1. Introduction
Wood, as one of the relatively renewable sources, occupies an important place in the balance of energy and resources. The situation varies extensively throughout the European Union. Forests cover between 60% and 72% with a total forest area of 182 million ha in the Scandinavian and Nordic-Baltic region, which accounts for around 5% of the total world’s forest area. Forests cover 43% of the EU land area. Sweden, Finland, Spain, France, Germany and Poland take the leading positions with two-thirds of the total EU forest area. The forest area of the European Union is increasing; for the period 1990–2010, the area increased by approximately 11 million ha due to natural forest development and afforestation [
1,
2,
3,
4].
The use of renewable energy sources in the development of the EU overall strategy is based on the guidelines set out in the Green Paper (COM (96) 576) [
5]. Starting from 1997, the Energy Strategy and Action Plan was set out in the publication of the EU Commission White Paper: Energy for the Future-Renewable Sources of Energy (COM (97) 599 of 26 November 1997) [
6,
7].
The aim of the study is to evaluate emissions from an alternative fuel or the mixture of alternative and conventional fuels and to compare the obtained results with the indicators of conventional fuel.
The global distribution of electricity/heat production by resources is shown in
Figure 1 [
8]. Data labels for renewable energy in terms of electricity and heat production are presented.
In 2020, the employment of all types of energy resulted in the generated 29.791 TWh of electricity and 16.328 PJ of thermal energy, 12.587 TWh and 7.398 PJ of which, respectively, were produced using solid fuel. The EU figures show that solid fuel consumption has fallen from 22% to 18% since 1995 due to an increase in energy from recycling and renewable energy. Fuel diversification has also led to a reduction in the consumption of fuel products and fuel itself [
2,
6,
9].
The EU Member States have committed themselves to achieving renewable energy targets throughout the energy balance. The national average is 6.7% for the transport sector, 28.8% for electricity and 18.6% for heating and cooling, as part of a total average of resources at 16.7%. The expected share of renewable energy in the total balance was 20% at the end of 2020.
Biomass and renewable waste are apparently the main energy sources the share of which will increase in the future [
4]. Similarly to the situation in the EU, the highest amount of biofuel in Lithuania is consumed by households and reaches 62%, whereas that used in the boiler rooms of district heating companies is approximately 24.8% of the total amount of wood fuel [
6,
10,
11,
12,
13].
Solid biomass potential was 1442 kt in 2011, and 50.8% of this potential was used in the heating sector. There was an increase of 14.6% by 2020, and 67% should be used in order to achieve national renewable energy targets (60% for district heating and 80% for heating individual houses). Achieving national targets should allow reduction of total greenhouse gas emissions by 6%. SO
2, NO
x and particulate matter emissions should decrease by 13.1%, 3.2% and 28.4% respectively. However, VOC and CO emissions should increase by 1.3% and 8.2% [
12,
14,
15].
Statistics suggests that since 1990 the gross available energy from renewables and biofuels in the EU increased more than 3.2 times. A detailed overview of changes is presented in
Figure 2 [
8].
Burnt straw is a type of fuel that does not increase the content of carbon dioxide (CO
2) in the atmosphere, because it is consumed as a nutrient by crops (such as cereals) growing the following year. Subject to calorific value, 1 t of straw can replace 0.28 t of fuel oil. After consuming all fuel available for 500,000 tons of straw, 140,000 tons of imported fuel could be saved nationwide annually [
16].
The substitution of fossil fuel by biofuel or the simultaneous combustion of various mixtures, the production of compressed fuel (pellets, briquettes), wider deforestation and the use of other types of waste are considered to be of utmost importance. The increased use of biofuels has been linked to the EU strategy for promoting the cogeneration of heat and electricity. The further development of applying biomass for heat production is indirectly supported by Directive 2004/8/EC on the promotion of cogeneration. A specific requirement for giving priority to renewable energy sources was also set out in Directive 2002/91/EC on the energy performance of buildings. Under the new Directive, coal and wood biofuel are taxed, but other solid energy sources, including wood and peat, remain exempt. The development of biofuel is also supported by the Directive 2003/87/EC of the European Parliament and of the Council of 13 October 2003, establishing a scheme for greenhouse gas emission allowance trading within the Union [
17].
Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources, amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC, supported the production of heat and cold from renewable energy sources. The Directive set the target of achieving renewable energy accounting for 20% of total Community energy consumption in the EU by 2020. The indicator for Lithuania is 23%. Biomass makes approximately two-thirds of all renewable energy consumption in Europe and is one of the fastest growing sectors in absolute terms [
18].
Wood is the main and most commonly used type of solid biofuel. Subject to the origin of the raw material, wood fuel can be divided into fuel produced from forests, fast-growing (energy) forests and reusable wood. Fuels originating from forests and energy forests, unlike reusable wood, can be considered ecologically acceptable, as the latter is usually impregnated and painted, contains various impurities (metal, glass, plastics, etc.), and is therefore treated in a complex way [
16]. Stricter requirements are imposed on combustion technology and emissions. Reusing wood in line with the balance of materials is equated with waste utilization. Waste from wood industry is generally of better quality compared to deforestation waste due to lower moisture, and thus is used for producing pressed biofuel, including pellets (most frequently 8–12 mm in diameter and 5–30 mm long), briquettes or wood chips. Biofuel also accounts for the bulk of RES at levels close to the EU-27 average and constitutes 86% [
17,
19].
