Synthesis of Mesoporous γ-Alumina Support for Water Composite Sorbents for Low Temperature Sorption Heat Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.1.1. The γ-Alumina Support Synthesis
2.1.2. Composite Preparation
2.2. Methods
3. Results and Discussion
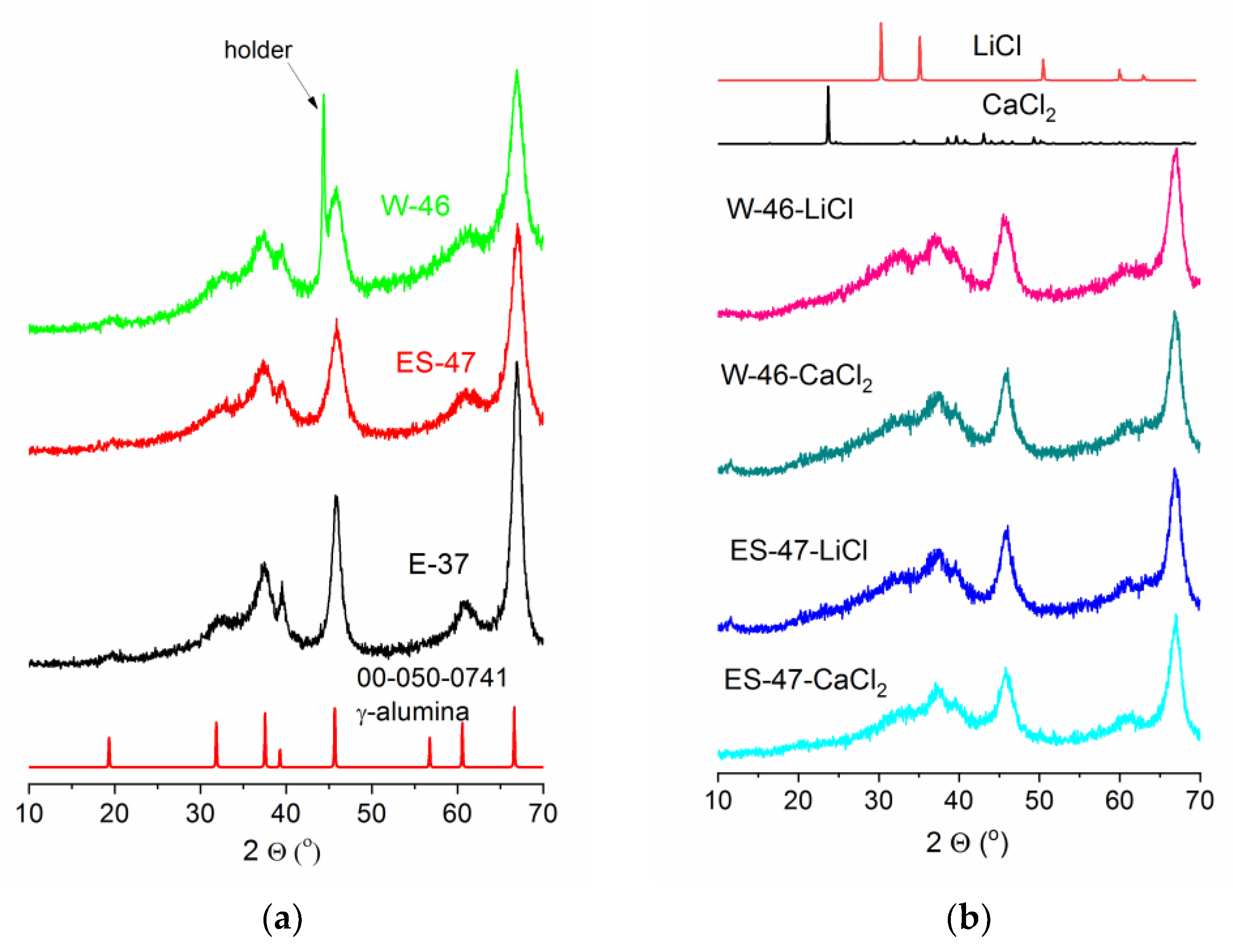
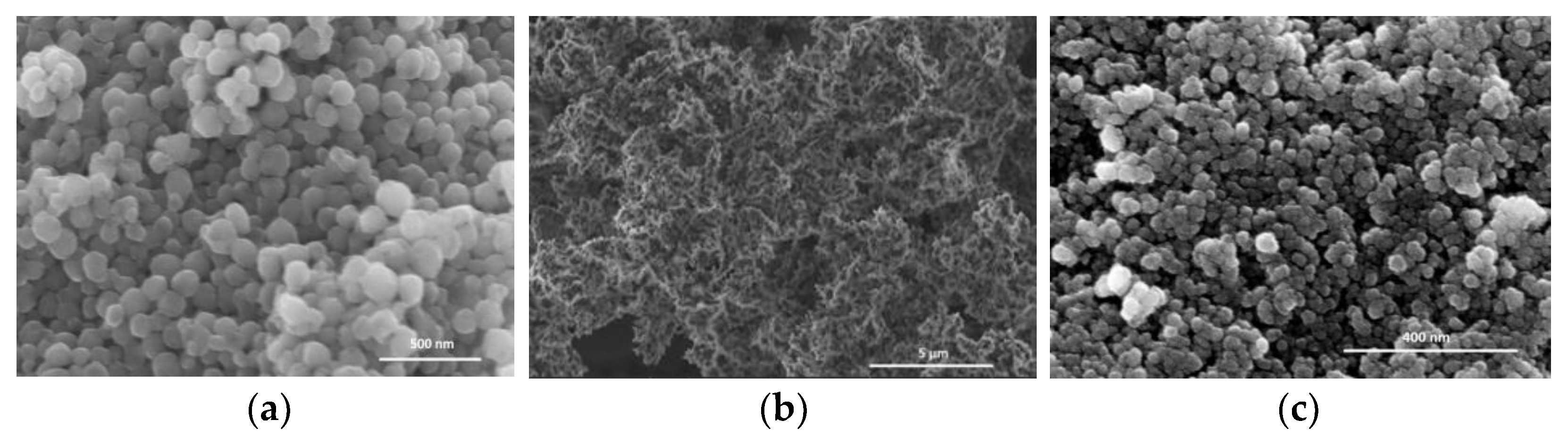
3.1. Structural Properties of All γ-Alumina Supports and Composites
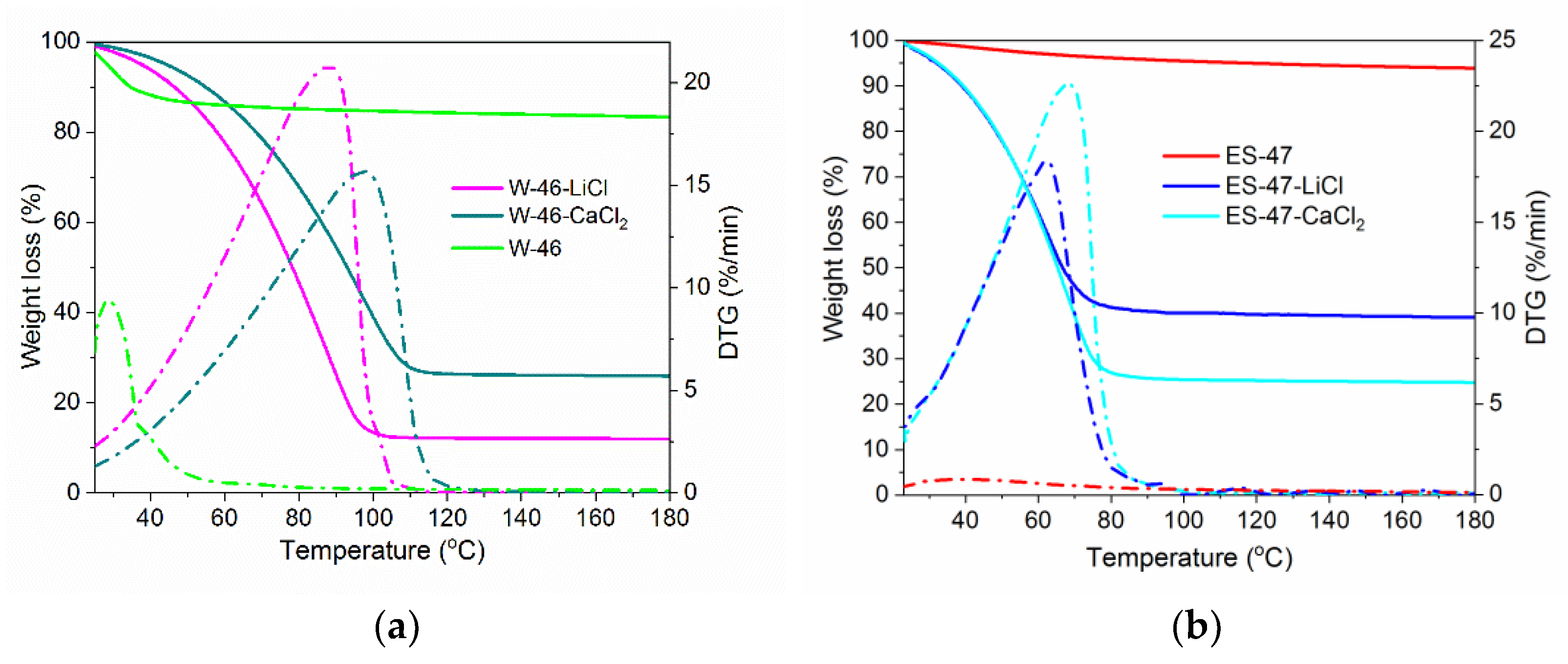
3.2. Water Sorption Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frazzica, A.; Brancato, V.; Caprì, A.; Cannilla, C.; Gordeeva, L.G.; Aristov, Y.I. Development of “Salt in Porous Matrix” Composites Based on LiCl for Sorption Thermal Energy Storage. Energy 2020, 208, 118338. [Google Scholar] [CrossRef]
- Gunasekara, S.N.; Barreneche, C.; Inés, A.; Díaz, A.C.; Ristić, A.; Weinberger, P.; Paksoy, H.Ö.; Koçak, B.; Rathgeber, C.; Chiu, N.; et al. Thermal Energy Storage Materials ( TESMs)—What Does It Take to Make Them Fly ? Crystals 2021, 11, 1276. [Google Scholar] [CrossRef]
- Gordeeva, L.G.; Aristov, Y.I. Composites “salt inside Porous Matrix” for Adsorption Heat Transformation: A Current State-of-the-Art and New Trends. Int. J. Low-Carbon Technol. 2012, 7, 288–302. [Google Scholar] [CrossRef] [Green Version]
- Zbair, M.; Bennici, S. Survey Summary on Salts Hydrates and Composites Used in Thermochemical Sorption Heat Storage: A Review. Energies 2021, 14, 3105. [Google Scholar] [CrossRef]
- Tokarev, M.; Gordeeva, L.; Romannikov, V.; Glaznev, I.; Aristov, Y. New Composite Sorbent CaCl2 in Mesopores for Sorption Cooling/Heating. Int. J. Therm. Sci. 2002, 41, 470–474. [Google Scholar] [CrossRef]
- Ristić, A.; Maučec, D.; Henninger, S.K.; Kaučič, V. New Two-Component Water Sorbent CaCl2-FeKIL2 for Solar Thermal Energy Storage. Microporous Mesoporous Mater. 2012, 164, 266–272. [Google Scholar] [CrossRef]
- Silvester, L.; Touloumet, Q.; Kamaruddin, A.; Chassagneux, F.; Postole, G.; Auroux, A.; Bois, L. Influence of Silica Functionalization on Water Sorption and Thermochemical Heat Storage of Mesoporous SBA-15/CaCl2 Composites. ACS Appl. Energy Mater. 2021, 4, 5944–5956. [Google Scholar] [CrossRef]
