141, 339–353. [Google Scholar] [CrossRef]
- Ngoie, W.I.; Oyekola, O.O.; Ikhu-Omoregbe, D.; Welz, P.J. Valorisation of Edible Oil Wastewater Sludge: Bioethanol and Biodiesel Production. Waste Biomass-Valorization 2019, 11, 2431–2440. [Google Scholar] [CrossRef]
- Tajuddin, N.; Lee, A.; Wilson, K. Production of biodiesel via catalytic upgrading and refining of sustainable oleagineous feedstocks. In Handbook of Biodiesel Production, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing Series in Energy: Cambridge, UK, 2016; pp. 121–164. [Google Scholar]
- García, I. Feedstocks and challenges to biofuel development. In Handbook of Biodiesel Production, 2nd ed.; Luque, R., Lin, C.S.K., Wilson, K., Clark, J., Eds.; Woodhead Publishing Series in Energy: Cambridge, UK, 2016; pp. 85–118. [Google Scholar]
- Le Anh, T.; Reksowardojo, I.; Wattanavichien, K. Utilization of biofuels in diesel engines. In Handbook of Biodiesel Production, 1st ed.; Luque, R., Campelo, J., Clark, J., Eds.; Woodhead Publishing: Cambridge, UK, 2011; pp. 611–646. [Google Scholar]
- Marchetti, J.; Miguel, V.; Errazu, A. Possible methods for biodiesel production. Renew. Sustain. Energy Rev. 2007, 11, 1300–1311. [Google Scholar] [CrossRef]
- Bateni, H.; Bateni, F.; Karimi, K. Effects of Oil Extraction on Ethanol and Biogas Production from Eruca sativa Seed Cake. Waste Biomass-Valorization 2016, 8, 1897–1905. [Google Scholar] [CrossRef]
- Demirbas, M.F. Biofuels from algae for sustainable development. Appl. Energy 2011, 88, 3473–3480. [Google Scholar] [CrossRef]
- Ajanovic, A. Biofuels versus food production: Does biofuels production increase food prices? Energy 2011, 36, 2070–2076. [Google Scholar] [CrossRef]
- Garlapati, V.K.; Tewari, S.; Ganguly, R. Life Cycle Assessment of First-, Second-Generation, and Microalgae Biofuels. In Advances in Feedstock Conversion Technologies for Alternative Fuels and Bioproducts, 1st ed.; Hosseini, M., Ed.; Woodhead Publishing Series in Energy: Cambridge, UK, 2019; pp. 355–371. [Google Scholar]
- Demirbas, A. Biodiesel production from vegetable oils via catalytic and non-catalytic supercritical methanol transesterification methods. Prog. Energy Combust. Sci. 2005, 31, 466–487. [Google Scholar] [CrossRef]
- Borges, M.; Díaz, L. Recent developments on heterogeneous catalysts for biodiesel production by oil esterification and transesterification reactions: A review. Renew. Sustain. Energy Rev. 2012, 16, 2839–2849. [Google Scholar] [CrossRef]
- Khan, T.M.Y.; Atabani, A.E.; Irfananjumbadruddin, I.; Badarudin, A.; Khayoon, M.S.; Triwahyono, S. Recent scenario and technologies to utilize non-edible oils for biodiesel production. Renew. Sustain. Energy Rev. 2014, 37, 840–851. [Google Scholar] [CrossRef]
- Joshi, G.; Pandey, J.K.; Rana, S.; Rawat, D.S. Challenges and opportunities for the application of biofuel. Renew. Sustain. Energy Rev. 2017, 79, 850–866. [Google Scholar] [CrossRef]
- Atabani, A.; Silitonga, A.; Badruddin, I.A.; Mahlia, T.; Masjuki, H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Marwaha, A.; Dhir, A.; Mahla, S.K.; Mohapatra, S.K. An overview of solid base heterogeneous catalysts for biodiesel production. Catal. Rev. 2018, 60, 594–628. [Google Scholar] [CrossRef]
- Tariq, M.; Ali, S.; Khalid, N. Activity of homogeneous and heterogeneous catalysts, spectroscopic and chromatographic characterization of biodiesel: A review. Renew. Sustain. Energy Rev. 2012, 16, 6303–6316. [Google Scholar] [CrossRef]
- Demirbas, A. Production of biodiesel fuels from linseed oil using methanol and ethanol in non-catalytic SCF conditions. Biomass-Bioenergy 2009, 33, 113–118. [Google Scholar] [CrossRef]
