Pr- and Sm-Substituted Layered Perovskite Oxide Systems for IT-SOFC Cathodes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Solid State Reaction, X-ray Diffraction Measurement, and Microstructure Analysis
2.2. Electrical Conductivity Analysis
2.3. Electrochemical Characterization
2.4. X-ray Photoelectron Spectroscopy (XPS) Analysis
3. Results and Discussion
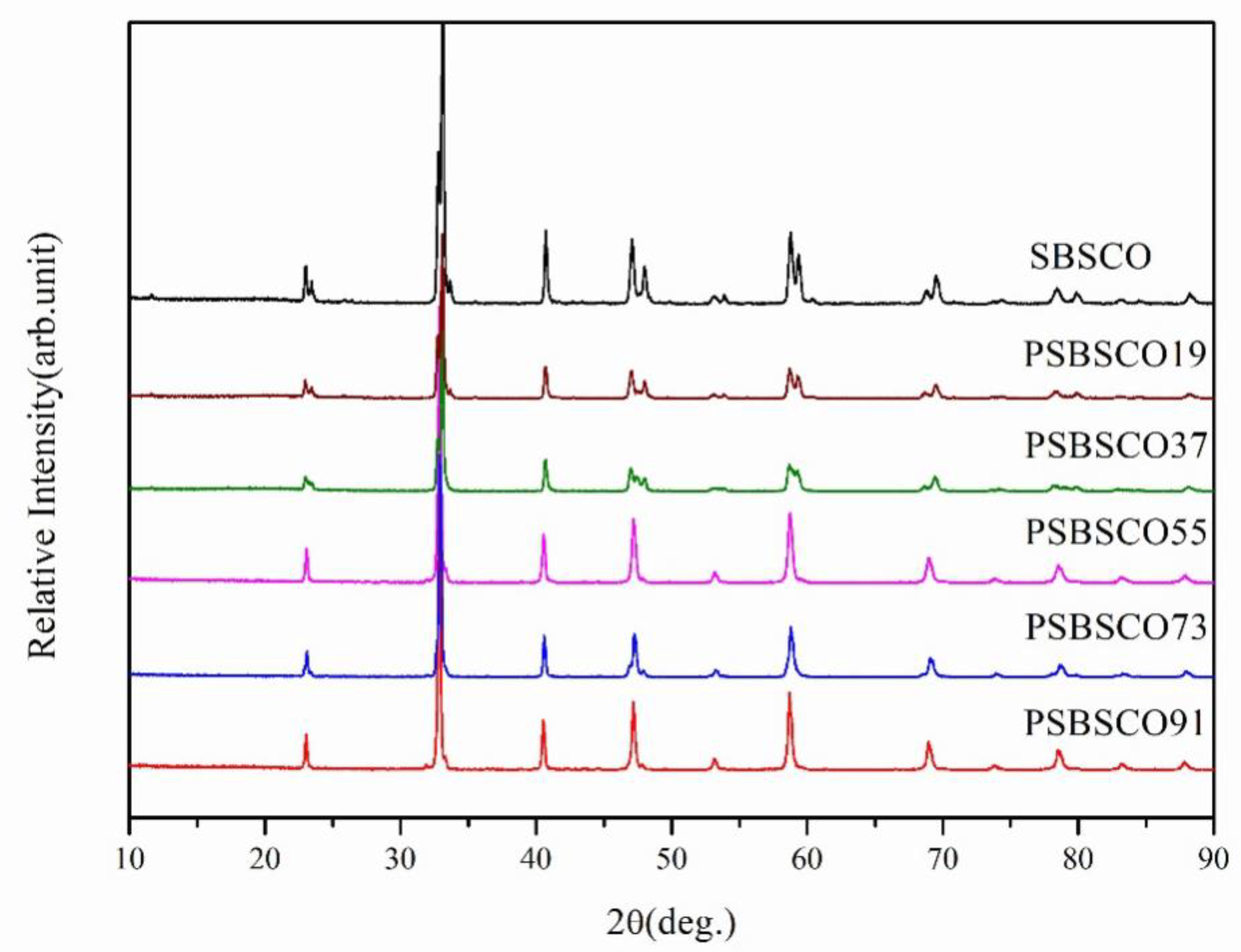
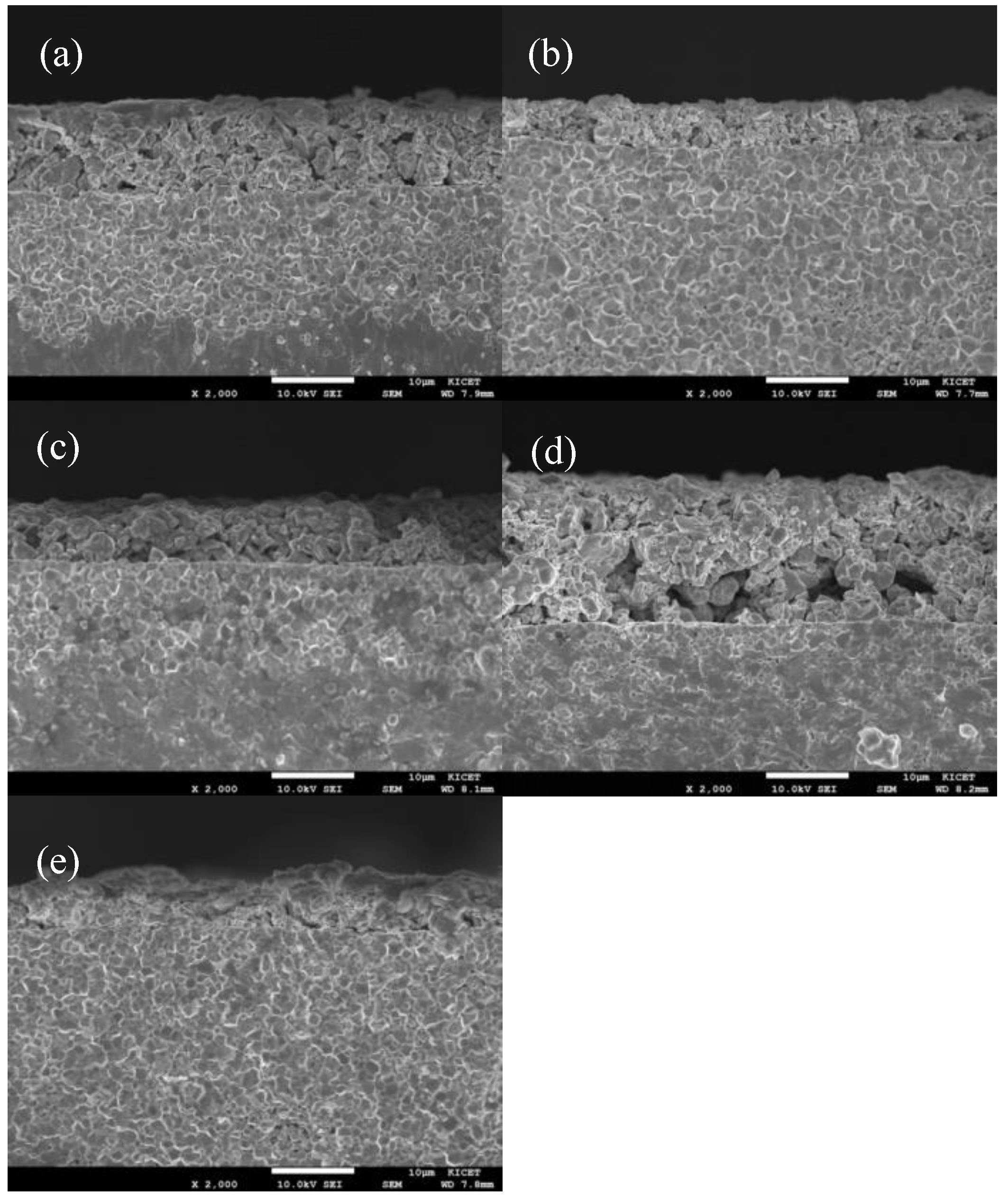
3.1. XRD Analysis and Microstructure
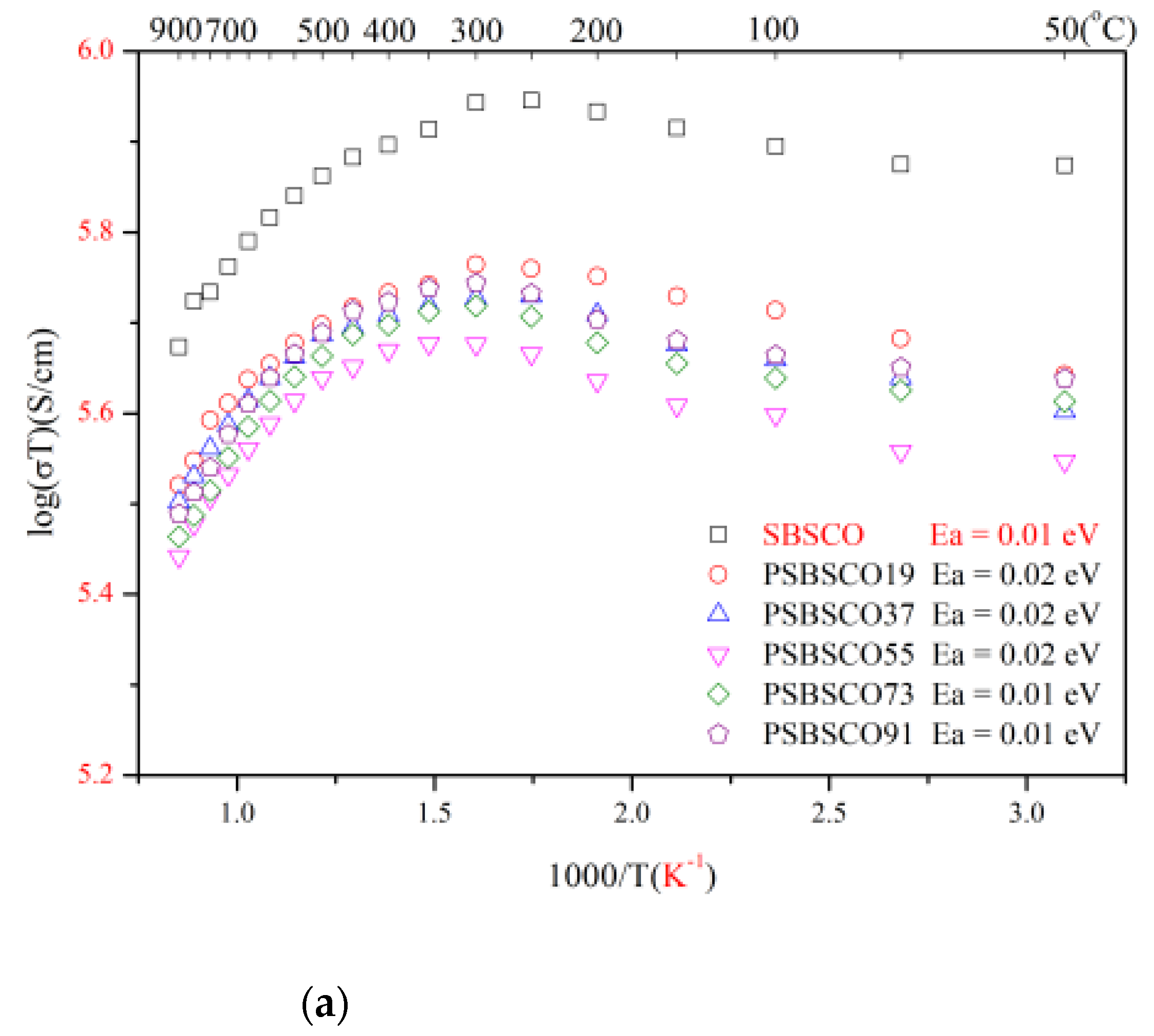
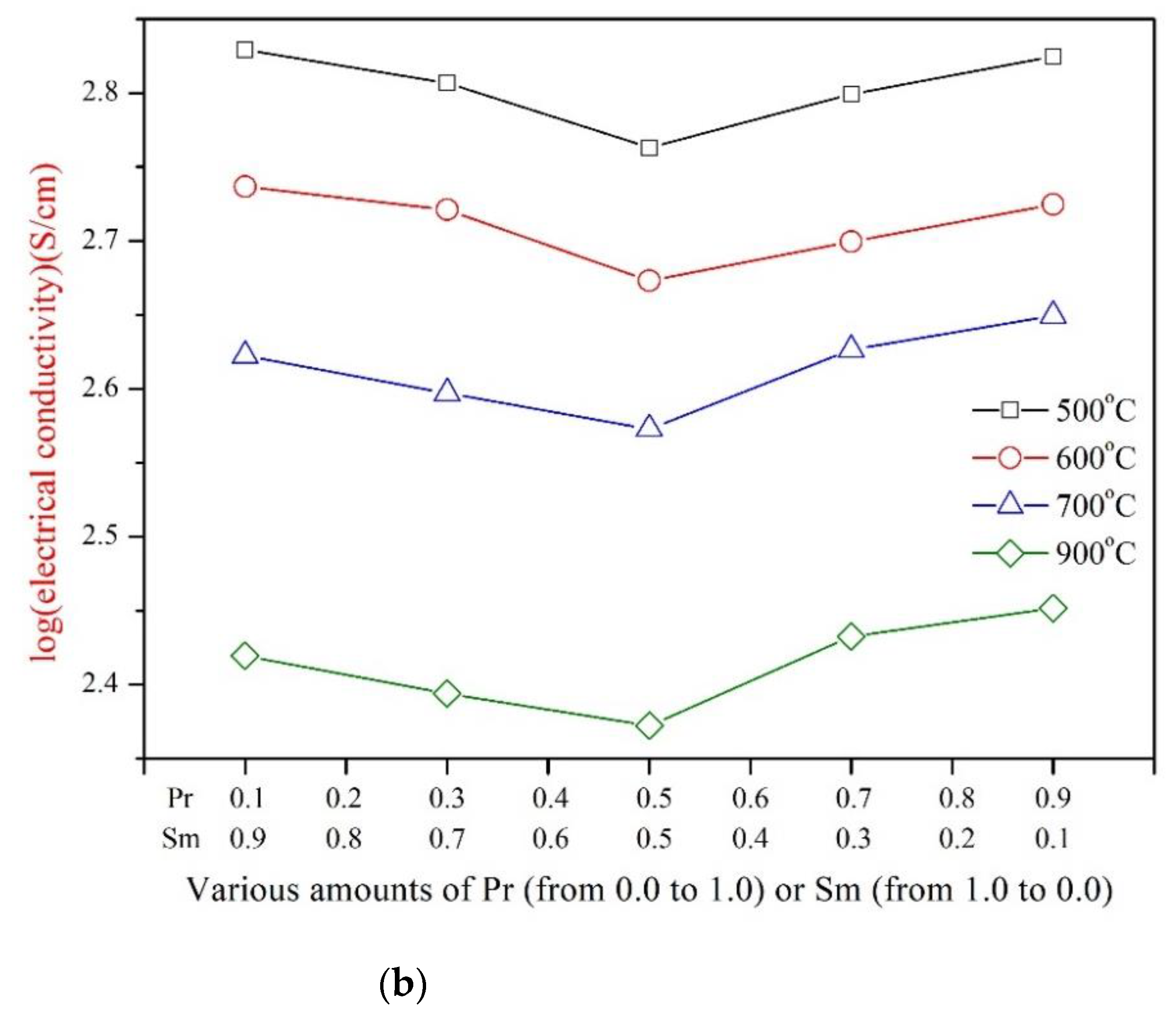
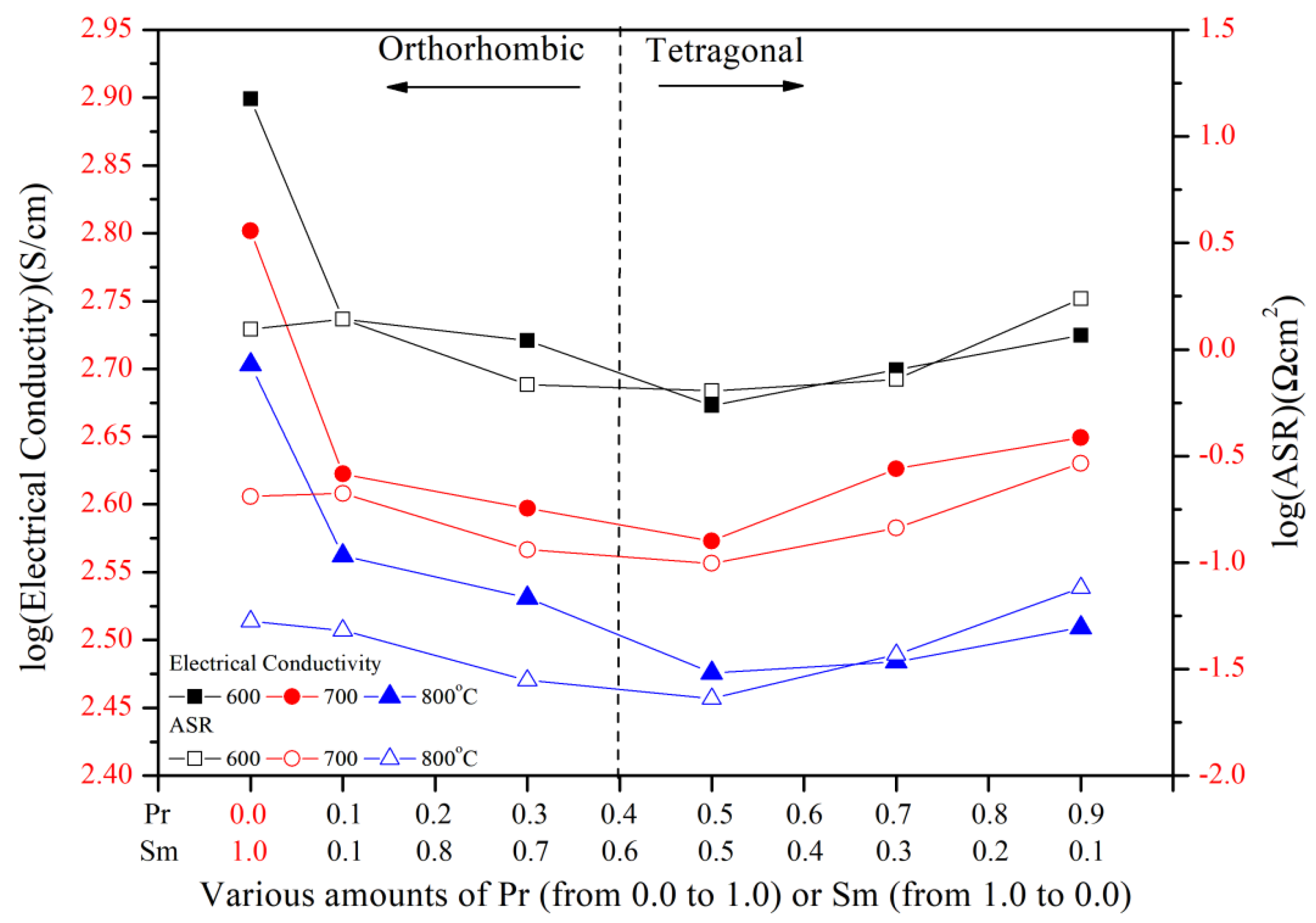
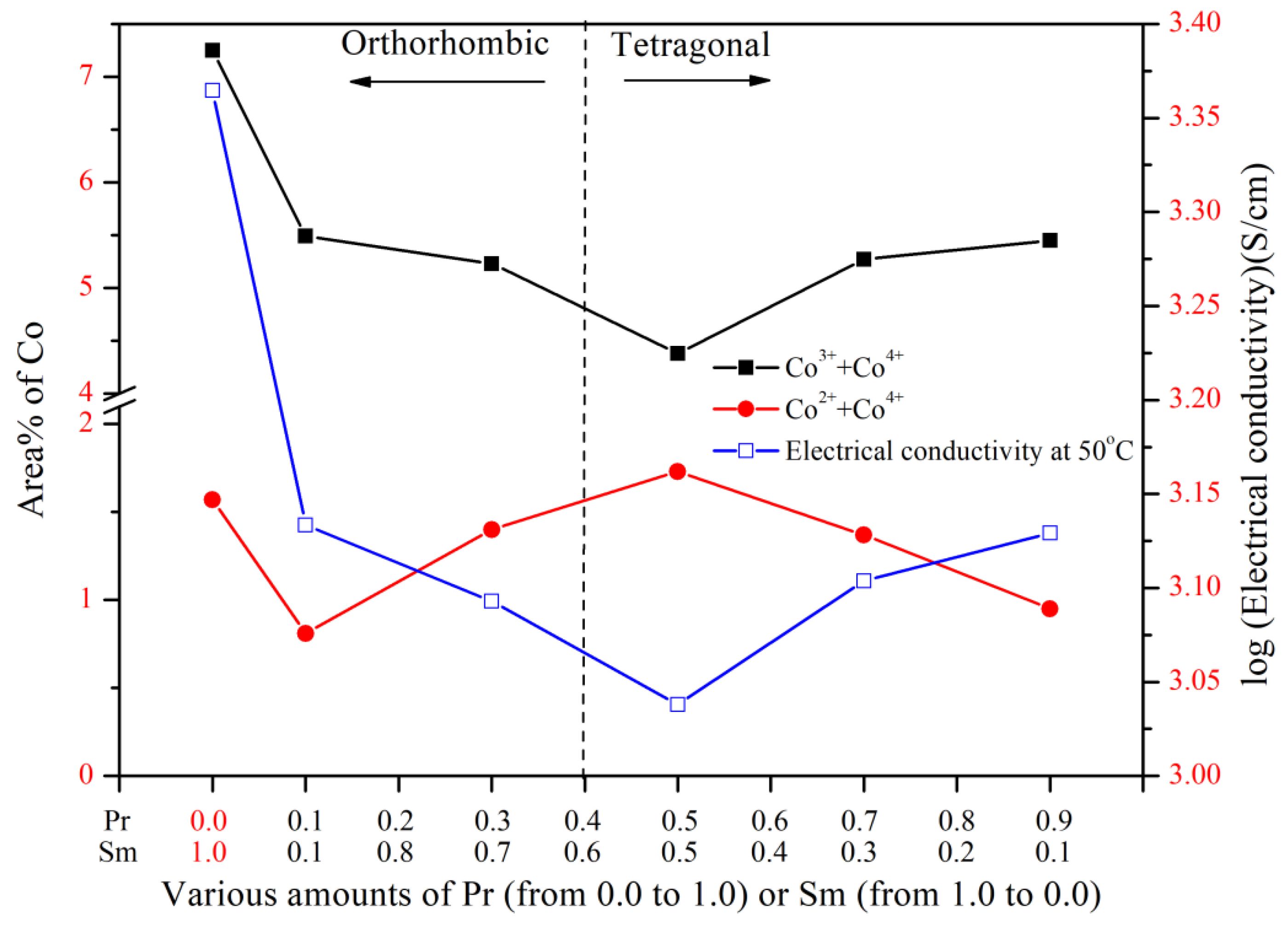
3.2. Electrical Conductivity of PrxSm1−xBa0.5Sr0.5Co2O5+d
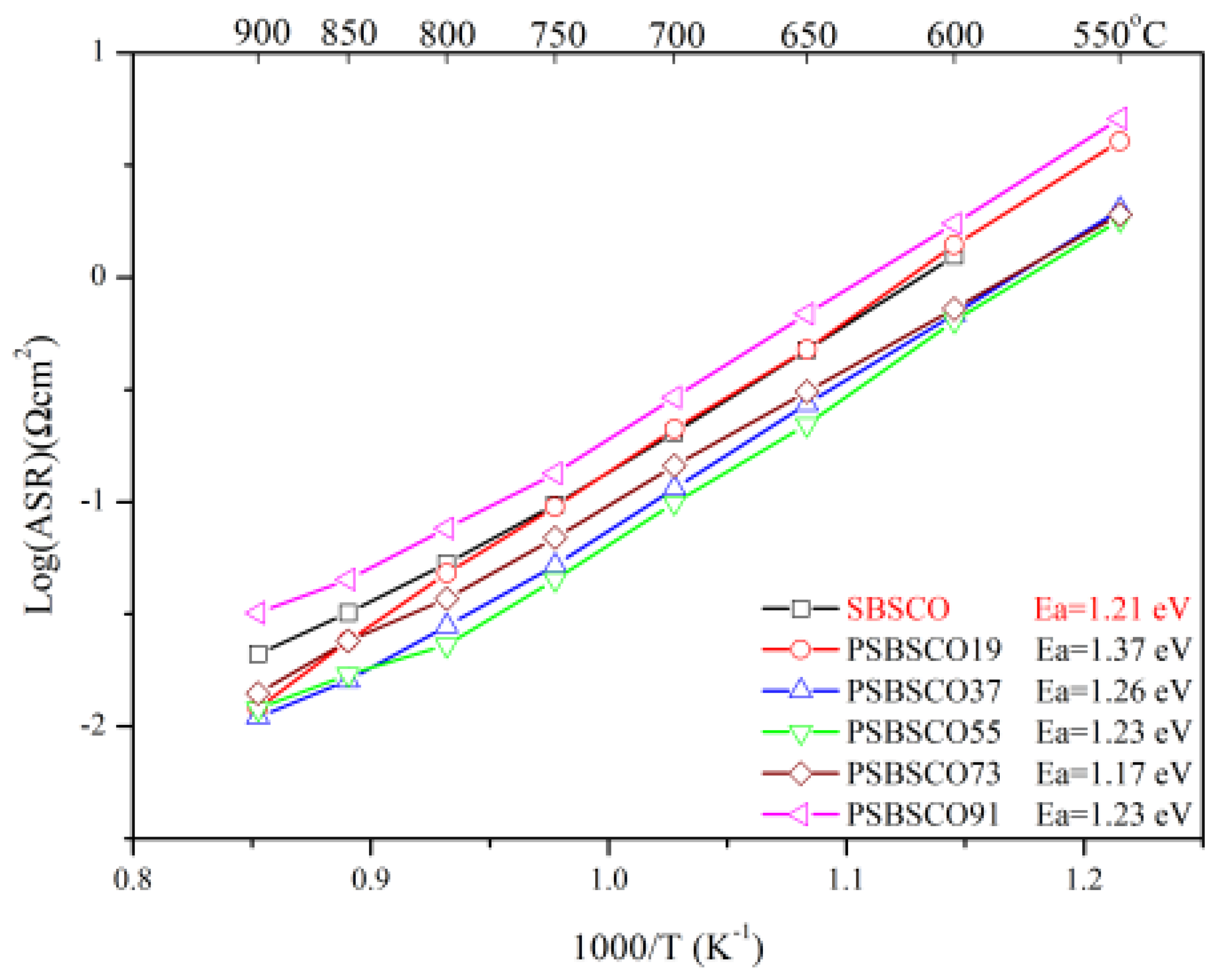
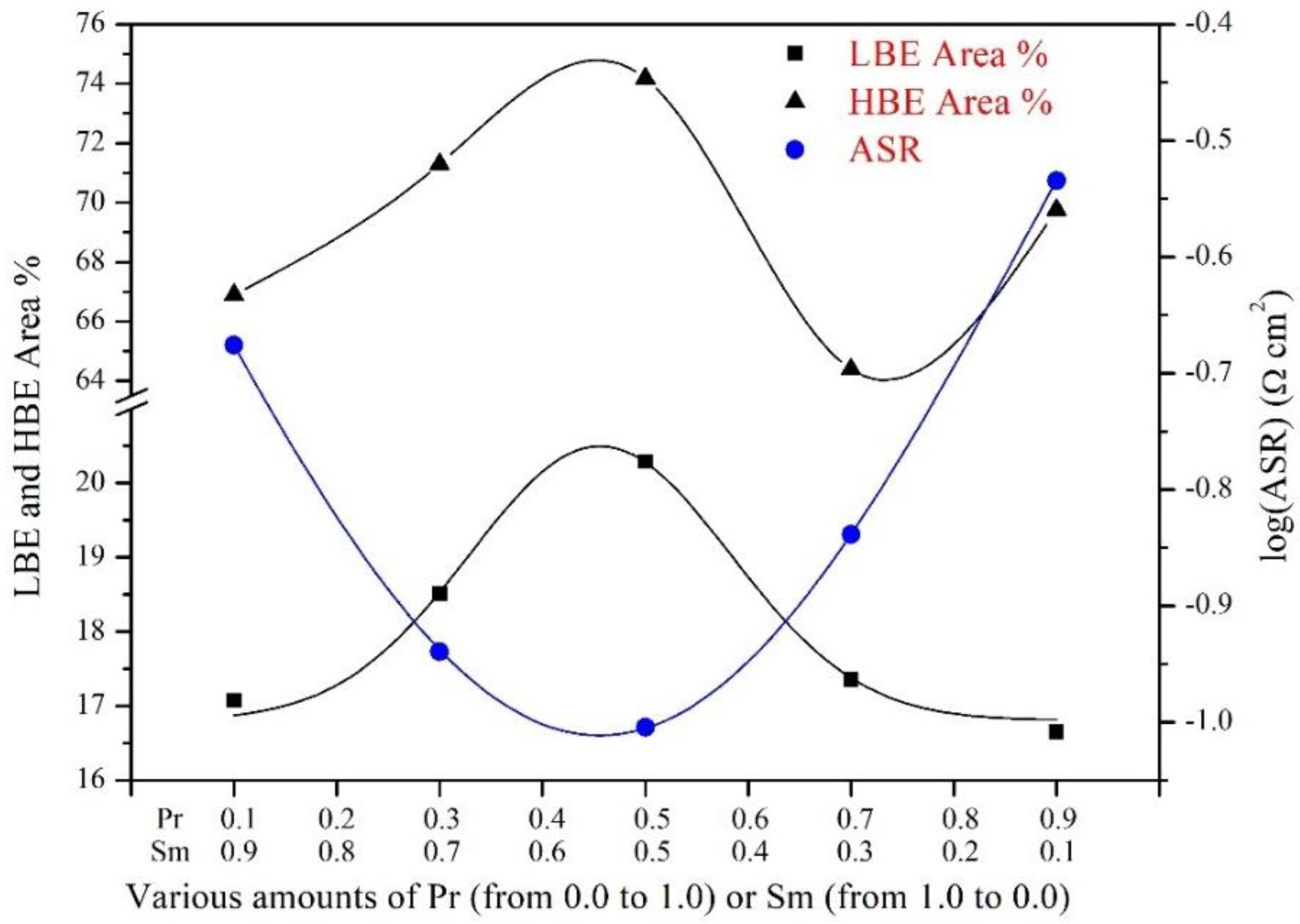
3.3. Electrochemical Characterization
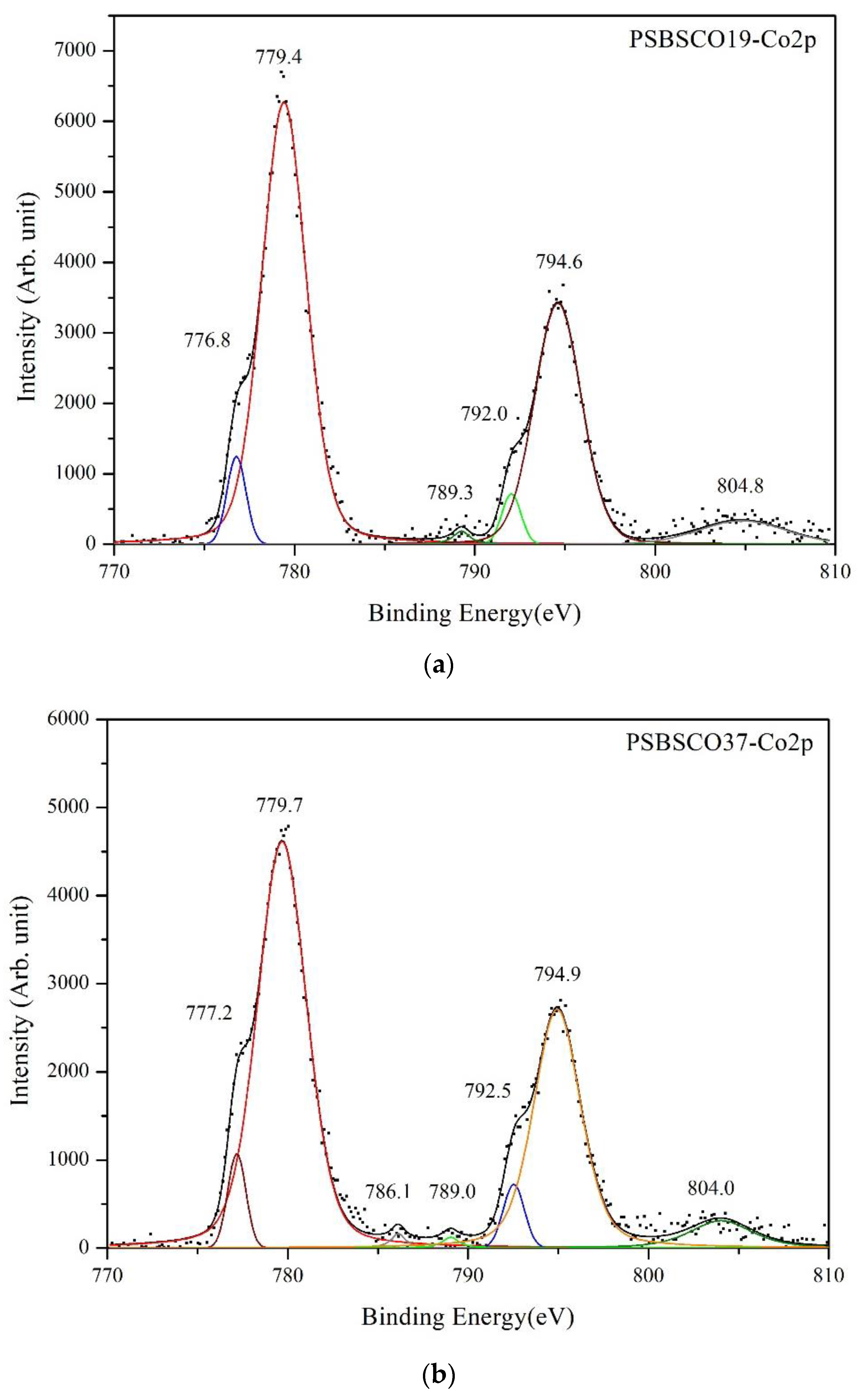
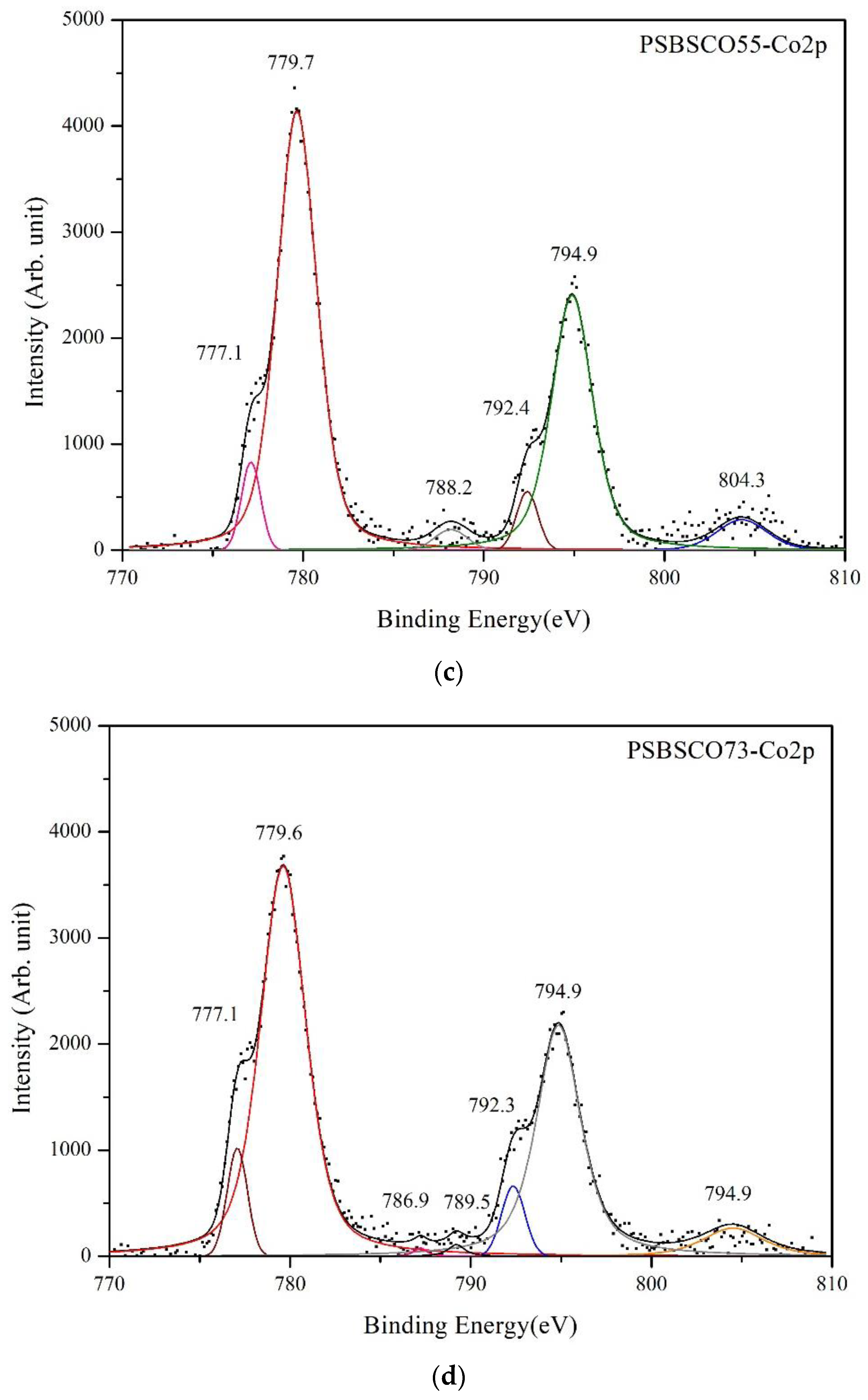
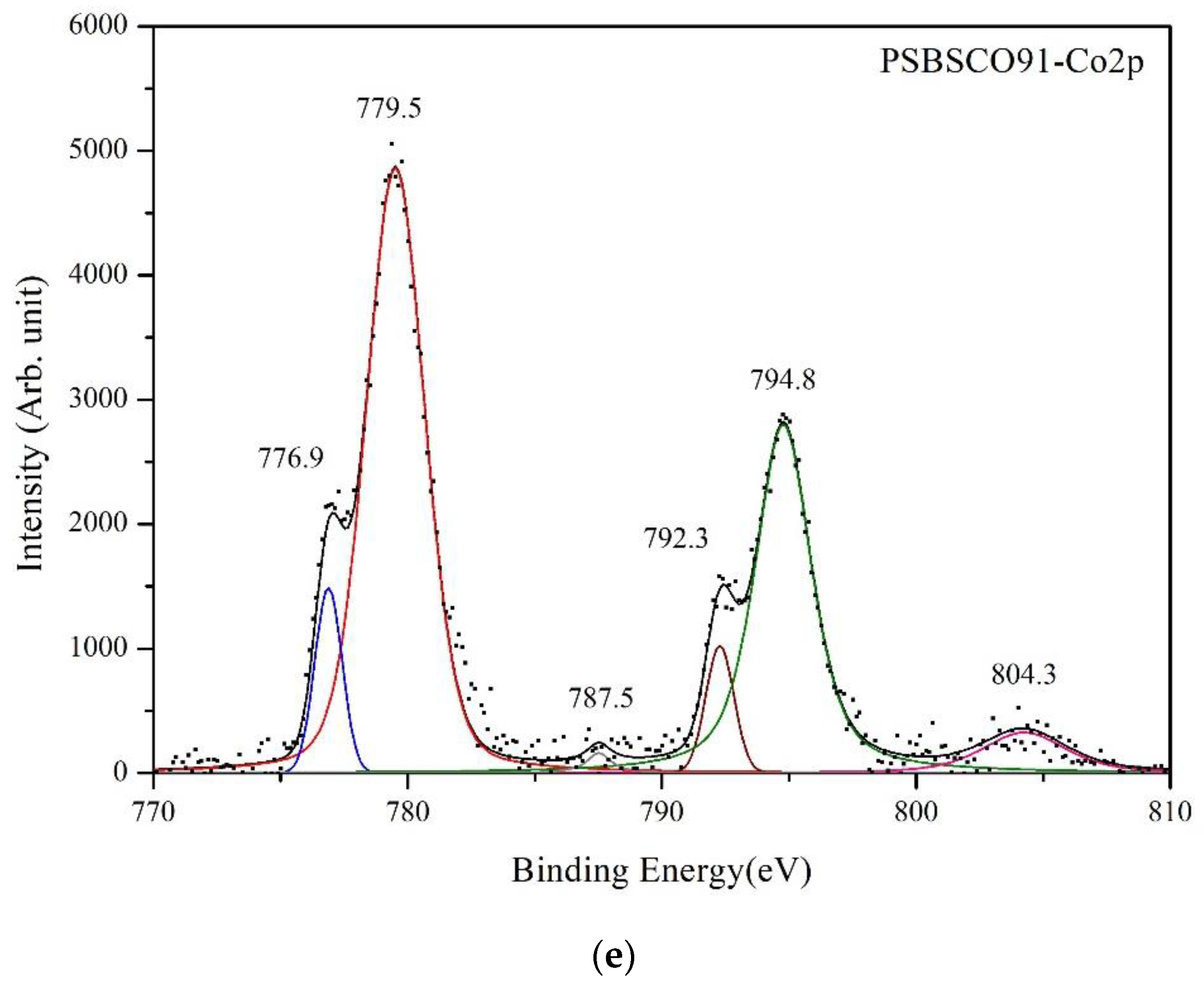
3.4. XPS Spectra of Cobalt
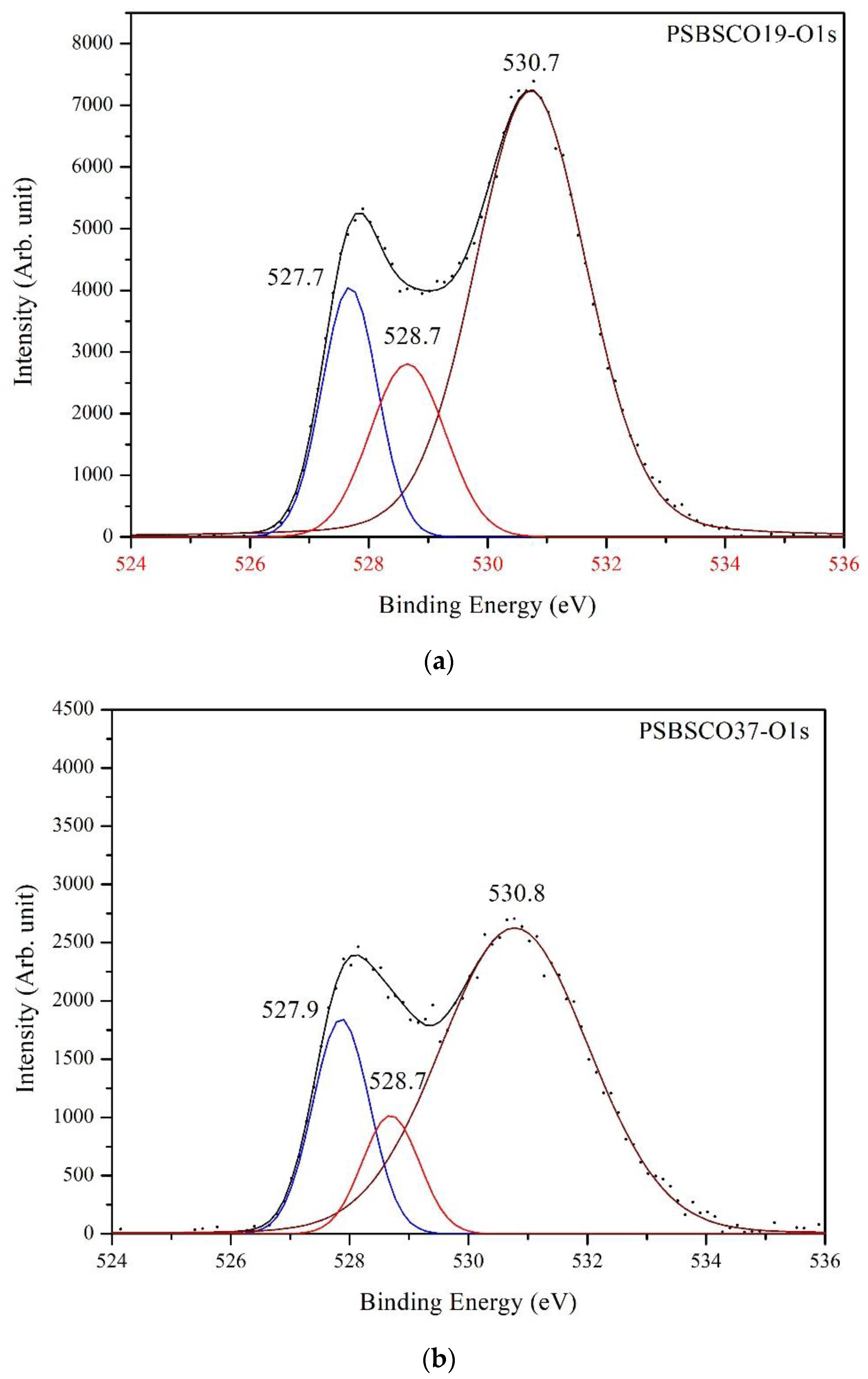
3.5. XPS Spectra of Oxygen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Hayre, R.; Cha, S.W.; Collella, W.; Prinz, F.B. Fuel Cell Fundamentals, 3rd ed.; Wiley: Hoboken, NJ, USA, 2006. [Google Scholar]
- Andersson, M.; Yuan, J.; Sunden, B. SOFC modeling considering hydrogen and carbon monoxide as electrochemical reactants. J. Power Sources 2013, 232, 42–54. [Google Scholar] [CrossRef] [Green Version]
