Abstract
This paper presents research on the development of pyrotechnic compositions producing an acoustic effect. These types of compositions are used in firecrackers to imitate a cannon shot—they are most frequently used during military exercises. The research was based on a mathematical model of an experiment. For environmental reasons, the replacement of the harmful oxidant Ba(NO3)2 by KClO4 and NH4ClO4 was modelled. The compositions were tested for reliability and evaluated in terms of friction sensitivity and burning rate. This allowed for the verification of the effectiveness of the modelling carried out. Optimum compositions were selected for further research.
1. Introduction
A pyrotechnic composition is a material or physical mixture of materials that may undergo a mutual, non-detonative, and self-sustaining chemical reaction producing an effect by heat, light, sound, and others [1,2].
Much of the original use of pyrotechnic compositions was for military applications [3]. The forerunners of the development of such products were the Chinese [4], who popularised their use in the USA. The first countries to use firecrackers for military exercises were the USA and Japan [5,6,7]. Potassium chlorate (VII) and (V), aluminium, and sulphur or antimony sulphide were used to make firecrackers. An alloy with 50% magnesium and 50% aluminium called “magnalium” (PAM) also became very popular [7]. The use of barium nitrate produced a very bright light, with firecrackers of this type being used in the German army [8].
Firecrackers were mainly made by hand by weighing out mixture components in selected proportions and putting them into paper casings [9].
Ternary graphs [10,11] are very useful in developing pyrotechnic compositions with optimum properties. They make it easier to discover relationships between different characteristics and the percentage of components. To obtain the planned final effect, mathematical models are used, which have been widely described in the literature for various three-component mixtures [12,13,14,15].
2. Materials and Methods
2.1. Selection of Components for Pyrotechnic Compositions
Components to create pyrotechnic compositions imitating the sound effect were selected based on the literature analysis and authors’ own concept. Such components most frequently contain an oxidant and a fuel.
Oxidants containing large amounts [16] of “active oxygen” (free oxygen released during decomposition) and not hygroscopic were included. The following components were selected:
- -
- from the group of oxidants: potassium chlorate (VII) (KClO4), iron (III) oxide (Fe2O3),
- -
- from the group of flammable substances: aluminium–magnesium powder (PAM), aluminium powder (Al), sulphur (S).
Ba(NO3)2 is the most commonly used in many pyrotechnic mixtures. This oxidizer contains 30.6% of active oxygen (free oxygen released during decomposition). It is less active than the salts of chloric or perchloric acid generally used in chemical technology, but mixtures containing it are less sensitive. From the group of perchlorates, KClO4 turned out to be the most useful. It occupied the leading place among oxidants due to its large amount, 46%, of active oxygen. It has a high density. In addition to potassium perchlorate, NH4ClO4 is an oxidant of great importance. It contains 34.2% active oxygen; it has a low molecular weight. The choice was mainly based on health aspects and the resulting necessity to replace the toxic barium nitrate with other oxidants. The oxidants that were taken into account contained a large amount of oxygen and were not hygroscopic.
In this study, a “reference composition” was adopted (M-0) containing [17,18]:
- -
- Ba(NO3)2 64%
- -
- PAM 18%
- -
- S 18%.
This is the basic composition of a firecracker producing a sound effect (imitating a cannon shot—with a hearing distance of 1.5 to 6 km) used during military exercises [9].
On the basis of the oxygen balance of this composition and in line with the rule for selecting components for pyrotechnic compositions, all components were classified into two groups (Table 1).