- Ristić, A.; Zabukovec Logar, N. New Composite Water Sorbents CaCl2-PHTS for Low-Temperature Sorption Heat Storage: Determination of Structural Properties. Nanomaterials 2018, 9, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonnen, T.; Beckert, S.; Gleichmann, K.; Brandt, A.; Unger, B.; Kerskes, H.; Mette, B.; Bonk, S.; Badenhop, T.; Salg, F.; et al. A Thermochemical Long-Term Heat Storage System Based on a Salt/Zeolite Composite. Chem. Eng. Technol. 2016, 39, 2427–2434. [Google Scholar] [CrossRef]
- D’Ans, P.; Skrylnyk, O.; Hohenauer, W.; Courbon, E.; Malet, L.; Degrez, M.; Descy, G.; Frère, M. Humidity Dependence of Transport Properties of Composite Materials Used for Thermochemical Heat Storage and Thermal Transformer Appliances. J. Energy Storage 2018, 18, 160–170. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Li, T.X. Thermochemical Characterizations of High-Stable Activated Alumina/LiCl Composites with Multistage Sorption Process for Thermal Storage. Energy 2018, 156, 240–249. [Google Scholar] [CrossRef]
- Brancato, V.; Gordeeva, L.G.; Caprì, A.; Grekova, A.D.; Frazzica, A. Experimental Comparison of Innovative Composite Sorbents for Space Heating and Domestic Hot Water Storage. Crystals 2021, 11, 476. [Google Scholar] [CrossRef]
- Permyakova, A.; Wang, S.; Courbon, E.; Nouar, F.; Heymans, N.; D’Ans, P.; Barrier, N.; Billemont, P.; De Weireld, G.; Steunou, N.; et al. Design of Salt-Metal Organic Framework Composites for Seasonal Heat Storage Applications. J. Mater. Chem. A 2017, 5, 12889–12898. [Google Scholar] [CrossRef]
- Calabrese, L.; Bonaccorsi, L.; Bruzzaniti, P.; Gullì, G.; Freni, A.; Proverbio, E. Zeolite Filled Siloxane Composite Foams: Compression Property. J. Appl. Polym. Sci. 2018, 135, 46145. [Google Scholar] [CrossRef]
- Kallenberger, P.A.; Posern, K.; Linnow, K.; Brieler, F.J.; Steiger, M.; Fröba, M. Alginate-Derived Salt/Polymer Composites for Thermochemical Heat Storage. Adv. Sustain. Syst. 2018, 2, 1–8. [Google Scholar] [CrossRef]
