Emission Characteristics for Swirl Methane–Air Premixed Flames with Ammonia Addition
Abstract
1. Introduction
- the determination of overall emission values and local parameters’ distribution for different swirl flames;
- the identification of the influence of the combustion process’ parameters, such as recirculation ratio, residence time and wall thermal condition on the NO concentration for ammonia doped lean methane–air swirl flames;
- the evaluation of the of the NO emission prediction with different kinetic reaction mechanisms for ammonia doped lean methane–air swirl flames.
2. Research Methods
2.1. Experimental Setup
2.2. Numerical Modelling
2.2.1. Reacting Flow Modelling
2.2.2. Reactor Network Setup
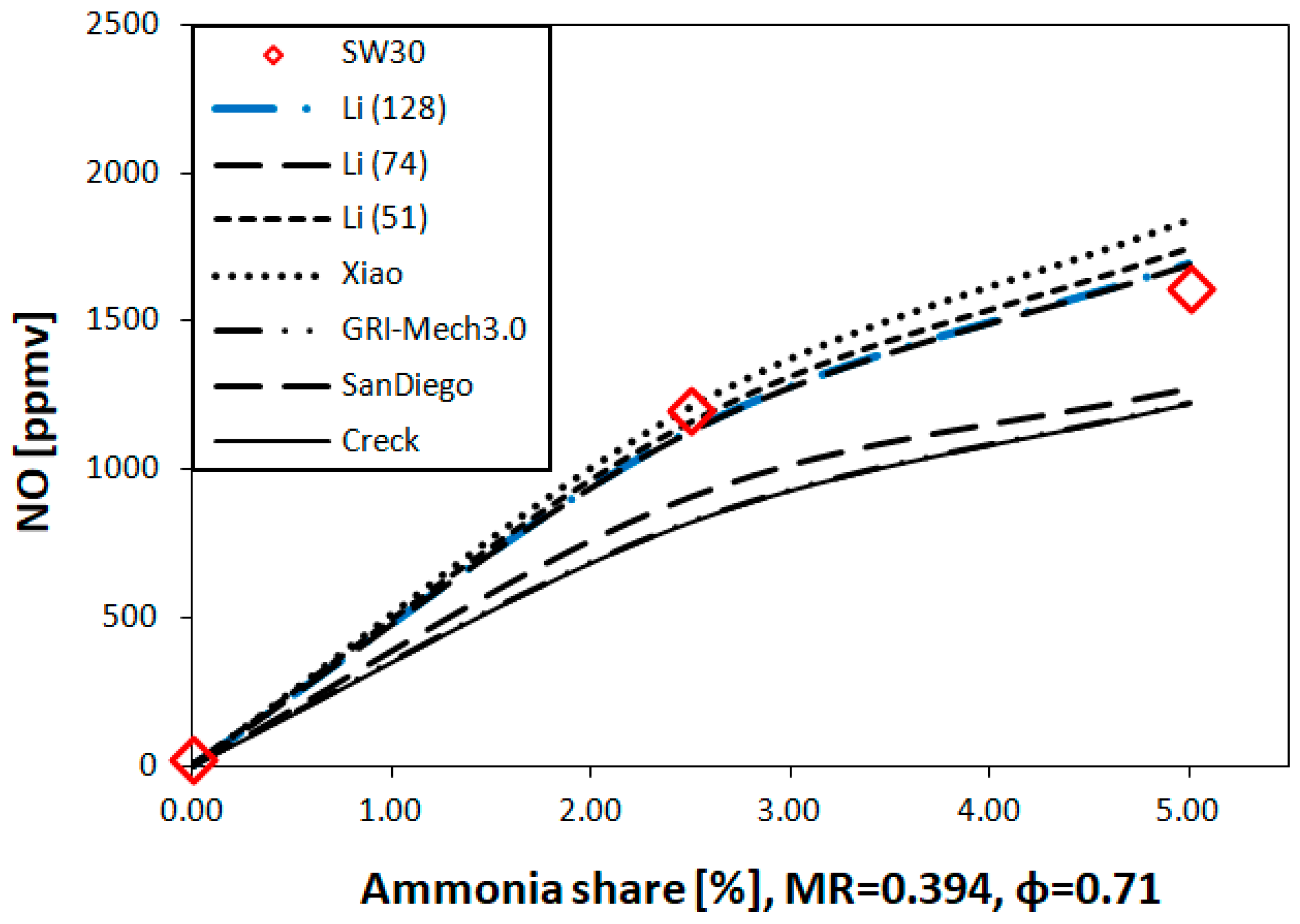
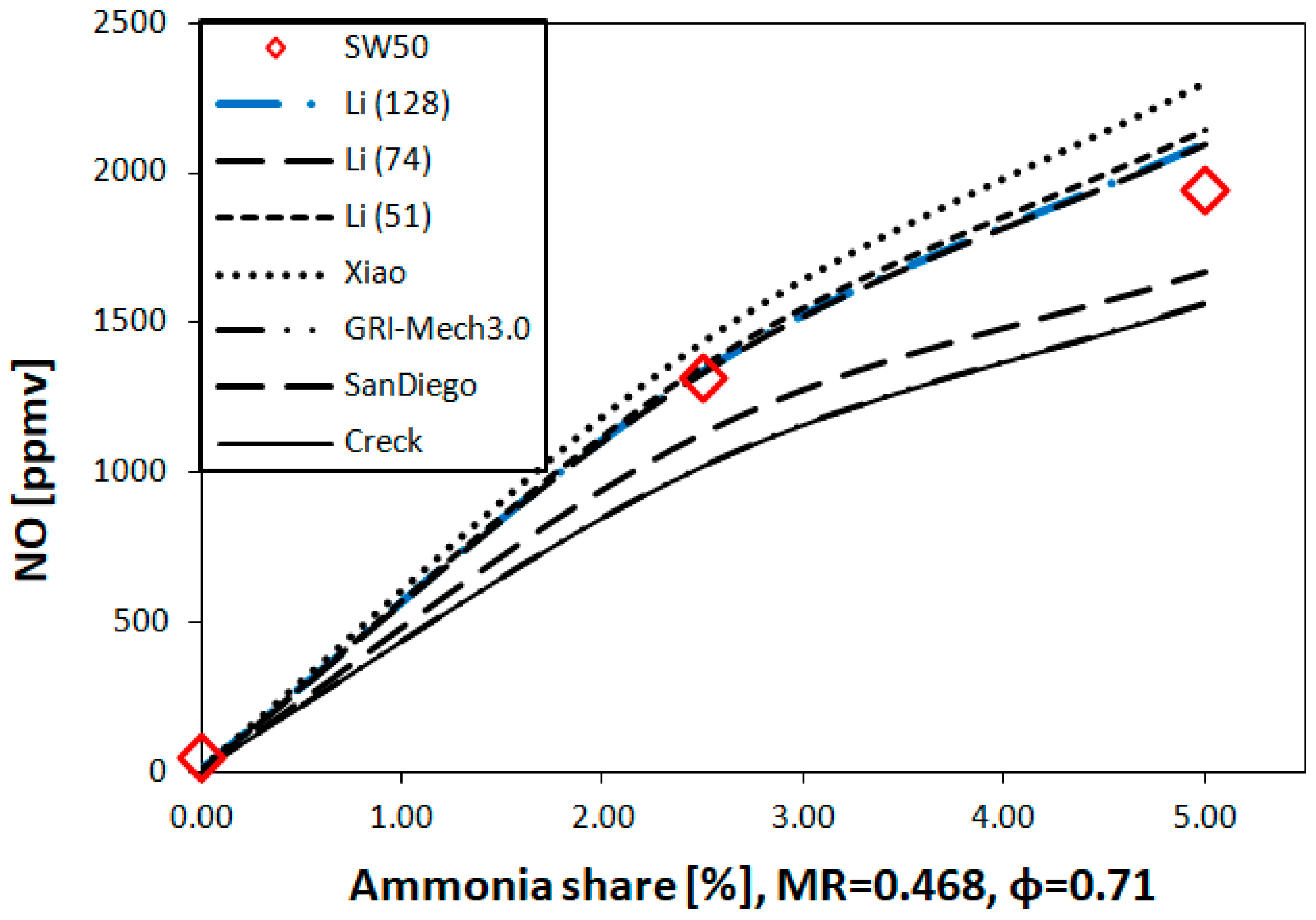
2.2.3. Kinetic Reaction Mechanisms
3. Results and Discussion
3.1. Experimental Tests
3.1.1. Flame Stability
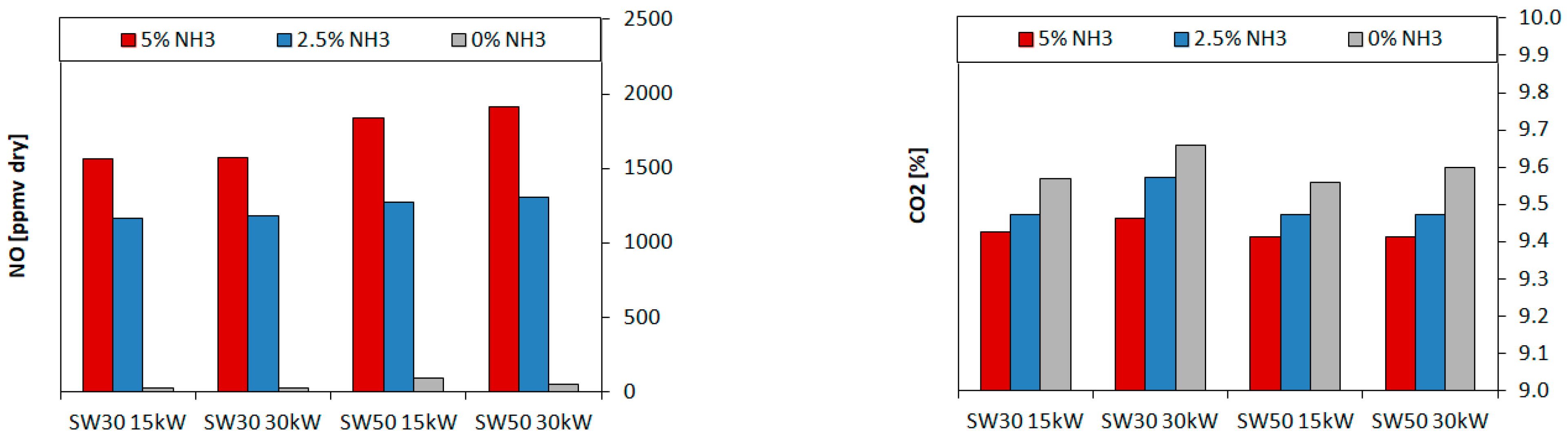
3.1.2. Overall Concentration
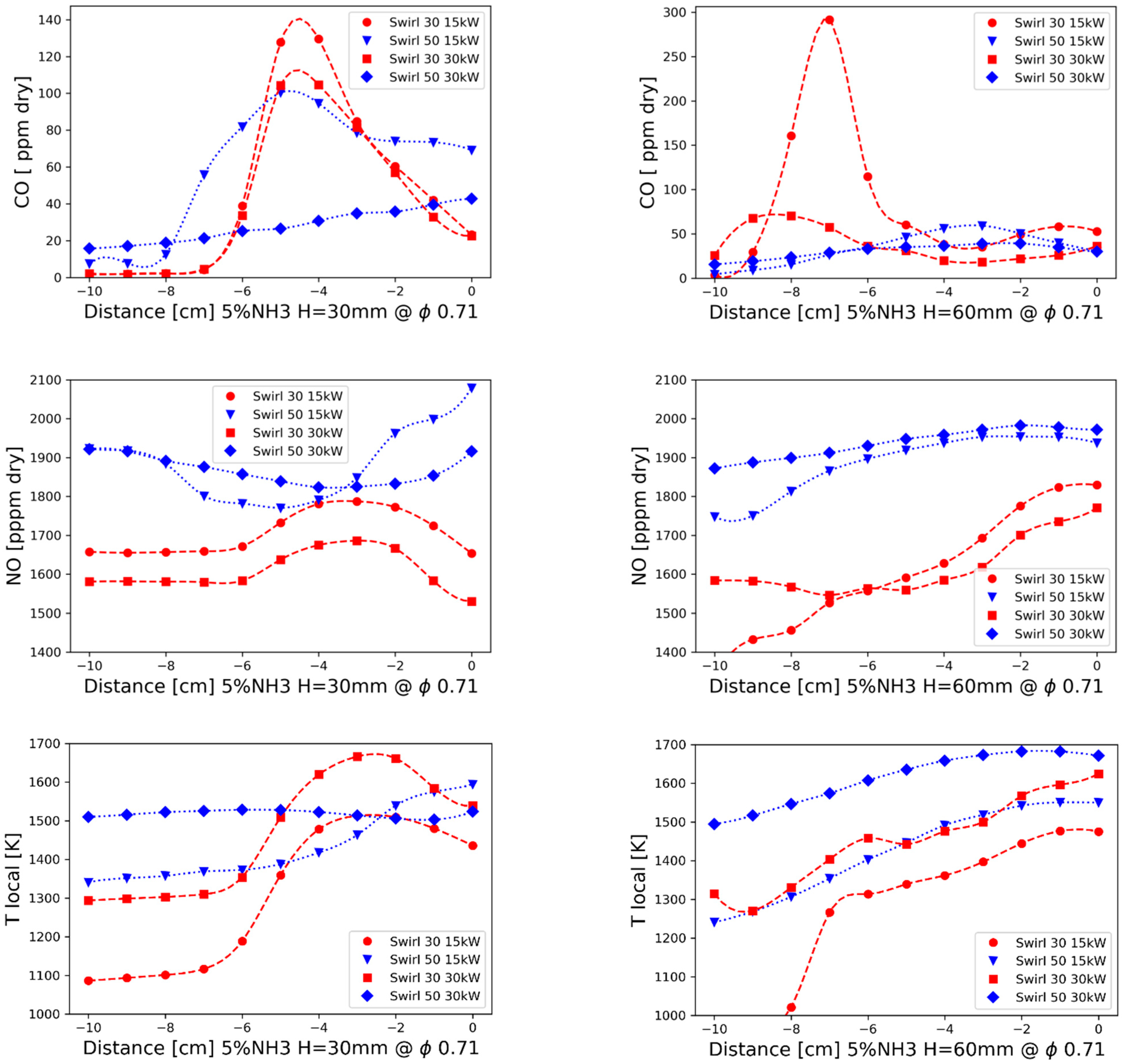
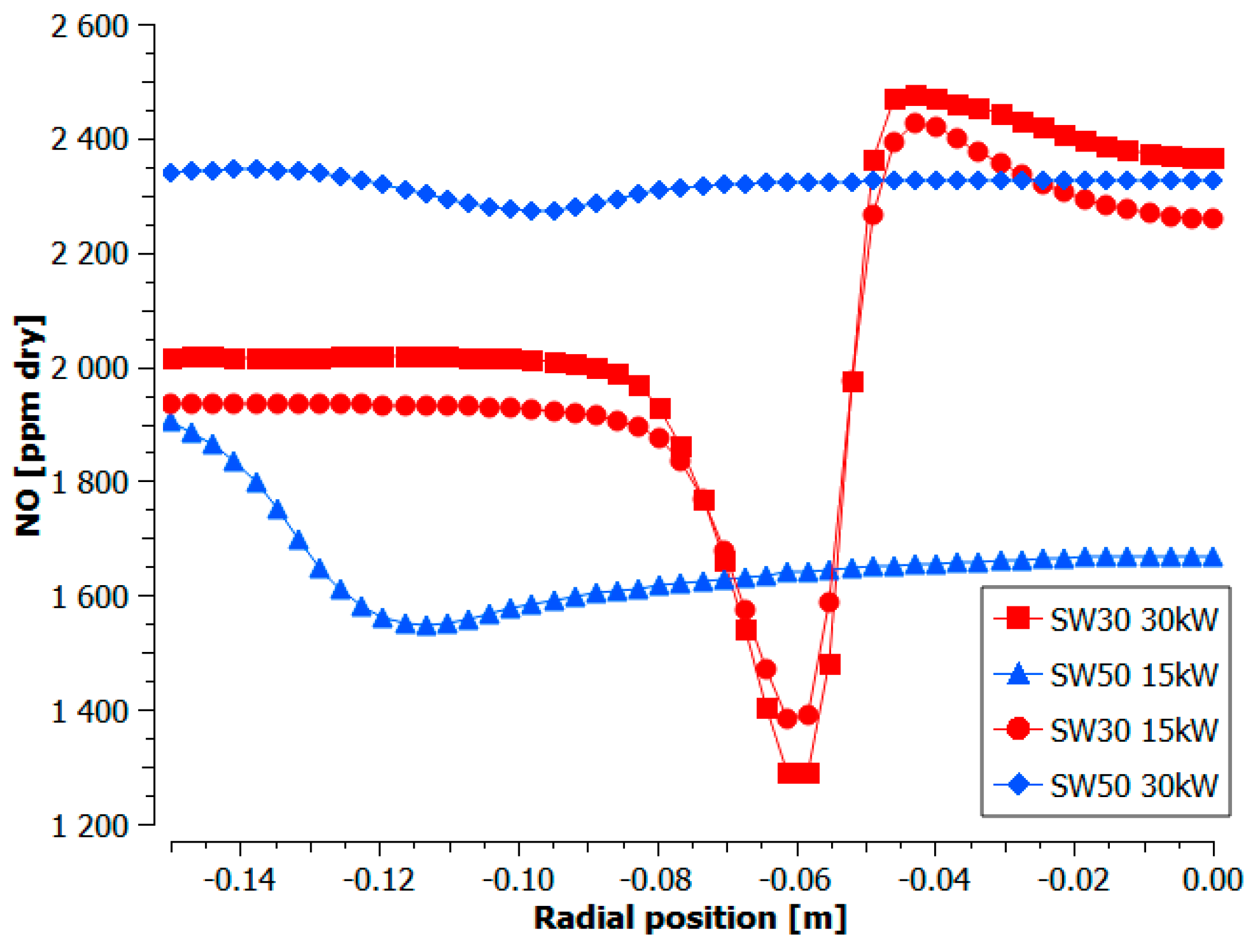
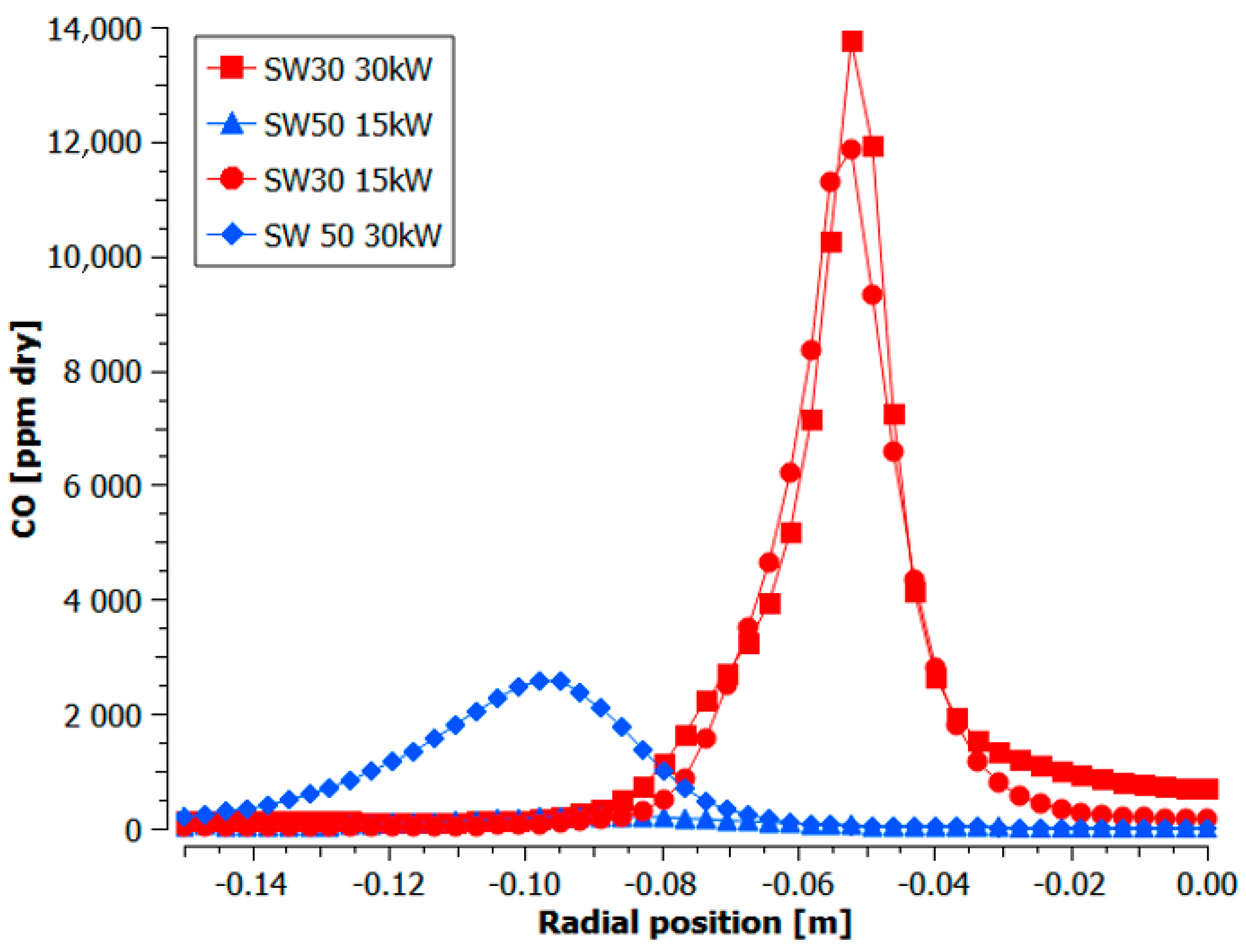
3.1.3. Distribution Measurements
3.2. Numerical Modelling
3.2.1. Concentration Profiles
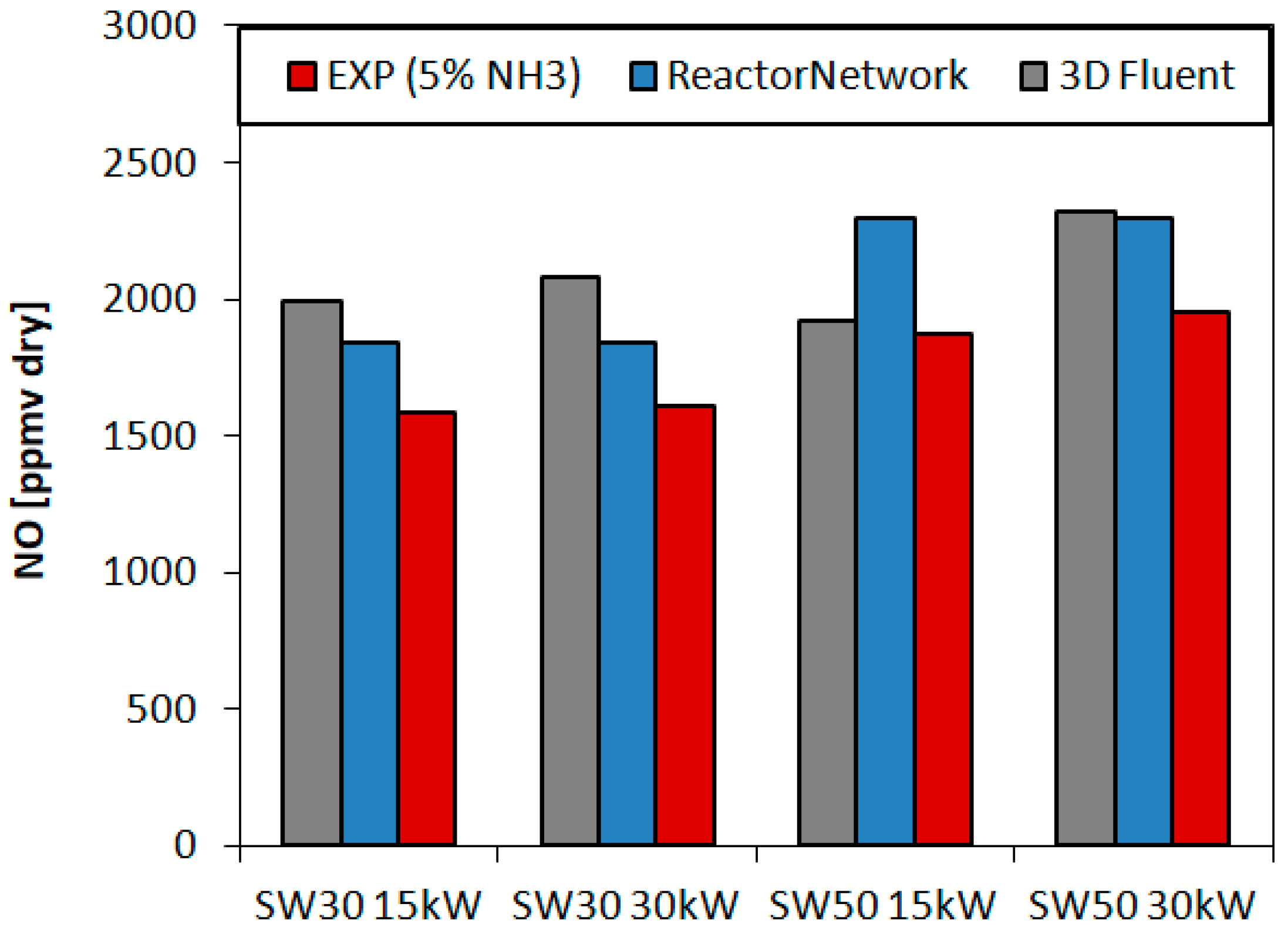
3.2.2. Overall Emission
4. Conclusions
- The lowest experimental emissions for 5% NH3 were achieved for burner 30SW (1585 ppmv) and it was comparable to the NO share for the doubled power in the fuel. The diameter of central recirculation zone for 30SW was 12 cm for the local CO measurements and the simulation outcome. The CTRZ location was corresponding to a significant rise in temperature and NO share. The presence of ORZ extended the residence time and lowered the local temperatures in the near-wall region due to heat losses, which decreased the resulting NO concentration