Table 1.
Oxidants and flammable substances selected for the study.
2.2. Thermodynamic Parameters of the Reference Composition
By knowing the oxygen balance and thermodynamic parameters of compositions, it is possible to conduct an approximate selection of components in order to obtain a composition with the desired parameters.
Theoretical calculations were based on the decomposition formula. The complete combustion of the components was assumed. By using the following formula:
the following thermodynamic parameters were obtained (Table 2). Calculations of thermodynamic parameters were made on the basis of Polish standards [19,20]:
7Ba(NO3)2 + 10Mg + 10Al + 5S = 7BaO + 10MgO + 5Al2O3 + 5SO2 + 7N2↑

Table 2.
Thermodynamic parameters of the reference composition.
The oxygen balance is an important parameter that determines the quality of compositions. Given that the majority of pyrotechnic compositions have a negative oxygen balance and that the reference composition is in line with the requirements to be met by imitating agents, this parameter served as a basis for selecting components for new compositions. Calculations for the groups specified in Table 1 were made. New compositions are presented in Table 3. The new compositions are made of three and four components. The catalytic amount of iron (III) oxide was used, i.e., 2–5%.

Table 3.
Components of new compositions.
All the compositions have a negative oxygen balance and similar values to the reference composition.
2.3. Preparation of Compositions
The pyrotechnic compositions were prepared by hand using 80 or 100 g portions. Individual components were weighed out in the amounts specified in the calculations. The components were mixed manually on igelit cloths placed on a special table. The next step involved filling the pyrotechnic composition. A brass funnel was inserted into holes in paper casings, which were placed on a special wooden table. Then the accurately weighed composition was poured into the casings. The casings filled with the composition were then handloaded. In the case of 100 g firecrackers, handloading involved gluing cardboard rings into the casings filled with the composition. Cardboard rings were glued with a semi-automatic tool. Handloading 80 g firecrackers was more complex. Carpenter’s glue was used to glue a fuse to the casings filled with the pyrotechnic composition. The inside of the casing chamber (from the side from which the composition was filled) was covered with carpenter’s glue. Then a semi-automatic tool was used to glue the fuse. Once the fuse was glued in place, an additional reinforcing ring was glued to the casing. Then the top part of the fuse ending with a head made of an incendiary and rubbing composition was rolled and placed in the chamber of the firecracker paper casing.
3. Results and Discussion
3.1. Initial Tests
When creating new compositions, the main issue is associated with the determination of the sensitivity of the composition to external stimuli. This is preceded by appropriate tests. Due to the lack of information on new compositions, this paper used sensitivity to heat as a criterion describing ignitability. Thermal tests for the new compositions were performed to identify this risk. The components were mixed in a 50/50 ratio and the resulting samples were heated using a sand bath. Notes were taken at temperatures of 293 K, 323 K, and 343 K. The test results are given in Table 4.

Table 4.
Testing two-component compositions for thermal resistance.
The tests revealed that at the mixing phase (the main step in the composition creation process), there is no risk of self-ignition associated with accidental rubbing.
Initial tests were performed after examining the main criterion. They involved the preparation of the new compositions and checking for reliability. These tests were qualitative and involved a visual and acoustic evaluation of the effects produced by individual compositions and their comparison with the reference composition (M-0). The reliability of the firecrackers was evaluated based on the following rules:
- -
- acoustic evaluation was performed by the same person,
- -
- the distance from the source of the sound and the place of noting the effect was always maintained,
- -
- appropriate time intervals were used, i.e., there was a break of several minutes after each series of three tests (this break was needed to improve auditory perception).
The following scale was applied to evaluate the pyrotechnic compositions producing the appropriate sound effect:
- - no effect (failure to ignite the composition due to a faulty fuse head, flash fire, or combustion),
- + bang effect comparable to or weaker than M-0 (good),
- ++ bang effect stronger than M-0 (better),
- +++ the strongest bang effect (best).
The test results are given in Table 5.

