Changes in Leachability of Selected Elements and Chemical Compounds in Residues from Municipal Waste Incineration Plants
Abstract
1. Introduction
Study Area
2. Research Methodology
2.1. Control Charts
- (A)
- The upper control limit (UCL) set at a distance of three standard deviations above the mean value. Observations higher than UCL are called extreme values;
- (B)
- Upper warning limit (UWL) set at a distance of two standard deviations above the mean value. Observations higher than UWL and lower than UCL are called outliers;
- (C)
- Center line (CL), determining the mean value;
- (D)
- The lower warning limit (LWL), which is set a distance of two standard deviations below the mean value, observations lower than UWL and higher than UCL are called outliers;
- (E)
- The lower control limit (LCL), which is set at a distance of three standard deviations below the mean value. Observations lower than UCL are called extreme values.
2.2. Time Series
- —sine and cosine coefficients;
- —frequency; and
- t—time (from 0 to n).
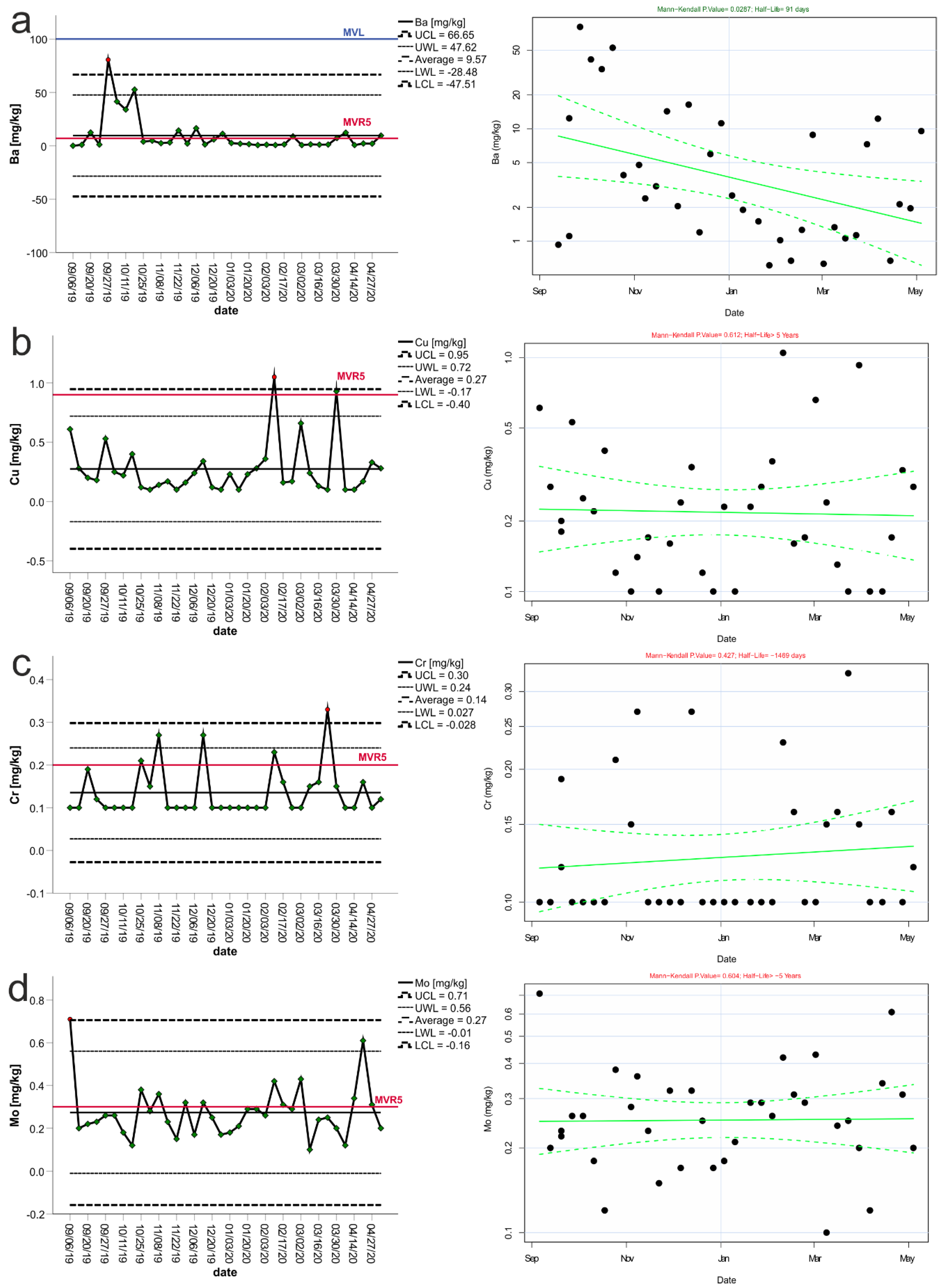