- Darnoko, D.; Cheryan, M. Kinetics of palm oil transesterification in a batch reactor. J. Am. Oil Chem. Soc. 2000, 77, 1263–1267. [Google Scholar] [CrossRef]
- Portha, J.-F.; Allain, F.H.T.; Coupard, V.; Dandeu, A.; Girot, E.; Schaer, E.; Falk, L. Simulation and kinetic study of transesterification of triolein to biodiesel using modular reactors. Chem. Eng. J. 2012, 207–208, 285–298. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar based solid acid catalyst for biodiesel production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Di Serio, M.; Tesser, R.; Pengmei, L.; Santacesaria, E. Heterogeneous Catalysts for Biodiesel Production. Energy Fuels 2008, 22, 207–217. [Google Scholar] [CrossRef]
- Konwar, L.J.; Boro, J.; Deka, D. Review on latest developments in biodiesel production using carbon-based catalysts. Renew. Sustain. Energy Rev. 2014, 29, 546–564. [Google Scholar] [CrossRef]
- Kulkarni, M.G.; Gopinath, R.; Meher, L.C.; Dalai, A.K. Solid acid catalyzed biodiesel production by simultaneous esterification and transesterification. Green Chem. 2006, 8, 1056–1062. [Google Scholar] [CrossRef]
- Gan, S.; Ng, H.K.; Ooi, C.W.; Motala, N.O.; Ismail, M.A.F. Ferric sulphate catalysed esterification of free fatty acids in waste cooking oil. Bioresour. Technol. 2010, 101, 7338–7343. [Google Scholar] [CrossRef]
- Meher, L.; Sagar, D.V.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Demirbaş, A. Biodiesel fuels from vegetable oils via catalytic and non-catalytic supercritical alcohol transesterifications and other methods: A survey. Energy Convers. Manag. 2003, 44, 2093–2109. [Google Scholar] [CrossRef]
- Zabeti, M.; Daud, W.M.A.W.; Aroua, M.K. Activity of solid catalysts for biodiesel production: A review. Fuel Process. Technol. 2009, 90, 770–777. [Google Scholar] [CrossRef]
- Furuta, S.; Matsuhashi, H.; Arata, K. Biodiesel fuel production with solid superacid catalysis in fixed bed reactor under atmospheric pressure. Catal. Commun. 2004, 5, 721–723. [Google Scholar] [CrossRef]
- Van Kasteren, J.H.; Nisworo, A. A process model to estimate the cost of industrial scale biodiesel production from waste cooking oil by supercritical transesterification. Resour. Conserv. Recycl. 2007, 50, 442–458. [Google Scholar] [CrossRef]
- Leung, D.Y.; Wu, X.; Leung, M. A review on biodiesel production using catalyzed transesterification. Appl. Energy 2010, 87, 1083–1095. [Google Scholar] [CrossRef]
- Helwani, Z.; Othman, M.; Aziz, N.; Kim, J.; Fernando, W. Solid heterogeneous catalysts for transesterification of triglycerides with methanol: A review. Appl. Catal. A Gen. 2009, 363, 1–10. [Google Scholar] [CrossRef]
- Ezebor, F.; Khairuddean, M.; Abdullah, A.Z.; Boey, P.L. Oil palm trunk and sugarcane bagasse derived solid acid catalysts for rapid esterification of fatty acids and moisture-assisted transesterification of oils under pseudo-infinite methanol. Bioresour. Technol. 2014, 157, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Takagaki, A.; Toda, M.; Okamura, M.; Kondo, J.N.; Hayashi, S.; Domen, K.; Hara, M. Esterification of higher fatty acids by a novel strong solid acid. Catal. Today 2006, 116, 157–161. [Google Scholar] [CrossRef]
- Su, F.; Guo, Y. Advancements in solid acid catalysts for biodiesel production. Green Chem. 2014, 16, 2934–2957. [Google Scholar] [CrossRef]
- Tavizón-Pozos, J.A.; Ibarra, I.S.; Guevara-Lara, A.; Galán-Vidal, C.A. Application of Design of Experiments in Biofuel Production: A Review. In Design of Experiments for Chemical, Pharmaceutical, Food, and Industrial Applications; IGI Global: Hershey, PA, USA, 2020; pp. 77–103. [Google Scholar]
