Optimization of Operating Conditions for CO2 Methanation Process Using Design of Experiments
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Preparation
2.2. Catalyst Activity Test
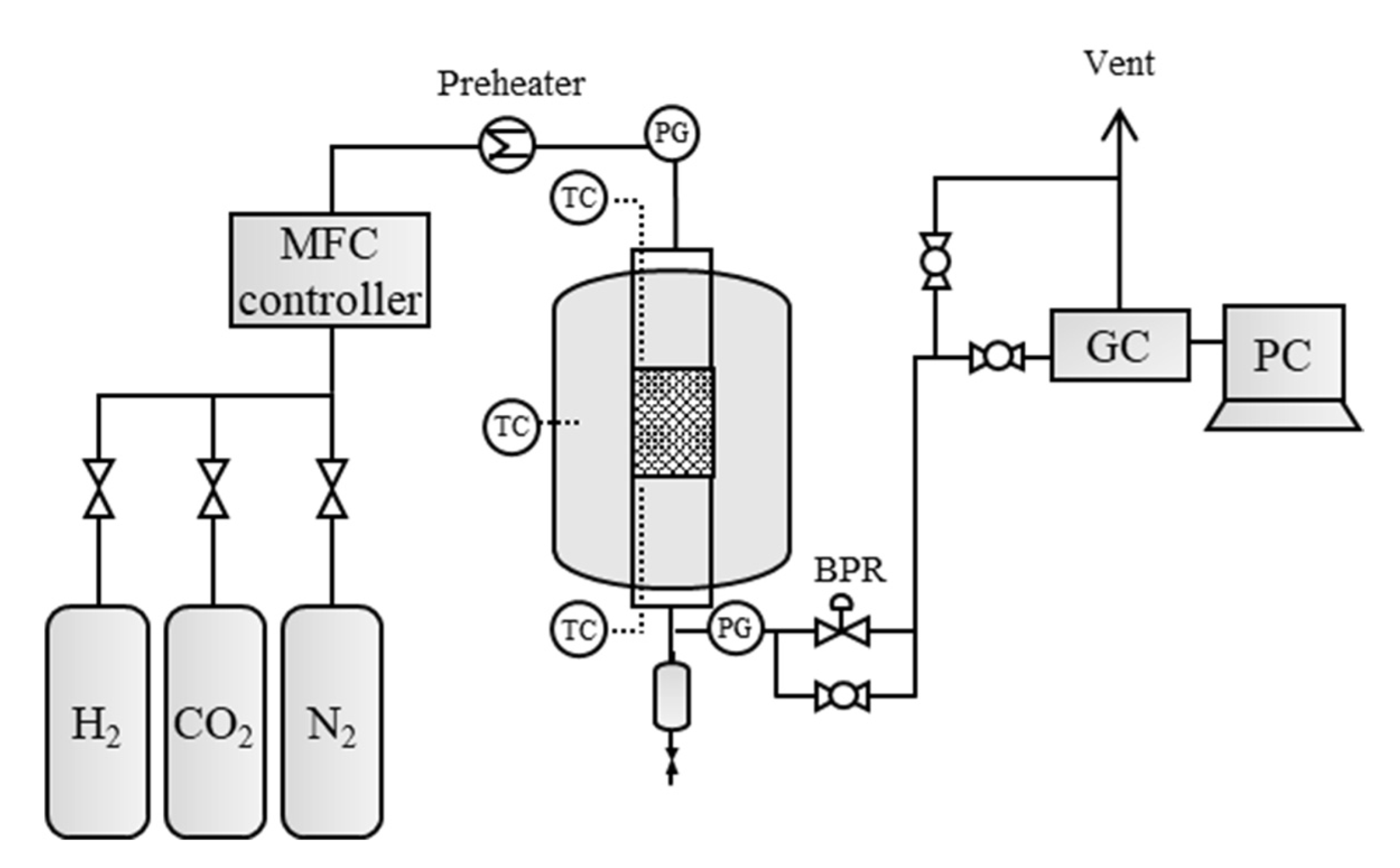
2.2.1. Experimental Apparatus
2.2.2. Data Analysis
2.3. Taguchi Experimental Design Method
2.4. Catalyst Characterization
2.4.1. Qualitative Analysis of Catalyst Characterization
2.4.2. Quantitative Analysis of Coke Production
3. Results and Discussion
3.1. Taguchi Approach
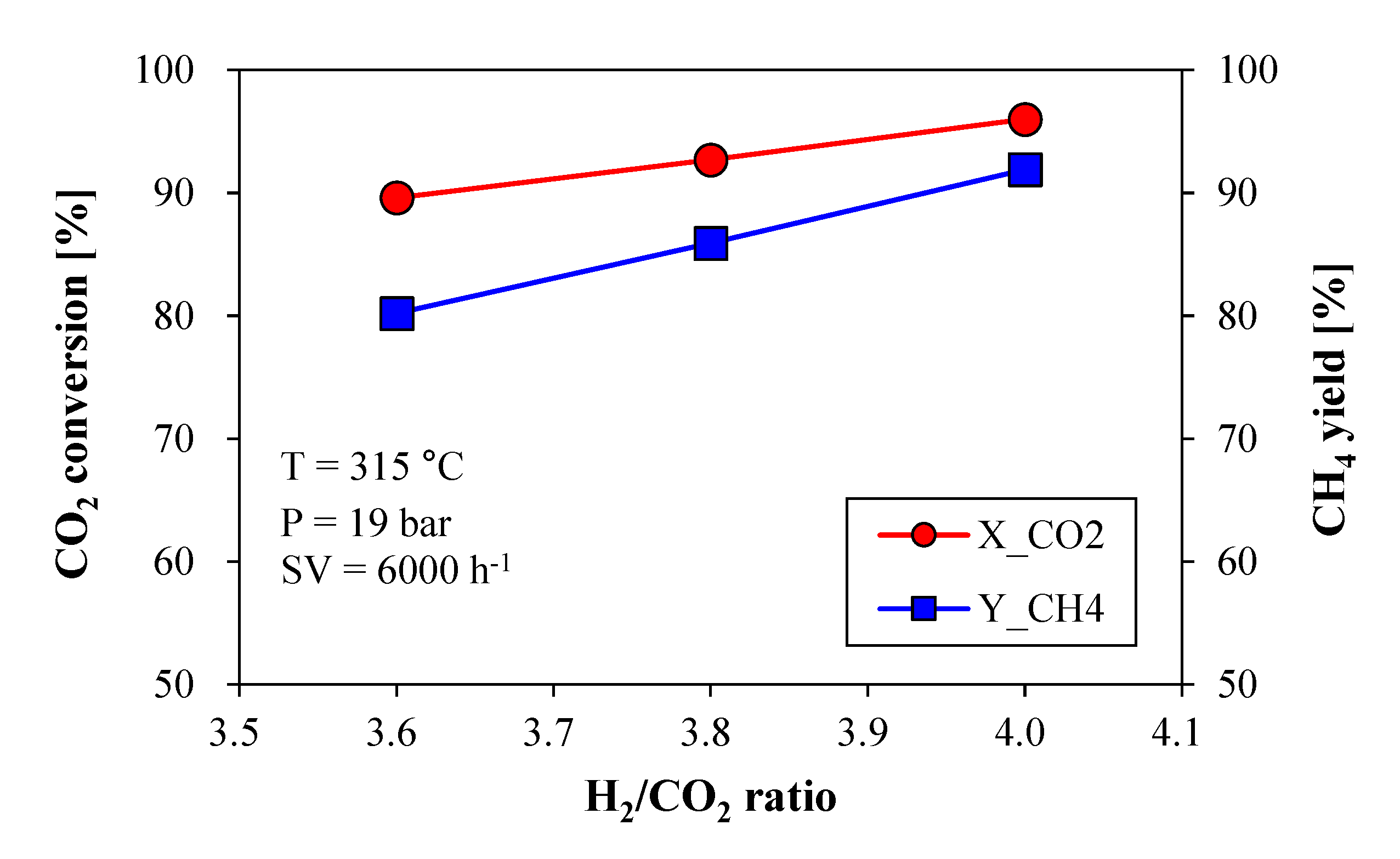
3.2. Catalytic Performance
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meylan, F.D.; Moreau, V.; Erkman, S. Material constraints related to storage of future European renewable electricity surpluses with CO2 methanation. Energy Policy 2016, 94, 366–376. [Google Scholar] [CrossRef]
- Momeni, M.; Soltani, M.; Hosseinpour, M.; Nathwani, J. A comprehensive analysis of a power-to-gas energy storage unit utilizing captured carbon dioxide as a raw material in a large-scale power plant. Energy Convers. Manag. 2021, 227, 113613. [Google Scholar] [CrossRef]
- Blanco, H.; Faaij, A. A review at the role of storage in energy systems with a focus on Power to Gas and long-term storage. Renew. Sustain. Energy Rev. 2018, 81, 1049–1086. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy. 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Dannesboe, C.; Hansen, J.B.; Johannsen, I. Catalytic methanation of CO2 in biogas: Experimental results from a reactor at full scale. React. Chem. Eng. 2020, 5, 183–189. [Google Scholar] [CrossRef]
- Schaaf, T.; Grünig, J.; Schuster, M.R.; Rothenfluh, T.; Orth, A. Methanation of CO2-storage of renewable energy in a gas distribution system. Energy Sustain. Soc. 2014, 4, 1–14. [Google Scholar] [CrossRef]