- The highest values of the experimental NO emissions were collected for 5% NH3 and SW50 (1950 ppmv), both for the experiments and the simulations. SW50 was characterized by an expanded internal recirculation zone, bounded to the combustor walls. Short residence time and high local temperatures near the walls increased the amount of formed NO, despite a greater ratio of the flow recirculation than that of SW30 (0.468 and 0.394).
- Boundary conditions connected with the heat exchange process need to be carefully defined in order to achieve a high accuracy of the NO predictions—both for 3D simulations and simplified reactor-based models.
- The presented reactor network has proven itself a useful and time-efficient method to investigate the influence of recirculation on NO emissions and to evaluate the mechanism’s prediction possibilities for the selected conditions. However, its stand-alone reliability is limited, so an input data set needs to be supplemented with a complimentary 3D modeling outcome.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| CSR | continuously stirred reactor |
| CTRZ | central recirculation zone |
| FGR | flue gas recirculation |
| LES | large eddy simulation |
| MFC | mass flow controller |
| MR | recirculation mass flow ratio |
| ORZ | outer recirculation zones |
| SNCR | Selective Non-Catalytic Reduction |
| VFD | variable frequency drive |
| BC | boundary condition |
References
- Czyzewski, P.; Slefarski, R.; Jojka, J.; Jankowski, R. Experimental and numerical calculations of two stage combustion process of syngas fuels with high content of ammonia. In Proceedings of the 25th European Biomass Conference and Exhibition, Stockholm, Sweden, 12–15 June 2017; Volume 2017. [Google Scholar] [CrossRef]
- Szewczyk, D.; Jankowski, R.; Ślefarski, R.; Chmielewski, J. Experimental study of the combustion process of gaseous fuels containing nitrogen compounds with the use of new, low-emission Zonal Volumetric Combustion technology. Energy 2015, 92, 3–12. [Google Scholar] [CrossRef]