- Veljković, V.B.; Veličković, A.V.; Avramović, J.M.; Stamenković, O.S. Modeling of biodiesel production: Performance comparison of Box–Behnken, face central composite and full factorial design. Chin. J. Chem. Eng. 2019, 27, 1690–1698. [Google Scholar] [CrossRef]
- Karmakar, B.; Dhawane, S.H.; Halder, G. Optimization of biodiesel production from castor oil by Taguchi design. J. Environ. Chem. Eng. 2018, 6, 2684–2695. [Google Scholar] [CrossRef]
- Dodo, R.; Ause, T.; Dauda, E.; Shehu, U.; Popoola, A. Multi-response optimization of transesterification parameters of mahogany seed oil using grey relational analysis in Taguchi method for quenching application. Heliyon 2019, 5, e02167. [Google Scholar] [CrossRef]
- Silitonga, A.; Shamsuddin, A.; Mahlia, T.; Milano, J.; Kusumo, F.; Siswantoro, J.; Dharma, S.; Sebayang, A.; Masjuki, H.; Ong, H.C. Biodiesel synthesis from Ceiba pentandra oil by microwave irradiation-assisted transesterification: ELM modeling and optimization. Renew. Energy 2020, 146, 1278–1291. [Google Scholar] [CrossRef]
- Kumar, S.A.; Sakthinathan, G.; Vignesh, R.; Banu, J.R.; Al-Muhtaseb, A.H. Optimized transesterification reaction for efficient biodiesel production using Indian oil sardine fish as feedstock. Fuel 2019, 253, 921–929. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M. Comparative analysis of effect of methanol and ethanol on Karanja biodiesel production and its optimisation. Fuel 2016, 180, 164–174. [Google Scholar] [CrossRef]
- Vinayaka, A.S.; Mahanty, B.; Rene, E.R.; Behera, S.K. Biodiesel production by transesterification of a mixture of pongamia and neem oils. Biofuels 2018, 1–9. [Google Scholar] [CrossRef]
- Endut, A.; Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Hamid, S.H.A.; Lananan, F.; Kamarudin, M.K.A.; Umar, R.; Juahir, H.; Khatoon, H. Optimization of biodiesel production by solid acid catalyst derived from coconut shell via response surface methodology. Int. Biodeterior. Biodegrad. 2017, 124, 250–257. [Google Scholar] [CrossRef]
- Abdullah, S.H.Y.S.; Hanapi, N.H.M.; Azid, A.; Umar, R.; Juahir, H.; Khatoon, H.; Endut, A. A review of biomass-derived heterogeneous catalyst for a sustainable biodiesel production. Renew. Sustain. Energy Rev. 2017, 70, 1040–1051. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.-S. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Dossin, T.F.; Reyniers, M.-F.; Berger, R.J.; Marin, G.B. Simulation of heterogeneously MgO-catalyzed transesterification for fine-chemical and biodiesel industrial production. Appl. Catal. B Environ. 2006, 67, 136–148. [Google Scholar] [CrossRef]
- Li, H.; Niu, S.; Lu, C.; Li, J. Calcium oxide functionalized with strontium as heterogeneous transesterification catalyst for biodiesel production. Fuel 2016, 176, 63–71. [Google Scholar] [CrossRef]
- D’Cruz, A.; Kulkarni, M.G.; Meher, L.C.; Dalai, A.K. Synthesis of Biodiesel from Canola Oil Using Heterogeneous Base Catalyst. J. Am. Oil Chem. Soc. 2007, 84, 937–943. [Google Scholar] [CrossRef]
- Chouhan, A.S.; Sarma, A. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Ling, J.S.J.; Tan, Y.H.; Mubarak, N.M.; Kansedo, J.; Saptoro, A.; Nolasco-Hipolito, C. A review of heterogeneous calcium oxide based catalyst from waste for biodiesel synthesis. SN Appl. Sci. 2019, 1, 810. [Google Scholar] [CrossRef]
- Yan, S.; Lu, H.; Liang, B. Supported CaO Catalysts Used in the Transesterification of Rapeseed Oil for the Purpose of Biodiesel Production. Energy Fuels 2008, 22, 646–651. [Google Scholar] [CrossRef]
- Chen, C.-L.; Huang, C.-C.; Tran, D.-T.; Chang, J.-S. Biodiesel synthesis via heterogeneous catalysis using modified strontium oxides as the catalysts. Bioresour. Technol. 2012, 113, 8–13. [Google Scholar] [CrossRef]
- Endalew, A.K.; Kiros, Y.; Zanzi, R. Inorganic heterogeneous catalysts for biodiesel production from vegetable oils. Biomass-Bioenergy 2011, 35, 3787–3809. [Google Scholar] [CrossRef]