Table 5.
Tests for the reliability of the Group 1 and Group 2 compositions.
In the case of composition 1-III, a negative effect was observed, i.e., the bang effect reoccurred after a short time—it was audible twice (in two stages). This could have resulted from poor pyrotechnic composition homogenisation.
The tests showed that Fe2O3 improves the sound effect of the compositions. It is noted that the compositions are reliable when the flammable substance is Al.
Following the observation that Fe2O3 has a positive impact on the quality of compositions, iron (III) oxide was tested in large quantities. However, it was no longer a catalyst but an oxidant in combination with potassium chlorate (VII).
For reliability tests, the results in which increasing the amount of Fe2O3 brought the oxygen balance of the composition closer to that of the reference composition were assumed. Iron (III) oxide was used in 50/50 and 30/70 ratios to the oxidant.
The test results and the calculated oxygen balance of compositions 1-I and 2-I containing Fe2O3 are presented in Table 6.

Table 6.
Tests for the reliability of compositions containing Fe2O3.
These compositions showed that adding Fe2O3 improved the sound effect of the modified products.
The exact percentage of individual components to be used to select the highest quality product is difficult to determine due to a large number of components.
A large number of components necessitates performing numerous experiments. Experiments involve testing one variable, but their number increases exponentially when four components need to be used. The interpretation of the results is also troublesome. For this reason, the use of the latest method for selecting composition components seems to be necessary. A mathematical method was used to obtain low-cost compositions that would all meet the expected requirements (simulating the sound effect).
3.2. Formulation of a Mathematical Plan for the Experiment
The initial tests made it possible to determine the boundary conditions for each component in order to prepare a mathematical plan for the experiment.
The coordinate points of the experiment plan were found in line with the algorithm proposed in Technometrics [21]. First, all possible combinations of lower (ai) and higher (bi) levels of components were made, omitting the level of one component in each such combination. The total number of different combinations was k·2k−1. Then, the resulting k·2k−1 combinations were used to select only those combinations for which the sum of pyrotechnic composition components was less than 100%. Omitted component xi was assigned values ci from the <ai, bi> range to bring the sum to 100%. If filling value ci was not within this range, the combination was omitted. The experimental plan points thus obtained satisfied the condition
and were located at the vertices of a polyhedron of dimension k − 1. The calculations were based on assuming variation limits for individual components. The variation limits were determined based on the reliability tests. The list of variation limits is provided in Table 7.

Table 7.
Variation range for individual components.
Based on the variability limits, the following compositions for individual groups were obtained through combinations. These compositions are given in Table 8 and Table 9.

Table 8.
Plan of M-I compositions acc. to the mathematical model of the experiment.

Table 9.
Plan of M-II compositions acc. to the mathematical model of the experiment.
3.3. Control Tests
To confirm the applicability of the mathematical model, the planned compositions were evaluated in terms of their practical use. Reliability was used as the main parameter, while the burning rate of the composition was used to confirm the quality of this parameter. The friction sensitivity of the composition was also evaluated to verify the safety of the mixing process and the process of filling firecracker casings.
3.4. Reliability
To check reliability, sound effect tests were conducted for the selected compositions. The firecrackers filled with the compositions were tested according to general requirements using a simplified two-level assessment scale (“yes” or “no”), i.e., in terms of their reliability or lack thereof. The 100 g firecrackers (ZT) were ignited by pulling the rubbing wire of the fuse, with the effect produced being noted down after 15–20 s (the time after which the paper casing of the firecracker should explode, imitating a cannon shot). The 80 g firecrackers (ZL) were ignited by rubbing the fuse head against the striking surface of a matchbox, with the effect produced being noted down after the time specified above. The effect was considered positive if a clear and rather loud bang was heard. If the sound was weak compared to M-0 or inaudible at all, the product was considered unreliable.
3.5. Koenen Friction Sensitivity Test Using the Peters Apparatus
In the technological process, special care is taken to ensure that compositions are not overly sensitive to mechanical stimuli. Therefore, for safety reasons, the pyrotechnic compositions were tested for friction sensitivity.
The Koenen friction sensitivity test [22] involves the determination of the lowest punch pressure in kg at which deflagration, flash fire, etc., occurs in six tests. Measurements are taken by placing the tested material (in a small specific amount) on a porcelain plate with grooves and applying an appropriate load to the apparatus arm.
3.6. Burning Rate
An important parameter affecting the performance of the product is the burning rate of the composition (the higher it is inside the firecracker casing, the more beneficial the effect). Therefore, for the sake of product quality, burning rate tests were conducted and the mean value was determined.
Appropriate weighted amounts of pyrotechnic compositions were placed in pipes (PVC, LG type, dimensions øext/øint 6.0/5.8 mm) with a flame retardant bed containing 40% antimony, 40% iron (III) oxide, and 20% potassium chlorate (VII). The tested pyrotechnic compositions were ignited by a Class 0.2 A fuse head. Time measurements were taken with an accuracy of 0.5 s. The length of the measurement section was 200 mm. The result was an arithmetic mean of three measurements calculated with a stopwatch, expressed in cm/s. The test results are presented in Table 10 and Table 11.