- Chein, R.Y.; Wang, C.C. Experimental Study on CO2 Methanation over Ni/Al2O3, Ru/Al2O3, and Ru-Ni/Al2O3 Catalysts. Catalysts 2020, 10, 1112. [Google Scholar] [CrossRef]
- Gruber, M.; Weinbrecht, P.; Biffar, L.; Harth, S.; Trimis, D.; Brabandt, J.; Blumentritt, R. Power-to-Gas through thermal integration of high-temperature steam electrolysis and carbon dioxide methanation-Experimental results. Fuel Process. Technol. 2018, 181, 61–74. [Google Scholar] [CrossRef]
- Brooks, K.P.; Hu, J.; Zhu, H.; Kee, R.J. Methanation of carbon dioxide by hydrogen reduction using the Sabatier process in microchannel reactors. Chem. Eng. Sci. 2007, 62, 1161–1170. [Google Scholar] [CrossRef]
- Gao, J.; Wang, Y.; Ping, Y.; Hu, D.; Xu, G.; Gu, F.; Su, F. A thermodynamic analysis of methanation reactions of carbon oxides for the production of synthetic natural gas. RSC Adv. 2012, 2, 2358–2368. [Google Scholar] [CrossRef]
- Stangeland, K.; Kalai, D.; Li, H.; Yu, Z. CO2 methanation: The effect of catalysts and reaction conditions. Energy Procedia 2017, 105, 2022–2027. [Google Scholar] [CrossRef]
- Chwoła, T.; Spietz, T.; Więcław-Solny, L.; Tatarczuk, A.; Krótki, A.; Dobras, S.; Zdeb, J. Pilot plant initial results for the methanation process using CO2 from amine scrubbing at the Łaziska power plant in Poland. Fuel 2020, 263, 116804. [Google Scholar] [CrossRef]
- Türks, D.; Mena, H.; Armbruster, U.; Martin, A. Methanation of CO2 on Ni/Al2O3 in a structured fixed-bed reactor—A scale-up study. Catalysts 2017, 7, 152. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Bassano, C.; Deiana, P.; Lietti, L.; Visconti, C.G. P2G movable modular plant operation on synthetic methane production from CO2 and hydrogen from renewables sources. Fuel 2019, 253, 1071–1079. [Google Scholar] [CrossRef]
- Pant, A.; Rai, J.P.N. Bioremediation of chlorpyrifos contaminated soil by two phase bioslurry reactor: Processes evaluation and optimization by Taguchi’s design of experimental (DOE) methodology. Ecotoxicol. Environ. Saf. 2018, 150, 305–311. [Google Scholar] [CrossRef]
