Environmental Life Cycle Assessment of Ammonia-Based Electricity
Abstract
:1. Introduction
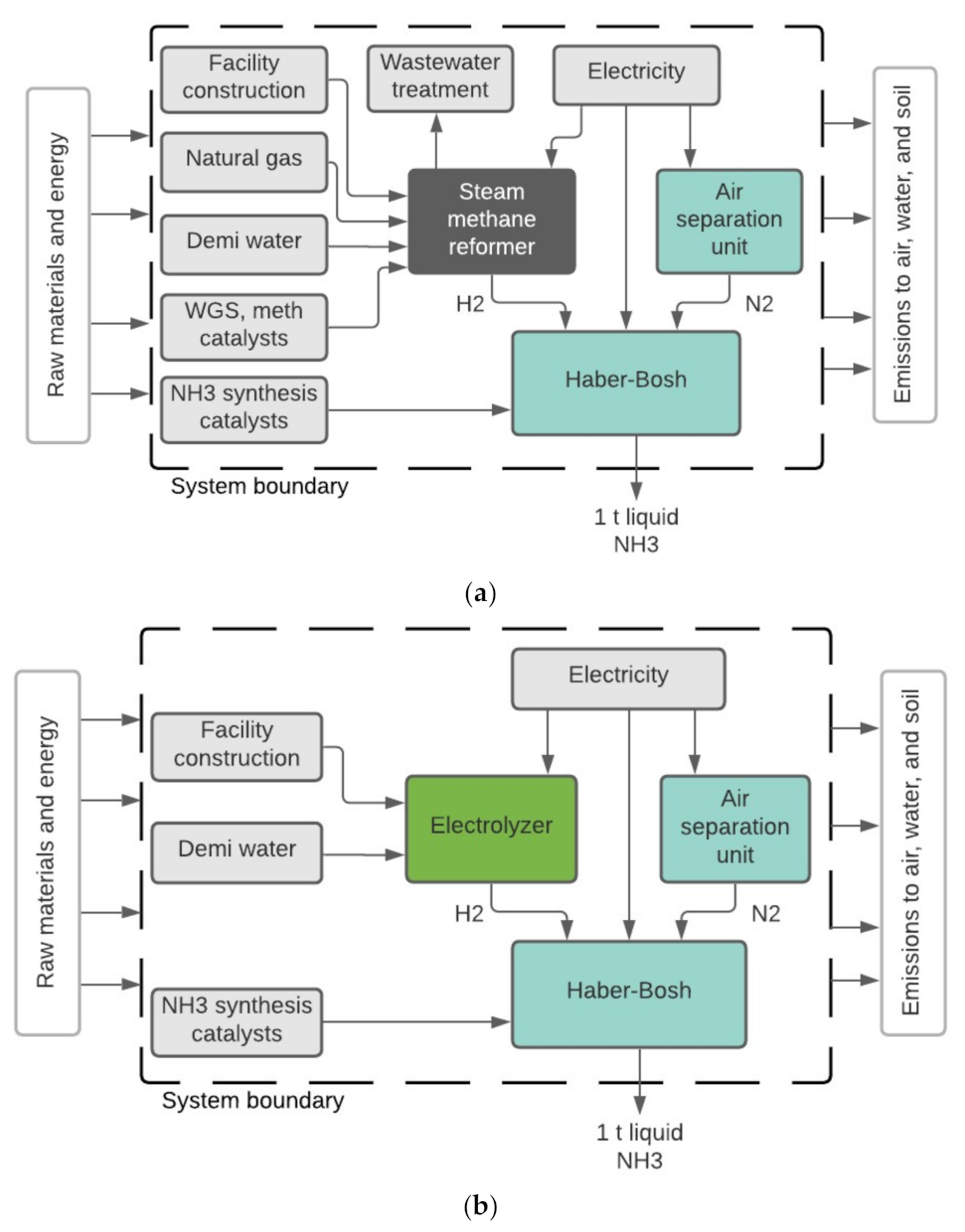