Lithuanian national documents for the energy sector, i.e., The National Energy Independence Strategy [
20], provide that the share of district heating produced from renewable energy sources is expected to grow by no less than 60% and that of households by at least 80% by 2020. Scientists estimate that the rational share of biofuel could reach 53–62% in 2020 [
21].
It should be noted that 100% of the available amount of biofuel prepared for producing heat in the sectors of firewood, waste from wood processing industry and woody energy plantations is utilized in Lithuania. Thus, it is necessary to expand more quickly energy plantations focusing on woody and perennial herbaceous plants as well as on the more effective use of felling waste [
22].
The chemical composition in the dry matter of wood and bark include 48–50% and 51–66% of carbon (C), 6.0–6.5% and 5.9–8.4% of hydrogen, 38–42% and 24.3–40.2% of oxygen, 0.5–2.3% and 0.3–0.8% of nitrogen, 0.05% of sulfur and less than 0.01% and 0.01–0.03% of chlorine, respectively.
Plants consist of cellulose, hemicellulose and lignin. Lignin is made of C, H and O atoms, is a mixed polymer containing aromatic and aliphatic components [
23] and is extracted as a secondary raw material in the wood industry by hydrolysis, removing polysaccharides (cellulose) from the shredded wood [
24]. Lignin is non-toxic, chemically inactive, and therefore has several uses: specifically, prepared lignin is employed as a type of biofuel or as a raw material for manufacturing pellets or briquettes [
25]. Lignin yields 20% more energy than wood [
26] and has a characteristic high calorific value (lignin contains approximately 64% carbon) [
1,
27].
Around 4 million tons of straw are produced annually in Lithuania [
28]. However, utilizing all produced straw for energy purposes is hardly possible because straw is only partially harvested due to natural conditions, and some straw is used for livestock litter or is incorporated into the soil. The content of dry straw comprises 45–47% carbon (C), 5.8–6.0% hydrogen, 0.4–0.6% oxygen, 39–41% nitrogen, 0.01–0.13% sulfur and 0.14–0.97% chlorine. The use of straw as a type of renewable fuel in the heat sector requires a solution to certain technological problems related to burning straw containing a considerable amount of sulfur and accumulating 7–10 times more chlorine and 10–12 times more nitrogen compared to wood. These introduced chemical elements stimulate equipment corrosion, and high ash content and low melting temperatures increase the likelihood of boiler wear [
29].
Chlorine present in biofuel can cause problems when burning coniferous chips because the content of needles in fuel is high. Although the concentration of heavy metals in wood fuel are not dangerously high, their levels should be considered in the case of sufficiently severe environmental restrictions. Small amounts of nickel, arsenic, cadmium, chromium, copper, mercury, lead and zinc may be found in the composition of the different parts of firewood [
30].
Fuel moisture is usually characterized by relative humidity, i.e., moisture content expressed as a percentage in terms of the mass of the fuel used. The relative humidity of fuel may vary over a wide range from 0 to ≥60%. Fuel is more difficult to be ignited at high humidity and the calorific value of fuel is lower because a part of the heat released during combustion is consumed to evaporate the amount of water in the fuel. The calorific value of 30% moisture content in wood leads to approximately 3.4 MWh/t.
The contribution of solid biofuel combustion to climate change is considered to be a neutral process [
10], i.e., the amount of CO
2 emitted during biofuel combustion is absorbed by vegetation growing in the environment. Although this is a positive feature from an environmental point of view, it is hardly an expedient objective to focus on the above provided indicator alone. The extraction of wood resources does not have a significant negative impact on the components of the environment or their quality, but the process of preparing wood must take place in line with prescribed standards and environmental requirements.
Different authors suggest the mean comparison values of greenhouse gas emissions from varying fuels throughout the life cycle (gCO
2/kWh) (
Table 1). These emissions evaluate the full journey of fuel from origin (extraction) to use or utilization. The obtained data differ quite strongly due to the specifics of each of the fuels, transportation, refining, distribution and conversion. In contrast to traditional fossil fuel such as coal, wood biofuel as solid biofuel emits on average 16.7 times less greenhouse gases (CO
2 equivalent) per unit of energy produced over the total life cycle. A comparison of emissions from different fuels demonstrates that the ratio of oil to solid biofuels is 14.6 times, and the ratio of natural gas to solid biofuels is 10.7 times. Coal is a more polluting fuel compared to oil and natural gas, but the extraction of the latter types of fuel causes no less pollution, while wood biofuels are the most environmentally friendly in both cases [
8].
Replacing all used fuel oil in Lithuania and 55% of the natural gas employed for district heating should reduce the amount of greenhouse gases by 1 million tons of CO2, and that in the household sector should equal 0.2 million tons of CO2, i.e., 28.8% and 6.7%, respectively. The increased use of solid biofuels in the district heating sector should reduce 4.9% of total greenhouse gas emissions nationwide, with the household sector contributing 1.1%. Thus, the overall reduction in greenhouse gas emissions should equal 6%, and that in the individual heating sector as much as 35.5%.
The data provided by scientific research and international energy organizations on air pollutant emission rates (g/kWh) illustrate the process of burning different fuels and are presented in
Table 2. Emissions from the same type of fuel in the air have been observed to vary across the EU Member States. The authors of the conducted research point out that the reasons for such variations most frequently involve different technological, engineering and pollutant trapping equipment, diverse boiler control solutions and the quality, moisture and calorific value of biofuel feedstock.
