Waste-Based Intermediate Bioenergy Carriers: Syngas Production via Coupling Slow Pyrolysis with Gasification under a Circular Economy Model
Abstract
1. Introduction
Scope and Objective of the Concept
- Reviewing the pyro-oil characteristics derived from a slow pyrolysis required for being suitable fuel for the gasification.
- Gasification parameters impacting syngas quality.
- Depicting the circular economy approach’s advantages.
- Screening the economic feasibility of such a project.
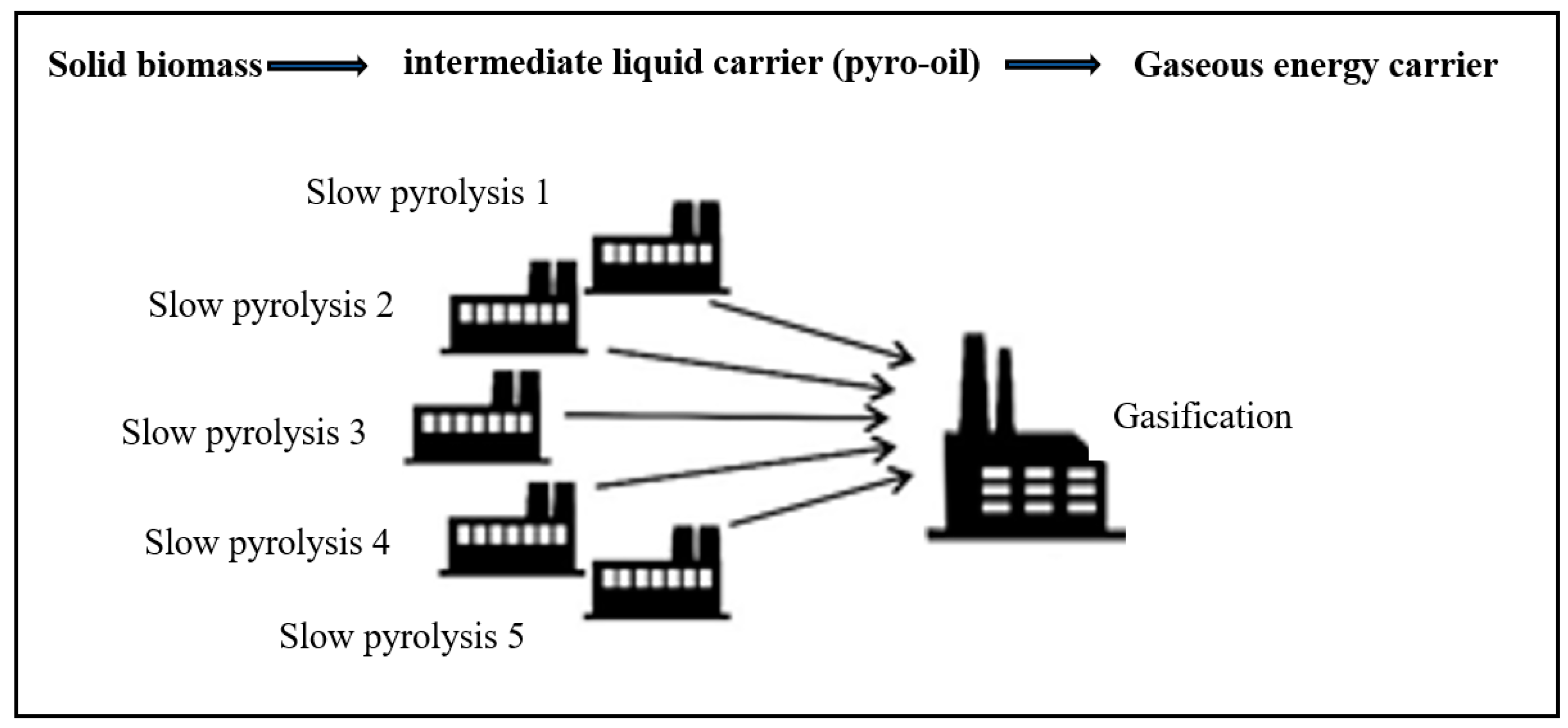
- It is a two-stage system suitable in a circular economy for bioenergy recovery from agro-industrial and agricultural waste, for the logistically optimized production of synthesis gas (syngas).
- Through the gasification of bio-oil, a better-quality synthesis gas is produced, while at the same time the transportation costs are minimized compared to solid biomass, due to the higher energy density of the pyro-oil (biooil).
- Agricultural residues, agro-industrial wastes, forestry residues, biodegradable municipal waste, can be treated regionally in decentralized plants of slow pyrolysis for biochar production and the produced pyro-oils can be further used in a central large scale gasification system, creating a symbiotic strategy for a circular economy.
- This proposed system can combine the two seemingly opposing concepts of bioenergy carrier’s production via slow pyrolysis from biomass and waste in the form of bio-oil, with carbon sequestration in the form of biochar.
2. Methods and Materials
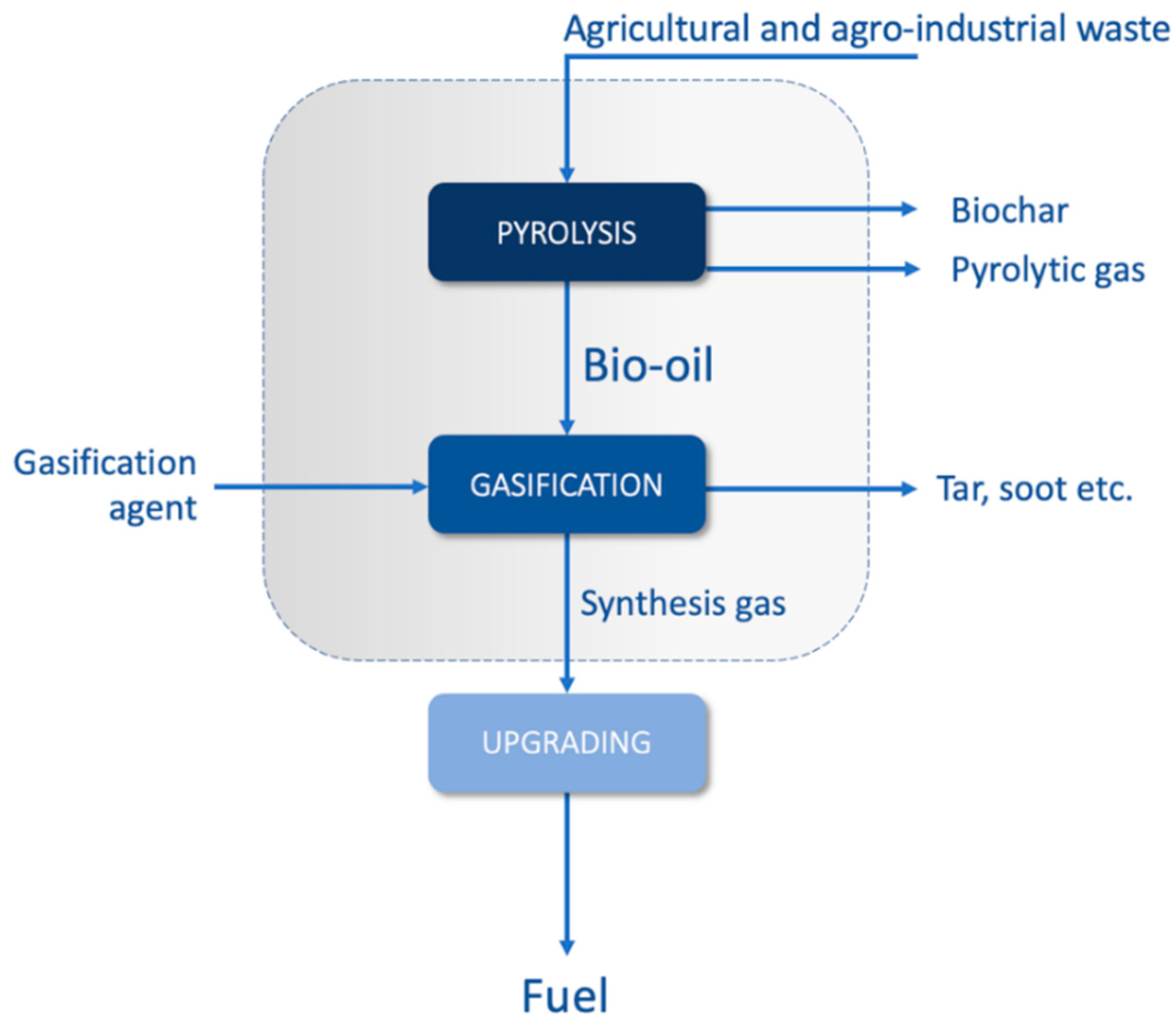
2.1. The Thermochemical System
2.2. Research Questions
- Through which thermochemical methods can biomass be converted into energy?
