Study on the Growth Kinetics and Morphology of Methane Hydrate Film in a Porous Glass Microfluidic Device
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
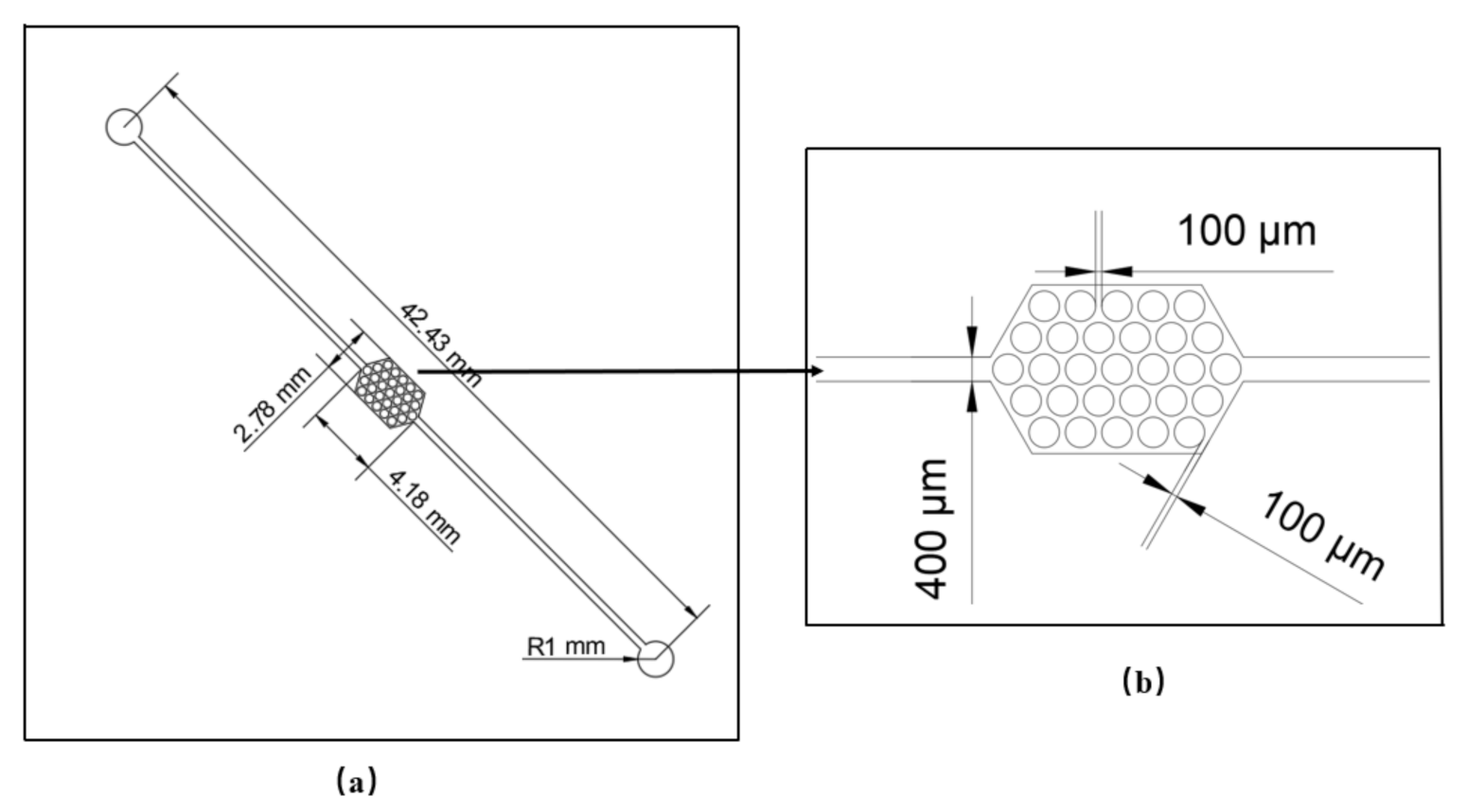
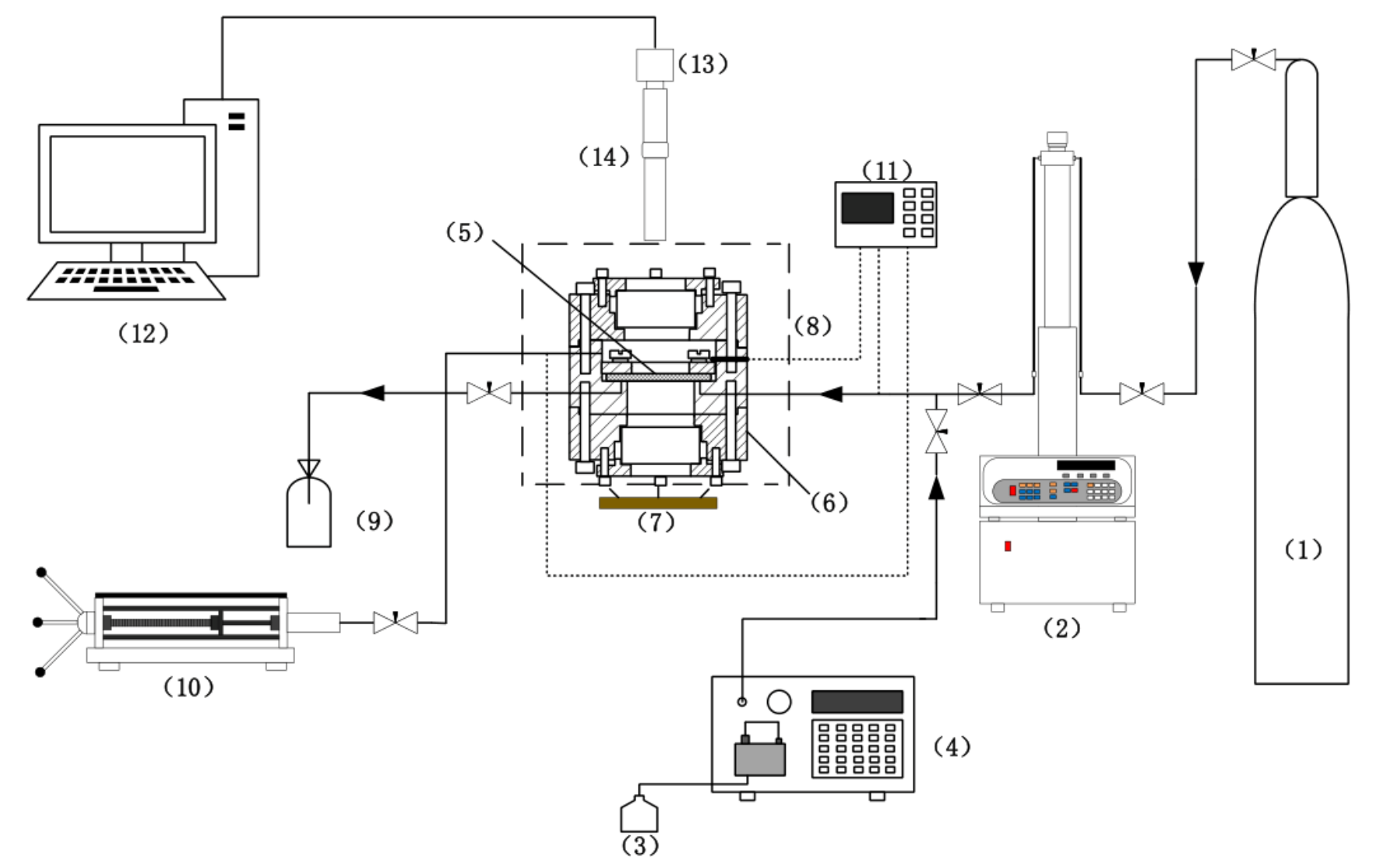
2.2. Methods
3. Results and Discussion
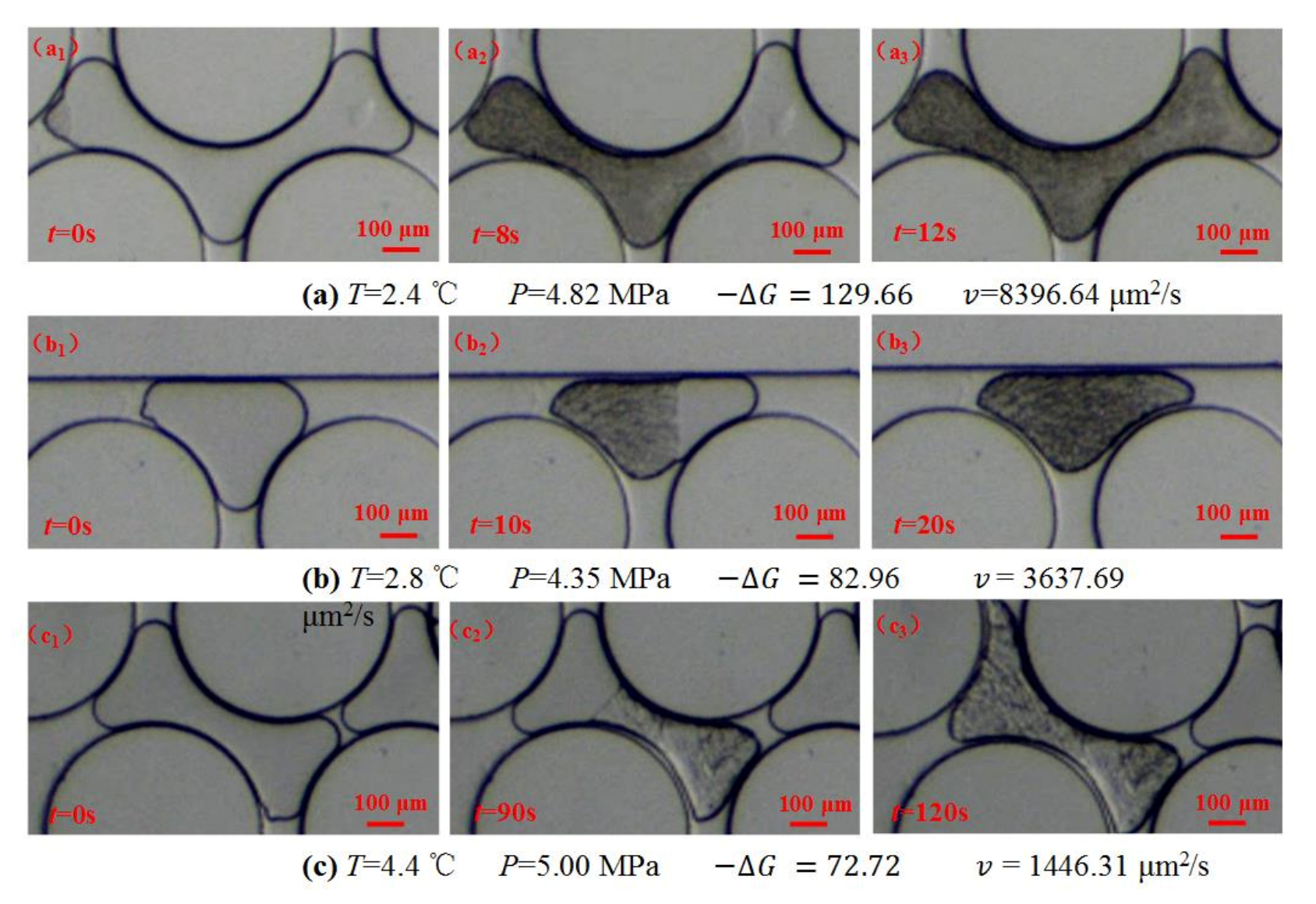
3.1. The Formation Process of Methane Hydrate Induced by Hydrate Seed in Pores
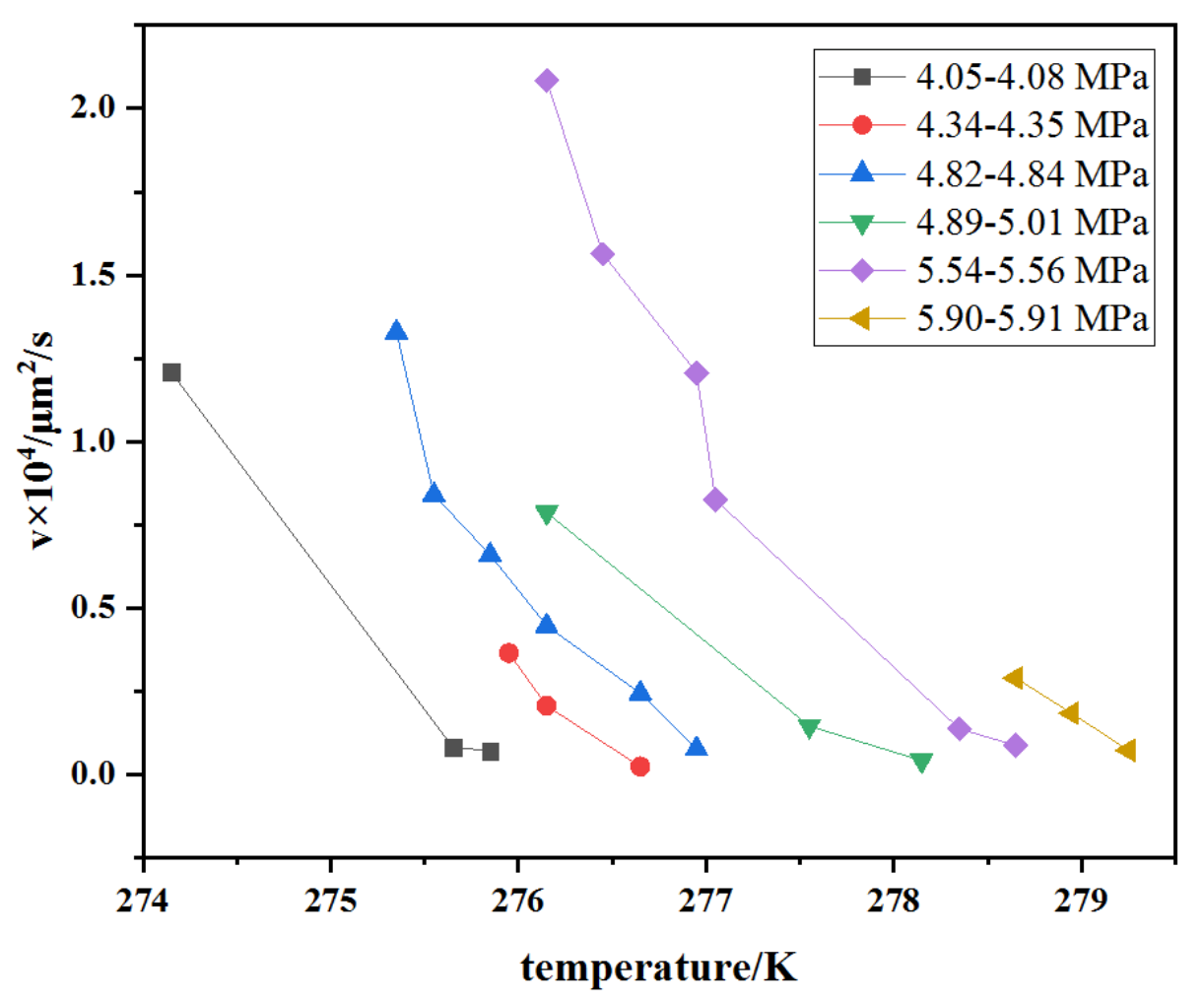
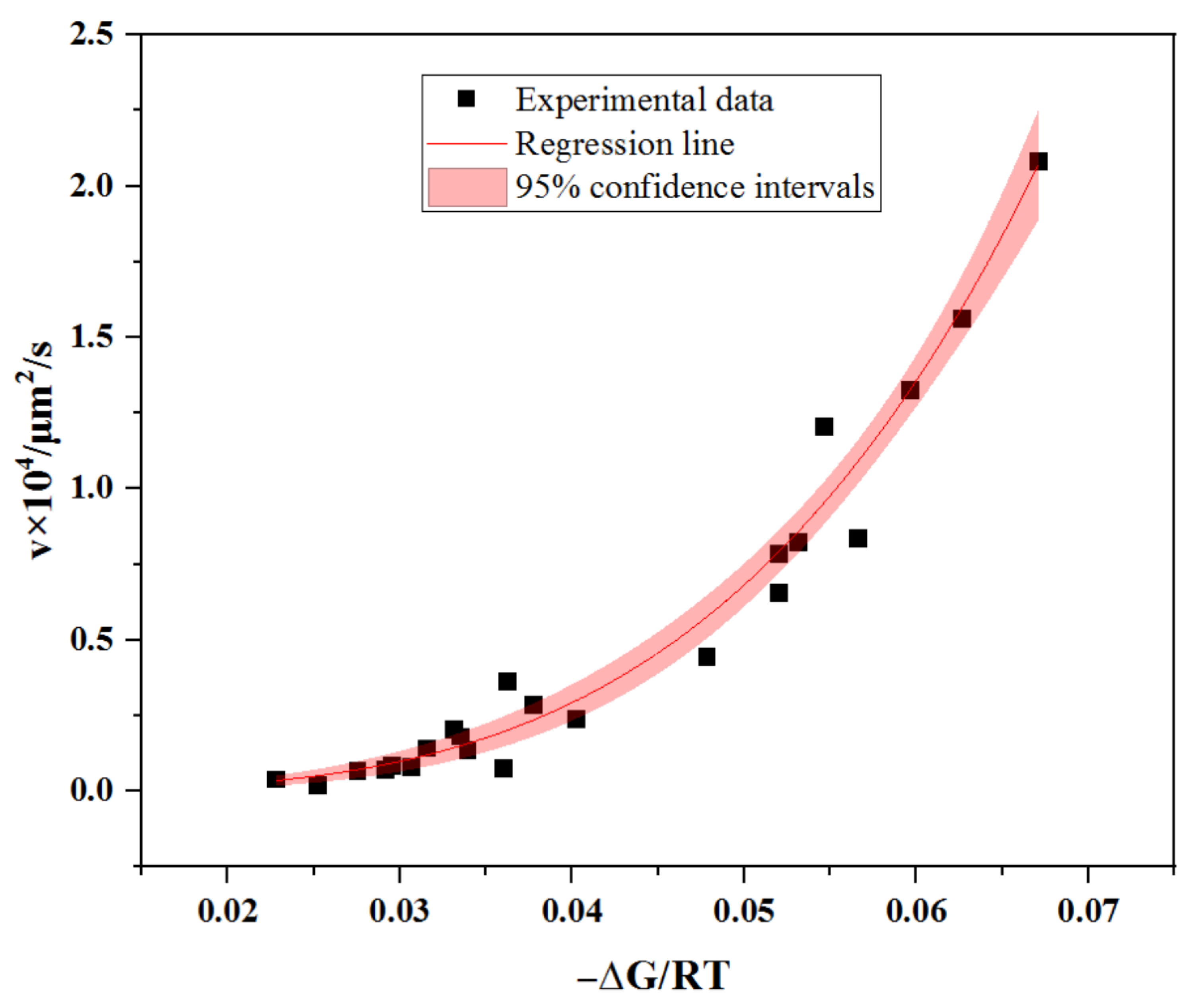
3.2. The Kinetics of the Lateral Growth of Methane Hydrate Film in Pores
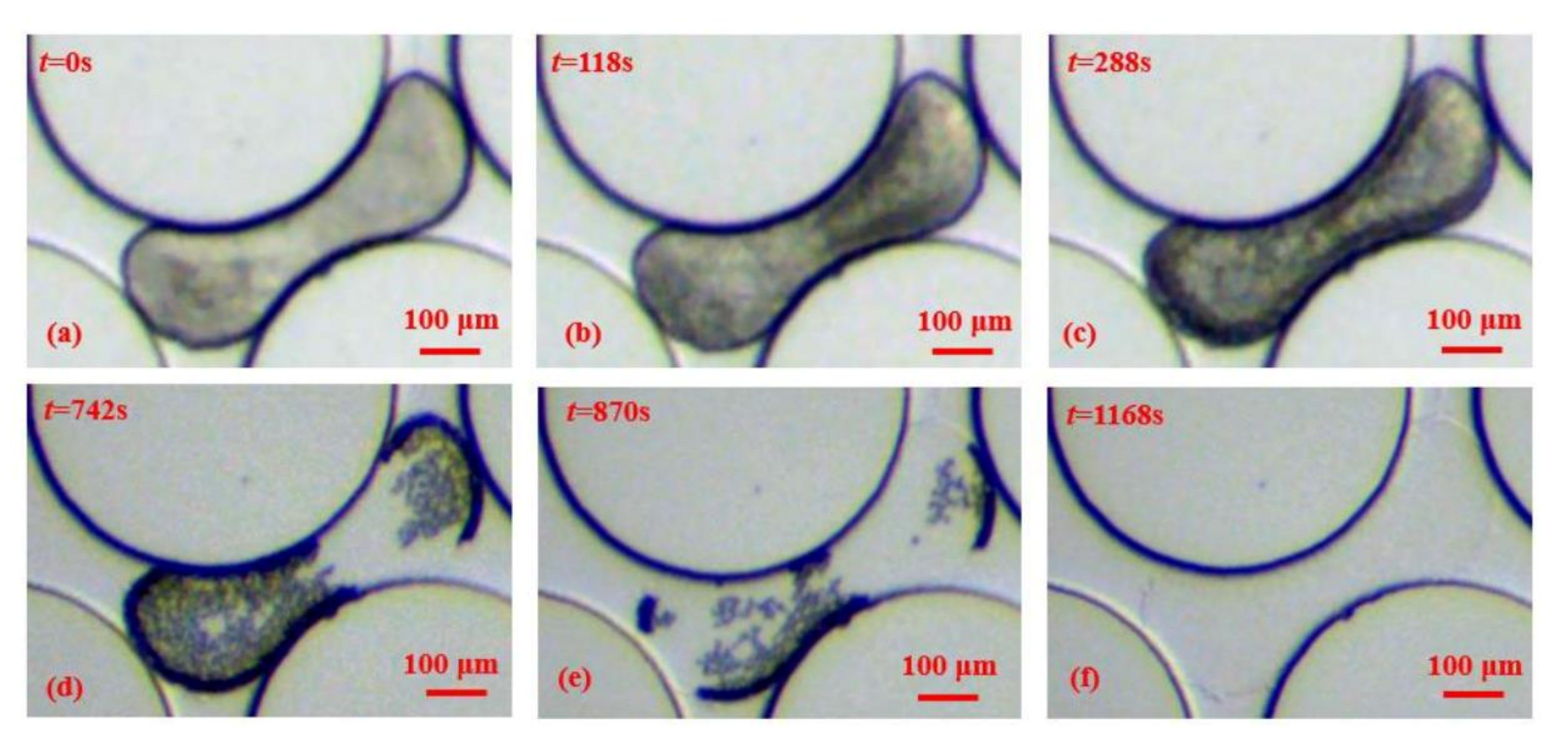
3.3. The Morphological Characteristics of Film Thickening Growth in Pores
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, C.-Y.; Chen, G.-J.; Ma, C.-F.; Huang, Q.; Luo, H.; Li, Q.-P. The growth kinetics of hydrate film on the surface of gas bubble suspended in water or aqueous surfactant solution. J. Cryst. Growth 2007, 306, 491–499. [Google Scholar] [CrossRef]