- Watkins, R.S.; Lee, A.; Wilson, K. Li/CaO catalysed tri-glyceride transesterification for biodiesel applications. Green Chem. 2004, 6, 335–340. [Google Scholar] [CrossRef]
- Hattori, H.; Shima, M.; Kabashima, H. Alcoholysis of ester and epoxide catalyzed by solid bases. In Studies in Surface Science and Catalysis; Corma, A., Melo, F.V., Mendioroz, S., Fierro, J.L.G., Eds.; Elsevier BV: Granada, Spain, 2000; Volume 130, pp. 3507–3512. [Google Scholar]
- Ruppert, A.M.; Meeldijk, J.D.; Kuipers, B.W.M.; Erné, B.H.; Weckhuysen, B.M. Glycerol Etherification over Highly Active CaO-Based Materials: New Mechanistic Aspects and Related Colloidal Particle Formation. Chem. Eur. J. 2008, 14, 2016–2024. [Google Scholar] [CrossRef]
- Patil, P.D.; Deng, S. Transesterification of Camelina Sativa Oil Using Heterogeneous Metal Oxide Catalysts. Energy Fuels 2009, 23, 4619–4624. [Google Scholar] [CrossRef]
- Gryglewicz, S. Rapeseed oil methyl esters preparation using heterogeneous catalysts. Bioresour. Technol. 1999, 70, 249–253. [Google Scholar] [CrossRef]
- Hattori, H. Heterogeneous Basic Catalysis. Chem. Rev. 1995, 95, 537–558. [Google Scholar] [CrossRef]
- Montero, J.M.; Gai, P.; Wilson, K.; Lee, A. Structure-sensitive biodiesel synthesis over MgO nanocrystals. Green Chem. 2009, 11, 265–268. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, D.J.; Kim, S.J.; Kim, J.H. Synthesis of mesoporous MgO catalyst templated by a PDMS–PEO comb-like copolymer for biodiesel production. Fuel Process. Technol. 2013, 116, 325–331. [Google Scholar] [CrossRef]
- Dossin, T.; Reyniers, M.; Marin, G. Kinetics of heterogeneously MgO-catalyzed transesterification. Appl. Catal. B Environ. 2006, 62, 35–45. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from Vegetable Oils with MgO Catalytic Transesterification in Supercritical Methanol. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 1645–1651. [Google Scholar] [CrossRef]
- Ilgen, O.; Akin, A.N. Transesterification of Canola Oil to Biodiesel Using MgO Loaded with KOH as a Heterogeneous Catalyst. Energy Fuels 2009, 23, 1786–1789. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, K.; Chen, L.; Kübel, C.; Richards, R.; Kuebel, C. MgO(111) Nanosheets with Unusual Surface Activity. J. Phys. Chem. C 2007, 111, 12038–12044. [Google Scholar] [CrossRef]
- Klabunde, K.J.; Erickson, L.E.; Koper, O.; Richards, R.M. Review of Nanoscale Materials in Chemistry: Environmental Applications. In ACS Symposium Series; Erickson, L., Koodali, R., Richards, R.M., Eds.; American Chemical Society (ACS): Washington, DC, USA, 2010; Volume 1045, pp. 1–13. [Google Scholar]
- Li, W.-C.; Lu, A.-H.; Weidenthaler, A.C.; Schüth, F. Hard-Templating Pathway to Create Mesoporous Magnesium Oxide. Chem. Mater. 2004, 16, 5676–5681. [Google Scholar] [CrossRef]
- Richards, R.; Li, W.; Decker, S.; Davidson, C.; Koper, O.; Zaikovski, V.; Volodin, A.; Rieker, T.; Klabunde, K.J. Consolidation of Metal Oxide Nanocrystals. Reactive Pellets with Controllable Pore Structure That Represent a New Family of Porous, Inorganic Materials. J. Am. Chem. Soc. 2000, 122, 4921–4925. [Google Scholar] [CrossRef]
- Verziu, M.; Cojocaru, B.; Hu, J.; Richards, R.; Ciuculescu, C.; Filip, P.I.; Pârvulescu, V.I. Sunflower and rapeseed oil transesterification to biodiesel over different nanocrystalline MgO catalysts. Green Chem. 2008, 10, 373–381. [Google Scholar] [CrossRef]
- Rasouli, H.; Esmaeili, H. Characterization of MgO nanocatalyst to produce biodiesel from goat fat using transesterification process. 3 Biotech 2019, 9, 429. [Google Scholar] [CrossRef] [PubMed]