- Szewczyk, D.; Ślefarski, R.; Jankowski, R. Analysis of the combustion process of syngas fuels containing high hydrocarbons and nitrogen compounds in Zonal Volumetric Combustion technology. Energy 2017, 121, 716–725. [Google Scholar] [CrossRef]
- Chiaramonti, D.; Marsh, R.; Runyon, J.; Pugh, D.; Beasley, P.; Hughes, T.; Bowen, P. Ammonia–methane combustion in tangential swirl burners for gas turbine power generation. Appl. Energy 2017, 185, 1362–1371. [Google Scholar] [CrossRef]
- Kurata, O.; Iki, N.; Matsunuma, T.; Inoue, T.; Tsujimura, T.; Furutani, H.; Kobayashi, H.; Hayakawa, A. Performances and emission characteristics of NH3–air and NH3CH4–air combustion gas-turbine power generations. Proc. Combust. Inst. 2017, 36, 3351–3359. [Google Scholar] [CrossRef]
- Vigueras-Zuniga, M.; Tejeda-Del-Cueto, M.-E.; Vasquez-Santacruz, J.-A.; Herrera-May, A.L.; Valera-Medina, A. Numerical Predictions of a Swirl Combustor Using Complex Chemistry Fueled with Ammonia/Hydrogen Blends. Energies 2020, 13, 288. [Google Scholar] [CrossRef]
- Mathieu, O.; Petersen, E.L. Experimental and modeling study on the high-temperature oxidation of Ammonia and related NOx chemistry. Combust. Flame 2015, 162, 554–570. [Google Scholar] [CrossRef]
- Xiao, H.; Howard, M.; Valera-Medina, A.; Dooley, S.; Bowen, P.J. Study on Reduced Chemical Mechanisms of Ammonia/Methane Combustion under Gas Turbine Conditions. Energy Fuels 2016, 30, 8701–8710. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Gutesa, M.; Xiao, H.; Pugh, D.; Giles, A.; Goktepe, B.; Marsh, R.; Bowen, P. Premixed ammonia/hydrogen swirl combustion under rich fuel conditions for gas turbines operation. Int. J. Hydrogen Energy 2019, 44, 8615–8626. [Google Scholar] [CrossRef]