- Which bio-oil production method is the best?
- What physical and chemical properties of bio-oil are important for its subsequent processing?
- What factors affect these properties?
- Which bio-oil can be used in the gasification process to produce gas fuel?
- How does the quality of bio-oil and the conditions of gasification affect the production of gaseous fuel?
- Is the syngas production by slow pyrolysis bio-oil gasification system economically feasible?
2.3. Bibliographic Search
3. Discussing Pyrolysis
3.1. Slow Pyrolysis
3.2. Fast Pyrolysis
3.3. Flash Pyrolysis
3.4. Comparison of Pyrolysis Methods
4. Properties of Bio-Oils
4.1. Physical Properties
4.1.1. Density
4.1.2. Viscosity
4.1.3. Pour Point
4.1.4. Flash Point
4.1.5. Heating Values
4.2. Chemical Properties
4.2.1. Moisture Content
4.2.2. Acidity
4.2.3. Elemental Analysis (C, H, O, N)
4.2.4. Ash Content
4.3. Parameters That Affect the Properties of Bio-Oil
4.3.1. Effect of Temperature
4.3.2. Effect of Heating Rate
4.3.3. Effect of Particle Size
4.3.4. Effect of Carrier Gas Flow
4.3.5. Effect of Residence Time
4.3.6. Effect of Biomass Composition
4.4. Combined Implications of Pyrolysis Parameters
| Pyrolysis Operating Conditions | Bio-Oil Characteristics | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Feedstock | Temperature (°C) | Heating Rate (°C/min) | Carrier Gas Flow (L/min) | Particle Size (mm) | Residence Time (s) | Bio-Oil Yield (wt.%) | Pour Point (°C) | Flash Point (°C) | Higher Heating Value (MJ/kg) | Kinematic Viscosity (mm2/s) | Density (g/mL) | pH | Ref. |
| Agro-industrial waste | |||||||||||||
| Rice husk | 300 400 500 300 400 500 300 400 500 | 5 5 5 10 10 10 20 20 20 | - | ≈0.231 | 3600 5400 7200 7200 3600 5400 5400 7200 3600 | 28.70 ± 4.50 32.14 ± 0.06 36.82 ± 0.5 15.82 ± 5.0 39.84 ± 1.26 38.20 ± 0.71 6.03 ± 2.35 34.94 ± 0.15 39.80 ± 2.4 | - | - | - | - | - | ≈3 | [32] |
| Corn cob | 300 350 400 450 | 20 | 0.05 | 0.5–2 | - | 42.8 45.0 45.6 47.3 | - | - | - | - | - | - | [31] |
| Processed sesame seeds | 350 | 25 | 0.00151 | - | - | 18.6 | −5 | 182 | 25.5 | 39.6 at 40 °C | 1.029 | - | [41] |
| Sunflower husk | 400 | 10 | 0.1 | - | - | 34 | - | - | - | - | - | - | [42] |
| Wheat husk | 550 | 15 | 0.02 | - | 3600 | 31.8 | - | - | 6.02 | - | 1.06 | 6 | [33] |
| Apricot kernel after oils extraction | 450 | 20 | 0.05–0.2 | 0.25 | 3600 | 43.66 | −6 | 96 | 39.12 | 37.9 at 40 °C | 1.0012 at 20 °C | 3.2 | [43] |
| Apricot kernel | 400 | 10 | 0.05–0.2 | 0.425–0.600 | - | ≈21 | - | - | 27.19 | - | - | - | [44] |
| Pomegranate peel | 750 | 25 | 0.1 | 1.5–5 | 3600 | 35 | - | - | 20.4 | - | - | - | [45] |
| Pomegranate seed | 400 800 | 5 | ≈0.01 | ≈3.2 | 3600 | 8.88 21.54 | - | - | 34.76 33.96 | - | - | - | [46] |
| Apricot pulp | 550 | 5 | 0.1 | 0.85–1.25 | - | 23.3 | - | - | 26.82 | - | - | - | [47] |
| Peach pulp | 550 | 5 | 0.1 | 0.85–1.25 | - | 23.2 | - | - | 25.76 | - | - | - | [47] |
| Potato peel | 550 | 5 | 0.2 | 0.81 | - | 27.11 | - | - | 32 | - | - | - | [48] |
| Tomato peel | 600 | 20 | - | - | - | 40 | - | 94 | 33.04 | 11.82 at 40 °C | 0.973 | - | [49] |
| Flaxseed residues | 350 500 650 | 5 | 0.06 | - | 1800 | 43.3 52.7 55.0 | - | - | - | - | - | - | [50] |
| Sunflower residues | 400 | 0.67 | 0.05 | 1 | - | 21 | - | - | - | - | - | - | [51] |
| Date kernel | 500 | 20 | 0.01 | 0.05–0.1 | ≈1800 | 66.5 | - | - | 29.06 | 1.4179 at 25 °C | 1.029 | 2–4 | [52] |
| Cherry kernel | 500 | 5 | 0.025 | - | - | ≈20 | - | - | 32.46 | - | - | - | [53] |
| Grape seed | 500 | 10 | 0.2 | - | - | ≈20 | - | - | - | - | - | [54] | |
| Winery wastes | 600 | ≈50 | - | - | - | 47.7 | - | - | - | - | - | - | [55] |
| Olive residues | 500 | 20 | 1 | - | 1800 | 45 | - | - | - | - | - | - | [56] |
| Coffee residues | 450 | 10 | - | - | 3600 | 27.77 | - | - | - | - | - | - | [57] |
| Agricultural waste | |||||||||||||
| Mushroom substrate | 470 | 20 | ≈0.27 | - | - | 14.4 | - | - | 24.82 | - | - | - | [58] |