- Amirthavalli, V.; Warrier, A.R. Production of biodiesel from waste cooking oil using MgO nanocatalyst. In Dae Solid State Physics Symposium 2018; Biswas, A., Veerendra, K., Eds.; AIP Publishing Conference Proceedings: Hisar, Haryana, India, 2019; Volume 2115. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B.; Korstad, J. Latest developments on application of heterogenous basic catalysts for an efficient and eco friendly synthesis of biodiesel: A review. Fuel 2011, 90, 1309–1324. [Google Scholar] [CrossRef]
- Ngamcharussrivichai, C.; Nunthasanti, P.; Tanachai, S.; Bunyakiat, K. Biodiesel production through transesterification over natural calciums. Fuel Process. Technol. 2010, 91, 1409–1415. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Shao, W.-L.; Zhou, W.-X.; Liu, Y.; Han, Y.-Y.; Zheng, Y.; Liu, Y.-J. Biodiesel Production by Esterification Reaction on K+ Modified MgAl-Hydrotalcites Catalysts. Catalysts 2019, 9, 742. [Google Scholar] [CrossRef]
- Jindapon, W.; Ngamcharussrivichai, C. Heterogeneously catalyzed transesterification of palm oil with methanol to produce biodiesel over calcined dolomite: The role of magnesium oxide. Energy Convers. Manag. 2018, 171, 1311–1321. [Google Scholar] [CrossRef]
- Rabie, A.M.; Shaban, M.; Abukhadra, M.R.; Hosny, R.; Ahmed, S.A.; Negm, N.A. Diatomite supported by CaO/MgO nanocomposite as heterogeneous catalyst for biodiesel production from waste cooking oil. J. Mol. Liq. 2019, 279, 224–231. [Google Scholar] [CrossRef]
- Modiba, E.; Enweremadu, C.; Rutto, H. Production of biodiesel from waste vegetable oil using impregnated diatomite as heterogeneous catalyst. Chin. J. Chem. Eng. 2015, 23, 281–289. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S.; Piao, X. Transesterification of soybean oil to biodiesel using CaO as a solid base catalyst. Fuel 2008, 87, 216–221. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Sugimoto, Y.; Yamanaka, S.; Hidaka, J. Calcium oxide as a solid base catalyst for transesterification of soybean oil and its application to biodiesel production. Fuel 2008, 87, 2798–2806. [Google Scholar] [CrossRef]
- Iizuka, T.; Hattori, H.; Ohno, Y.; Sohma, J.; Tanabe, K. Basic sites and reducing sites of calcium oxide and their catalytic activities. J. Catal. 1971, 22, 130–139. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Yamanaka, S.; Hidaka, J. Active phase of calcium oxide used as solid base catalyst for transesterification of soybean oil with refluxing methanol. Appl. Catal. A Gen. 2008, 334, 357–365. [Google Scholar] [CrossRef]
- Kouzu, M.; Hidaka, J.-S.; Wakabayashi, K.; Tsunomori, M. Solid base catalysis of calcium glyceroxide for a reaction to convert vegetable oil into its methyl esters. Appl. Catal. A Gen. 2010, 390, 11–18. [Google Scholar] [CrossRef]
- Meher, L.C.; Kulkarni, M.G.; Dalai, A.K.; Naik, S.N. Transesterification of karanja(Pongamia pinnata) oil by solid basic catalysts. Eur. J. Lipid Sci. Technol. 2006, 108, 389–397. [Google Scholar] [CrossRef]
- Kaur, M.; Ali, A. Lithium ion impregnated calcium oxide as nano catalyst for the biodiesel production from karanja and jatropha oils. Renew. Energy 2011, 36, 2866–2871. [Google Scholar] [CrossRef]
- Wen, L.; Wang, Y.; Lu, D.; Hu, S.; Han, H. Preparation of KF/CaO nanocatalyst and its application in biodiesel production from Chinese tallow seed oil. Fuel 2010, 89, 2267–2271. [Google Scholar] [CrossRef]
- Kumar, D.; Ali, A. Nanocrystalline K–CaO for the transesterification of a variety of feedstocks: Structure, kinetics and catalytic properties. Biomass-Bioenergy 2012, 46, 459–468. [Google Scholar] [CrossRef]
- Schaafsma, A.; Pakan, I.; Hofstede, G.J.H.; Muskiet, F.A.; Van Der Veer, E.; De Vries, P.J.F. Mineral, amino acid, and hormonal composition of chicken eggshell powder and the evaluation of its use in human nutrition. Poult. Sci. 2000, 79, 1833–1838. [Google Scholar] [CrossRef]