- Koide, H.; Someya, Y.; Yoshida, T.; Maruyama, T. Properties of Ni/YSZ cermet as anode for SOFC. Solid State Ion. 2000, 132, 253–260. [Google Scholar] [CrossRef]
- Jin, S.B.; Kim, K.S.; Baek, S.W.; Kim, H.S.; Kang, H.I.; Choi, W.S.; Kim, J.H. Characterization of Layered Perovskite Nanofibers using Electrospinning for Cathode Materials of Low Temperature-operating Solid Oxide Fuel Cell. New. Renew. Energy 2017, 13, 50–58. [Google Scholar] [CrossRef]
- Frontera, C.; Garcia-Munoz, J.L.; Llobet, A.; Manosa, L.; Aranda, M.A.G. Selective spin-state and metal–insulator transitions in GdBaCo2O5.5. J. Solid State Chem. 2003, 171, 349–352. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Kim, J.H.; Cassidy, M.; Irvine, J.T.S.; Bea, J.M. Advanced Electrochemical Properties of LnBa0.5Sr0.5Co2O5+d (Ln = Pr, Sm, and Gd) as Cathode Materials for IT-SOFC. J. Electrochem. Soc. 2009, 156, B682–B689. [Google Scholar] [CrossRef]
- Kim, J.H.; Cassidy, M.; Irvine, J.T.S.; Bea, J.M. Electrochemical investigation of composite cathodes with SmBa0.5Sr0.5Co2O5+d cathodes for intermediate temperature-operating solid oxide fuel cell. Chem. Mater. 2010, 22, 883–892. [Google Scholar] [CrossRef]