- Ichikawa, A.; Hayakawa, A.; Kitagawa, Y.; Somarathne, K.K.A.; Kudo, T.; Kobayashi, H. Laminar burning velocity and Markstein length of ammonia/hydrogen/air premixed flames at elevated pressures. Int. J. Hydrogen Energy 2015, 40, 9570–9578. [Google Scholar] [CrossRef]
- Da Rocha, R.C.; Costa, M.; Bai, X.-S. Chemical kinetic modelling of ammonia/hydrogen/air ignition, premixed flame propagation and NO emission. Fuel 2019, 246, 24–33. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.; Bowen, P. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Xiao, H.; Chiaramonti, D.; Bowen, P.J. Study on premixed combustion characteristics of co-firing ammonia/methane fuels. Energy 2017, 140, 125–135. [Google Scholar] [CrossRef]
- Henshaw, P.F.; D’Andrea, T.; Mann, K.R.C.; Ting, D.S.-K. Premixed ammonia-methane-air combustion. Combust. Sci. Technol. 2005, 177, 2151–2170. [Google Scholar] [CrossRef]
- Okafor, E.C.; Naito, Y.; Colson, S.; Ichikawa, A.; Kudo, T.; Hayakawa, A.; Kobayashi, H. Experimental and numerical study of the laminar burning velocity of CH4–NH3–air premixed flames. Combust. Flame 2018, 187, 185–198. [Google Scholar] [CrossRef]
- Honzawa, T.; Kai, R.; Okada, A.; Valera-Medina, A.; Bowen, P.J.; Kurose, R. Predictions of NO and CO emissions in ammonia/methane/air combustion by LES using a non-adiabatic flamelet generated manifold. Energy 2019, 186, 115771. [Google Scholar] [CrossRef]
- Li, J.; Huang, H.; Kobayashi, N.; He, Z.; Osaka, Y.; Zeng, T. Numerical study on effect of oxygen content in combustion air on ammonia combustion. Energy 2015, 93, 2053–2068. [Google Scholar] [CrossRef]
- Xiao, H.; Wang, Z.; Chiaramonti, D.; Bowen, P.J. Study on Characteristics of Co-firing Ammonia/Methane Fuels under Oxygen Enriched Combustion Conditions. J. Therm. Sci. 2018, 27, 270–276. [Google Scholar] [CrossRef]