| Lemon leaves | 350 450 550 | 10 | 0.1 | 0.125–0.250 | - | 39.3 32.8 27.7 | - | - | - | - | - | - | [34] |
| Grape residues | 600 | 5 | 0.15 | <2 | 1800 | 41.4 | - | - | - | - | - | - | [59] |
| Grape bagasse | 550 | 10 | - | 0.425–0.600 | >1800 | ≈23 | - | 61 | 32.95 | 23 at 40 °C | 0.992 at 20 °C | - | [60] |
| Wheat straw | 300 350 400 450 | 20 | 0.05 | 0.5–2 | - | 32.5 36.0 36.7 29.2 | - | - | - | - | - | - | [31] |
| Date residues mix | 500 | 20 | 0.01 | 0.05–0.1 | ≈1800 | 30.1 | - | - | 24.35 | 1.367 at 25 °C | 1.011 | 2–4 | [52] |
| Cotton stalk | 400 | 13 | 0.5 | 1–3 | 3600 | 23.63 | - | - | - | - | - | - | [61] |
| Energy crops | |||||||||||||
| Cotton thistle | 550 | 40 | - | 0.6–0.85 | >1800 | ≈17 | - | - | 32.6 | - | - | - | [62] |
| Canola | 500 | 10 | 0.0012 | 0.425–1.25 | - | 32.7 | - | - | 34.75 | - | - | - | [63] |
| Flaxseed | 550 | 5 | 0.1 | 0.425–2.8 | ≈1800 | 46.4 | - | - | 34.58 | - | - | - | [64] |
| Castor seed | 550 | 20 | 0.1 | - | <3000 | 64.4 | <5 | 31 | ≈35 | 83.19 at 40 °C | 0.966 | 3.7 | [65] |
| Feedstock | T (°C) | wt.% Dry Ash Free (daf) | HHV (MJ/kg) | Ref. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | Β | Cl | Fe | Cu | Zn | Al | ||||
| Agro-industrial waste | ||||||||||||||
| Processed sesame seeds | 350 | 55.8 | 8.34 | 28.91 | 7.0 | 0.17 | - | - | - | - | - | - | 25.5 | [41] |
| Wheat husk | 550 | 60.9 | 9.7 | 8.8 | 11.5 | - | - | - | - | - | - | - | 6.02 | [33] |
| Apricot kernel after oils extraction | 450 | 74.19 | 11.18 | 13.60 | 1.03 | - | - | - | - | - | - | - | 39.12 | [43] |
| Apricot kernel | 400 | 64.45 | 8.24 | 26.5 | 0.81 | - | - | - | - | - | - | - | 27.19 | [44] |
| Pomegranate seed | 400 800 | 64.26 67.99 | 8.21 8.07 | 25.43 21.17 | 2.06 2.66 | 0.04 0.11 | - | - | - | - | - | - | 34.76 33.96 | [46] |
| Apricot pulp | 550 | 61.5 | 7.8 | 28.96 | 1.76 | - | - | - | - | - | - | - | 26.82 | [47] |
| Peach pulp | 550 | 59.58 | 7.88 | 31.96 | 0.58 | - | - | - | - | - | - | - | 25.76 | [47] |
| Potato peel | 550 | 58.82 | 8.54 | 31.41 | 1.22 | - | - | - | - | - | - | - | 32.00 | [48] |
| Tomato peel | 600 | 75 | 9.28 | 11.2 | 4.4 | 0.12 | - | - | - | - | - | - | 33.04 | [49] |
| Cherry kernel | 500 | 67.18 | 8.48 | 21.86 | 2.45 | 0.03 | - | - | - | - | - | - | 32.46 | [53] |
| Agricultural waste | ||||||||||||||
| Mushroom substrate | 470 | 65.29 | 7.16 | 21.72 | 5.83 | - | - | - | - | - | - | - | 24.82 | [58] |
| Grape bagasse | 550 | 71.72 | 8.69 | 16.90 | 2.69 | - | - | - | - | - | - | - | 32.95 | [60] |
| Energy crops | ||||||||||||||
| Cotton thistle | 550 | 68.9 | 8.9 | 20.3 | 1.7 | - | - | - | - | - | - | - | 32.6 | [62] |
| Canola | 500 | - | - | - | 10.87 | 1.08 | - | 0.157 | 0.0036 | 0.0004 | 0.0007 | 0.0077 | 34.75 | [63] |
| Flaxseed | 550 | 74.2 | 10.5 | 13.8 | 1.5 | - | - | - | - | - | - | - | 34.58 | [64] |
| Castor seed | 550 | 69.33 | - | 2.25 | - | - | 28.25 | - | - | - | - | - | ≈35 | [65] |
5. Gasification
5.1. What Kind of Bio-Oil Is Suitable for Gasification?
5.2. Bio-Oil Feed to the Gasifier
5.3. Stages of Gasification
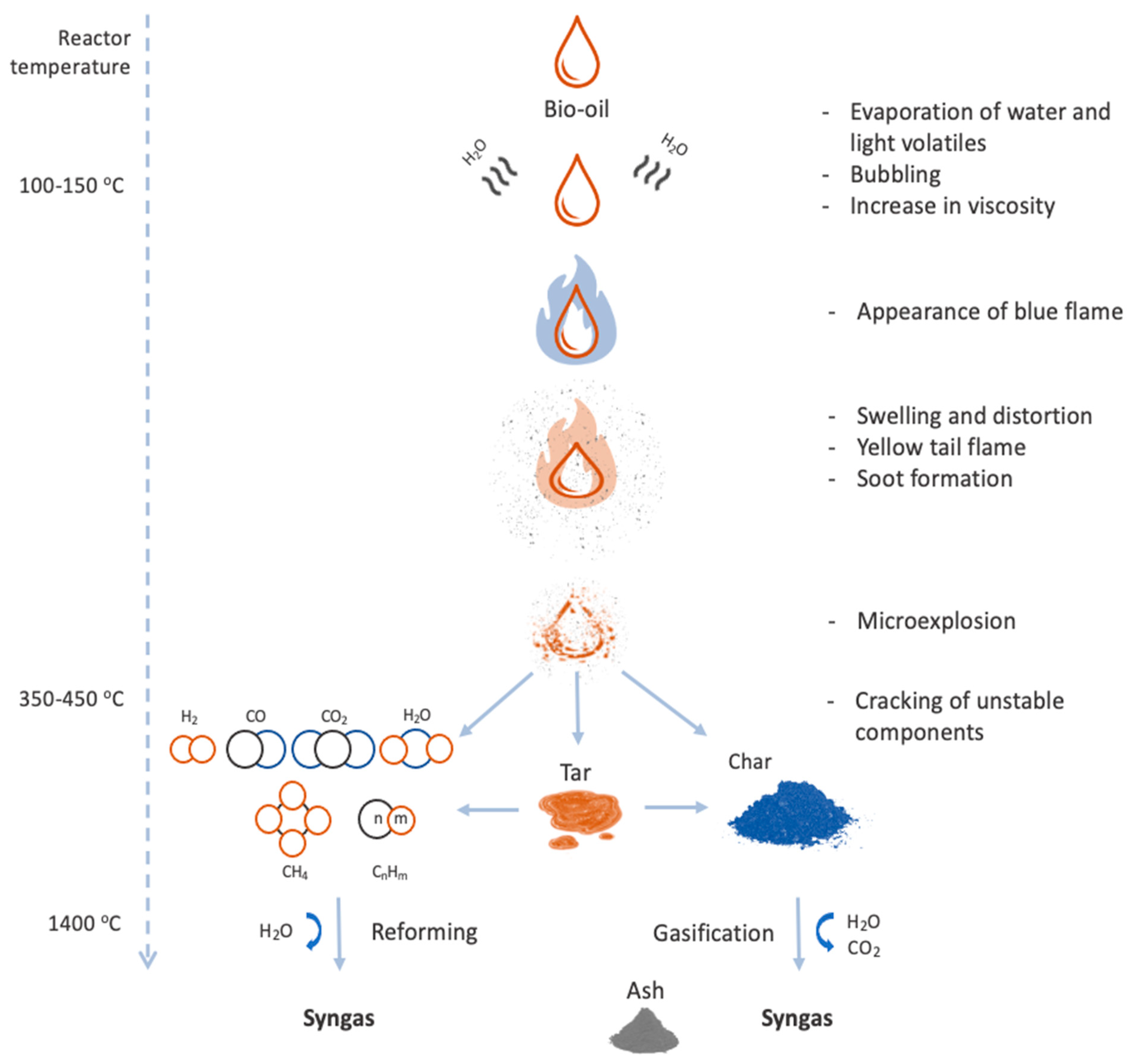
5.4. Steam Gasification of Bio-Oil
5.5. Factors That Affect Syngas Quality
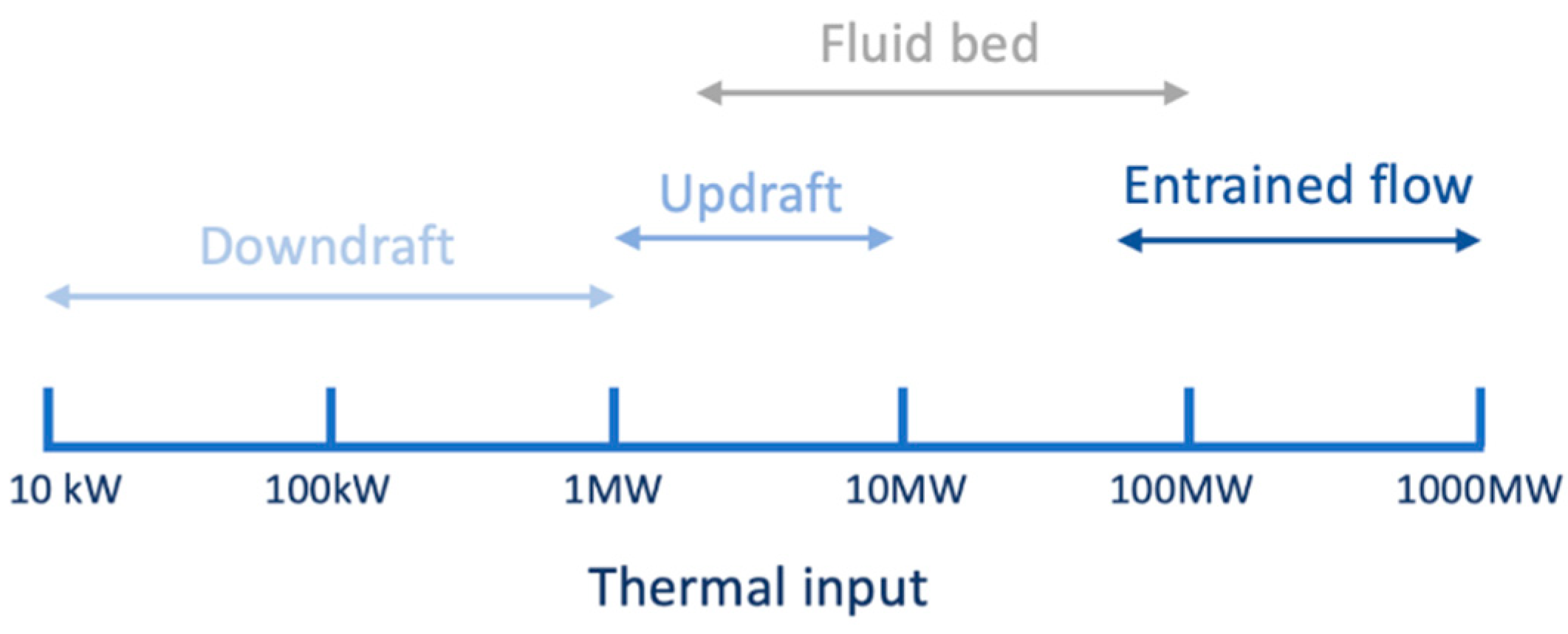
5.5.1. Effect of the Gasifier Type
Fixed Bed Gasifiers
Fluidized Bed Gasifiers
Entrained Flow Gasifier
5.6. Effect of Gasification Agent on Syngas Composition
5.7. Effect of Equivalence Ratio (ER)
| Gasification Parameters | Gas Characteristics | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Feedstock for Bio-Oil | Τ (°C) | Gasifying Agent | Steam/ Bio-Oil | Gasifier Type | ER (kg/kg) | Bio-Oil Flow Rate (kg/h) | Air Flow Rate (kg/h) | Gas Yield (m3/ kg bio-oil) | CGE (100%) | CCE (100%) | LHV (MJ/Nm3) | Oxidation Degree | Tar (mg/Nm3) | Ref. |
| Agro-industrial waste | ||||||||||||||