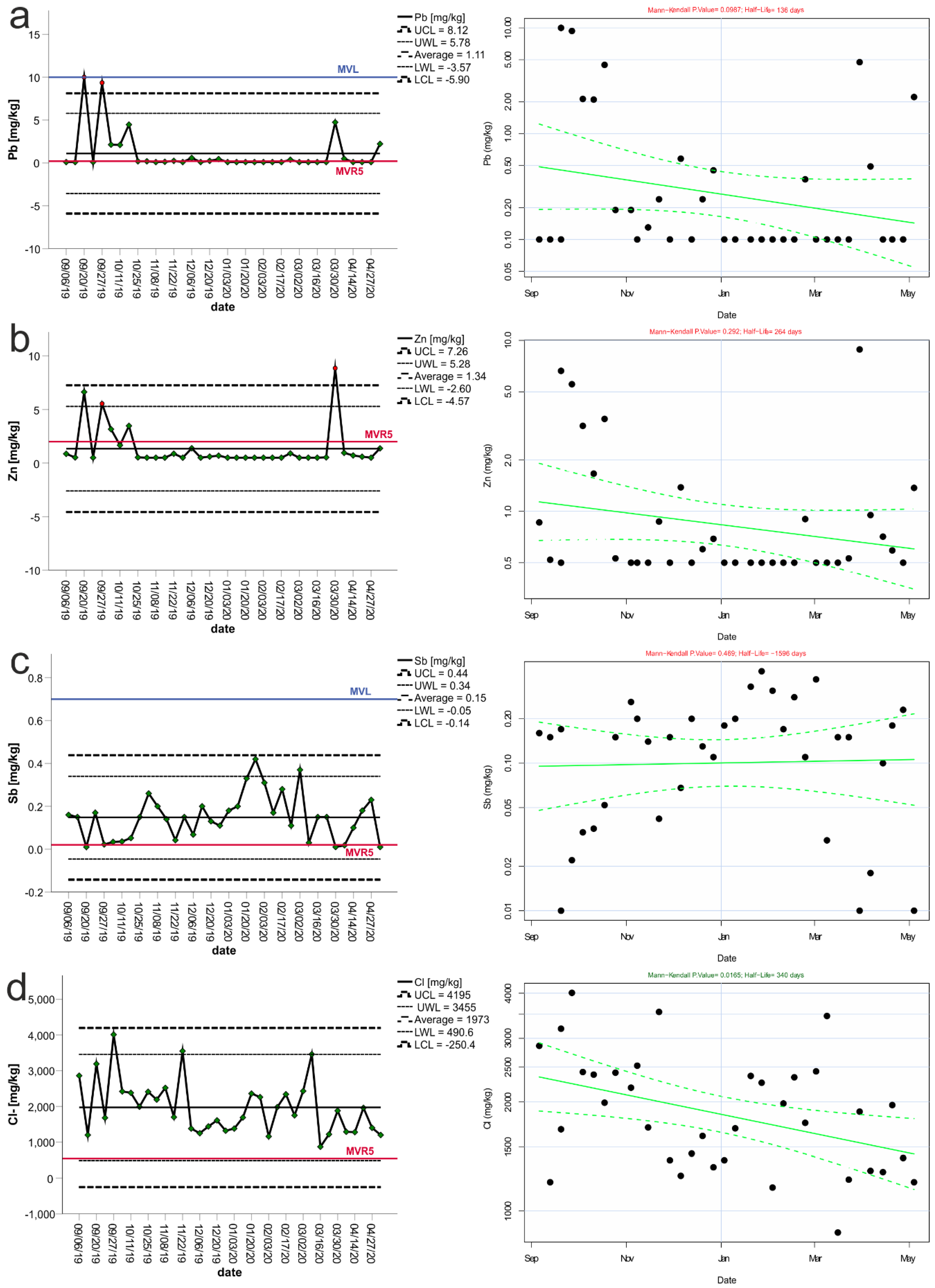
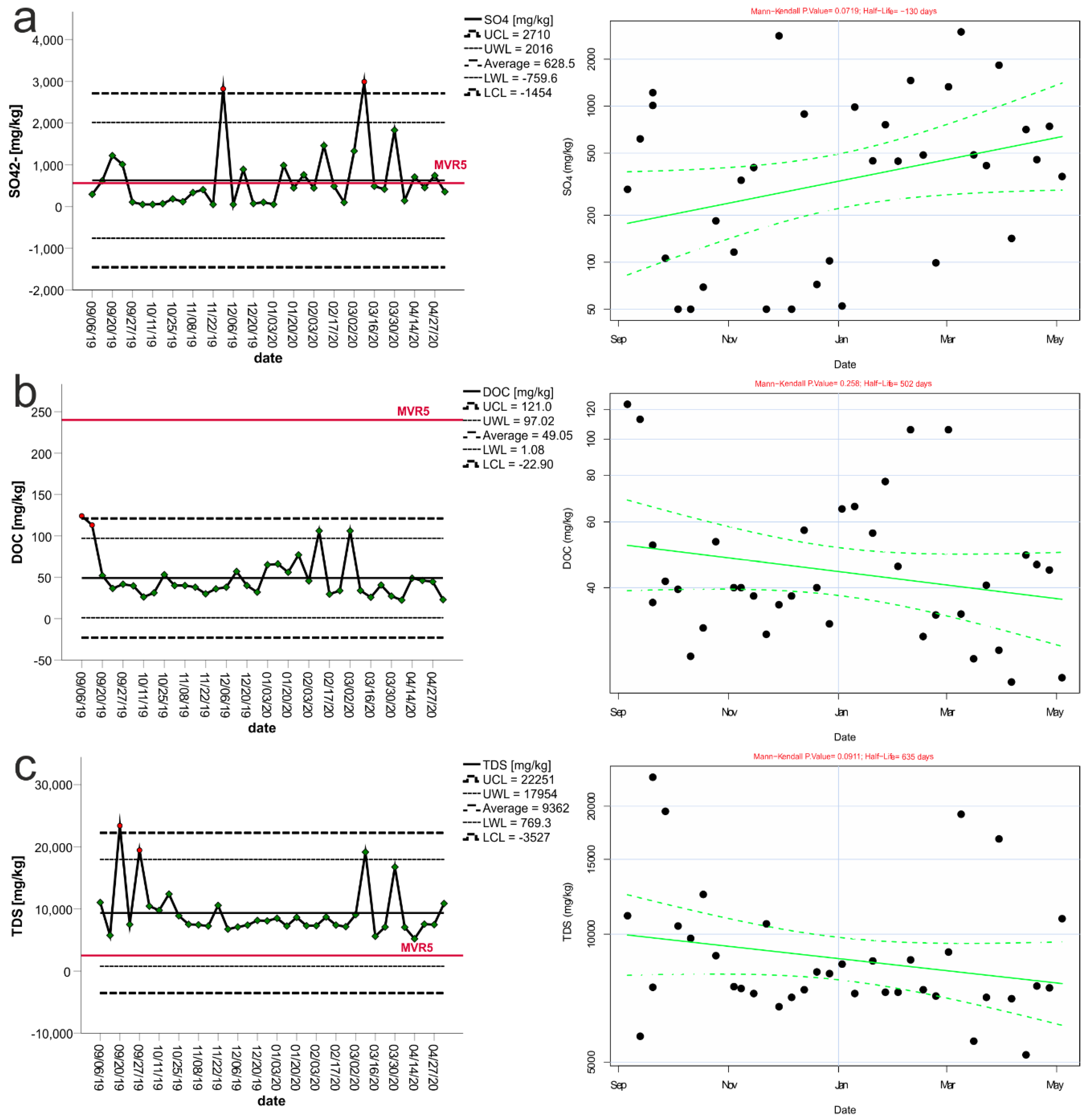
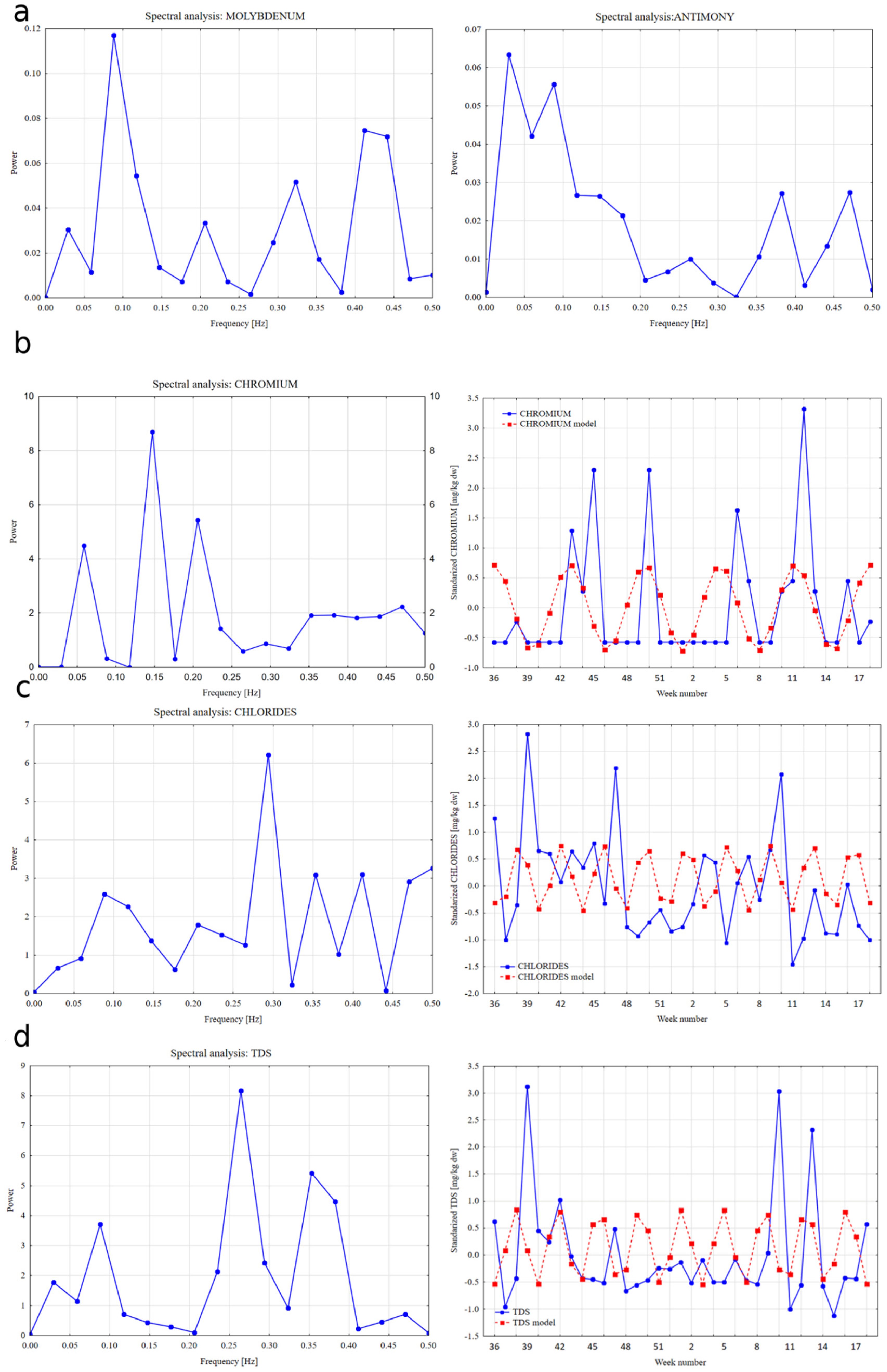
3. Results
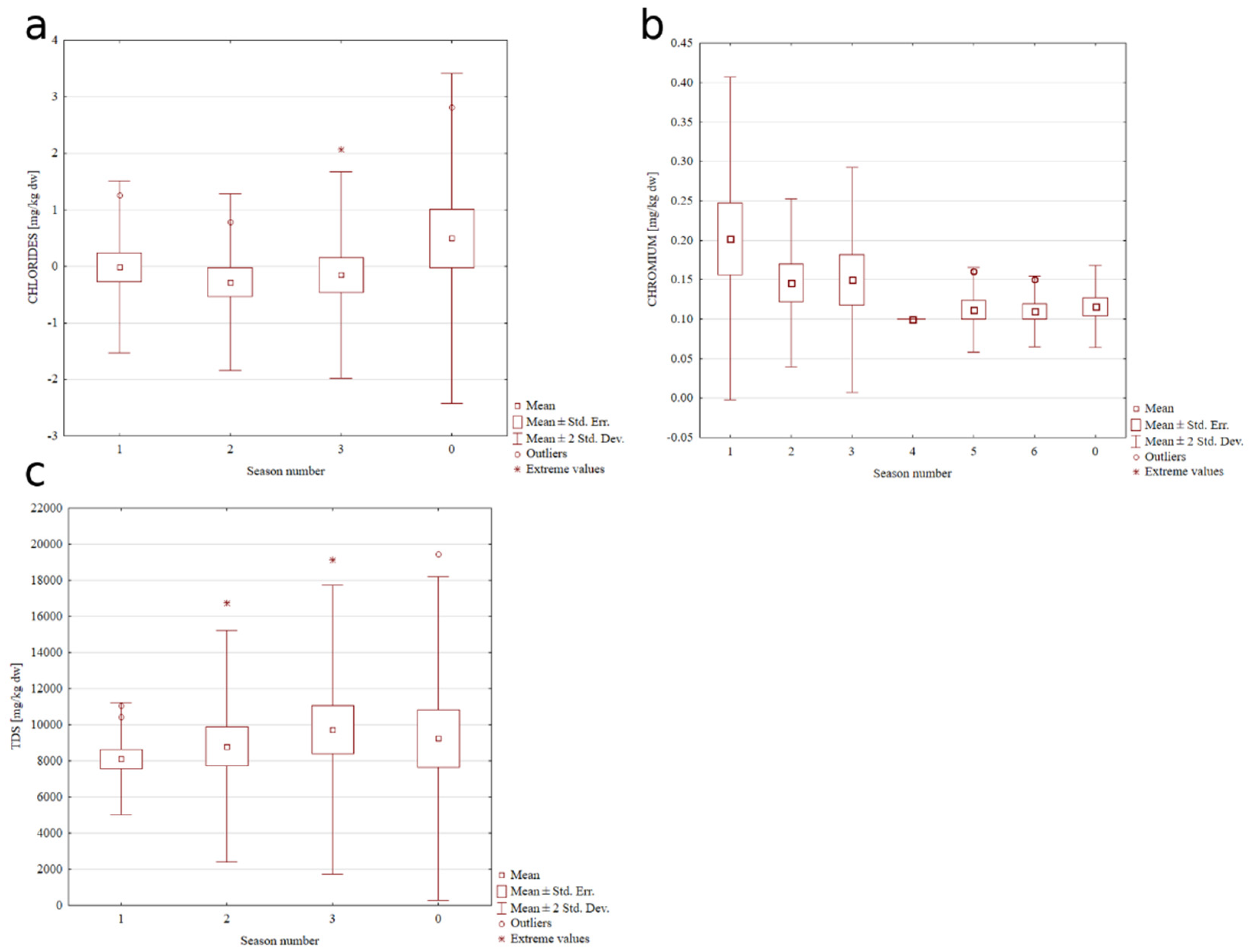
3.1. Control Charts and Trend Analysis
3.2. Assessment of Seasonality and Trend
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alba, N.; Gassó, S.; Lacorte, T.; Baldasano, J.M. Characterization of municipal solid waste incineration residues from facilities with different air pollution control systems. J. Air Waste Manag. Assoc. 1997, 47, 1170–1179. [Google Scholar] [CrossRef]
- European Commission. Council Directive 1999/31/EC of 26 April 1999 on the Landfill of Waste; European Commission: Luxembourg, 1999. [Google Scholar]
- European Commission. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on waste and repealing certain Directives. Off. J. Eur. Union 2008, 51, 47. [Google Scholar]
- Ministerstwo Środowiska. Rozporządzenie Ministra Gospodarki z Dnia 16 Lipca 2015 r. w Sprawie Dopuszczania Odpadów do Składowania na Składowiskach, Dz.U. 2015 poz. 1277; Ministerstwo Środowiska: Warsaw, Poland, 2015.