- Di Sarli, V.; Cammarota, F.; Salzano, E.; Di Benedetto, A. Explosion behavior of ammonia and ammonia/methane in oxygen-enriched air. Process. Saf. Prog. 2017, 36, 368–371. [Google Scholar] [CrossRef]
- Jójka, J.; Ślefarski, R. Dimensionally reduced modeling of nitric oxide formation for premixed methane-air flames with ammonia content. Fuel 2018, 217, 98–105. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2019, 37, 109–133. [Google Scholar] [CrossRef]
- Skreiberg, Ø.; Kilpinen, P.; Glarborg, P. Ammonia chemistry below 1400 K under fuel-rich conditions in a flow reactor. Combust. Flame 2004, 136, 501–518. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Harding, L.B.; Glarborg, P.; Miller, J.A. The role of NNH in NO formation and control. Combust. Flame 2011, 158, 774–789. [Google Scholar] [CrossRef]
- Sullivan, N.; Jensen, A.; Glarborg, P.; Day, M.S.; Grcar, J.F.; Bell, J.B.; Pope, C.J. Ammonia Conversion and NO x Formation in Laminar Coflowing Nonpremixed Methane-Air Flames. Combust. Flame 2002, 131, 285–298. [Google Scholar] [CrossRef]
- Song, Y.; Hashemi, H.; Christensen, J.M.; Zou, C.; Marshall, P.; Glarborg, P. Ammonia oxidation at high pressure and intermediate temperatures. Fuel 2016, 181, 358–365. [Google Scholar] [CrossRef]
- Konnov, A.A.; De Ruyck, J. Kinetic Modeling of the Thermal Decomposition of Ammonia. Combust. Sci. Technol. 2000, 152, 23–37. [Google Scholar] [CrossRef]
- Konnov, A.A. Implementation of the NCN pathway of prompt-NO formation in the detailed reaction mechanism. Combust. Flame 2009, 156, 2093–2105. [Google Scholar] [CrossRef]
- Li, B.; He, Y.; Li, Z.; Konnov, A.A. Measurements of NO concentration in NH3-doped CH4+air flames using saturated laser-induced fluorescence and probe sampling. Combust. Flame 2013, 160, 40–46. [Google Scholar] [CrossRef]
- Mendiara, T.; Glarborg, P. Ammonia chemistry in oxy-fuel combustion of methane. Combust. Flame 2009, 156, 1937–1949. [Google Scholar] [CrossRef]