Pollution from wood biofuel combustion is only slightly higher evaluated by CO and PM, and only comparable to highly flammable fuels (natural gas and fuel oil). In other cases, the combustion of wood biofuels based on SO2 is the same as that of natural gas, but more than 19 and 33 times lower than coal and fuel oil. In terms of NOX pollution, wood biofuels emit at least 1.7 times less than any of these fuels, and in terms of VOCs slightly more than natural gas, but less than 1.5 times than that of other types of fuel.
Balance sheet calculations carried out by different authors demonstrate that SO
2 emissions should decrease more in households (HH), by 7.2%, rather than in the production of district heating (DH) at 5.9%, although growth in the use of solid biofuels in households is not expected to be very high. The complete replacement of coal by solid biofuels in households and the abandonment of heavy fuel oil in the district heating sector should have a positive environmental effect. The employment of solid biofuels should reduce NO
x emissions by 3.2% of the total generated nationwide. In this case, the variation is slighter than that of SO
2 emissions due to a smaller difference in NO
x emissions generated from fuel oil combustion, coal, natural gas and solid biofuels [
31,
32]. Woody energy plants, as opposed to forest wood, accumulate higher amounts of nitrogen, phosphorus, potassium, and cadmium and have a higher wood ash content than woody energy plants. The content of nitrogen and chlorine in energy crops can be reduced by applying agrotechnical measures, optimizing fertilization and using fewer chemical preservatives. Particularly high levels of pollutants are accumulated when plantations are grown in reclaimed areas, sanitary industrial zones and sewage sludge storage areas.
VOC (volatile organic compound) emissions are expected to increase to 1.3% of total VOC emissions countrywide. The amount of VOCs produced during natural gas combustion is 2.4 times less than that produced by burning solid biofuels. Despite the fact that this type of fuel is cleaner than fuel oil or coal in this particular case, the latter make up a relatively small part of the heating sector and replacing natural gas with solid biofuels has a negative effect on VOC emissions. VOCs are mainly generated by the incineration of wood processing waste often contaminated with chemicals such as melamine-urea-formaldehyde resins, poly-chlorovinyl, synthetic varnishes and paints used in technological processes.
However, an increase in CO content by 8.2% should have the biggest negative effect. The amounts of emitted CO are determined by the unevenness of biomass quality parameters (humidity, calorific value) and the poorly set operating mode of the boiler. Therefore, the largest increase (5.5%) in CO emissions should be observed in the district heating sector.
In the case of particulate matter (PM), a rather atypical situation is monitored, because solid biofuels should reduce the content of PM emissions by as much as 27.1% in households and increase PM emissions by 1.3% in the district heating sector. Households use coal for heating, which is as much as 83.5 times more polluting in the case of PM than solid biofuels. Thus, the use of solid biofuels instead of coal should exert a very tangible positive effect. It must be noted that the above discussed significant positive environmental impact would only be achieved if coal used in households was 100% replaced by biofuels. As for district heating, the situation is more complex due to PM emissions that are lower than in the case of fuel oil combustion but higher than in the case of natural gas combustion. The implementation of biofuel development plans should increase PM emissions by around 1.3% in the district heating sector.
The wider use of solid biofuels in the heat production sector should reduce SO2 emissions by 13.1%, NOx by 3.2% and PM by 28.4% and could improve urban air quality (for instance, by reducing particulate pollution).
This article discusses the potential of biofuels employed for energy production, looks at the problems of atmospheric air pollution caused by different biofuel blends and explores the possibilities of replacing the current traditional biofuels with other effective alternatives with a focus on the analysis of emissions.
The paper analyses the current use of solid biomass and its potential for heating, different wood products and/or waste biofuel mixes and further development opportunities for changing common wood biofuel (chips, briquettes, pellets and wooden waste), as well as environmental impact.
2. Materials and Methods
Experimental research was carried out in the district-type (area of around 100,000 inhabitants) cogeneration boilers and was aimed at investigating emissions from different biofuels burned in boilers at different smoke emission points.
Boiler 1 (Compact C-500 DH) represented a water heating boiler with grate heating. The boiler burned SM3 biofuel, the share of lignin in which accounted for one third of the total flow of fuel. The average thermal power of the boiler was 3.2–4 MW. The research involved a biofuel-lignin mixture fed to the kiln without further processing. The other case included mechanical mixing.
Boiler 2 (Compact 500 DH) represented a water heating boiler with grate heating. The boiler was examined using SM3 biofuel, the share of lignin in which was 100%. The other case included 30% of the total flow of fuel. The average thermal power of the boiler was 2.5 MW. In all cases, the biofuel-lignin mixture was mechanically mixed before being fed to the grate.
Boiler 3 (BFB 25-45-460) included a steam boiler with a single drum, a vertical water tube and natural water recirculation. Biofuel combustion in the fluidized bed was investigated under 100% presence of lignin. The average thermal power of the boiler was 10 MW. Lignin was supplied to the grate without further processing.
Boiler 4 (Compact C-500 DH) represented a water heating boiler with grate heating. The boiler burned SM3 biofuel, the share of lignin in which accounted for one third of the total flow of fuel. The average thermal power of the boiler was 3.2–4 MW, the maximum possible thermal power up to 5.1 MW. The research involved a biofuel-lignin mixture fed to the kiln without further processing. Boiler 4 and boiler 1 are similar by work principle, but the latter has lower thermal power.