- Sabbas, T.; Polettini, A.; Pomi, R.; Astrup, T.; Hjelmar, O.; Mostbauer, P.; Cappai, G.; Magel, G.; Salhofer, S.; Speiser, C.; et al. Management of municipal solid waste incineration residues. Waste Manag. 2003, 23, 61–88. [Google Scholar] [CrossRef]
- Jung, C.H.; Matsuto, T.; Tanaka, N.; Okada, T. Metal distribution in incineration residues of municipal solid waste (MSW) in Japan. Waste Manag. 2004, 24, 381–391. [Google Scholar] [CrossRef]
- Abramov, S.; He, J.; Wimmer, D.; Lemloh, M.L.; Muehe, E.M.; Gann, B.; Roehm, E.; Kirchhof, R.; Babechuk, M.G.; Schoenberg, R.; et al. Heavy metal mobility and valuable contents of processed municipal solid waste incineration residues from Southwestern Germany. Waste Manag. 2018, 79, 735–743. [Google Scholar] [CrossRef]
- Yao, Q.; Samad, N.B.; Keller, B.; Seah, X.S.; Huang, L.; Lau, R. Mobility of heavy metals and rare earth elements in incineration bottom ash through particle size reduction. Chem. Eng. Sci. 2014, 118, 214–220. [Google Scholar] [CrossRef]
- Šyc, M.; Simon, F.G.; Hykš, J.; Braga, R.; Biganzoli, L.; Costa, G.; Funari, V.; Grosso, M. Metal recovery from incineration bottom ash: State-of-the-art and recent developments. J. Hazard. Mater. 2020, 393, 122433. [Google Scholar] [CrossRef]
- Forteza, R.; Far, M.; Seguí, C.; Cerdá, V. Characterization of bottom ash in municipal solid waste incinerators for its use in road base. Waste Manag. 2004, 24, 899–909. [Google Scholar] [CrossRef]
- Blasenbauer, D.; Huber, F.; Lederer, J.; Quina, M.J.; Blanc-Biscarat, D.; Bogush, A.; Bontempi, E.; Blondeau, J.; Chimenos, J.M.; Dahlbo, H.; et al. Legal situation and current practice of waste incineration bottom ash utilisation in Europe. Waste Manag. 2020, 102, 868–883. [Google Scholar] [CrossRef]
- Lynn, C.J.; OBE, R.K.; Ghataora, G.S. Municipal incinerated bottom ash characteristics and potential for use as aggregate in concrete. Constr. Build. Mater. 2016, 127, 504–517. [Google Scholar] [CrossRef]
- Van Dr Wegen, G.; Hofstra, U.; Speerstra, J. Upgraded MSWI bottom ash as aggregate in concrete. Waste Biomass Valorization 2013, 4, 737–743. [Google Scholar] [CrossRef]
- Yin, K.; Chan, W.P.; Dou, X.; Ren, F.; Wei-Chung Chang, V. Cr, Cu, Hg and Ni release from incineration bottom ash during utilization in land reclamation—based on lab-scale batch and column leaching experiments and a modeling study. Chemosphere 2018, 197, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Tanaka, N.; Matsuto, T. Solubility and adsorption characteristics of Pb in leachate from MSW incinerator bottom ash. Waste Manag. Res. 2002, 20, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, J.J.; Van Der Sloot, H.A.; Comans, R.N.J. The leaching of major and trace elements from MSWI bottom ash as a function of pH and time. Appl. Geochem. 2006, 21, 335–351. [Google Scholar] [CrossRef]
- Van Der Sloot, H.A.; Kosson, D.S.; Hjelmar, O. Characteristics, treatment and utilization of residues from municipal waste incineration. Waste Manag. 2001, 21, 753–765. [Google Scholar] [CrossRef]
- Vateva, I.; Laner, D. Grain-size specific characterisation and resource potentials of municipal solid waste incineration (MSWI) bottom ash: A German case study. Resources 2020, 9, 66. [Google Scholar] [CrossRef]
- Caviglia, C.; Confalonieri, G.; Corazzari, I.; Destefanis, E.; Mandrone, G.; Pastero, L.; Boero, R.; Pavese, A. Effects of particle size on properties and thermal inertization of bottom ashes (MSW of Turin’s incinerator). Waste Manag. 2019, 84, 340–354. [Google Scholar] [CrossRef]
- Del Valle-Zermeño, R.; Gómez-Manrique, J.; Giro-Paloma, J.; Formosa, J.; Chimenos, J.M. Material characterization of the MSWI bottom ash as a function of particle size. Effects of glass recycling over time. Sci. Total Environ. 2017, 581–582, 897–905. [Google Scholar] [CrossRef]
- Loginova, E.; Volkov, D.S.; van de Wouw, P.M.F.; Florea, M.V.A.; Brouwers, H.J.H. Detailed characterization of particle size fractions of municipal solid waste incineration bottom ash. J. Clean. Prod. 2019, 207, 866–874. [Google Scholar] [CrossRef]