- Di Sarli, V.; Marra, F.S.; Di Benedetto, A. Spontaneous oscillations in lean premixed combustors: Cfd simulation. Combust. Sci. Technol. 2007, 179, 2335–2359. [Google Scholar] [CrossRef]
- Somarathne, K.D.K.A.; Okafor, E.C.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Kobayashi, H. Emission characteristics of turbulent non-premixed ammonia/air and methane/air swirl flames through a rich-lean combustor under various wall thermal boundary conditions at high pressure. Combust. Flame 2019, 210, 247–261. [Google Scholar] [CrossRef]
- Xiao, H.; Howard, M.; Valera-Medina, A.; Dooley, S.; Bowen, P. Reduced Chemical Mechanisms for Ammonia/Methane Co-firing for Gas Turbine Applications. Energy Procedia 2017, 105, 1483–1488. [Google Scholar] [CrossRef]
- Pugh, D.; Bowen, P.; Valera-Medina, A.; Giles, A.; Runyon, J.; Marsh, R. Influence of steam addition and elevated ambient conditions on NOx reduction in a staged premixed swirling NH3/H2 flame. Proc. Combust. Inst. 2019, 37, 5401–5409. [Google Scholar] [CrossRef]
- Khateeb, A.A.; Guiberti, T.F.; Zhu, X.; Younes, M.; Jamal, A.; Roberts, W.L. Stability limits and exhaust NO performances of ammonia-methane-air swirl flames. Exp. Therm. Fluid Sci. 2020, 114, 110058. [Google Scholar] [CrossRef]
- Baej, H.; Syred, N.; Bowen, P.; Valera-Medina, A.; March, R. CFD predictions of Swirl burner aerodynamics with variable outlet configurations. Int. J. Energy Technol. 2019, 31–43. [Google Scholar] [CrossRef]
- Degeneve, A.; Jourdaine, P.; Mirat, C.; Caudal, J.; Vicquelin, R.; Schuller, T. Effects of a Diverging Cup on Swirl Number, Flow Pattern, and Topology of Premixed Flames. J. Eng. Gas. Turbines Power 2018, 141, 031022. [Google Scholar] [CrossRef]
- Goodwin, D.G.; Moffat, H.K.; Speth, R.L.; Weber, B.W. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes. Version 2.4.0. 2018. Available online: https://www.cantera.org (accessed on 22 January 2021). [CrossRef]