- Kang, K.; Azargohar, R.; Dalai, A.K.; Wang, H. Hydrogen production from lignin, cellulose and waste biomass via supercritical water gasification: Catalyst activity and process optimization study. Energy Convers. Manag. 2016, 117, 528–537. [Google Scholar] [CrossRef]
- Özdemir, C.; Akın, A.N.; Yıldırım, R. Low temperature CO oxidation in hydrogen rich streams on Pt-SnO2/Al2O3 catalyst using Taguchi method. Appl. Catal. A Gen. 2004, 258, 145–152. [Google Scholar] [CrossRef]
- Chen, W.H.; Chiu, G.L.; Ong, H.C.; Lam, S.S.; Lim, S.; Ok, Y.S.; Kwon, E.E. Optimization and analysis of syngas production from methane and CO2 via Taguchi approach, response surface methodology (RSM) and analysis of variance (ANOVA). Fuel 2021, 296, 120642. [Google Scholar] [CrossRef]
- Tamimi, K.; Alavi, S.M.; Rezaei, M.; Akbari, E. Preparation of the Mn-Promoted NiO–Al2O3 nanocatalysts for low temperature CO2 methanation. J. Energy Inst. 2021, 99, 48–58. [Google Scholar] [CrossRef]
- Ocampo, F.; Louis, B.; Kiennemann, A.; Roger, A.C. CO2 methanation over Ni-Ceria-Zirconia catalysts: Effect of preparation and operating conditions. IOP Conf. Ser. Mater. Sci. Eng. 2011, 19, 012007. [Google Scholar] [CrossRef]
- Han, D.; Kim, Y.; Byun, H.; Cho, W.; Baek, Y. CO2 methanation of biogas over 20 wt% Ni-Mg-Al catalyst: On the effect of N2, CH4, and O2 on CO2 conversion rate. Catalysts 2020, 10, 1201. [Google Scholar] [CrossRef]
- Guilera, J.; Boeltken, T.; Timm, F.; Mallol, I.; Alarcón, A.; Andreu, T. Pushing the limits of SNG process intensification: High GHSV operation at pilot scale. ACS Sustain. Chem. Eng. 2020, 8, 8409–8418. [Google Scholar] [CrossRef]
- Shah, M.; Mondal, P. Optimization of CO2 reforming of methane process for the syngas production over Ni–Ce/TiO2–ZrO2 catalyst using the Taguchi method. Int. J. Hydrog. 2021, 46, 22799–22812. [Google Scholar] [CrossRef]