- Boey, P.-L.; Maniam, G.P.; Hamid, S.A. Utilization of waste crab shell (Scylla serrata) as a catalyst in palm olein transesterification. J. Oleo Sci. 2009, 58, 499–502. [Google Scholar] [CrossRef]
- Al-Zaini, E.O.; Olsen, J.; Nguyen, T.H.; Adesina, A. Transesterification of Waste Cooking Oil in Presence of Crushed Seashell as a Support for Solid Heterogeneous Catalyst. SAE Int. J. Fuels Lubr. 2011, 4, 139–157. [Google Scholar] [CrossRef]
- Boro, J.; Konwar, L.J.; Deka, D. Transesterification of non edible feedstock with lithium incorporated egg shell derived CaO for biodiesel production. Fuel Process. Technol. 2014, 122, 72–78. [Google Scholar] [CrossRef]
- Viriya-Empikul, N.; Krasae, P.; Puttasawat, B.; Yoosuk, B.; Chollacoop, N.; Faungnawakij, K. Waste shells of mollusk and egg as biodiesel production catalysts. Bioresour. Technol. 2010, 101, 3765–3767. [Google Scholar] [CrossRef]
- Wei, Z.; Xu, C.; Li, B. Application of waste eggshell as low-cost solid catalyst for biodiesel production. Bioresour. Technol. 2009, 100, 2883–2885. [Google Scholar] [CrossRef]
- Chen, G.; Shan, R.; Shi, J.; Yan, B. Ultrasonic-assisted production of biodiesel from transesterification of palm oil over ostrich eggshell-derived CaO catalysts. Bioresour. Technol. 2014, 171, 428–432. [Google Scholar] [CrossRef]
- Tan, Y.H.; Abdullah, M.O.; Nolasco-Hipolito, C.; Zauzi, N.S.A. Application of RSM and Taguchi methods for optimizing the transesterification of waste cooking oil catalyzed by solid ostrich and chicken-eggshell derived CaO. Renew. Energy 2017, 114, 437–447. [Google Scholar] [CrossRef]
- Gollakota, A.; Volli, V.; Shu, C.-M. Transesterification of waste cooking oil using pyrolysis residue supported eggshell catalyst. Sci. Total Environ. 2019, 661, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Umdu, E.S.; Tuncer, M.; Seker, E. Transesterification of Nannochloropsis oculata microalga’s lipid to biodiesel on Al2O3 supported CaO and MgO catalysts. Bioresour. Technol. 2009, 100, 2828–2831. [Google Scholar] [CrossRef]
- Witoon, T.; Bumrungsalee, S.; Vathavanichkul, P.; Palitsakun, S.; Saisriyoot, M.; Faungnawakij, K. Biodiesel production from transesterification of palm oil with methanol over CaO supported on bimodal meso-macroporous silica catalyst. Bioresour. Technol. 2014, 156, 329–334. [Google Scholar] [CrossRef]
- Sawangkeaw, R.; Tejvirat, P.; Ngamcharassrivichai, C.; Ngamprasertsith, S. Supercritical Transesterification of Palm Oil and Hydrated Ethanol in a Fixed Bed Reactor with a CaO/Al2O3 Catalyst. Energies 2012, 5, 1062–1080. [Google Scholar] [CrossRef]
- Moradi, G.; Davoodbeygi, Y.; Mohadesi, M.; Hosseini, S. Kinetics of transesterification reaction using CAO/Al2O3 catalyst synthesized by sol-gel method. Can. J. Chem. Eng. 2015, 93, 819–824. [Google Scholar] [CrossRef]
- Kesserwan, F.; Ahmad, M.N.; Khalil, M.; El-Rassy, H. Hybrid CaO/Al2O3 aerogel as heterogeneous catalyst for biodiesel production. Chem. Eng. J. 2020, 385, 123834. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Alexandri, M.; Strati, A.; Gardeli, C.; Papanikolaou, S.; Komaitis, M.; Kookos, I.K.; Koutinas, A.A. Integrated sunflower-based biorefinery for the production of antioxidants, protein isolate and poly(3-hydroxybutyrate). Ind. Crop. Prod. 2015, 71, 106–113. [Google Scholar] [CrossRef]
- Aghilinategh, M.; Barati, M.; Hamadanian, M. Supercritical methanol for one put biodiesel production from chlorella vulgaris microalgae in the presence of CaO/TiO2 nano-photocatalyst and subcritical water. Biomass-Bioenergy 2019, 123, 34–40. [Google Scholar] [CrossRef]