- Irvine, J.T.S.; Bea, J.M.; Park, J.Y.; Choi, W.S.; Kim, J.H. Electrochemical properties and durability of in-situ composite cathodes with SmBa0.5Sr0.5Co2O5+δ for metal supported solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 1212–1220. [Google Scholar] [CrossRef] [Green Version]
- Song, S.W.; Choi, W.S.; Kang, H.; Baek, S.W.; Azad, A.K.; Park, J.Y.; Kim, J.H. Synthesis and electrochemical properties of layered perovskite substituted with heterogeneous lanthanides for intermediate temperature-operating solid oxide fuel cell. Int. J. Hydrogen Energy 2018, 43, 11378–11385. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.M.; Connor, P.A.; Irvine, J.Y.S.; Bae, J.M. Structural, thermal and electrochemical properties of layered perovskite SmBaCo2O5+d, a potential cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2009, 194, 704–711. [Google Scholar] [CrossRef]
- Xia, L.N.; He, Z.P.; Huang, Z.W.; Yu, Y. Synthesis and properties of SmBaCo2−xNixO5+δ perovskite oxide for IT-SOFC cathodes. Ceram. Int. 2016, 42, 1272–1280. [Google Scholar] [CrossRef]
- Aksenova, T.V.; Gavrilova, L.Y.; Yaremchenko, A.A.; Cherepanov, V.A.; Kharton, V.V. Oxygen nonstoichiometry, thermal expansion and high-temperature electrical properties of layered NdBaCo2O5+d and SmBaCo2O5+ d. Mater. Res. Bull. 2010, 45, 1288–1292. [Google Scholar] [CrossRef]
- Marrero-Jerez, J.; Pena-Martınez, J.; Nunez, P. Study of the oxygen desorption from GdBa1−xSrxCo2O5+δ (x = 0, 0.25, 0.5 and 1): Effect of the Sr-content on the oxidation state of cobalt ions. J. Alloys Compd. 2014, 606, 269–272. [Google Scholar] [CrossRef]