- Frazzica, A.; Brancato, V.; Dawoud, B. Unified Methodology to Identify the Potential Application of Seasonal Sorption Storage Technology. Energies 2020, 13, 1037. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.W.; Metcalf, S.J.; Critoph, R.E.; Tamainot-Telto, Z.; Thorpe, R. Two Types of Natural Graphite Host Matrix for Composite Activated Carbon Adsorbents. Appl. Therm. Eng. 2013, 50, 1652–1657. [Google Scholar]
- Yan, T.; Li, T.; Xu, J.; Chao, J.; Wang, R.; Aristov, Y.I.; Gordeeva, L.G.; Dutta, P.; Murthy, S.S. Ultrahigh-Energy-Density Sorption Thermal Battery Enabled by Graphene Aerogel-Based Composite Sorbents for Thermal Energy Harvesting from Air. ACS Energy Lett. 2021, 6, 1795–1802. [Google Scholar] [CrossRef]
- An, G.; Zhang, Y.; Wang, L.; Zhang, B. An Advanced Composite Sorbent with High Thermal Stability and Superior Sorption Capacity without Hysteresis for a Better Thermal Battery. J. Mater. Chem. A 2020, 8, 11849–11858. [Google Scholar] [CrossRef]
- Dawoud, B.; Aristov, Y. Experimental Study on the Kinetics of Water Vapor Sorption on Selective Water Sorbents, Silica Gel and Alumina under Typical Operating Conditions of Sorption Heat Pumps. Int. J. Heat Mass Transf. 2003, 46, 273–281. [Google Scholar] [CrossRef]
- Jabbari-Hichri, A.; Bennici, S.; Auroux, A. CaCl2-Containing Composites as Thermochemical Heat Storage Materials. Sol. Energy Mater. Sol. Cells 2017, 172, 177–185. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Wang, R.Z.; Li, T.X. Experimental Investigation on an Open Sorption Thermal Storage System for Space Heating. Energy 2017, 141, 2421–2433. [Google Scholar] [CrossRef]
- Xu, S.Z.; Wang, R.Z.; Wang, L.W.; Zhu, J. Performance Characterizations and Thermodynamic Analysis of Magnesium Sulfate-Impregnated Zeolite 13X and Activated Alumina Composite Sorbents for Thermal Energy Storage. Energy 2019, 167, 889–901. [Google Scholar] [CrossRef]