Table 10.
Qualitative and quantitative evaluation of M-I pyrotechnic compositions.

Table 11.
Qualitative and quantitative evaluation of M-II pyrotechnic compositions.
The tests revealed that the compositions obtained through mathematical calculations produced the desired effects. The applicability of the mathematical plan for the experiment was confirmed.
3.7. Optimization of Pyrotechnic Compositions
To achieve the optimum composition, the objective is to develop a product with good performance.
The composition has to have little friction sensitivity, but it must also burn at the right rate in order to meet the requirements for safety and performance.
Based on the tests and the M-0 composition, boundary parameters were adopted as a compromise between safety and performance.
The following boundary parameters were determined:
- -
- friction equal to or higher than 2 kg,
- -
- mean burning rate equal to or less than 0.8 cm/s.
The aforementioned boundary parameters were used to assume the following designations (Table 12) for optimization purposes:

Table 12.
Designations used for optimization.
Designations for tested properties (identical for all compositions):
Y1—mean burning rate,
Y2—friction.
The following mathematical model was used to describe the experimental data
where:
- Yn—designated composition property,
- zi,zj—i-th and j-th composition component in coded units,
- ai,aj—regression coefficients,
- e—the so-called random component resulting from failure to adapt the model to the experimental data.
The transition from natural components Xi to coded ones Zi is defined by the following relationship.

Table 13.
Values for Xoi and ΔX.
The calculated values of regression coefficients and basic statistical evaluation parameters of the obtained models:
- R—coefficient of multiple correlation,
- sR—standard deviation of the residual component,
- sR(%)—standard deviation as a plan of the value and mean of the optimised value.
are shown in Table 14, i.e.,