In the Baltic Sea region, as in other countries, shredded wood is classified according to its quality, from the highest quality SM1, which is produced by shredding firewood and its waste, to SM3, which is obtained by collecting and shredding forest waste. As a result, SM3 fuel contains many leaves, bark, earth and other impurities, which means that such fuel is relatively moist, has a low calorific value, and when stored for a long time it can even ignite. Burning SM3 category of fuel, more ash and smoke condensate are generated, which must be disposed of properly, which is costly. In addition, non-combustible impurities tend to slag, and the risk of corrosion and erosion of boiler surfaces increases, all of which shortens the operating time of the equipment, so repair costs increase. Thus, considering these issues and given that the price differences between SM1, SM2 and SM3 fuels are relatively small, it is understandable that it is often more economically viable to choose a higher quality fuel.
Included were the single-drum, the vertical water tube and a natural water circulation boiler of Ո-shaped arrangement intended for wood fuel combustion in the fluidized bed.
The investigated parameters for boilers are provided in
Table 3.
The concentration of gaseous compounds (O2, CO, NO, NO2 and SO2) in the emissions was measured applying Testo 350-M/XL equipment.
Investigation into the concentrations of gaseous compounds was performed before discharging pollutants into the environment in the closed-type system of heating network. The study was conducted under industrial conditions during autumn-winter-spring seasons.
Fuel and ash quality indicators were determined by independent experts prior and following boiler emission, respectively. The concentrations of total chlorine, fluorine and heavy metals (cadmium, arsenic, chromium, nickel, lead, copper, zinc and mercury) were clarified before fuel combustion. The indicators were developed in consonance to international standards EN ISO 16994, ISO 10359, EN ISO 16968, EN ISO 15586, EN ISO 11885, EN ISO 13657, EN ISO 7980, EN ISO 8288, EN ISO 12846 and EN ISO 15936 for analysing solid fuels.
The chemical composition of ash was determined in the course of investigation into the burned fuel. Analogous to pre-combustion tests, the concentrations of heavy metals (cadmium, arsenic, nickel, lead, chromium, copper, zinc and mercury) as well as total chlorine and fluorine were specified. In addition, the concentrations of alkali and alkaline earth metals such as potassium, sodium, magnesium were discovered. Experimental research established the total concentrations of sulfur and silicon determining fuel quality indicators and defining the proportion of the remaining chemical elements in the non-volatile part. The share of organic carbon was found to control the degree of fuel combustion subject to the type of boiler and the fuel mixture.
Ash was taken from each hopper of the boiler after cooling at a room temperature of 10–15 °C. Six ash samples (30 kg) were taken from the different piles of ash and dried at a temperature of 100–105 °C to constant weight, ground in a soil mill, homogenized by stirring and sieved through 1 mm mesh sieves. The composition of ash was tested in line with international standards EN ISO 16994, ISO 10359, EN ISO 16968, EN ISO 15586, EN ISO 11885, EN ISO 13657, EN ISO 7980, EN ISO 8288, EN ISO 12846 and EN ISO 15936 for analysing solid fuels.
3. Results and Analysis
Investigation into emissions from different types of biofuel is based on the possibility of using more energy-effective or equivalent fuels thus reducing the concentration of the emitted pollutants and/or replacing the composition of exhaust gas with a more environmentally acceptable structure.
Prior to then investigation, chemical elemental analysis identified the composition of the samples of different fuel mixtures. Studies have shown that lignin contains the highest levels of total chlorine (138.50 mg/kg) and zinc (98.95 mg/kg), and a lesser amount of copper (54 mg/kg) and chromium (32.35 mg/kg). The descending order of chemical elements is as follows: lead, nickel, fluorine, boron, arsenic, cadmium and mercury.
The fuel mixture of lignin and wood biofuel SM3 was prepared in the ratio of 33 to 67 (% by weight) respectively. The chemical composition of bottom ash was determined using ISO standards given in
Table 4.
Quality indicators applied to fuels:
The ash content of SM3 biofuel does not exceed 5% (EN 14775), that of sulfur (% by weight) 0.04% (CEN/TS 15289) and that of chlorine (% by weight) 0.03% (CEN/TS 15289);
The ash content of lignin does not exceed 8% (class LBM) and that of sulfur 0.3% (CEN/TS 15289);
Chlorine in both fuels (% by mass) equals 0.03% (CEN/TS 15289);
Wood pellets: arsenic does not exceed 1.0 mg/kg in dry weight, cadmium does not exceed 0.5 mg/kg in dry weight, chromium, copper, lead and nickel do not exceed 10.0 mg/kg in dry weight, mercury does not exceed 0.1 mg/kg in dry weight and zinc does not exceed 100.0 mg/kg in dry weight.
Compared to the chemical composition of biofuel, the share of heavy metals in ash increased in all cases. The concentration of cadmium increased by almost 40 times when the share of lignin was 50% in the biofuel blend. The concentration of arsenic was higher at 33% of lignin in fuel rather than in the pure lignin sample. However, the concentrations were close to the detection limit in all samples.