- Huber, F.; Blasenbauer, D.; Aschenbrenner, P.; Fellner, J. Complete determination of the material composition of municipal solid waste incineration bottom ash. Waste Manag. 2020, 102, 677–685. [Google Scholar] [CrossRef]
- Hyks, J.; Astrup, T.; Christensen, T.H. Long-term leaching from MSWI air-pollution-control residues: Leaching characterization and modeling. J. Hazard. Mater. 2009, 162, 80–91. [Google Scholar] [CrossRef] [PubMed]
- European Commission. 2000/532/EC, COMMISSION DECISION of 3 May 2000 Replacing Decision 94/3/EC Establishing a List of Wastes Pursuant to Article 1(a) of Council Directive 75/442/EEC on Waste and Council Decision 94/904/EC Establishing a List of Hazardous Waste Pursuant to Art; European Commission: Luxembourg, 2020. [Google Scholar]
- Ministerstwo Środowiska. Rozporządzenie Ministra Środowiska z Dnia 11 Maja 2015 r. w Sprawie Odzysku Odpadów Poza Instalacjami i Urządzeniami. Dz.U. 2015 poz. 796; Ministerstwo Środowiska: Warsaw, Poland, 2015.
- EN 12457-1:2002. Characterisation of Waste—Leaching—Compliance Test for Leaching of Granular Waste Materials and Sludges—Part 1: One Stage Batch Test at a Liquid to Solid Ratio of 2 L/kg for Materials with High Solid Content and with Particle Size Below 4 mm; European Standards: Brussels, Belgium, 2002. [Google Scholar]
- EN 12457-2. Characterisation of Waste LEACHING Compliance Test for Leaching of Granular Waste Materials and Sludges Part 2: One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 4 mm (without or with Size Reduction); European Standards: Brussels, Belgium, 2002. [Google Scholar]
- EN 12457-3. Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. Two Stage batch Test at a Liquid to Solid Ratio of 2 L/kg and 8 L/kg for Materials with a High Solid Content and with a Particle Size Below 4 mm (without or with Size Reduction); European Standards: Brussels, Belgium, 2002. [Google Scholar]
- EN 12457-4. Characterisation of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludges. One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size Below 10 mm (without or with size reduction); European Standards: Brussels, Belgium, 2002. [Google Scholar]
- ISO/IEC 17025:2017. General Requirements for the Competence of Testing and Calibration Laboratories; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- UNE. EN 15934:2012. Sludge, Treated Biowaste, Soil and Waste—Calculation of Dry Matter Fraction after Determination of Dry Residue or Water Content (Endorsed by AENOR in October of 2012); European Standards: Brussels, Belgium, 2012. [Google Scholar]
- ISO. ISO 17294-2:2016. Water Quality—Application of Inductively Coupled Plasma Mass Spectrometry (ICP-MS)—Part 2: Determination of Selected Elements Including Uranium Isotopes; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- ISO. ISO 12846:2012. Water Quality—Determination of Mercury—Method Using Atomic Absorption Spectrometry (AAS) with and without Enrichment; ISO: Geneva, Switzerland, 2012. [Google Scholar]
- ISO. ISO 15682:2000. Water Quality—Determination of Chloride by Flow Analysis (CFA and FIA) and Photometric or Potentiometric Detection; ISO: Geneva, Switzerland, 2000. [Google Scholar]
- ISO. ISO 22743:2006. Water Quality—Determination of Sulfates—Method by Continuous Flow Analysis (CFA); ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO. ISO/TS 17951-2:2016. Water Quality—Determination of Fluoride Using Flow Analysis (FIA and CFA)—Part 2: Method Using Continuous Flow Analysis (CFA) with Automated In-line Distillation; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- BS EN 1484:1997. Water Analysis. Guidelines for the Determination of Total Organic Carbon (TOC) and Dissolved Organic Carbon (DOC); European Standards: Brussels, Belgium, 1997. [Google Scholar]
- NEMI Method Summary—2540 C. Available online: https://www.nemi.gov/methods/method_summary/9818/ (accessed on 14 January 2021).