Table 14.
Values of regression coefficients and evaluation parameters of the obtained mathematical models.
Identical values of the regression coefficients reflect a good fit of the models to the experimental data. Examples of calculation results are illustrated in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6. These figures show the relationship between the mean burning rate (cm/s) and friction (kg) and the content of composition components (%).
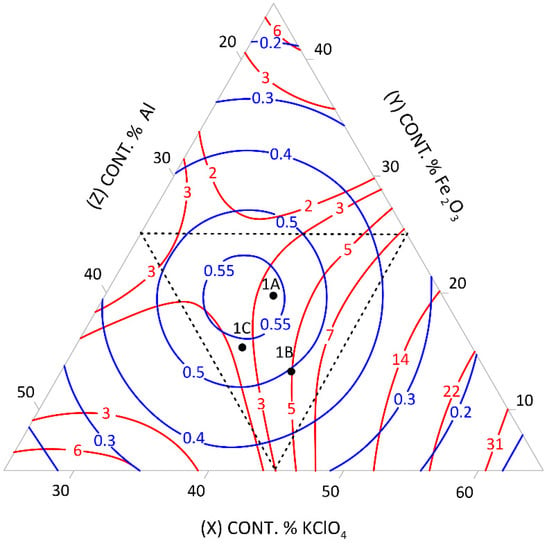
Figure 1.
Relationship between the mean burning rate (cm/s)—blue line, friction (kg)—red line, and the content of pyrotechnic composition components (%)—triangle sides, 1A, 1B, 1C—selected control pyrotechnic compositions for which friction and burning rate tests were performed, at the fixed sulphur weight content of 15%.
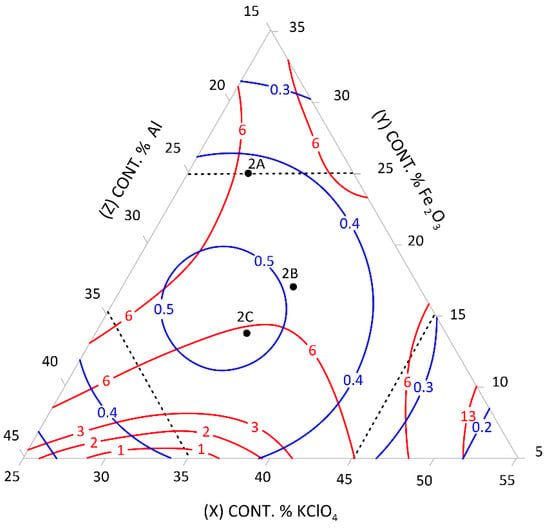
Figure 2.
Relationship between the mean burning rate (cm/s)—blue line, friction (kg)—red line, and the content of pyrotechnic composition components (%)—triangle sides, 2A, 2B, 2C—selected control pyrotechnic compositions for which friction and burning rate tests were performed at the fixed sulphur weight content of 25%.
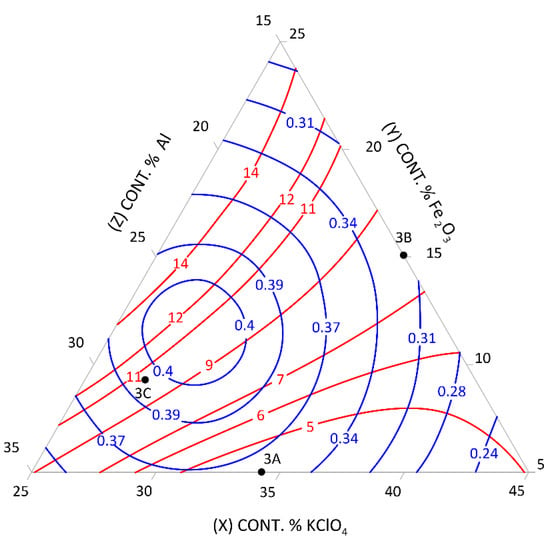
Figure 3.
Relationship between the mean burning rate (cm/s)—blue line, friction (kg)—red line, and the content of pyrotechnic composition components (%)—triangle sides, 3A, 3B, 3C—selected control pyrotechnic compositions for which friction and burning rate tests were performed at the fixed sulphur weight content of 35%.