The concentrations of nickel and lead in both lignin before incineration and ash were 10 to 60 times higher than those of cadmium and arsenic. Nickel detected in ash was approximately 1.7–3.1 times more and lead up to 1.5 times more, while in the case of 100% of lignin fuel, this was 7 and 2.5 times more, respectively, than in lignin before incineration. The concentration of lead in fuel is similar to that of Ni, but ash contains much less lead than nickel. An increase in chromium concentration was the highest, reaching 4.2 times when fuel was purely made of 100% of lignin. Copper concentration increased from 2.2 to 6.4 times and peaked at 100% of lignin. Potassium concentration in the samples was the largest, reaching 1010 mg/kg in pure lignin. Sodium values increased by 7.6 times (in the cases of 100% of lignin and when 50% of lignin and 50% of biofuel were burned). The concentrations of zinc and magnesium were 100 mg/kg and 950 mg/kg, respectively, while a rise in zinc was not significant (1.2–2.1 times) in ash. Magnesium concentration increased from 8.3 to 12.6 times and was the highest at 33% and 50% of lignin, boron rose from 5.8 to 11.5 times and was the largest at 25% and 50% of lignin and mercury was close to the limit of detection, slightly higher at 25% of lignin in fuel.
Variations in the total concentrations of chlorine and sulfur are similar, but the absolute values of this total sulfur are approximately 5 times higher. The concentration of the total chlorine increases on average 3.1–8.5 times after incineration.
The average organic carbon content of lignin was 63.1 mg/kg, whereas decreased from 8.7 to 1.6 times (which defines the degree of fuel combustion) in ash.
The obtained trends remain in all cases. One of the lowest concentrations of heavy metals are formed at a lignin ratio of 25% in the mixture, and the highest concentrations were found when lignin had a share of 33% or 100% of the total mixture. A smaller share of lignin in the mixture results in the lower proportion of organic carbon; however, under the presence of 100% of lignin in fuel, the concentration established in ash differed by only 1.1–to 1.3 times. It is assumed that, for biofuel combustion, a lignin content of more than 25% could disrupt the combustion process in the boiler combustion zone. Still, using only lignin as a type of fuel makes combustion more effective. The content of silicon in fuel is an indicator of the residue of the coarse fraction settling in ash, while the fine fraction is removed through gas flow. Concentration values after combustion increase from 3 to 8 times compared to pure lignin before combustion. The smallest change was observed under lignin content equalling 33%, and a slightly higher variation was noticed at 50% of lignin.
Investigation into emissions identified trends, subject to the composition of the fuel mixture and different loads for boilers. The obtained results show that, under the lowest content of lignin in the fuel mixture (30%), the composition of emissions in the flue installed next to the boiler, having a rated power of 5 MW and a set power of 3.2 MW, was as follows: CO–24.7–28.6 mg/Nm3, NOx–590.0–618.5 mg/Nm3, SO2–123.9–132.9 mg/Nm3 and PM–150.1–162.3 mg/Nm3. Share of oxygen reached 8.05–8.52%. Gas flow temperature in the flue ranged between 141 and 145 °C and the volumetric flow rate was equal to 1.54 Nm3/s. The boiler used the mass of SM3 biofuel as a type of fuel and recorded the following emissions: CO–12.1–14.9 mg/Nm3, NOx–505.8–552.8 mg/Nm3, SO2–5.5–11.5 mg/Nm3 and PM–480.5–540.6 mg/Nm3 when the share of oxygen reached 7.84–7.94%, temperature fluctuated between 136 and 141 °C and the volumetric flow rate was equal to 1.56 Nm3/s.
The aerosol pollution increased by 8% with an increase in crop residue burning by 4% [
33,
34,
35]. The carbonaceous aerosols were mostly contributed by the fossil fuel emissions during pre-monsoon and in winter at the pick of biomass burning. Research [
36] has shown that the emission of particulate matter (including black carbon aerosol) was about four times higher over an urban atmosphere from the burning process of fossil fuels comparing to biomass.
The findings demonstrate that the savings of the one third of SM3 biofuel replaced by lignin only increase the concentration of sulfur dioxide but reduce PM concentrations by up to 3.5 times. The biofuel-lignin mixture, unlike pure biofuel SM3, slightly increases thermal efficiency by raising combustion temperature by a little less than 10%.
Increasing power from 3.2 MW to 4 MW by burning the mixture containing 30% of lignin in fuel elevated exhaust gas temperature up to 152.4–155.6 °C, the volumetric flow rate remained constant at 1.57 Nm3/s and oxygen concentration was 5.71–6.92%. Hereupon, CO concentration ranged from 28.4 to 31.6 mg/Nm3, NOx–from 694.3 to 709.5 mg/Nm3, SO2–from 91.8 to 96.8 mg/Nm3 and PM–from 156.1 to 178 mg/Nm3.
The results were compared to their raw coal analogues to evaluate the emission performance of each fuel type. All the NOx emission factors vary from 0.25 to 0.55 g/MJ and the values do not correlate with particle size.
Under the present mixture of SM3 biofuel and lignin, an increase in the boiler load does not cause additional technological barriers, and a higher temperature achieved compared to the case of burning SM3 biofuel only proves the potential (high calorific value) of lignin for energy application. It should be noted simultaneously that a rising temperature results in better combustion of the fuel mixture and a reduction in sulfur dioxide emissions to 1.5 times, while carbon monoxide and PM remain at a similar level. A higher temperature increases the concentration of nitrogen oxide, but this occurs at the reduced oxygen content of around 6.3%.