- Chavez, E.; Mueller, M.; Mogni, L.; Caneiro, A. Study of LnBaCo2O6-δ (Ln = Pr, Nd, Sm and Gd) double perovskites as new cathode material for IT-SOFC. J. Phys. Conf. Ser. 2009, 167, 012043. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Choi, S.H.; Park, S.H.; Kim, C.M.; Shin, J.Y.; Kim, G.T. Effect of Mn on the electrochemical properties of a layered perovskite NdBa0.5Sr0.5Co2−xMnxO5+δ (x = 0, 0.25, and 0.5) for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2013, 112, 712–718. [Google Scholar] [CrossRef]
- Subardi, A.; Liao, K.Y.; Fua, Y.P. Oxygen transport, thermal and electrochemical properties of NdBa0.5Sr0.5Co2O5+δ cathode for SOFCs. J. Eur. Ceram. Soc. 2019, 39, 30–40. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X. PrBa0.5Sr0.5Co2O5+d layered peroyskite cathode for intermediate temperature solid oxide fuel cells. Electrochim. Acta 2010, 55, 3812–3816. [Google Scholar] [CrossRef]
- Lu, S.; Long, G.; Meng, X.; Ji, Y.; Lu, B.; Zhao, H. PrBa0.5Sr0.5Co2O5+x as cathode material based on LSGM and GDC electrolyte for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 5914–5919. [Google Scholar] [CrossRef]