| Rice husk | 1000 | air-steam | 0 1 2 2.5 3 4 5 | entrained flow | 0.4 | 9.0 | 21.32 | 1.30 ± 0.002 1.75 ± 0.01 1.81 ± 0.01 1.90 ± 0.01 1.86 ± 0.02 1.65 ± 0.02 1.47 ± 0.02 | 0.54 ± 0.007 0.72 ± 0.012 0.79 ± 0.014 0.90 ± 0.012 0.84 ± 0.009 0.51 ± 0.007 0.40 ± 0.006 | 0.62 ± 0.02 0.75 ± 0.02 0.82 ± 0.05 0.93 ± 0.05 0.89 ± 0.06 0.87 ± 0.02 0.81 ± 0.02 | 6.50 ± 0.10 7.20 ± 0.11 7.63 ± 0.13 8.26 ± 0.12 7.88 ± 0.01 5.34 ± 0.04 4.78 ± 0.08 | 0.17 ± 0.002 0.20 ± 0.002 0.25 ± 0.005 0.31 ± 0.001 0.43 ± 0.005 0.46 ± 0.003 0.54 ± 0.003 | 280 ± 8.89 180 ± 3.61 100 ± 1.73 52 ± 1.0 58 ± 1.00 67 ± 2.65 90 ± 4.00 | [88] |
| 700 750 800 850 900 950 1000 | air-steam | 2.5 | entrained flow | 0.4 | 9.0 | 21.32 | 1.10 ± 0.01 1.22 ± 0.01 1.32 ± 0.01 1.43 ± 0.01 1.60 ± 0.02 1.71 ± 0.02 1.90 ± 0.03 | 0.37 ± 0.005 0.44 ± 0.008 0.52 ± 0.007 0.58 ± 0.007 0.66 ± 0.008 0.78 ± 0.012 0.90 ± 0.014 | 0.69 ± 0.01 0.74 ± 0.01 0.80 ± 0.03 0.84 ± 0.03 0.87 ± 0.05 0.87 ± 0.02 0.93 ± 0.04 | 5.85 ± 0.09 6.23 ± 0.09 6.83 ± 0.12 7.02 ± 0.10 7.21 ± 0.10 7.9 ± 0.05 8.26 ± 0.14 | 0.19 ± 0.002 0.22 ± 0.003 0.26 ± 0.005 0.31 ± 0.001 0.32 ± 0.004 0.31 ± 0.003 0.31 ± 0.003 | 270 ± 8.88 230 ± 4.36 200 ± 3.46 150 ± 2.65 93 ± 3.00 65 ± 2.65 52 ± 2.00 | ||
| Rice husk | 1000 | air air-oxygen oxygen | - | entrained flow | 0.3 | - | - | - | 0.747 0.732 0.749 | - | 8.00 11.10 13.80 | - | 1300 820 490 | [95] |
| Agricultural waste | ||||||||||||||
| Corn stalk | 500–800 | steam | - | dual fixed beds | - | 0.0144 | - | - | - | 0.87 | - | - | - | [97] |
| Wheat straw | 1272 | oxygen | - | entrained flow | - | - | - | - | - | 0.89 | - | - | - | [98] |
| Other | ||||||||||||||
| Hard wood | 1000–1400 | steam | - | entrained flow | - | 0.018 | - | - | - | - | 10.6 | - | - | [99] |
| Birch wood | 800 | steam | - | entrained flow | - | - | - | ≈0.72 | - | 0.59 | - | - | - | [99] |
| Coal/bio-oil slurry | 1300 | steam | - | entrained flow | - | - | - | - | - | 0.924 | 10.7 | - | - | [96] |
| Poplar wood | 725 | oxygen | - | entrained flow | 0.27 | - | - | - | - | 0.80 | 12.48 | - | - | [100] |
| Pine wood | 1321 | oxygen | - | entrained flow | - | - | - | - | - | 0.96 | - | - | - | [98] |
| Bio-Oil | Gasification Agent | T | H2 (vol%) | CO (vol%) | CH4 (vol%) | CO2 (vol%) | N2 (vol%) | S/C (mol/mol) | H2/CO | CO/CO2 | LHV (MJ/Nm3) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agro-industrial waste | ||||||||||||
| Rice husk | air-steam | 1000 | 30.6 | 15.3 | 0.6 | 20.6 | 32.2 | 4.36 | 2.00 | 0.74 | 8.26 | [88] |
| Rice husk | air air-steam oxygen | 1000 | 25.0 30.1 37.3 | 22.3 28.1 32.5 | 3.4 5.2 6.7 | 5.6 12.5 18.3 | 41.6 20.3 - | 0.4 | 1.12 1.07 1.15 | 3.96 2.25 1.78 | 8.00 11.10 13.80 | [95] |
| Agricultural waste | ||||||||||||
| Corn stalk | steam | 500–800 | 72.5 | 0.75 | 0.06 | 27.3 | - | 10.6 | - | - | - | [97] |
| Wheat straw | oxygen | 1272 | 30.3 | 46.4 | 1.98 | 23.1 | - | - | - | - | - | [98] |
| Other | ||||||||||||
| Hard wood | steam | 1200 | 55.3 | 16.0 | 4.7 | 22.4 | - | 7.39 | 3.45 | 0.71 | 10.60 | [99] |
| Coal/bio-oil slurry | steam | 1300 | 55.44 | 18.97 | 3.1 | 22.49 | - | 5 | 2.92 | 0.84 | 10.7 | [96] |
| Poplar wood | oxygen | 725 | 48.3 | 42.6 | 5.3 | 3.8 | - | 0.023 | 1.13 | 11.2 | 12.48 | [100] |
| Pine wood | oxygen | 1321 | 30.1 | 45.6 | 2.0 | 22.5 | - | - | - | - | - | [98] |
5.8. Effect of Steam/Carbon Ratio (SC)
5.9. Effect of Temperature
5.10. Effect of Catalysts
6. Syngas Characteristics for the Downstream Part of the Combined Process
6.1. Gas Lower Heating Value (LHV)
6.2. Wobbe Index
6.3. Tar and Particulates in Syngas
6.4. Soot and Coke
6.5. Comparison of Bio-Oil and Heavy Fuel Oils Gasification
7. Looking at the Economic Viability of Bio-Oil Gasification
8. Discussion
Advantages of Coupling Pyrolysis and Gasification for Syngas Production
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- World Bioenergy Association. Global Bioenergy Statistics 2019. 2019. Available online: https://worldbioenergy.org/uploads/191129%20WBA%20GBS%202019_LQ.pdf (accessed on 9 March 2021).