- Ranzi, E.; Frassoldati, A.; Grana, R.; Cuoci, A.; Faravelli, T.; Kelley, A.P.; Law, C. Hierarchical and comparative kinetic modeling of laminar flame speeds of hydrocarbon and oxygenated fuels. Prog. Energy Combust. Sci. 2012, 38, 468–501. [Google Scholar] [CrossRef]
- Li, R.; Konnov, A.A.; He, G.; Konnov, A.A.; Zhang, D. Chemical mechanism development and reduction for combustion of NH3/H2/CH4 mixtures. Fuel 2019, 257, 116059. [Google Scholar] [CrossRef]
- Xiao, H.; Valera-Medina, A.; Bowen, P.; Dooley, S. 3D Simulation of Ammonia Combustion in a Lean Premixed Swirl Burner. Energy Procedia 2017, 142, 1294–1299. [Google Scholar] [CrossRef]
- Okafor, E.C.; Somarathne, K.K.A.; Ratthanan, R.; Hayakawa, A.; Kudo, T.; Kurata, O.; Iki, N.; Tsujimura, T.; Furutani, H.; Kobayashi, H. Control of NOx and other emissions in micro gas turbine combustors fuelled with mixtures of methane and ammonia. Combust. Flame 2020, 211, 406–416. [Google Scholar] [CrossRef]
- Slefarski, R.; Czyzewski, P.; Golebiewski, M. Experimental study on combustion of CH4/NH3 fuel blends in an industrial furnace operated in flameless conditions. Therm. Sci. 2020, 24, 3625–3635. [Google Scholar] [CrossRef]




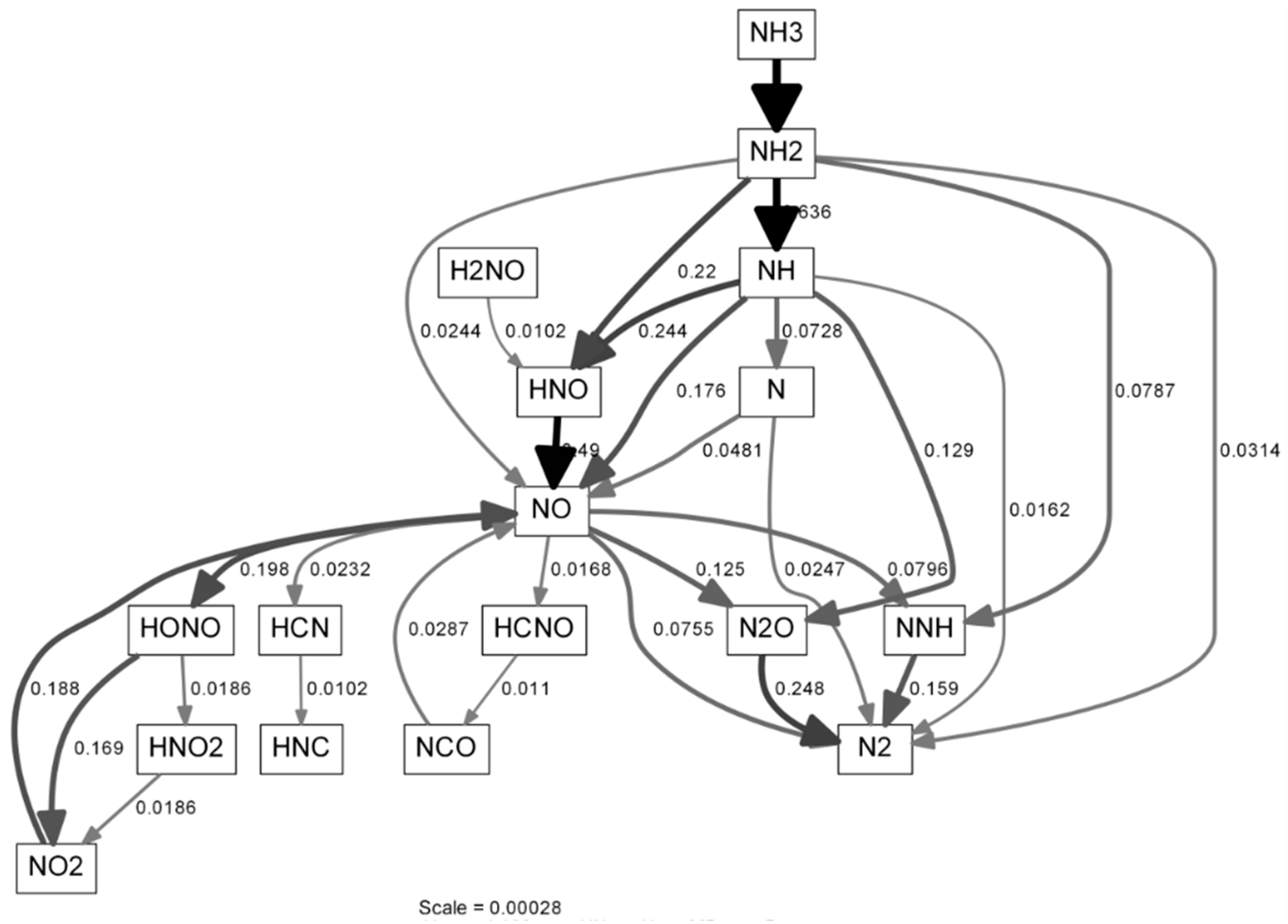
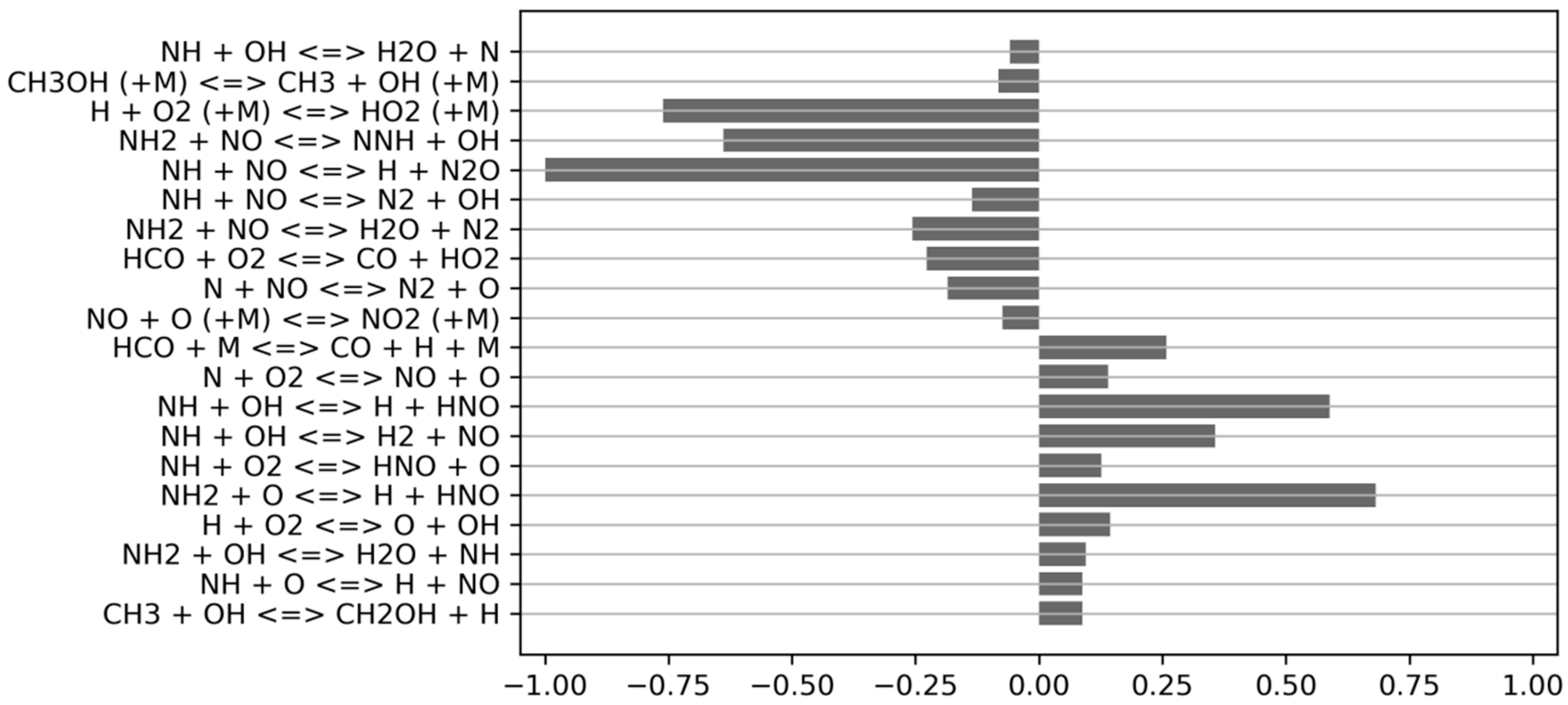
| Parameter | Range | Unit |
|---|---|---|
| Inlet temperature | 473 | K |
| Burners | Swirl 30° (SW30), Swirl 50° (SW50) | deg |
| Power | 15, 30 | kW |
| Axial position | 30, 60, 110 | mm |
| Equivalence ratio | 0.71 | - |
| Fuel composition | 0.0, 2.5, 5.0 NH3 (CH4 balance) | % |
| Parameter | Range | Unit |
|---|---|---|
| Model | 0D IdealGasReactor Network 3D EDC RSM Fluent | - |
| Fuel | 0.0–5.0 NH3, CH4 balance | % |
| Inlet temperature | 473 | K |
| Equivalence ratio ϕ | 0.71 | - |