- Chen, C.J.; Hung, C.I. Optimization of co-gasification process in an entrained-flow gasifier using the Taguchi method. J. Therm. Sci. Technol. 2013, 8, 190–208. [Google Scholar] [CrossRef][Green Version]
- Aziz, M.A.A.; Jalil, A.A.; Triwahyono, S.; Mukti, R.R.; Taufiq-Yap, Y.H.; Sazegar, M.R. Highly active Ni-promoted mesostructured silica nanoparticles for CO2 methanation. Appl. Catal. B Environ. 2014, 147, 359–368. [Google Scholar] [CrossRef]
- Johnson, M.F. Surface area stability of aluminas. J. Catal. 1990, 123, 245–259. [Google Scholar] [CrossRef]




| Operating Parameters | Reactor Type | Catalyst | Reference |
|---|---|---|---|
| Temperature: 250–550 °C Reaction cycle, Metal loading amount (H2/CO2 = 5; SV = 5835 h−1) | Packed-bed reactor | Ni/Al2O3, Ru/Al2O3, Ru-Ni/Al2O3 | [7] |
| H2/CO2: 1–5 Pressure: 1–100 atm Temperature: 200–600 °C H2/CO2/H2O: 4/1/(0–0.5) H2/CO2/O2: 4/1/(0–0.5) | Fixed-bed reactor | Ni-based catalyst | [10] |
| Gas flow rate: 9.9–23 Nm3/h H2/CO2: 3.72–4.2 Pressure: 1.5–3.0 bar Temperature: 280–350 °C | Microchannel reactor | Ni-based catalyst | [12] |
| Pressure: 1–10 bar Temperature: 250–400 °C | Triple fixed-bed reactor | Ni/Al2O3 catalyst | [13] |
| GHSV: 5760–23,000 L kg−1 h−1 H2/CO2: 1.0–4.2 Temperature: 100–600 °C | Fixed-bed reactor | Ni-based catalyst (Ni-MCM-41) | [14] |
| WHSV: 6000–11,500 Ncm3/g∙h H2/CO2: 4.3–4.5 Pressure: 1–5 bar | Multi-tubular fixed-bed reactor | 0.5 wt.% Ru on Al2O3 | [15] |
| Factor | Unit | Level 1 | Level 2 | Level 3 | Level 4 | |
|---|---|---|---|---|---|---|
| A | Temperature | °C | 280 | 315 | 350 | 385 |
| B | Pressure | bar | 1 | 7 | 13 | 19 |
| C | Space velocity | h−1 | 6000 | 13,500 | 21,000 | 28,500 |
| No. | Factor A | Factor B | Factor C |
|---|---|---|---|