The power of boiler 2 ranged between 2.3 and 2.5 MW; the lignin-biofuel SM3 mixture was mechanically supplied at the ratio of 30 to 70. The test bench had the possibility of determining the concentrations of pollutants next to the boiler: CO–238.1–258.2 mg/Nm3, NOx–482.8–499.0 mg/Nm3, SO2–10.5–12.5 mg/Nm3 and PM–297.4–311.3 mg/Nm3. Oxygen concentration equalled 9.58–9.60%. Gas flow temperature in the flue ranged from 154.3 to 159.7 °C, and the volumetric flow rate was equal to 1.85 Nm3/s.
Using a mixture of both biofuels in the medium power boiler and maintaining an oxygen concentration of around 9.5% assisted in achieving the lowest emissions among all cases analysed in this study. Although the concentrations of carbon monoxide and PM are higher in this case than under the maintained oxygen concentration of around 6.3%, the values of sulfur and nitrogen oxides are significantly reduced. Thus, it can be assumed that at the oxygen concentration of 7–8% carbon monoxide concentration will be reduced to 100 mg/m3. It has also been noticed that the mechanical supply of the mixture to the combustion chamber potentially increases the concentration of ultrafine PM in the air. It should therefore be worthwhile to use a semi-enclosed combustion chamber in which PM should burn and remain in the form of ash rather than be lifted by the flows formed due to convection and/or forced movement through smoke pumps.
Boiler 2 involved studies performed under the thermal power of 2.5 MW when lignin mass only was supplied to the fuel combustion chamber. The test bench had the possibility of determining the concentrations of pollutants next to the boiler: CO–444.3–545.0 mg/Nm3, NOx–448.0–564.3 mg/Nm3, SO2–299.9–321.9 mg/Nm3 and PM–392.6–419.7 mg/Nm3. Oxygen content reached 7.96–9.12%. Gas flow temperature in the flue ranged from 151.6 to 158.9 °C, and the volumetric flow rate was equal to 1.41 Nm3/s.
In line with the results of emissions where pure lignin was burned in the medium power boiler, concentrations increased quite significantly to around 1.8 times CO, approximately 10 times SO2 and 1.3 times particulate matter. However, NOx values were approximate. Thus, the use of the pure lignin mixture in the low load boiler poses problems of maintaining the complete combustion process, which is reflected in particularly high concentrations of carbon monoxide and variable oxygen as well as in higher concentrations of other pollutants than in the other cases under consideration. Therefore, the application of such a process is hardly an example of the rational use of lignin for combustion to obtain energy.
Boiler 3 employed automatic mixture preparation and feed to the boiler. In all cases, the weight ratio of lignin to SM3 biofuel was 50/50. The thermal power of the boiler was the highest of all examined cases and reached 10 MW. Emission and gas flow parameters were investigated at the point at which the distance of the flue from the boiler grate was 15 m. The determined emissions contained 55.0–60.1 mg/Nm3 of CO, 702.4–711.7 mg/Nm3 of NOX, 0 mg/Nm3 of SO2 and 2447.8–2539.4 mg/Nm3 of particulate matter. Oxygen content ranged from 8.60 to 8.92%. Gas flow temperature in the flue fluctuated from 248.9 to 249.8 °C, and the volumetric flow rate was equal to 10.65 Nm3/s.
Regarding the examined emissions from the burned biofuel mixtures, the highest power boiler discloses that concentrations are emitted subject to the type of pollutants, for example, carbon monoxide emissions are low and no sulphur dioxide is detected. However, despite high gas flow temperatures, the concentrations of nitrogen oxides are high. PM concentrations are also large. To implement this particular case, gas flow treatment is mandatory. On the other hand, the treatment method does not require complex technologies due to fairly large lignin solids, and thus it should be sufficient to install conventional treatment equipment such as a heat-resistant sleeve filter or a cyclone for the effective deposition of particulate matter. The research has also proved that a lower temperature in the combustion chamber significantly reduces the concentration of nitrogen oxides [
32].
Polyoxymethylene dimethyl ether 3 (PODE
3) as a highly promising renewable fuel has been found to be capable of offering soot reduction across all engine loads. In particular, 20% of PODE
3 addition to diesel has demonstrated soot reduction at all loads, with a remarkable particulate matter reduction factor of 3.55 at 50% load. However, despite such an impressive 71.8% soot reduction at medium load, it can cause a spike increase of 71.2% in particle number concentration, owing largely to ultrafine particles [
33].
Hence, depending on the operating conditions of the boilers and parameters for the fuel burned, the preliminary dynamics of the pollutants emitted can be shown (
Figure 3).
The indicators for boiler performance were analysed considering the temperature of the air duct in the combustion modes of the boilers, thus establishing the correlation with variations in the concentrations of pollutants specific to each combustion process.
The correlation between gas flow temperature in the flue and the concentrations of gaseous pollutants shown in
Figure 4 allows optimization of the combustion process subject to the output of the boiler. The concentrations of carbon monoxide have been found to remain relatively constant at around 10–30 mg/Nm
3 at a gas flow temperature of 140–150 °C and at a 30% share of lignin in fuel. In the case of the 10 MW boiler containing 50% of lignin in fuel, emissions are relatively low at slightly less than 60 mg/Nm
3. When burning fuel containing no lignin, CO concentrations were also low at around 15 mg/Nm
3.