- Kim, Y.M.; Schlegl, H.; Kim, K.S.; Irvine, J.T.S.; Kim, J.H. X-ray photoelectron spectroscopy of Sm-doped layered perovskite for intermediate temperature-operating solid oxide fuel cell. Appl. Surf. Sci. 2018, 288, 695–701. [Google Scholar] [CrossRef]
- Huang, K.; Lee, H.Y.; Goodenough, J.B. Sr- and Ni- Doped LaCoO3 and LaFeO3 Perovskites: New Cathode Materials for Solid-Oxide Fuel Cells. J. Electrochem. Soc. 1998, 145, 3220–3227. [Google Scholar] [CrossRef]
- Joung, Y.H.; Kang, H.I.; Choi, W.S.; Kim, J.H. Investigation of X-ray photoelectron spectroscopy and electrical conductivity properties of the layered perovskite LnBaCo2O5+d (Ln = Pr, Nd, Sm, and Gd) for IT-SOFC. Electron. Mater. Lett. 2013, 9, 463–465. [Google Scholar] [CrossRef]
- Azad, A.K.; Kim, J.H.; Irvine, J.T.S. Structural, electrochemical and magnetic characterization of the layered-type PrBa0.5Sr0.5Co2O5+δ perovskite. J. Solid State Chem. 2014, 213, 268–274. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.; Zhang, H.; Liu, X.; Meng, J.; Zhang, X.; Meng, F.; Meng, J. Investigation of layered perovskite NdBa0.5Sr0.25Ca0.25Co2O5+δ as cathode for solid oxide fuel cells. Ceram. Int. 2018, 44, 12048–12054. [Google Scholar] [CrossRef]
- Galenda, A.; Natile, M.M.; Krishnan, V.; Bertagnolli, H.; Glisenti, A. LaSrCoFeO and Fe2O3/LaSrCoFeO Powders: Synthesis and Characterization. Chem. Mater. 2007, 19, 2796–2808. [Google Scholar] [CrossRef] [Green Version]