- Thitsartarn, W.; Kawi, S. An active and stable CaO–CeO2 catalyst for transesterification of oil to biodiesel. Green Chem. 2011, 13, 3423–3430. [Google Scholar] [CrossRef]
- Lee, H.; Juan, J.; Taufiq-Yap, Y. Preparation and application of binary acid–base CaO–La2O3 catalyst for biodiesel production. Renew. Energy 2015, 74, 124–132. [Google Scholar] [CrossRef]
- Liu, X.; He, H.; Wang, Y.; Zhu, S. Transesterification of soybean oil to biodiesel using SrO as a solid base catalyst. Catal. Commun. 2007, 8, 1107–1111. [Google Scholar] [CrossRef]
- Singh, A.K.; Fernando, S.D. Reaction Kinetics of Soybean Oil Transesterification Using Heterogeneous Metal Oxide Catalysts. Chem. Eng. Technol. 2007, 30, 1716–1720. [Google Scholar] [CrossRef]
- Viola, E.; Blasi, A.; Valerio, V.; Guidi, I.; Zimbardi, F.; Braccio, G.; Giordano, G. Biodiesel from fried vegetable oils via transesterification by heterogeneous catalysis. Catal. Today 2012, 179, 185–190. [Google Scholar] [CrossRef]
- Koberg, M.; Gedanken, A. Direct Transesterification of Castor and Jatropha Seeds for FAME Production by Microwave and Ultrasound Radiation Using a SrO Catalyst. BioEnergy Res. 2012, 5, 958–968. [Google Scholar] [CrossRef]
- Ali, S.D.; Javed, I.N.; Rana, U.A.; Nazar, M.F.; Ahmed, W.; Junaid, A.; Pasha, M.; Nazir, R.; Nazir, R. Novel SrO-CaO Mixed Metal Oxides Catalyst for Ultrasonic-Assisted Transesterification of Jatropha Oil into Biodiesel. Aust. J. Chem. 2017, 70, 258–264. [Google Scholar] [CrossRef]
- Mierczynski, P.; Ciesielski, R.; Kedziora, A.; Maniukiewicz, W.; Shtyka, O.; Kubicki, J.; Albinska, J.; Maniecki, T.P. Biodiesel Production on MgO, CaO, SrO and BaO Oxides Supported on (SrO)(Al2O3) Mixed Oxide. Catal. Lett. 2015, 145, 1196–1205. [Google Scholar] [CrossRef]
- Anderson, J.A.; Beaton, A.; Galadima, A.; Wells, R.P.K. Role of Baria Dispersion in BaO/Al2O3 Catalysts for Transesterification. Catal. Lett. 2009, 131, 213–218. [Google Scholar] [CrossRef]
- Tonetto, G.M.; Marchetti, J.M. Transesterification of Soybean Oil Over Me/Al2O3 (Me = Na, Ba, Ca, and K) Catalysts and Monolith K/Al2O3-Cordierite. Top. Catal. 2010, 53, 755–762. [Google Scholar] [CrossRef]
- Mootabadi, H.; Abdullah, A.Z. Response Surface Methodology for Simulation of Ultrasonic-assisted Biodiesel Production Catalyzed by SrO/Al2O3 Catalyst. Energy Sources Part A Recover. Util. Environ. Eff. 2015, 37, 1747–1755. [Google Scholar] [CrossRef]
- Photaworn, S.; Tongurai, C.; Kungsanunt, S. Process development of two-step esterification plus catalyst solution recycling on waste vegetable oil possessing high free fatty acid. Chem. Eng. Process. Process. Intensif. 2017, 118, 1–8. [Google Scholar] [CrossRef]
- Tangy, A.; Pulidindi, I.N.; Gedanken, A. SiO2 Beads Decorated with SrO Nanoparticles for Biodiesel Production from Waste Cooking Oil Using Microwave Irradiation. Energy Fuels 2016, 30, 3151–3160. [Google Scholar] [CrossRef]
- Shahbazi, F.; Mahdavi, V.; Zolgharnein, J. Preparation and characterization of SrO/MgO nanocomposite as a novel and efficient base catalyst for biodiesel production from waste cooking oil: A statistical approach for optimization. J. Iran. Chem. Soc. 2020, 17, 333–349. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, S.; Han, K.; Li, Y.; Lu, C. Synthesis of the SrO–CaO–Al2O3 trimetallic oxide catalyst for transesterification to produce biodiesel. Renew. Energy 2021, 168, 981–990. [Google Scholar] [CrossRef]





| Oil System | Design of Experiment | Optimal Conditions | Yield (%) | Catalysts | Reference |
|---|---|---|---|---|---|
| Sunflower Oil | Box-Behnken, Face Centered Central Composite, Full Factorial | Temperature: 75 °C Ethanol/Oil: 12:1 Catalyst loading: 1.25 wt.% | 97.6 | NaOH | [56] |