SO2 emissions for the latter case are also among the lowest, i.e., 0 mg/Nm3. Zero concentrations were also obtained when burning fuel containing 30% of lignin in the lower power (2.5 MW) boiler and when burning pure SM3 biofuel containing no lignin. A high temperature is known to have a fairly strong effect on the concentration of nitrogen oxides, and in this case the temperatures ranged from 140 to 150 °C in medium power boilers and 250 °C–in higher power (10 MW) boilers. The findings demonstrate that the most favourable cases of the estimated lowest concentrations of other pollutants remain in the fuel burned in lower power boilers (2.5–3.2 MW) and containing a 30% share of lignin under NOx emissions of approximately 500–600 mg/Nm3. NOx emissions from the higher power boiler (10 MW) were found to be relatively small and equal to around 700 mg/Nm3 assuming that the temperature in the latter case was approximately 1.7 times higher than in other cases and affected the obtained results.
The results were compared to their raw coal counterparties to assess the emission characteristics of each fuel type. The NO
x and SO
2 emission factors were found to depend on the fuel nitrogen and sulfur contents in the coal and the combustion conditions used during pyrolysis. The PM, SO
2 and VOC emissions show a strong dependence on the ash percentage and volatile matter yields, which both increased along with increasing particle size. The work [
37] shows that the PM, SO
2 and VOC emissions were found to depend only on particle size on a mechanistic level. The VOCs and PM emission factors are inversely correlated with particle size.
Authors [
38] studied the emissions of fine particles up to 20 µm, and, additionally, NO, NO
2 and N
2O pollutants from pulverized fuel (pf) using a fixed bed reactor. An increase of N
2O emission depending on the increase in mean particle size was performed, while NO emission decreased.
The emission factors of CO and CO2 are not only influenced by the fuel C-content, but also by the combustion conditions. The relative amounts of these two gases give an indication of the quality of the combustion.
Based on the research in [
39], as the combustion temperature of the clean wood biofuel increased, the maximum NO
x emitted significantly decreased, in contrast with the trend observed for SO
2 emissions, and the NO
x concentrations corresponding to the peak at 800 °C were less than 90 mg/m
3. The SO
2 concentrations were equal to 835 mg/m
3 using clean coal and up to 80 mg/m
3 using clean wood biofuel. This is similar to the results of this work in the case of wood biofuel mixture with 30% of lignin using 4 MW boiler. Similar to the SO
2 emissions, the maximum NO
X and total concentrations of NO
x emitted from clean coke combustion were lower than the maximum and total concentrations of NO
x emitted from household coal combustion and power plant coal boilers with air pollution control devices, which meet the requirements of NO
x emissions to protect the atmospheric environment.
Particulate matter emissions can appear in various ways [
40]: (1) Soot, or black fine carbon, is produced from the incomplete combustion of the coal (i.e., instead of forming CO
2). Soot is made up of chainlike aggregates of primary carbon-enriched particles, or poly-aromatic hydrocarbons. (2) Mineral matter associated with the coal can form ash particles depending on their mode of occurrence [
41]. The transformed products of inherent minerals become free after the carbon structure around them burns, and coalesce in the liquid or solid phase [
42]. (3) Transformed products of submicron non-mineral inorganics and inherent minerals in the coal may vaporize in the flame and subsequently undergo homogeneous and/or heterogeneous nucleation to form ultrafine (10–30 nm) aerosols which can condense and coagulate in the post flame region, where temperatures are low [
43].
PM concentrations varied over a wide range from 150 mg/Nm3 to 2500 mg/Nm3 on average. As in other cases, the least polluted gas was generated burning biofuel containing 30% of lignin in lower power (2.5–3.2 MW) boilers, while PM emissions fluctuated from 150–305 mg/Nm3. In this case, the gas flow most polluted with PM was formed in the higher power boiler of 10 MW.
The analysis of the findings included the correlation between variations in the concentrations of the emitted pollutants such as CO–NO
x, CO–PM and CO-SO
2, PM–SO
2 shown in
Figure 5.
CO concentration is low in the boilers with a power of 2.3–4 MW and correlates with NOx where direct dependence is observed. However, NOx concentration decreases when CO emission exceeds approximately 200 mg/Nm3. PM concentrations decrease under an increase in CO concentration, which may be related to the formation of a smaller content of soot in exhaust gas when CO content is lower. The highest concentrations of SO2 were established in the cases when the average CO content was 20–30 mg/Nm3 and when fuel contained 100% of lignin and was burned in the 2.5 MW boiler. In other cases, SO2 emission was equal to 0 mg/Nm3. A similar situation was found in PM–SO2 dependence when SO2 levels were determined only at low PM concentrations or in the case of pure lignin combustion.
Oxygen concentration in gas flow plays an important role in optimizing combustion processes.