- Leopércio, B.C.; de Souza Mendes, P.R.; Fuller, G.G. Growth Kinetics and Mechanics of Hydrate Films by Interfacial Rheology. Langmuir 2016, 32, 4203–4209. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-L.; Sun, C.-Y.; Liu, B.; Li, Z.-Y.; Chen, G.-J.; Sum, A.K. New Observations and Insights into the Morphology and Growth Kinetics of Hydrate Films. Sci. Rep. 2014, 4, 4129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlasov, V.A.; Nesterov, A.N.; Reshetnikov, A.M. Kinetics of Gas Hydrate Film Growth along the Water–Gas Interface. Russ. J. Phys. Chem. A 2020, 94, 1949–1951. [Google Scholar] [CrossRef]
- Zeng, X.; Wu, G.; Wang, J.; Yang, C.; Meng, Q.; Chen, G.; Chen, D. Effects of inhibitors on the morphology and kinetics of hydrate growth on surface of bubble. J. Nat. Gas Sci. Eng. 2020, 74, 103096. [Google Scholar] [CrossRef]
- Mori, Y.H. Estimating the thickness of hydrate films from their lateral growth rates: Application of a simplified heat transfer model. J. Cryst. Growth 2001, 223, 206–212. [Google Scholar] [CrossRef]
- Mochizuki, T.; Mori, Y.H. Clathrate-hydrate film growth along water/hydrate-former phase boundaries—Numerical heat-transfer study. J. Cryst. Growth 2006, 290, 642–652. [Google Scholar] [CrossRef]
- Liu, Z.; Li, H.; Chen, L.; Sun, B. A New Model of and Insight into Hydrate Film Lateral Growth along the Gas–Liquid Interface Considering Natural Convection Heat Transfer. Energy Fuels 2018, 32, 2053–2063. [Google Scholar] [CrossRef]
- Kar, A.; Bhati, A.; Acharya, P.V.; Mhadeshwar, A.; Venkataraman, P.; Barckholtz, T.A.; Bahadur, V. Diffusion-based modeling of film growth of hydrates on gas-liquid interfaces. Chem. Eng. Sci. 2021, 234, 116456. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, H.C.; Lee, J.D. Morphology study of methane–propane clathrate hydrates on the bubble surface in the presence of SDS or PVCap. J. Cryst. Growth 2014, 402, 249–259. [Google Scholar] [CrossRef]
- Zeng, X.-Y.; Wu, G.; Zhong, J.-R.; Chen, D.-Y.; Sun, C.-Y.; Chen, G.-J. Three-Scale in Situ Investigation on the Film Morphology and Mass Transfer Channels during the Thickening Growth of Hydrates on Gas Bubble. Cryst. Growth Des. 2019, 19, 3158–3165. [Google Scholar] [CrossRef]
- Li, S.-L.; Sun, C.-Y.; Liu, B.; Feng, X.-J.; Li, F.-G.; Chen, L.-T.; Chen, G.-J. Initial thickness measurements and insights into crystal growth of methane hydrate film. AIChE J. 2013, 59, 2145–2154. [Google Scholar] [CrossRef]
- Uchida, T.; Ebinuma, T.; Kawabata, J.; Narita, H. Microscopic observations of formation processes of clathrate-hydrate films at an interface between water and carbon dioxide. J. Cryst. Growth 1999, 204, 348–356. [Google Scholar] [CrossRef]
- Li, S.-L.; Wang, Y.-F.; Sun, C.-Y.; Chen, G.-J.; Liu, B.; Li, Z.-Y.; Ma, Q.-L. Factors controlling hydrate film growth at water/oil interfaces. Chem. Eng. Sci. 2015, 135, 412–420. [Google Scholar] [CrossRef]
- Beltrán, J.G.; Servio, P. Morphological Investigations of Methane−Hydrate Films Formed on a Glass Surface. Cryst. Growth Des. 2010, 10, 4339–4347. [Google Scholar] [CrossRef]
- Nagashima, H.D.; Oshima, M.; Jin, Y. Film-growth rates of methane hydrate on ice surfaces. J. Cryst. Growth 2020, 537, 125595. [Google Scholar] [CrossRef]
- Kishimoto, M.; Iijima, S.; Ohmura, R. Crystal Growth of Clathrate Hydrate at the Interface between Seawater and Hydrophobic-Guest Liquid: Effect of Elevated Salt Concentration. Ind. Eng. Chem. Res. 2012, 51, 5224–5229. [Google Scholar] [CrossRef]