- Cao, L.; Yu, I.K.; Xiong, X.; Tsang, D.C.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef]
- Situmorang, Y.A.; Zhao, Z.; Chaihad, N.; Wang, C.; Anniwaer, A.; Kasai, Y.; Abudula, A.; Guan, G. Steam gasification of co-pyrolysis chars from various types of biomass. Int. J. Hydrogen Energy 2020, 46, 3640–3650. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Aravind, P.; de Jong, W. Evaluation of high temperature gas cleaning options for biomass gasification product gas for Solid Oxide Fuel Cells. Prog. Energy Combust. Sci. 2012, 38, 737–764. [Google Scholar] [CrossRef]
- Yun, Y. Gasification for Practical Applications; InTech: Rijeka, Croatia, 2014. [Google Scholar]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Manyà, J.J.; Azuara, M.; Manso, J.A. Biochar production through slow pyrolysis of different biomass materials: Seeking the best operating conditions. Biomass Bioenergy 2018, 117, 115–123. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Bin Johan, R. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; IntechOpen: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Brewer, C. Biochar Characterization and Engineering. Ph.D. Thesis, Iowa State University, Ames, IA, USA, 2012. [Google Scholar]
- Tippayawong, N.; Kinorn, J.; Thavornun, S. Yields and Gaseous Composition from Slow Pyrolysis of Refuse-derived Fuels. Energy Sources Part A Recover. Util. Environ. Eff. 2008, 30, 1572–1580. [Google Scholar] [CrossRef]
- Pecha, B.; Garcia-Perez, M. Pyrolysis of Lignocellulosic Biomass; U.S. Department of Energy: Washington, DC, USA, 2015; pp. 413–442.
- Veses, A.; Aznar, M.; López, J.; Callén, M.; Murillo, R.; García, T. Production of upgraded bio-oils by biomass catalytic pyrolysis in an auger reactor using low cost materials. Fuel 2015, 141, 17–22. [Google Scholar] [CrossRef]
- Remón, J.; Arcelus-Arrillaga, P.; García, L.; Arauzo, J. Simultaneous production of gaseous and liquid biofuels from the synergetic co-valorisation of bio-oil and crude glycerol in supercritical water. Appl. Energy 2018, 228, 2275–2287. [Google Scholar] [CrossRef]
- Nowrouzi, M.; Behin, J.; Younesi, H.; Bahramifar, N.; Charpentier, P.; Rohani, S. An enhanced counter-current approach towards activated carbon from waste tissue with zero liquid discharge. Chem. Eng. J. 2017, 326, 934–944. [Google Scholar] [CrossRef]
- Gupta, S.; Mondal, P.; Borugadda, V.B.; Dalai, A.K. Advances in upgradation of pyrolysis bio-oil and biochar towards improvement in bio-refinery economics: A comprehensive review. Environ. Technol. Innov. 2020, 21, 101276. [Google Scholar] [CrossRef]
- Heidari, A.; Khaki, E.; Younesi, H.; Lu, H.R. Evaluation of fast and slow pyrolysis methods for bio-oil and activated carbon production from eucalyptus wastes using a life cycle assessment approach. J. Clean. Prod. 2019, 241, 118394. [Google Scholar] [CrossRef]
- Karunanithy, C.; Muthukumarapp, K. Rheological Characterization of Bio-Oils from Pilot Scale Microwave Assisted Pyrolysis. In Biofuel’s Engineering Process Technology; BoD—Books on Demand: Norderstedt, Germany, 2011. [Google Scholar]
- Kumar, R.; Strezov, V. Thermochemical production of bio-oil: A review of downstream processing technologies for bio-oil upgrading, production of hydrogen and high value-added products. Renew. Sustain. Energy Rev. 2020, 135, 110152. [Google Scholar] [CrossRef]
- Selvaganapathy, T.; Muthuvelayudham, R.; Jayakumar, M.; Lebbai, S.M.M.; Murugesan, M. Rheological property analysis of pyrolytic liquid fuel (PLF) using ASTM and APHA standards. Mater. Today Proc. 2020, 26, 3030–3036. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Fuel Oil Quality and Combustion of Fast Pyrolysis Bio-Oils; VTT Technical Research Centre of Finland: Kuopio, Finland, 2013. [Google Scholar]
- Oasmaa, A.; Källi, A.; Lindfors, C.; Elliott, D.C.; Springer, D.; Peacocke, C.; Chiaramonti, D. Guidelines for Transportation, Handling, and Use of Fast Pyrolysis Bio-Oil. 1. Flammability and Toxicity. Energy Fuels 2012, 26, 3864–3873. [Google Scholar] [CrossRef]
- Gupta, S.; Kawale, H.D.; Ahmed, G.; Acharya, S.; Kishore, N. Effect of temperature on catalytic pyrolysis of Polyalthia Longifolia leaves solid waste and characterization of their products. Curr. Res. Green Sustain. Chem. 2021, 4, 100062. [Google Scholar] [CrossRef]
- Shan Ahamed, T.; Anto, S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Upgrading of bio-oil from thermochemical conversion of various biomass—Mechanism, challenges and opportunities. Fuel 2021, 287, 119329. [Google Scholar] [CrossRef]
- Yi, W.; Wang, X.; Zeng, K.; Yang, H.; Shao, J.; Zhang, S.; Chen, H. Improving bio-oil stability by fractional condensation and solvent addition. Fuel 2021, 290, 119929. [Google Scholar] [CrossRef]
- Choi, Y.; Johnston, P.; Brown, R.; Shanks, B.; Lee, K. Detailed characterization of red oak-derived pyrolysis oil: Integrated use of GC, HPLC, IC, GPC and Karl-Fischer. J. Anal. Appl. Pyrolysis 2014, 110, 147–154. [Google Scholar] [CrossRef]
- Sadaka, S.; Boateng, A. Pyrolysis and Bio Oil; University of Arkansas Cooperative Extension Service Printing Services: Little Rock, AR, USA, 2017. [Google Scholar]
- Park, J.; Lee, Y.; Ryu, C.; Park, Y.-K. Slow pyrolysis of rice straw: Analysis of products properties, carbon and energy yields. Bioresour. Technol. 2014, 155, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Lin, J.; Ma, R.; Chen, X.; Sun, S.; Zhang, P.; Liu, X. Effect of different ash/organics and C/H/O ratios on characteristics and reaction mechanisms of sludge microwave pyrolysis to generate bio-fuels. Waste Manag. 2020, 117, 188–197. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.S. A review on operating parameters for optimum liquid oil yield in biomass pyrolysis. Renew. Sustain. Energy Rev. 2012, 16, 5101–5109. [Google Scholar] [CrossRef]
- Biswas, B.; Pandey, N.; Bisht, Y.; Singh, R.; Kumar, J.; Bhaskar, T. Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour. Technol. 2017, 237, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.R.; Luna, C.M.R.; Arce, G.L.; Ávila, I. Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy 2020, 132, 105412. [Google Scholar] [CrossRef]
- Bertero, M.; de la Puente, G.; Sedran, U. Fuels from bio-oils: Bio-oil production from different residual sources, characterization and thermal conditioning. Fuel 2012, 95, 263–271. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Ahmed, A.; Jeffery, D.M.; Hidayat, S.; Sukri, R.S.; Mahlia, T.M.I.; Jamil, F.; Khurrum, M.S.; Inayat, A.; Moogi, S.; et al. Pyrolysis of solid waste residues from Lemon Myrtle essential oils extraction for bio-oil production. Bioresour. Technol. 2020, 318, 123913. [Google Scholar] [CrossRef]