- Ivanovskaya, M.I.; Kotikov, D.A.; Pan’kov, V.V.; Zyryanov, V.V. Structure of SrCo0.5Fe0.5O3 − δ-based composites prepared by sol-gel and mechanochemical processes. Inorg. Mater. 2009, 45, 910–915. [Google Scholar] [CrossRef]
- Tan, B.J.; Klabunde, K.J.; Sherwood, P.M. XPS studies of solvated metal atom dispersed (SMAD) catalysts. Evidence for layered cobalt-manganese particles on alumina and silica. J. Am. Chem. Soc. 1991, 113, 855–861. [Google Scholar] [CrossRef]
- Nitadori, T.; Muramatsu, M.; Misono, M. Valence control, reactivity of oxygen, and catalytic activity of lanthanum strontium cobalt oxide (La2-xSrxCoO4). Chem. Mater. 1989, 1, 215–220. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Eloy, P.; Cadus, L.E. La1−xCaxCoO3 perovskite-type oxides: Identification of the surface oxygen species by XPS. Appl. Surf. Sci. 2006, 253, 1489–1493. [Google Scholar] [CrossRef]
- Noboru, Y.; Yasutake, T.; Tetsuro, S. TPD and XPS study on thermal behavior of absorbed oxygen in La1−xSrxCoO3. Chem. Lett. 1981, 10, 1767–1770. [Google Scholar]
- Yi, K.; Sun, L.; Li, Q.; Xia, T.; Huo, L.; Zha, H.; Li, J.; Lü, Z.; Bassat, J.M.; Rougier, A.; et al. Effect of Nd-deficiency on electrochemical properties of NdBaCo2O6−δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 10228–10238. [Google Scholar] [CrossRef]



| Chemical Compositions | Abbreviations |
|---|---|
| SmBa0.5Sr0.5Co2O5+d | SBSCO |