| Power in the fuel | 15, 30 | kW |
| Mass flow recirculation ratio (MR) | 0.0, 0.1, 0.2, 0.4 | - |
| Name | Li | Xiao | Creck | SanDiego | GRI-Mech3.0 | ||
|---|---|---|---|---|---|---|---|
| Species | 128 | 74 | 51 | 48 | 115 | 66 | 52 |
| Reactions | 957 | 634 | 420 | 500 | 2141 | 321 | 325 |
| Test Case | τ [s] | MR [-] | Snr N [-] | Snr T [-] | Snr S [-] | Snr ISO [-] |
|---|---|---|---|---|---|---|
| SW50 30 kW | 0.506 | 0.513 | 0.506 | 0.653 | 0.654 | 0.61 |
| SW50 15 kW | 1.190 | 0.424 | 0.730 | 0.660 | 0.640 | 0.61 |
| SW30 30 kW | 0.756 | 0.414 | 0.347 | 0.678 | 1.296 | 1.26 |
| SW30 15 kW | 1.420 | 0.373 | 0.384 | 0.700 | 1.300 | 1.26 |
| Test Case | τRN ave [s] | Thot wall [K] | MRave [-] | τR0 [ms] | TR0 [K] | TR OUT [K] | NO [ppm dry] | N2O, NO2 [ppm dry] | NOexp [ppm dry] |
|---|---|---|---|---|---|---|---|---|---|
| SW50 | 0.848 | 1230 | 0.0 | 87 | 1707 | 515 | 2246 | 18 | - |
| 0.468 | 59 | 1651 | 520 | 2098 | 19 | 1914 | |||
| SW30 | 1.088 | 1050 | 0.0 | 109 | 1597 | 435 | 1863 | 30 | - |
| 0.394 | 79 | 1546 | 439 | 1703 | 32 | 1598 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jójka, J.; Ślefarski, R. Emission Characteristics for Swirl Methane–Air Premixed Flames with Ammonia Addition. Energies 2021, 14, 662. https://doi.org/10.3390/en14030662
Jójka J, Ślefarski R. Emission Characteristics for Swirl Methane–Air Premixed Flames with Ammonia Addition. Energies. 2021; 14(3):662. https://doi.org/10.3390/en14030662
Chicago/Turabian StyleJójka, Joanna, and Rafał Ślefarski. 2021. "Emission Characteristics for Swirl Methane–Air Premixed Flames with Ammonia Addition" Energies 14, no. 3: 662. https://doi.org/10.3390/en14030662
APA StyleJójka, J., & Ślefarski, R. (2021). Emission Characteristics for Swirl Methane–Air Premixed Flames with Ammonia Addition. Energies, 14(3), 662. https://doi.org/10.3390/en14030662