- Huang, X.; Cao, J.; Shi, P.; Zhao, X.; Feng, X.; Zhao, Y.; Fan, X.; Wei, X.; Takarada, T. Influences of pyrolysis conditions in the production and chemical composition of the bio-oils from fast pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 110, 353–362. [Google Scholar] [CrossRef]
- Ly, H.V.; Kim, S.-S.; Woo, H.C.; Choi, J.H.; Suh, D.J.; Kim, J. Fast pyrolysis of macroalga Saccharina japonica in a bubbling fluidized-bed reactor for bio-oil production. Energy 2015, 93, 1436–1446. [Google Scholar] [CrossRef]
- Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Bio-oil production from rice husk fast pyrolysis in a conical spouted bed reactor. Fuel 2014, 128, 162–169. [Google Scholar] [CrossRef]
- Bartoli, M.; Rosi, L.; Giovannelli, A.; Frediani, P.; Frediani, M. Production of bio-oils and bio-char from Arundo donax through microwave assisted pyrolysis in a multimode batch reactor. J. Anal. Appl. Pyrolysis 2016, 122, 479–489. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Omar, R.; Idris, A.; Yunus, R.; Khalid, K.; Aida Isma, M. Characterization of empty fruit bunch for microwave-assisted pyrolysis. Fuel 2011, 90, 1536–1544. [Google Scholar] [CrossRef]
- Volli, V.; Singh, R. Production of bio-oil from de-oiled cakes by thermal pyrolysis. Fuel 2012, 96, 579–585. [Google Scholar] [CrossRef]
- Casoni, A.I.; Bidegain, M.; Cubitto, M.A.; Curvetto, N.; Volpe, M.A. Pyrolysis of sunflower seed hulls for obtaining bio-oils. Bioresour. Technol. 2015, 177, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Fadhil, A.B. Evaluation of apricot (Prunus armeniaca L.) seed kernel as a potential feedstock for the production of liquid bio-fuels and activated carbons. Energy Convers. Manag. 2017, 133, 307–317. [Google Scholar] [CrossRef]
- Demiral, I.; Kul, Ş.Ç. Pyrolysis of apricot kernel shell in a fixed-bed reactor: Characterization of bio-oil and char. J. Anal. Appl. Pyrolysis 2014, 107, 17–24. [Google Scholar] [CrossRef]
- Saadi, W.; Rodríguez-Sánchez, S.; Ruiz, B.; Souissi-Najar, S.; Ouederni, A.; Fuente, E. Pyrolysis technologies for pomegranate (Punica granatum L.) peel wastes. Prospects in the bioenergy sector. Renew. Energy 2019, 136, 373–382. [Google Scholar] [CrossRef]
- Uçar, S.; Karagöz, S. The slow pyrolysis of pomegranate seeds: The effect of temperature on the product yields and bio-oil properties. J. Anal. Appl. Pyrolysis 2009, 84, 151–156. [Google Scholar] [CrossRef]
- Özbay, N.; Apaydın-Varol, E.; Uzun, B.B.; Pütün, A.E. Characterization of bio-oil obtained from fruit pulp pyrolysis. Energy 2008, 33, 1233–1240. [Google Scholar] [CrossRef]
- Önal, E.P.; Uzun, B.B.; Pütün, A.E. Steam pyrolysis of an industrial waste for bio-oil production. Fuel Process. Technol. 2011, 92, 879–885. [Google Scholar] [CrossRef]
- Prasad, K.M.; Murugavelh, S. Experimental investigation and kinetics of tomato peel pyrolysis: Performance, combustion and emission characteristics of bio-oil blends in diesel engine. J. Clean. Prod. 2020, 254, 120115. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Li, Q.; Zhang, S.; Xu, L.; Gholizadeh, M.; Hu, X. Pyrolysis of flaxseed residue: Exploration of characteristics of the biochar and bio-oil products. J. Energy Inst. 2021, 97, 1–12. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kantarelis, E.; Theodoropoulos, D. Sunflower shells utilization for energetic purposes in an integrated approach of energy crops: Laboratory study pyrolysis and kinetics. Bioresour. Technol. 2008, 99, 3174–3181. [Google Scholar] [CrossRef]
- Bharath, G.; Hai, A.; Rambabu, K.; Banat, F.; Jayaraman, R.; Taher, H.; Bastidas-Oyanedel, J.-R.; Ashraf, M.T.; Schmidt, J.E. Systematic production and characterization of pyrolysis-oil from date tree wastes for bio-fuel applications. Biomass-Bioenergy 2020, 135, 105523. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stahl, R.; Yanik, J. The slow and fast pyrolysis of cherry seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef]
- Părpăriţă, E.; Brebu, M.; Uddin, A.; Yanik, J.; Vasile, C. Pyrolysis behaviors of various biomasses. Polym. Degrad. Stab. 2014, 100, 1–9. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Kamaterou, P.; Pavlou, A.; Panayiotou, C. Sustainable bioeconomy transitions: Targeting value capture by integrating pyrolysis in a winery waste biorefinery. J. Clean. Prod. 2018, 172, 3387–3397. [Google Scholar] [CrossRef]
- Caballero, B.; López-Urionabarrenechea, A.; Pérez, B.; Solar, J.; Acha, E.; de Marco, I. Potentiality of “orujillo” (olive oil solid waste) to produce hydrogen by means of pyrolysis. Int. J. Hydrogen Energy 2020, 45, 20549–20557. [Google Scholar] [CrossRef]
- Setter, C.; Borges, F.; Cardoso, C.; Mendes, R.; Oliveira, T. Energy quality of pellets produced from coffee residue: Characterization of the products obtained via slow pyrolysis. Ind. Crop. Prod. 2020, 154, 112731. [Google Scholar] [CrossRef]
- Jiang, H.; Cheng, Z.; Zhao, T.; Liu, M.; Zhang, M.; Li, J.; Hu, M.; Zhang, L.; Li, J. Pyrolysis kinetics of spent lark mushroom substrate and characterization of bio-oil obtained from the substrate. Energy Convers. Manag. 2014, 88, 259–266. [Google Scholar] [CrossRef]
- Mortari, D.; Perondi, D.; Rossi, G.; Bonato, J.; Godinho, M.; Pereira, F. The influence of water-soluble inorganic matter on combustion of grape pomace and its chars produced by slow and fast pyrolysis. Fuel 2020, 284, 118880. [Google Scholar] [CrossRef]
- Demiral, İ.; Ayan, E. Pyrolysis of grape bagasse: Effect of pyrolysis conditions on the product yields and characterization of the liquid product. Bioresour. Technol. 2011, 102, 3946–3951. [Google Scholar] [CrossRef] [PubMed]
- Al Afif, R.; Anayah, S.S.; Pfeifer, C. Batch pyrolysis of cotton stalks for evaluation of biochar energy potential. Renew. Energy 2019, 147, 2250–2258. [Google Scholar] [CrossRef]
- Gerçel, H.F. Bio-oil production from Onopordum acanthium L. by slow pyrolysis. J. Anal. Appl. Pyrolysis 2011, 92, 233–238. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Saricaoğlu, B. Pressurized pyrolysis of dried distillers grains with solubles and canola seed press cake in a fixed-bed reactor. Bioresour. Technol. 2015, 177, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Acikgoz, C.; Kockar, O. Characterization of slow pyrolysis oil obtained from linseed (Linum usitatissimum L.). J. Anal. Appl. Pyrolysis 2009, 85, 151–154. [Google Scholar] [CrossRef]
- Singh, R.; Shadangi, K. Liquid fuel from castor seeds by pyrolysis. Fuel 2011, 90, 2538–2544. [Google Scholar] [CrossRef]
- Pereira, E.; da Silva, J.; de Oliveira, J.; Machado, C. Sustainable energy: A review of gasification technologies. Renew. Sustain. Energy Rev. 2012, 16, 4753–4762. [Google Scholar] [CrossRef]
- Nanou, P. Biomass Gasification for the Production of Methane. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 7 May 2013. [Google Scholar] [CrossRef]