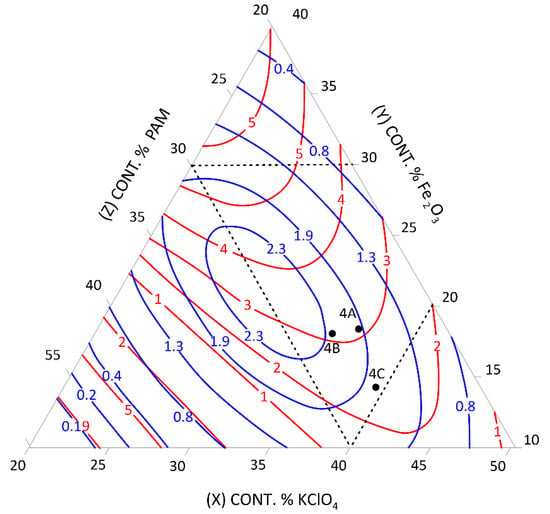
Figure 4.
Relationship between the mean burning rate (cm/s)—blue line, friction (kg)—red line, and the content of pyrotechnic composition components (%)—triangle sides, 4A, 4B, 4C—selected control pyrotechnic compositions for which friction and burning rate tests were performed at the fixed sulphur weight content of 20%.
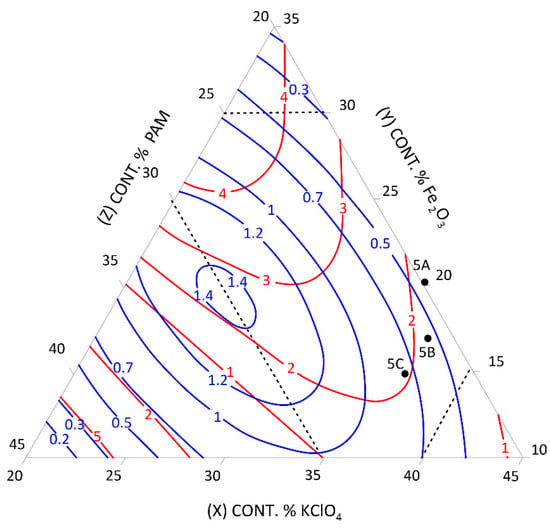
Figure 5.
Relationship between the mean burning rate (cm/s)—blue line, friction (kg)—red line, and the content of pyrotechnic composition components (%)—triangle sides, 5A, 5B, 5C—selected control pyrotechnic compositions for which friction and burning rate tests were performed at the fixed sulphur weight content of 25%.
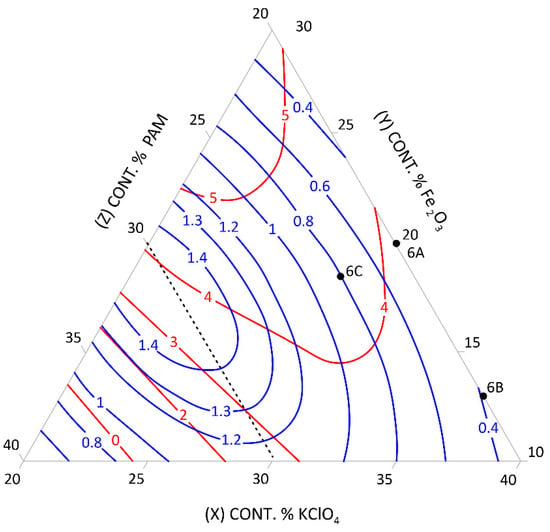
Figure 6.
Relationship between the mean burning rate (cm/s)—blue line, friction (kg)—red line, and the content of pyrotechnic composition components (%)—triangle sides, 6A, 6B, 6C—selected control pyrotechnic compositions for which friction and burning rate tests were performed at the fixed sulphur weight content of 30%.
For the sake of clarity, the following designations were used:
- -
- mean burning rate—blue line,
- -
- friction—red line,
- -
- percentage of individual composition components—triangle side.
One of the components (sulphur) was at a fixed level, but it varied between the compositions (Table 15).