- Massart, D.L.; Vandeginste, B.G.M.; Buydens, L.M.C.; de Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A; Data Handling in Science and Technology Volume 20A; Elsevier: Amsterdam, The Netherlands, 1997; p. Xvii. ISBN 0-444-89724-0. [Google Scholar]
- Jones, W.; Spence, M. GroundWater Spatio-Temporal Data Analysis Tool (GWSDAT Version 2.0) User Manual; Shell Global Solutions: Manchester, UK, 2013. [Google Scholar]
- Chatfield, C. The Analysis of Time Series: An Introduction, 6th ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Ramsey, F.L. Characterization of the Partial Autocorrelation Function. Ann. Stat. 1974, 2, 1296–1301. [Google Scholar] [CrossRef]
- Shumway, R.H.; Stoffer, D.S. Time Series Analysis and Its Applications: With R Examples; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Bloomfield, P. Fourier Analysis of Time Series; Wiley Series in Probability and Statistics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000; Volume 22, ISBN 9780471722236. [Google Scholar]
- Kowalski, P.; Michalik, M. Slags and Ashes from Municipal Waste Incineration in Poland-Mineralogical and Chemical Composition. Geophys. Res. Abstr. 2013, 15, 12642. [Google Scholar]
- Eusden, J.D.; Eighmy, T.T.; Hockert, K.; Holland, E.; Marsella, K. Petrogenesis of municipal solid waste combustion bottom ash. Appl. Geochem. 1999, 14, 1073–1091. [Google Scholar] [CrossRef]
- Zevenbergen, C.; Vander Wood, T.; Bradley, J.P.; Van der Broeck, P.F.C.W.; Orbons, A.J.; Van Reeuwijk, L.P. Morphological and chemical properties of MSWI bottom ash with respect to the glassy constituents. Hazard. Waste Hazard. Mater. 1994, 11, 371–383. [Google Scholar] [CrossRef]
- Chang, F.Y.; Wey, M.Y. Comparison of the characteristics of bottom and fly ashes generated from various incineration processes. J. Hazard. Mater. 2006, 138, 594–603. [Google Scholar] [CrossRef]
- Kowalski, P.R.; Kasina, M.; Michalik, M. Metallic Elements Fractionation in Municipal Solid Waste Incineration Residues. Energy Procedia 2016, 97, 31–36. [Google Scholar] [CrossRef]
- Krzanowski, J.E.; Crannell, B.S.; Krzanowski, J.E.; Eighmy, T.T.; Crannell, B.S.; Dykstra Eusden, J.; Eusden, J.D. An analytical electron microscopy investigation of municipal solid waste incineration bottom ash. J. Mater. Res. 1998, 13, 28–36. [Google Scholar] [CrossRef]
- Wei, Y.; Shimaoka, T.; Saffarzadeh, A.; Takahashi, F. Mineralogical characterization of municipal solid waste incineration bottom ash with an emphasis on heavy metal-bearing phases. J. Hazard. Mater. 2011, 187, 534–543. [Google Scholar] [CrossRef]
- Bayuseno, A.P.; Schmahl, W.W. Understanding the chemical and mineralogical properties of the inorganic portion of MSWI bottom ash. Waste Manag. 2010, 30, 1509–1520. [Google Scholar] [CrossRef]
- Rambaldi, E.; Esposito, L.; Andreola, F.; Barbieri, L.; Lancellotti, I.; Vassura, I. The recycling of MSWI bottom ash in silicate based ceramic. Ceram. Int. 2010, 36, 2469–2476. [Google Scholar] [CrossRef]
- Seniunaite, J.; Vasarevicius, S. Leaching of Copper, Lead and Zinc from Municipal Solid Waste Incineration Bottom Ash. In Energy Procedia; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; Volume 113, pp. 442–449. [Google Scholar]
- Feng, S.; Wang, X.; Wei, G.; Peng, P.; Yang, Y.; Cao, Z. Leachates of municipal solid waste incineration bottom ash from Macao: Heavy metal concentrations and genotoxicity. Chemosphere 2007, 67, 1133–1137. [Google Scholar] [CrossRef]
- Stamps, B.W.; Lyles, C.N.; Suflita, J.M.; Masoner, J.R.; Cozzarelli, I.M.; Kolpin, D.W.; Stevenson, B.S. Municipal Solid Waste Landfills Harbor Distinct Microbiomes. Front. Microbiol. 2016, 7, 534. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Steenari, B.M. Leaching optimization of municipal solid waste incineration ash for resource recovery: A case study of Cu, Zn, Pb and Cd. Waste Manag. 2016, 48, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Seniūnaitė, J.; Vasarevičius, S.; Grubliauskas, R.; Zigmontienė, A.; Vaitkus, A. Characteristics of Bottom Ash from Municipal Solid Waste Incineration. Rocznik Ochrona Środowiska 2018, 20 Pt 1, 123–144. [Google Scholar]