- Chhiti, Y.; Salvador, S. Gasification of Wood Bio-Oil. In Gasification for Practical Applications; BoD—Books on Demand: Norderstedt, Germany, 2012. [Google Scholar]
- Zheng, J.-L.; Zhu, Y.-H.; Zhu, M.-Q.; Kang, K.; Sun, R.-C. A review of gasification of bio-oil for gas production. Sustain. Energy Fuels 2019, 3, 1600–1622. [Google Scholar] [CrossRef]
- Venderbosch, R.; Prins, W. Fast pyrolysis technology development. Biofuels Bioprod. Biorefin. 2010, 4, 178–208. [Google Scholar] [CrossRef]
- Gollakota, A.R.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sustain. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Zhang, F.; Cai, Q.; Wang, Y.; Luo, Z. Bio-oil catalytic reforming without steam addition: Application to hydrogen production and studies on its mechanism. Int. J. Hydrogen Energy 2013, 38, 16038–16047. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Chhiti, Y.; Peyrot, M.; Salvador, S. Soot formation and oxidation during bio-oil gasification: Experiments and modeling. J. Energy Chem. 2013, 22, 701–709. [Google Scholar] [CrossRef]
- Koido, K.; Iwasaki, T. Biomass Gasification: A Review of Its Technology, Gas Cleaning Applications, and Total System Life Cycle Analysis; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Sharma, A. Assessing the Suitability of Various Feedstocks for Biomass Gasification; Louisiana State University and Agricultural and Mechanical College: Baton Rouge, LA, USA, 2011. [Google Scholar]
- Pfeifer, C.; Koppatz, S.; Hofbauer, H. Steam gasification of various feedstocks at a dual fluidised bed gasifier: Impacts of operation conditions and bed materials. Biomass Convers. Biorefin. 2011, 1, 39–53. [Google Scholar] [CrossRef]
- Remón, J.; Broust, F.; Volle, G.; García, L.; Arauzo, J. Hydrogen production from pine and poplar bio-oils by catalytic steam reforming. Influence of the bio-oil composition on the process. Int. J. Hydrogen Energy 2015, 40, 5593–5608. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Zawawi, N.A.; Kasim, F.; Inayat, A.; Khasri, A. Assessing the gasification performance of biomass: A review on biomass gasification process conditions, optimization and economic evaluation. Renew. Sustain. Energy Rev. 2016, 53, 1333–1347. [Google Scholar] [CrossRef]
- Pandey, A. Recent Advances in Thermochemical Conversion of Biomass; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Antonopoulos, I. An LCA Methodological Tool for the Dynamic Planning and Environmental Assessment of Solid Waste Treatment Systems with Emphasis on Advanced Conversion Technologies. Ph.D. Thesis, Aristotle University, Thessaloniki, Greece, 2012. [Google Scholar]
- Oakey, J. Fuel Flexible Energy Generation; Woodhead Publishing: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Abraham, M. Encyclopedia of Sustainable Technologies; Elsevier: Dayton, OH, USA, 2017. [Google Scholar]
- Luque, R.; Lin, C.; Wilson, K.; Clark, J. Handbook of Biofuels Production; Woodhead Publishing: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Wan, Z.; Hu, J.; Qi, X. Numerical analysis of hydrodynamics and thermochemical property of biomass gasification in a pilot-scale circulating fluidized bed. Energy 2021, 225, 120254. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification; Academic Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Foscolo, P.; Materazzi, M. Substitute Natural Gas from Waste; Elsevier: London, UK, 2019. [Google Scholar]
- Zheng, J.-L.; Zhu, Y.-H.; Zhu, M.-Q.; Wu, H.-T.; Sun, R.-C. Bio-oil gasification using air—Steam as gasifying agents in an entrained flow gasifier. Energy 2017, 142, 426–435. [Google Scholar] [CrossRef]
- Nelson, L.; Park, S.; Hubbe, M.A. Thermal Depolymerization of Biomass with Emphasis on Gasifier Design and Best Method for Catalytic Hot Gas Conditioning. BioResources 2018, 13, 4630–4727. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Santos, R.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Chen, T.; Wu, C.; Liu, R. Steam reforming of bio-oil from rice husks fast pyrolysis for hydrogen production. Bioresour. Technol. 2011, 102, 9236–9240. [Google Scholar] [CrossRef]
- Otto, A.; Grube, T.; Schiebahn, S.; Stolten, D. Closing the loop: Captured CO2 as a feedstock in the chemical industry. Energy Environ. Sci. 2015, 8, 3283–3297. [Google Scholar] [CrossRef]
- Zheng, J.-L.; Zhu, M.-Q.; Wen, J.-L.; Sun, R.-C. Gasification of bio-oil: Effects of equivalence ratio and gasifying agents on product distribution and gasification efficiency. Bioresour. Technol. 2016, 211, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Lin, W.; Jensen, P.A.; Song, W.; Hao, L.; Raffelt, K.; Dam-Johansen, K. Entrained flow gasification of coal/bio-oil slurries. Energy 2016, 111, 793–802. [Google Scholar] [CrossRef]
- Kan, T.; Xiong, J.; Li, X.; Ye, T.; Yuan, L.; Torimoto, Y.; Yamamoto, M.; Li, Q. High efficient production of hydrogen from crude bio-oil via an integrative process between gasification and current-enhanced catalytic steam reforming. Int. J. Hydrogen Energy 2010, 35, 518–532. [Google Scholar] [CrossRef]
- Leijenhorst, E.; Assink, D.; van de Beld, L.; Weiland, F.; Wiinikka, H.; Carlsson, P.; Öhrman, O. Entrained flow gasification of straw- and wood-derived pyrolysis oil in a pressurized oxygen blown gasifier. Biomass Bioenergy 2015, 79, 166–176. [Google Scholar] [CrossRef]
- Chhiti, Y. Non Catalytic Steam Gasification of Wood Bio-Oil. Ph.D. Thesis, Institut National Polytechnique de Toulouse, Toulouse, France, 2011. [Google Scholar]
- Marda, J.R.; DiBenedetto, J.; McKibben, S.; Evans, R.J.; Czernik, S.; French, R.J.; Dean, A.M. Non-catalytic partial oxidation of bio-oil to synthesis gas for distributed hydrogen production. Int. J. Hydrogen Energy 2009, 34, 8519–8534. [Google Scholar] [CrossRef]
- Watson, J.; Zhang, Y.; Si, B.; Chen, W.-T.; de Souza, R. Gasification of biowaste: A critical review and outlooks. Renew. Sustain. Energy Rev. 2018, 83, 1–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Haynes, D.; Baltrus, J.; Roy, A.; Shekhawat, D.; Spivey, J.J. Methane steam reforming at low steam-to-carbon ratio: The effect of Y doping in Rh substituted lanthanum zirconates. Appl. Catal. A Gen. 2020, 606, 117802. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Benito, P.L.; Bilbao, J.; Gayubo, A.G. Biomass to hydrogen-rich gas via steam reforming of raw bio-oil over Ni/La2O3-αAl2O3 catalyst: Effect of space-time and steam-to-carbon ratio. Fuel 2018, 216, 445–455. [Google Scholar] [CrossRef]
- Das, B. Effect of Temperature on Gasification Performance of Biomass in a Bubbling Fluidized Bed Gasifier. In International Asian Congress on Contemporary Science—IV; Khazar University: Baku, Azerbaijan, 2020. [Google Scholar]