- Wu, H.; Salles, F.; Zajac, J. A Critical Review of Solid Materials for Low-Temperature Thermochemical Storage of Solar Energy Based on Solid-Vapour Adsorption in View of Space Heating Uses. Molecules 2019, 24, 945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grekova, A.D.; Girnik, I.S.; Nikulin, V.V.; Tokarev, M.M.; Gordeeva, L.G.; Aristov, Y.I. New Composite Sorbents of Water and Methanol “Salt in Anodic Alumina”: Evaluation for Adsorption Heat Transformation. Energy 2016, 106, 231–239. [Google Scholar] [CrossRef]
- Henninger, S.K.; Ernst, S.J.; Gordeeva, L.; Bendix, P.; Fröhlich, D.; Grekova, A.D.; Bonaccorsi, L.; Aristov, Y.; Jaenchen, J. New Materials for Adsorption Heat Transformation and Storage. Renew. Energy 2017, 110, 59–68. [Google Scholar] [CrossRef]
- Afshar Taromi, A.; Kaliaguine, S. Synthesis of Ordered Mesoporous Γ-Alumina—Effects of Calcination Conditions and Polymeric Template Concentration. Microporous Mesoporous Mater. 2017, 248, 179–191. [Google Scholar] [CrossRef]
- Trueba, M.; Trasatti, S.P. γ-Alumina as a Support for Catalysts: A Review of Fundamental Aspects. Eur. J. Inorg. Chem. 2005, 2005, 3393–3403. [Google Scholar] [CrossRef]
- Xu, X.; Megarajan, S.K.; Zhang, Y.; Jiang, H. Ordered Mesoporous Alumina and Their Composites Based on Evaporation Induced Self-Assembly for Adsorption and Catalysis. Chem. Mater. 2020, 32, 3–26. [Google Scholar] [CrossRef]
- Žilková, N.; Zukal, A.; Čejka, J. Synthesis of Organized Mesoporous Alumina Templated with Ionic Liquids. Microporous Mesoporous Mater. 2006, 95, 176–179. [Google Scholar] [CrossRef]
- Grant, S.M.; Vinu, A.; Pikus, S.; Jaroniec, M. Adsorption and Structural Properties of Ordered Mesoporous Alumina Synthesized in the Presence of F127 Block Copolymer. Colloids Surf. A: Physicochem. Eng. Asp. 2011, 385, 121–125. [Google Scholar] [CrossRef]
- Gonçalves, A.A.S.; Costa, M.J.F.; Zhang, L.; Ciesielczyk, F.; Jaroniec, M. One-Pot Synthesis of MeAl2O4 (Me = Ni, Co, or Cu) Supported on γ-Al2O3 with Ultralarge Mesopores: Enhancing Interfacial Defects in γ-Al2O3 to Facilitate the Formation of Spinel Structures at Lower Temperatures. Chem. Mater. 2018, 30, 436–446. [Google Scholar] [CrossRef] [Green Version]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Sayari, A. Application of Large Pore MCM-41 Molecular Sieves to Improve Pore Size Analysis Using Nitrogen Adsorption Measurements. Langmuir 1997, 13, 6267–6273. [Google Scholar] [CrossRef]