- Santos, R.M.; Mertens, G.; Salman, M.; Cizer, Ö.; Van Gerven, T. Comparative study of ageing, heat treatment and accelerated carbonation for stabilization of municipal solid waste incineration bottom ash in view of reducing regulated heavy metal/metalloid leaching. J. Environ. Manag. 2013, 128, 807–821. [Google Scholar] [CrossRef]
- Meima, J.A.; Comans, R.N.J. The leaching of trace elements from municipal solid waste incinerator bottom ash at different stages of weathering. Appl. Geochem. 1999, 14, 159–171. [Google Scholar] [CrossRef]
- Meima, J.A.; Comans, R.N.J. Geochemical modeling of weathering reactions in municipal solid waste incinerator bottom ash. Environ. Sci. Technol. 1997, 31, 1269–1276. [Google Scholar] [CrossRef]
- Freyssinet, P.; Piantone, P.; Azaroual, M.; Itard, Y.; Clozel-Leloup, B.; Guyonnet, D.; Baubron, J.C. Chemical changes and leachate mass balance of municipal solid waste bottom ash submitted to weathering. Waste Manag. 2002, 22, 159–172. [Google Scholar] [CrossRef]


| Element/Compound | Analytical Method | Limit of Quantification (LOQ) | Uncertainty (U) * (%) |
|---|---|---|---|
| Dry mass | Weight method [31] | 0.10% | 25 |
| As | Inductively Coupled Plasma Mass Spectrometry ICP-MS [32] | 0.10 mg/kg | 35 |
| Cd | 0.010 mg/kg | 35 | |
| Ba | 0.10 mg/kg | 35 | |
| Cu | 0.10 mg/kg | 35 | |
| Cr | 0.10 mg/kg | 35 | |
| Mo | 0.10 mg/kg | 35 | |
| Ni | 0.10 mg/kg | 35 | |
| Pb | 0.10 mg/kg | 35 | |
| Se | 0.010 mg/kg | 40 | |
| Sb | 0.010 mg/kg | 35 | |
| Zn | 0.50 mg/kg | 35 | |
| Hg | Atomic Absorption Spectrometry with the amalgamation technique [33] | 0.005 mg/kg | 35 |
| Cl− | Continuous flow analysis method (CFA) with spectrophotometric detection [34] | 25 mg/kg | 29 |
| SO42− | Continuous flow analysis method (CFA) with spectrophotometric detection [35] | 50 mg/kg | 29 |
| F− | Continuous flow analysis method (CFA) with spectrophotometric detection [36] | 5 mg/kg | 29 |
| TOC | Infrared spectroscopy [37] | 1.0 mg/L | 29 |
| TDS | Weight method [38] | 3.0 mg/L | 29 |
| Variable | Mean | Median | Min | Max | Std. Dev. | The Coefficient of Variation | “−2 Std. Dev.” | “+2 Std. Dev.” | “−3 Std. Dev.” | “+3 Std. Dev.” | LLV | LLVR5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Barium | 9.58 | 2.48 | 0.61 | 80.60 | 17.17 | 175.29 | −24.76 | 43.92 | −41.9 | 61.1 | ≤100 | 7 |
| Copper | 0.28 | 0.21 | 0.10 | 1.05 | 0.23 | 81.97 | −0.18 | 0.73 | −0.41 | 0.96 | ≤50 | 0.9 |
| Chromium | 0.13 | 0.10 | 0.10 | 0.33 | 0.06 | 43.66 | 0.02 | 0.25 | −0.04 | 0.31 | ≤10 | 0.2 |
| Molybdenum | 0.28 | 0.26 | 0.10 | 0.71 | 0.13 | 45.43 | 0.02 | 0.53 | −0.10 | 0.65 | ≤10 | 0.3 |
| Lead | 0.85 | 0.10 | 0.10 | 10.00 | 1.88 | 216.20 | −2.90 | 4.60 | −4.77 | 6.48 | ≤10 | 0.2 |
| Antimony | 0.15 | 0.15 | 0.01 | 0.42 | 0.10 | 71.49 | −0.06 | 0.36 | −0.16 | 0.47 | ≤0.7 | 0.02 |
| Zinc | 1.19 | 0.53 | 0.50 | 8.85 | 1.70 | 141.77 | −2.20 | 4.59 | −3.90 | 6.28 | ≤50 | 2 |
| Chlorides | 1938 | 1815.00 | 871.00 | 4010.00 | 735 | 38.20 | 468 | 3407 | −266 | 4142 | ≤15,000 | 550 |
| Sulfates | 612 | 430.00 | 50.00 | 2990.00 | 722 | 114.31 | −832 | 2055 | −1553 | 2776 | ≤20,000 | 560 |
| DOC | 49.0 | 40.00 | 22.40 | 124.00 | 26.3 | 52.79 | −3.6 | 101.5 | −29.8 | 127.8 | ≤800 | 240 |
| TDS | 8961 | 7537.50 | 5200.00 | 23,400.00 | 3357 | 43.70 | 2247 | 15,674 | −1110 | 19031 | ≤60,000 | 2500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielowicz, B.; Chuchro, M.; Jędrusiak, R.; Wątor, K. Changes in Leachability of Selected Elements and Chemical Compounds in Residues from Municipal Waste Incineration Plants. Energies 2021, 14, 771. https://doi.org/10.3390/en14030771
Bielowicz B, Chuchro M, Jędrusiak R, Wątor K. Changes in Leachability of Selected Elements and Chemical Compounds in Residues from Municipal Waste Incineration Plants. Energies. 2021; 14(3):771. https://doi.org/10.3390/en14030771
Chicago/Turabian StyleBielowicz, Barbara, Monika Chuchro, Radosław Jędrusiak, and Katarzyna Wątor. 2021. "Changes in Leachability of Selected Elements and Chemical Compounds in Residues from Municipal Waste Incineration Plants" Energies 14, no. 3: 771. https://doi.org/10.3390/en14030771
APA StyleBielowicz, B., Chuchro, M., Jędrusiak, R., & Wątor, K. (2021). Changes in Leachability of Selected Elements and Chemical Compounds in Residues from Municipal Waste Incineration Plants. Energies, 14(3), 771. https://doi.org/10.3390/en14030771