| Pr0.1Sm0.9Ba0.5Sr0.5Co2O5+d | PSBSCO19 |
| Pr0.3Sm0.7Ba0.5Sr0.5Co2O5+d | PSBSCO37 |
| Pr0.5Sm0.5Ba0.5Sr0.5Co2O5+d | PSBSCO55 |
| Pr0.7Sm0.3Ba0.5Sr0.5Co2O5+d | PSBSCO73 |
| Pr0.9Sm0.1Ba0.5Sr0.5Co2O5+d | PSBSCO91 |
| PrBa0.5Sr0.5Co2O5+d | PBSCO |
| Compositions | Binding Energy (BE) Distribution | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Co 2p3/2 | Co 2p1/2 | Co2+ + Co4+ | Co3+ + Co4+ | |||||||||
| BE (eV) | FWHM | Area % | BE (eV) | FWHM | Area % | BE (eV) | FWHM | Area % | BE (eV) | FWHM | Area % | |
| PSBSCO19 | 779.4 | 2.93 | 56.01 | 794.6 | 3.08 | 30.93 | 789.3 | 1.08 | 0.81 | 804.8 | 5.65 | 5.49 |
| PSBSCO37 | 779.7 | 3.22 | 54.50 | 794.9 | 3.08 | 31.48 | 786.1, 789.0 | 0.95/1.09 | 0.76, 0.65 | 804.0 | 4.09 | 5.23 |
| PSBSCO55 | 779.7 | 2.63 | 53.35 | 794.9 | 2.69 | 32.95 | 788.2 | 2.13 | 1.73 | 804.3 | 3.28 | 4.38 |
| PSBSCO73 | 779.6 | 3.07 | 55.88 | 794.9 | 2.90 | 27.35 | 786.9, 789.5 | 1.45/1.20 | 0.90, 0.47 | 804.6 | 3.70 | 5.27 |
| PSBSCO91 | 779.5 | 2.73 | 50.67 | 794.8 | 2.62 | 31.88 | 787.5 | 1.17 | 0.95 | 804.3 | 3.90 | 5.45 |
| Compositions | Binding Energy (BE) Distribution | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| LBE | IBE | HBE | |||||||
| BE (eV) | FWHM | Area % | BE (eV) | FWHM | Area % | BE (eV) | FWHM | Area % | |
| PSBSCO19 | 527.7 | 1.10 | 17.07 | 528.7 | 1.49 | 16.03 | 530.7 | 2.23 | 66.90 |