- Jaroniec, M.; Solovyov, L.A. Improvement of the Kruk-Jaroniec-Sayari Method for Pore Size Analysis of Ordered Silicas with Cylindrical Mesopores. Langmuir 2006, 22, 6757–6760. [Google Scholar] [CrossRef]
- Ristić, A.; Fischer, F.; Hauer, A.; Zabukovec Logar, N. Improved Performance of Binder-Free Zeolite y for Low-Temperature Sorption Heat Storage. J. Mater. Chem. A 2018, 6, 11521–11530. [Google Scholar] [CrossRef] [Green Version]
- Nuhnen, A.; Janiak, C. A Practical Guide to Calculate the Isosteric Heat/Enthalpy of Adsorption: Via Adsorption Isotherms in Metal-Organic Frameworks, MOFs. Dalton Trans. 2020, 49, 10295–10307. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, A.; Varlec, J.; Mazaj, M.; Ristić, A.; Logar, N.Z.; Mali, G. Superior Performance of Microporous Aluminophosphate with LTA Topology in Solar-Energy Storage and Heat Reallocation. Adv. Energy Mater. 2017, 7, 1601815. [Google Scholar] [CrossRef]
- Calvin, J.J.; Asplund, M.; Zhang, Y.; Huang, B.; Woodfield, B.F. Heat Capacity and Thermodynamic Functions of Γ-Al2O3. J. Chem. Thermodyn. 2017, 112, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Frazzica, A.; Brancato, V. Verification of Hydrothermal Stability of Adsorbent Materials for Thermal Energy Storage. Int. J. Energy Res. 2019, 43, 6161–6170. [Google Scholar] [CrossRef]
- Šuligoj, A.; Ristić, A.; Dražić, G.; Pintar, A.; Logar, N.Z.; Tušar, N.N. Bimetal Cu-Mn Porous Silica-Supported Catalyst for Fenton-like Degradation of Organic Dyes in Wastewater at Neutral PH. Catal. Today 2020, 358, 270–277. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Tušar, N.N.; Ristić, A.; Mali, G.; Mazaj, M.; Arčon, I.; Arčon, D.; Kaučič, V.; Logar, N.Z. MnO x Nanoparticles Supported on a New Mesostructured Silicate with Textural Porosity. Chem. A Eur. J. 2010, 16, 5783–5793. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Spieß, A.; Jansen, C.; Nuhnen, A.; Gökpinar, S.; Wiedey, R.; Ernst, S.J.; Janiak, C. Tunable LiCl@UiO-66 Composites for Water Sorption-Based Heat Transformation Applications. J. Mater. Chem. A 2020, 8, 13364–13375. [Google Scholar] [CrossRef]





| Sample Support | Solvent | SBET (m2/g) | Vp (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|---|
| E-37-γ-Al2O3 | Ethanol | 213 ± 1 | 0.542 | 10.7 |
| W-46-γ-Al2O3 | Water | 147 ± 1 | 0.414 | 9.1 |
| ES-47-γ-Al2O3 | Ethanolic solution | 148 ± 1 | 0.367 | 11.3 |
| Sample Support | SBET (m2/g) | Vp (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| W-46-LiCl | 68 ± 1 | 0.196 | 8.7 |
| W-46-CaCl2 | 81 ± 1 | 0.252 | 8.3 |
| ES-47-LiCl | 96 ± 1 | 0.204 | 9.3 |
| ES-47-CaCl2 | 83 ± 1 | 0.207 | 10.2 |
| Sample | ∆w (kg/kg) | Qsor (kWh/m3) | Qsor (GJ/m3) |
|---|---|---|---|
| W-46-CaCl2 | 0.127 | 350 | 1.26 |
| W-46-LiCl | 0.514 | 1437 | 5.17 |
| ES-47-LiCl | 0.137 | 380 | 1.37 |
| ES-47-CaCl2 | 0.135 | 377 | 1.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ocvirk, M.; Ristić, A.; Zabukovec Logar, N. Synthesis of Mesoporous γ-Alumina Support for Water Composite Sorbents for Low Temperature Sorption Heat Storage. Energies 2021, 14, 7809. https://doi.org/10.3390/en14227809
Ocvirk M, Ristić A, Zabukovec Logar N. Synthesis of Mesoporous γ-Alumina Support for Water Composite Sorbents for Low Temperature Sorption Heat Storage. Energies. 2021; 14(22):7809. https://doi.org/10.3390/en14227809
Chicago/Turabian StyleOcvirk, Manca, Alenka Ristić, and Nataša Zabukovec Logar. 2021. "Synthesis of Mesoporous γ-Alumina Support for Water Composite Sorbents for Low Temperature Sorption Heat Storage" Energies 14, no. 22: 7809. https://doi.org/10.3390/en14227809
APA StyleOcvirk, M., Ristić, A., & Zabukovec Logar, N. (2021). Synthesis of Mesoporous γ-Alumina Support for Water Composite Sorbents for Low Temperature Sorption Heat Storage. Energies, 14(22), 7809. https://doi.org/10.3390/en14227809