Table 15.
List of designations.
To select the desired mixture, the figure can be used to determine the optimum composition by reading percentages of component contents from individual sides of the triangle at the right friction and mean burning rate.
3.8. Checking the Optimum Area
The calculation results presented graphically in Figure 1, Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 were used to select new compositions meeting the requirements specified in Section 3.7 for further tests.
The data from the figures were read only for the area covered by the plan for the experiment. This area is specified on the figures by dotted lines. The appropriateness of limiting the considerations to this area resulted from the mathematical model accuracy—the further away from the experimental area, the lower the mathematical reliability.
Three variants were selected from each figure to verify the quality of the calculations. The selected compositions were also subjected to the tests described in Section 3.3 to verify the quality of the calculations. The test results and the values read from the figures (including the content of pyrotechnic composition components) are presented in Table 16 and Table 17.

Table 16.
Test results concerning friction and mean burning rate of M-1 compositions obtained through the mathematical model.

Table 17.
Test results concerning friction and mean burning rate of M-2 compositions obtained through the mathematical model.
The results of the tests show some deviations from the values read from the figures. Inaccurate drying of KClO4 or inaccurate component mixing may have had a negative effect on the repeatability of the results for compositions M-1 and M-2. Components have different fineness levels so their inaccurate mixing could have caused coarser particles not to be distributed in the entire mass, thus increasing sensitivity. The effect of fineness on combustion is also significant. If components are ground well, they can come into close contact, facilitating the stimulation of combustion. In this case, components of different thicknesses and their inaccurate mixing may have caused the opposite, affecting the test results.
To sum up, it can be said that despite certain deviations resulting from a large measurement error, the mathematical selection of compositions allows their properties to be predicted well.
4. Conclusions
The paper attempts to apply a mathematical model in order to develop pyrotechnic compositions used to imitate a sound effect. New compositions were selected for tests in order to replace Ba(NO3)2 used in this type of firecracker.
The selected components of the new compositions formed four-component systems, which made it necessary to increase the number of tests used to evaluate the effect produced by firecrackers. As a result, it was useful to apply mathematical modelling, which allowed the number of combinations to be minimised and the number of experiments to be reduced. For optimisation purposes, performance values were used, i.e.,
- -
- friction sensitivity—this makes it possible to determine manufacturing process safety (provides information on the sensitivity of compositions),
- -
- burning rate of the composition—this phenomenon makes it possible to evaluate its impact on the effect imitating an explosion process (makes it possible to evaluate the dynamics of the combustion process).
The final result of the experiments was the graphical preparation, in the form of ternary graphs, of the relationship between the mean burning rate and friction, depending on the percentage composition components.
To select the desired mixture, the figure can be used to determine the optimum composition by reading percentages of component contents from individual sides of the triangle at the right friction and mean burning rate. The most important conclusions resulting from this article can be summarized as follows:
- The method of the mathematical development of pyrotechnic compositions while determining other parameters allowed the number of tests to be simplified greatly because the correlations between the four components were determined in a programmed manner.
- Pyrotechnic compositions containing newly developed percentages and amounts of components proved useful due to their quality and meeting safety requirements.
- The method of mathematical selection of the mixture composition allows for a good prediction of the burning velocity and friction of the modelled pyrotechnic mixtures.
Author Contributions