- Adamova, T.P.; Stoporev, A.S.; Manakov, A.Y. Visual Studies of Methane Hydrate Formation on the Water–Oil Boundaries. Cryst. Growth Des. 2018, 18, 6713–6722. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, B.; Xu, L.; Zhang, R.; He, Y.; Wang, F. How porous surfaces influence the nucleation and growth of methane hydrates. Fuel 2021, 291, 120142. [Google Scholar] [CrossRef]
- Vysniauskas, A.; Bishnoi, P.R. A kinetic study of methane hydrate formation. Chem. Eng. Sci. 1983, 38, 1061–1072. [Google Scholar] [CrossRef]
- Skovborg, P.; Ng, H.J.; Rasmussen, P.; Mohn, U. Measurement of induction times for the formation of methane and ethane gas hydrates. Chem. Eng. Sci. 1993, 48, 445–453. [Google Scholar] [CrossRef]
- Natarajan, V.; Bishnoi, P.R.; Kalogerakis, N. Induction phenomena in gas hydrate nucleation. Chem. Eng. Sci. 1994, 49, 2075–2087. [Google Scholar] [CrossRef]
- Chen, G.-J.; Guo, T.-M. A new approach to gas hydrate modelling. Chem. Eng. J. 1998, 71, 145–151. [Google Scholar] [CrossRef]
- Henning, R.W.; Schultz, A.J.; Thieu, V.; Halpern, Y. Neutron Diffraction Studies of CO2 Clathrate Hydrate: Formation from Deuterated Ice. J. Phys. Chem. A 2000, 104, 5066–5071. [Google Scholar] [CrossRef]
- Davies, S.R.; Sloan, E.D.; Sum, A.K.; Koh, C.A. In Situ Studies of the Mass Transfer Mechanism across a Methane Hydrate Film Using High-Resolution Confocal Raman Spectroscopy. J. Phys. Chem. C 2010, 114, 1173–1180. [Google Scholar] [CrossRef]
- Liang, S.; Kusalik, P.G. The Mobility of Water Molecules through Gas Hydrates. J. Am. Chem. Soc. 2011, 133, 1870–1876. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wang, C.; Li, Q.; Fan, Q.; Chen, G.; Sun, C. Study on the Growth Kinetics and Morphology of Methane Hydrate Film in a Porous Glass Microfluidic Device. Energies 2021, 14, 6814. https://doi.org/10.3390/en14206814
Li X, Wang C, Li Q, Fan Q, Chen G, Sun C. Study on the Growth Kinetics and Morphology of Methane Hydrate Film in a Porous Glass Microfluidic Device. Energies. 2021; 14(20):6814. https://doi.org/10.3390/en14206814
Chicago/Turabian StyleLi, Xingxun, Cunning Wang, Qingping Li, Qi Fan, Guangjin Chen, and Changyu Sun. 2021. "Study on the Growth Kinetics and Morphology of Methane Hydrate Film in a Porous Glass Microfluidic Device" Energies 14, no. 20: 6814. https://doi.org/10.3390/en14206814
APA StyleLi, X., Wang, C., Li, Q., Fan, Q., Chen, G., & Sun, C. (2021). Study on the Growth Kinetics and Morphology of Methane Hydrate Film in a Porous Glass Microfluidic Device. Energies, 14(20), 6814. https://doi.org/10.3390/en14206814