| Temperature (°C) | Pressure (Bar) | Space Velocity (H−1) | |
| 1 | 280 | 1 | 6000 |
| 2 | 280 | 7 | 13,500 |
| 3 | 280 | 13 | 21,000 |
| 4 | 280 | 19 | 28,500 |
| 5 | 315 | 1 | 13,500 |
| 6 | 315 | 7 | 6000 |
| 7 | 315 | 13 | 28,500 |
| 8 | 315 | 19 | 21,000 |
| 9 | 350 | 1 | 21,000 |
| 10 | 350 | 7 | 28,500 |
| 11 | 350 | 13 | 6000 |
| 12 | 350 | 19 | 13,500 |
| 13 | 385 | 1 | 28,500 |
| 14 | 385 | 7 | 21,000 |
| 15 | 385 | 13 | 13,500 |
| 16 | 385 | 19 | 6000 |
| Parameter Combination | Predicted | Experimental Confirmation | Relative Difference (%) |
|---|---|---|---|
| S/N Ratio | S/N Ratio | ||
| A2B4C1 | 39.80 | 39.45 | 0.87 |
| H2/CO2 | Bet Surface Area (m2/g) | 1.7 nm–300 nm Pore Volume (cm3/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|
| 3.6 | 129.2 | 0.24 | 0.298 |
| 3.8 | 130.1 | 0.24 | 0.300 |
| 4.0 | 132.8 | 0.25 | 0.298 |
| Fresh catalyst | 161.1 | 0.26 | 0.301 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeo, C.-E.; Seo, M.; Kim, D.; Jeong, C.; Shin, H.-S.; Kim, S. Optimization of Operating Conditions for CO2 Methanation Process Using Design of Experiments. Energies 2021, 14, 8414. https://doi.org/10.3390/en14248414
Yeo C-E, Seo M, Kim D, Jeong C, Shin H-S, Kim S. Optimization of Operating Conditions for CO2 Methanation Process Using Design of Experiments. Energies. 2021; 14(24):8414. https://doi.org/10.3390/en14248414
Chicago/Turabian StyleYeo, Chae-Eun, Minhye Seo, Dongju Kim, Cheonwoo Jeong, Hye-Sun Shin, and Suhyun Kim. 2021. "Optimization of Operating Conditions for CO2 Methanation Process Using Design of Experiments" Energies 14, no. 24: 8414. https://doi.org/10.3390/en14248414
APA StyleYeo, C.-E., Seo, M., Kim, D., Jeong, C., Shin, H.-S., & Kim, S. (2021). Optimization of Operating Conditions for CO2 Methanation Process Using Design of Experiments. Energies, 14(24), 8414. https://doi.org/10.3390/en14248414