- Mohammed, M.; Salmiaton, A.; Azlina, W.W.; Amran, M.M.; Fakhru’L-Razi, A. Air gasification of empty fruit bunch for hydrogen-rich gas production in a fluidized-bed reactor. Energy Convers. Manag. 2011, 52, 1555–1561. [Google Scholar] [CrossRef]
- Wongsiriamnuay, T.; Kannang, N.; Tippayawong, N. Effect of Operating Conditions on Catalytic Gasification of Bamboo in a Fluidized Bed. Int. J. Chem. Eng. 2013, 2013, 297941. [Google Scholar] [CrossRef]
- Garche, J.; Dyer, C. Encyclopedia of Electrochemical Power Sources; Academic Press: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Gómez, H.; Calleja, M.; Fernández, L.; Kiedrzyńska, A.; Lewtak, R. Application of the CFD simulation to the evaluation of natural gas replacement by syngas in burners of the ceramic sector. Energy 2019, 185, 15–27. [Google Scholar] [CrossRef]
- Knoef, H.; Sanchez Angrill, L. Handbook Biomass Gasification; BTG Biomass Technology Group: Enschede, The Netherlands, 2012. [Google Scholar]
- Xie, H.; Yu, Q.; Zuo, Z.; Han, Z.; Yao, X.; Qin, Q. Hydrogen production via sorption-enhanced catalytic steam reforming of bio-oil. Int. J. Hydrogen Energy 2016, 41, 2345–2353. [Google Scholar] [CrossRef]
- Vaezi, M.; Passandideh-Fard, M.; Moghiman, M.; Charmchi, M. Gasification of heavy fuel oils: A thermochemical equilibrium approach. Fuel 2011, 90, 878–885. [Google Scholar] [CrossRef]
- Braimakis, K.; Atsonios, K.; Panopoulos, K.D.; Karellas, S.; Kakaras, E. Economic evaluation of decentralized pyrolysis for the production of bio-oil as an energy carrier for improved logistics towards a large centralized gasification plant. Renew. Sustain. Energy Rev. 2014, 35, 57–72. [Google Scholar] [CrossRef]
- Dabros, T.M.; Stummann, M.Z.; Høj, M.; Jensen, P.A.; Grunwaldt, J.-D.; Gabrielsen, J.; Mortensen, P.M.; Jensen, A.D. Transportation fuels from biomass fast pyrolysis, catalytic hydrodeoxygenation, and catalytic fast hydropyrolysis. Prog. Energy Combust. Sci. 2018, 68, 268–309. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-economic comparison of biomass-to-transportation fuels via pyrolysis, gasification, and biochemical pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Y.; Hu, G. Techno-economic analysis of advanced biofuel production based on bio-oil gasification. Bioresour. Technol. 2015, 191, 88–96. [Google Scholar] [CrossRef]
- Sarkar, S.; Kumar, A. Large-scale biohydrogen production from bio-oil. Bioresour. Technol. 2010, 101, 7350–7361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J. Hydrogen Generation, Storage and Utilization; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]

| Screening Process | Number of Publications |
|---|---|
| (1) 1st sample | 8155 |
| (2) Sample after exclusion of irrelevant publications | 903 |
| (3) Final screening | 52 |
| (4) Books | 3 |
| (5) Other (reports, studies) | 4 |
| Total | 59 |
| Screening Process | Number of Publications |
|---|---|
| (1) 1st sample | 10,870 |
| (2) Sample after exclusion of irrelevant publications | 426 |
| (3) Final screening | 43 |
| (4) Books | 12 |
| (5) Other (thesis, studies) | 3 |
| Total | 58 |
| Screening Process | Number of Publications |
|---|---|
| (1) 1st sample | 19,025 |
| (2) Sample after exclusion of irrelevant publications | 1329 |
| (3) Final screening | 95 |
| (4). Books | 15 |
| (5) Other (thesis, studies, reports) | 7 |
| Total | 117 |
| Pyrolysis Type | Temperature (°C) | Heating Rate | Pressure | Residence Time | Primary Product | Ref. |
|---|---|---|---|---|---|---|
| Slow pyrolysis | 350–800 | slow (≈10 °C/min) | atmospheric | 30–60 min | biochar | [9,10,16] |
| Fast pyrolysis | 700–1200 | very fast (10–100 °C/s) | vacuum-atmospheric | 10 s | bio-oil | [9,10,11,12] |
| Flash pyrolysis | 800–1150 | >1000 °C/s | atmospheric | 1 s | bio-oil | [9,12] |
| Pyrolysis Type | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| Slow pyrolysis |
|
| [9,10,16,17] |
| Fast pyrolysis |
|
| [10,11,12,17] |
| Flash pyrolysis |
|
| [9,12,17] |
| Reaction | Mechanism | ΔH°r(298) (kJ/mol) | ΔG°r(298) (kJ/mol) |
|---|---|---|---|
| Carbon reactions | |||
| R1 (Boudouard) | C + CO2 ↔ 2CO | 205.3 | 140.1 |
| R2 (heterogeneous WGS) | C + H2O ↔ CO + H2 | 130.4 | 89.8 |
| R3 (methanisation) | C + 2H2 ↔ CH4 | 123.7 | 168.6 |
| R4 (partial oxidation) | C + 0.5O2 ↔ CO | −111 | |
| Oxidation reactions | |||
| R5 | C + O2 ↔ CO2 | −394 | |
| R6 | CO + 0.5O2 ↔ CO2 | ||
| R7 | CH4 + 2O2 ↔ CO2 + 2H2O | ||
| R8 | H2 + 0.5O2 ↔ H2O | ||
| Shift reaction | |||
| R9 (WGS) | CO + H2O ↔ CO2 + H2 | −41.47 | −28.5 |
| Methanization | |||
| R10 | 2CO + 2H2 ↔ CH4 + CO2 | ||
| Steam reforming reactions | |||
| R11 (methane reforming) | CH4 + H2O ↔ CO + 3H2 | 172.6 | 118.4 |
| R12 | CnHmOk + (n − k)H2O ↔ nCO +(n + m/2 − k)H2 | ||
| Characteristic | Fixed Bed | Fluidized Bed | Entrained Flow |
|---|---|---|---|
| Feed size | <51 mm | <6 mm | <0.15 mm |
| Tolerance for fines | limited | good | great |
| Tolerance for coarse | very good | good | poor |
| Gas exit temperature | 450–650 °C | 800–1000 °C | >1260 °C |
| Feedstock tolerance | suitable for biomass | suitable for biomass (especially MSW) | unsuitable for biomass |
| Oxidant requirments | low | moderate | high |
| Reaction zone temperature | 1090 °C | 800–1000 °C | 1990 °C |
| Steam requirment | high | moderate | low |
| Nature of ash produced | dry | dry | slagging |
| CGE | 80% | 89% | 80% |
| Capacity | small | medium | large |
| Bio-oil application | steam gasification | steam gasification | non-catalytic oxidation |
| Problems | tar production and utilization of fines | carbon conversion | gas cooling |
| Range for power applicability of each biomass gasifier type | 10 kW–10 MW | 1–100 MW | 70–1000 MW |
| Ref. | [9,79,80,81,82] | [9,80,83,84,85] | [9,81,86,87,88,89,90] |
| Advantages | Disadvantages |
|---|---|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frantzi, D.; Zabaniotou, A. Waste-Based Intermediate Bioenergy Carriers: Syngas Production via Coupling Slow Pyrolysis with Gasification under a Circular Economy Model. Energies 2021, 14, 7366. https://doi.org/10.3390/en14217366
Frantzi D, Zabaniotou A. Waste-Based Intermediate Bioenergy Carriers: Syngas Production via Coupling Slow Pyrolysis with Gasification under a Circular Economy Model. Energies. 2021; 14(21):7366. https://doi.org/10.3390/en14217366
Chicago/Turabian StyleFrantzi, Danai, and Anastasia Zabaniotou. 2021. "Waste-Based Intermediate Bioenergy Carriers: Syngas Production via Coupling Slow Pyrolysis with Gasification under a Circular Economy Model" Energies 14, no. 21: 7366. https://doi.org/10.3390/en14217366
APA StyleFrantzi, D., & Zabaniotou, A. (2021). Waste-Based Intermediate Bioenergy Carriers: Syngas Production via Coupling Slow Pyrolysis with Gasification under a Circular Economy Model. Energies, 14(21), 7366. https://doi.org/10.3390/en14217366