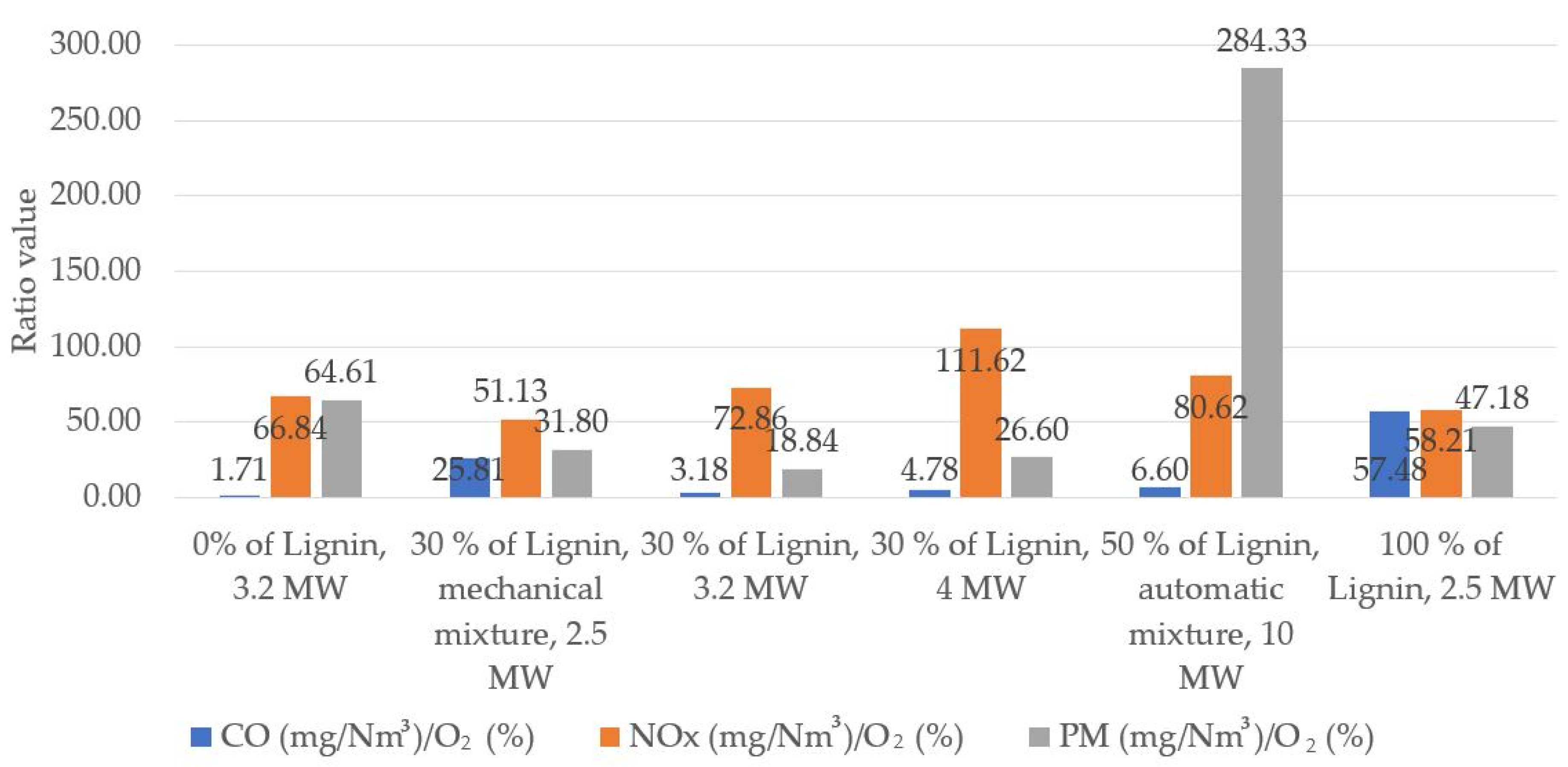
Figure 6 shows the generalized relationship between pollutants and oxygen ratios for each of the boilers at different compositions of fuel. In line with the bar graph, the ratio of CO to O
2 remains very similar in all situations, except for the case of burning pure lignin when the value of the ratio of CO to O
2 increased approximately 15 times and the amounts of pollutants around 6.8 times. This is due to a higher content of O
2 than in other situations, which resulted in an approximately two-fold increase in CO concentration compared to the cases when lignin content in fuel was 0%, 30% or 50%.
Although the values of the ratio of nitrogen oxides to oxygen varied in a wide range of 51.1–111.6, changes were not significant and no specific trends were observed. The values of the particle-to-oxygen ratio showed a decreasing trend with the rising share of lignin in fuel. An exception included the case when the fuel made of 50% lignin was burned in the higher power boiler (10 MW). The value of the ratio increased when the share of lignin reached 100%. The latter two cases indicate that under a small amount of oxygen remaining in gas flow and at a rising share of lignin in the total flow of fuel, PM concentrations increase. This is explained by the fact that lignin as a fuel is more polluting than conventional SM3 biofuel because more volatile ultrafine PM is released and does not precipitate as ash but is removed through the boiler chimney when trapped in gas flow. It is also assumed that fuel combustion containing more than 50% of lignin must increase oxygen content in the boiler to generate smaller amounts of pollutants.
PM
2.5, PM
10, NO
2 and O
3 pollutant are analyzed as emission for the associations between outdoor exposures and human depressive symptoms. Indoor solid fuel use is predominantly derived from biomass burning; it also contains higher components of carbonaceous organics [
44,
45] polycyclic aromatic hydrocarbons, and benzo(a)pyrene [
46], which may cause higher cytotoxicity [
47].
The use of fossil fuels will be increasingly complicated in the future due to rapidly rising prices and limited supply as a non-renewable energy source. Renewable energy, whatever its nature, will be developed rapidly, especially with the development of energy-producing technologies. Biofuel has great potential, assuming that high-quality biofuels are kept to a minimum. Instead of this, the use of the second type of biofuel for combustion in heat and electricity production will make it possible to preserve valuable raw materials. Wood biofuel as the main object of this work can be partially used for energy production adopting the principles of the green energy and the circular economy. Possibilities for the use of other types of biofuels, such as pulp containing paper production, could be analyzed in future studies. There is also an important market share for the use of biofuel for low power (less than 1 MW) boilers.