Conceptualization, J.B.; Formal analysis, K.B.; Funding acquisition, K.B.; Methodology, J.B.; Writing—review & editing, J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author (K.B.).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Warchoł, R.; Nita, M. Mieszaniny pirotechniczne stosowane w pirotechnicznych układach opóźniających środków bojowych. (Pyrotechnic compositions for use in pyrotechnic delay devices of munitions). Probl. Tech. Uzbroj. 2014, 131, 57–66. [Google Scholar]
- Szydłowski, A. Podstawy Pirotechniki. (Basics of Pyrotechnics); Wydawnictwo MON: Warsaw, Poland, 1957. [Google Scholar]
- Conkling, J.A. Chemistry of Pyrotechnics: Basic Principles and Theory; Marcel Dekker: New York, NY, USA, 1985; ISBN 0-8247-7443-4. [Google Scholar]
- U.S. Army Material Command. Engineering Design Handbook. In Military Pyrotechnic Series, Part One, “Theory and Application”; U.S. Army Material Command: Washington, DC, USA, 1967; AMC Pamphlet 706–185. [Google Scholar]
- McIntyre, F.L. A Compilation of Hazard. and Test. Data for Pyrotechnic Compositions. In Report ARLCD-CR-80047; U.S. Army Armament Research and Development Command: Picatinny, NY, USA, 1980. [Google Scholar]
- Ellern, H. Military and Civilian Pyrotechnics; Chemical Publishing Company: New York, NY, USA, 1968. [Google Scholar]
- Shimizu, T. Fireworks—The Art, Science and Technique; Maruzen Publishing Co., Ltd.: Tokyo, Japan, 1981. [Google Scholar]
- Urbański, T.; Woźniak, S. Badanie pewnych własności mieszanek sygnalizacyjnych i oświetlających. (Testing some properties of signaling and lighting mixtures). Przegląd Artyl. 1937, 9, 43–65. [Google Scholar]
- Strona Internetowa Zakładu Produkcji Specjalnej ”Gamrat” Sp. z o.o., Jasło katalogi produktów (The Website of Specjal Production Plant ”Gamrat” Ltd., Product Catalogs). Available online: http://www.zpsgamrat.pl/ (accessed on 14 December 2021).
- Kosanke, K.; Kosanke, B. Selected Pyrotechnic Publications of K. L. and B. J. Kosanke, Part 1; Journal of Pyrotechnics: Whitewater, CO, USA, 1982; pp. 22–26. [Google Scholar]
- Glück, J. Development and Characterization of Environmentally Benign Light and Smoke-Producing Pyrotechnical Formulations. Unpublished Ph.D. Dissertation, Deutschland, Trostberg, Germany, 2018. [Google Scholar]
- Kurotori, I.S. Experiments with Mixtures of Components Having Lowerbounds. Ind. Qual. Control. 1966, 22, 592–596. [Google Scholar]
- Cornell, J.A. Experiments with Mixtures: Designs, Models, and the Analysis of Mixture Data, 3rd ed.; John Wiley: New York, NY, USA, 2002. [Google Scholar]
- Montgmery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley Sons: New York, NY, USA, 2012. [Google Scholar]
- Lawson, J. Design and Analysis of Experiments with R; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Krowicki, K.; Syczewski, M. Stałe Paliwa Rakietowe (Solid Rocket Propellants); MON: Warsaw, Poland, 1964. [Google Scholar]
- Goleniowski, T. Substancje i Przedmioty Niebezpieczne w Naszym Otoczeniu. Część 2. Petarda Wojskowa. (Hazardous Substances and Objects in Our Environment. Part 2. Military Firecracker). Available online: https://www.linkedin.com/pulse/substancje-i-przedmioty-niebezpieczne-w-naszym-tomasz-goleniowski-1c (accessed on 23 July 2019).
- Perzyk, B. Granaty Ręczne I Karabinowe Armii Czerwonej 1918–1945 I Radzieckie Granaty Ręczne w Wojsku Polskim 1945–1955. (Hand and Rifle Grenades of the Red Army 1918–1945 and Soviet Hand Grenades in the Polish Army 1945–1955); Wydawnictwo Militaria Bogusława Perzyka: Warsaw, Poland, 2009; ISBN 978-83-907405-2-2. [Google Scholar]
- PN-EN 13631-15 2007. Materiały wybuchowe do użytku cywilnego—Materiały wybuchowe kruszące—Część 15: Obliczanie właściwości termodynamicznych. In Explosives for Civil Uses—High. Explosives—Calculation of Thermodynamic Properties; Polish Committee for Standardization: Warsaw, Poland, 2007. [Google Scholar]
- BN 80 6091 42. Górnicze Materiały Wybuchowe—Obliczanie parametrów użytkowych. In Mining Explosives—Calculation of Utilities Parameters; Polish Committee for Standardization: Warsaw, Poland, 1980. [Google Scholar]
- McLean, R.A.; Anderson, V.L. Extreme Vertices Design of Mixture Experiments. Technometrics 1966, 8, 447–454. [Google Scholar] [CrossRef]
- PN-EN 13631-3:2006. Materiały wybuchowe do użytku cywilnego—Materiały wybuchowe kruszące—Część 3: Oznaczanie wrażliwości materiałów wybuchowych na tarcie. In Explosives for Civil Uses—High.s Explosives—Part. 3: Determination of Sensitiveness to Friction of Explosives; Polish Committee for Standardization: Warsaw, Poland, 2006. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).