| PSBSCO37 | 527.9 | 1.13 | 18.51 | 528.7 | 1.12 | 10.19 | 530.8 | 2.95 | 71.30 |
| PSBSCO55 | 527.9 | 1.35 | 20.29 | 528.9 | 1.03 | 5.53 | 530.7 | 2.44 | 74.18 |
| PSBSCO73 | 527.8 | 1.12 | 17.36 | 528.7 | 1.29 | 18.24 | 530.8 | 2.54 | 64.40 |
| PSBSCO91 | 527.6 | 1.14 | 16.65 | 528.4 | 1.21 | 13.59 | 530.7 | 2.55 | 69.76 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woo, S.H.; Song, K.E.; Baek, S.-W.; Kang, H.; Choi, W.; Shin, T.H.; Park, J.-Y.; Kim, J.H. Pr- and Sm-Substituted Layered Perovskite Oxide Systems for IT-SOFC Cathodes. Energies 2021, 14, 6739. https://doi.org/10.3390/en14206739
Woo SH, Song KE, Baek S-W, Kang H, Choi W, Shin TH, Park J-Y, Kim JH. Pr- and Sm-Substituted Layered Perovskite Oxide Systems for IT-SOFC Cathodes. Energies. 2021; 14(20):6739. https://doi.org/10.3390/en14206739
Chicago/Turabian StyleWoo, Sung Hun, Kyeong Eun Song, Seung-Wook Baek, Hyunil Kang, Wonseok Choi, Tae Ho Shin, Jun-Young Park, and Jung Hyun Kim. 2021. "Pr- and Sm-Substituted Layered Perovskite Oxide Systems for IT-SOFC Cathodes" Energies 14, no. 20: 6739. https://doi.org/10.3390/en14206739
APA StyleWoo, S. H., Song, K. E., Baek, S.-W., Kang, H., Choi, W., Shin, T. H., Park, J.-Y., & Kim, J. H. (2021). Pr- and Sm-Substituted Layered Perovskite Oxide Systems for IT-SOFC Cathodes. Energies, 14(20), 6739. https://doi.org/10.3390/en14206739