| Castor Oil | Taguchi | Time: 60 min Temperature: 50 °C Catalyst loading: 1 wt.% Methanol/oil: 20:1 Agitation speed: 700 rpm | 90.83 | H2SO4 | [57] |
| Mahogany Seed Oil | Taguchi | Methanol/oil: 9:1 Catalyst loading: 0.5 wt.% Temperature: 60 °C Agitation speed: 300 rpm | 96.8 | NaOH | [58] |
| Ceiba Pentandra Oil | Box-Behnken | Methanol/oil: 60:1 Catalyst loading: 0.84 wt.% Time: 6.46 min Agitation speed: 800 rpm Microwaved to 100 °C | 95.42 | KOH | [59] |
| Sardine Fish Oil | Box-Behnken | Temperature: 150 °C Methanol/oil: 6:1 Catalyst loading: 1.25 wt.% Time: 25 min | 98.1 | KOH | [60] |
| Karanja Oil | Box-Behnken | Methanol/oil: 10.44:1 Catalyst loading: 1.22 wt.% Time: 90.78 min Temperature: 66.8 °C | 91.05 | KOH | [61] |
| Mixtureof Pongamiaand Neem Oils | Central Composite | Time: 77 min Catalyst loading: 0.67 wt.% Methanol/oil: 6:1 | 86.3 | NaOH | [62] |
| Catalysts | Basicity |
|---|---|
| MgO | 8.2 ≥ H ≥ 6.8 |
| CaO | 15 ≥ H ≥ 13.4 |
| SrO | 17.2 ≥ H ≥ 15 |
| BaO | 17.2 ≥ H ≥ 15 |
| Catalyst | Oil | Temperature (°C) | Alcohol/Oil | Catalysts Loading (wt.%) | Time (Min) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| MgO | Sunflower Soybean Rapeseed | 252 (at 100 MPa) | 41:1 | 3 | 20 | 98 100 95 | [83] |
| KOH/MgO (20 wt.% KOH) | Canola | 65 | 6:1 | 3 | 540 | 95 | [84] |
| Copolymer templated MgO (PDMS-PEO) | Canola | 190 | 20:3 | 3 | 120 | 98.2 | [81] |
| MgO nanoparticles | Goat fat | 70 | 12:1 | 1 | 180 | 93 | [90] |
| MgO nanoparticles | Wasted cooking oil | 60 | 10:1 | 2 | 120 | 80 | [91] |
| Catalyst | Oil | Temperature (°C) | Alcohol/Oil | Catalysts Loading (wt.%) | Time (Min) | Yield (%) | Reference |
|---|---|---|---|---|---|---|---|
| Li, Na, K/CaO (1.25 wt.%) | Karanja | 65 | 12:1 | 2 | 360 | 97–98 | [103] |
| Li/CaO (1.75 wt.%) | Karanja and Jatropha | 65 | 12:1 | 5 | 120 | 98 | [104] |
| KF/CaO | Chinese Tallow Seed | 70 | 9:1 | 5 | 180 | 96 | [105] |
| K/CaO (3.5 wt.% K+) | Wasted Cotton Oil, Jatropha and Karanja | 65 | 12:1 | 7.5 | 75 | 98 | [106] |
| Catalyst | Basic Strength | Approximate Total Basic Sites (mmol CO2·g−1) | Biodiesel Yield (%) |
|---|---|---|---|
| Co-precipitated SrO/MgO | 27 ≥ H_ ≥ 22 | 71 | 79 |
| Wet Impregnated SrO/MgO | 18 ≥ H_ ≥ 15 | 34 | 52 |
| Strontium oxide | 15 ≥ H_ ≥ 9 | 37 | 48 |
| Magnesium oxide | 9 ≥ H | 10 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavizón-Pozos, J.A.; Chavez-Esquivel, G.; Suárez-Toriello, V.A.; Santolalla-Vargas, C.E.; Luévano-Rivas, O.A.; Valdés-Martínez, O.U.; Talavera-López, A.; Rodriguez, J.A. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies 2021, 14, 1031. https://doi.org/10.3390/en14041031
Tavizón-Pozos JA, Chavez-Esquivel G, Suárez-Toriello VA, Santolalla-Vargas CE, Luévano-Rivas OA, Valdés-Martínez OU, Talavera-López A, Rodriguez JA. State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies. 2021; 14(4):1031. https://doi.org/10.3390/en14041031
Chicago/Turabian StyleTavizón-Pozos, Jesús Andrés, Gerardo Chavez-Esquivel, Víctor Alejandro Suárez-Toriello, Carlos Eduardo Santolalla-Vargas, Oscar Abel Luévano-Rivas, Omar Uriel Valdés-Martínez, Alfonso Talavera-López, and Jose Antonio Rodriguez. 2021. "State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production" Energies 14, no. 4: 1031. https://doi.org/10.3390/en14041031
APA StyleTavizón-Pozos, J. A., Chavez-Esquivel, G., Suárez-Toriello, V. A., Santolalla-Vargas, C. E., Luévano-Rivas, O. A., Valdés-Martínez, O. U., Talavera-López, A., & Rodriguez, J. A. (2021). State of Art of Alkaline Earth Metal Oxides Catalysts Used in the Transesterification of Oils for Biodiesel Production. Energies, 14(4), 1031. https://doi.org/10.3390/en14041031