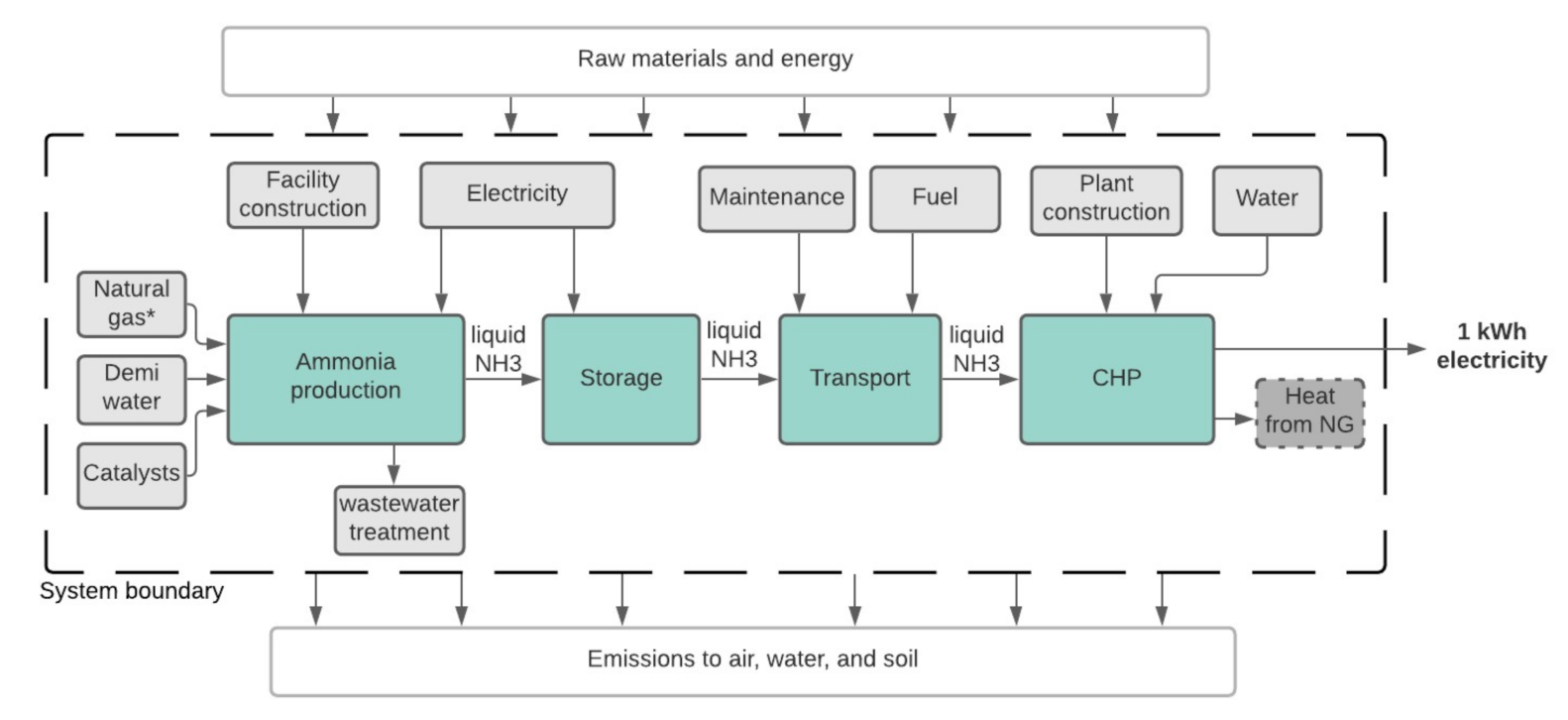
2. Materials and Methods
3. Results and Discussion
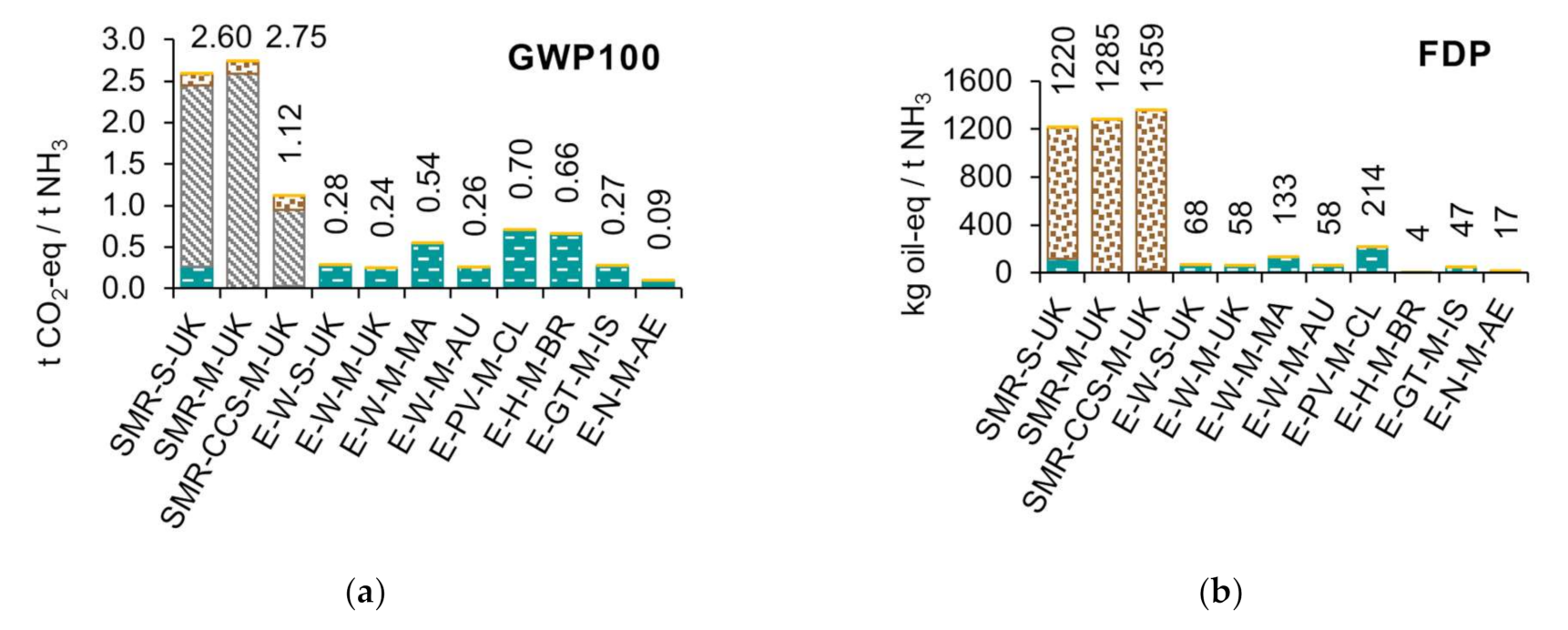
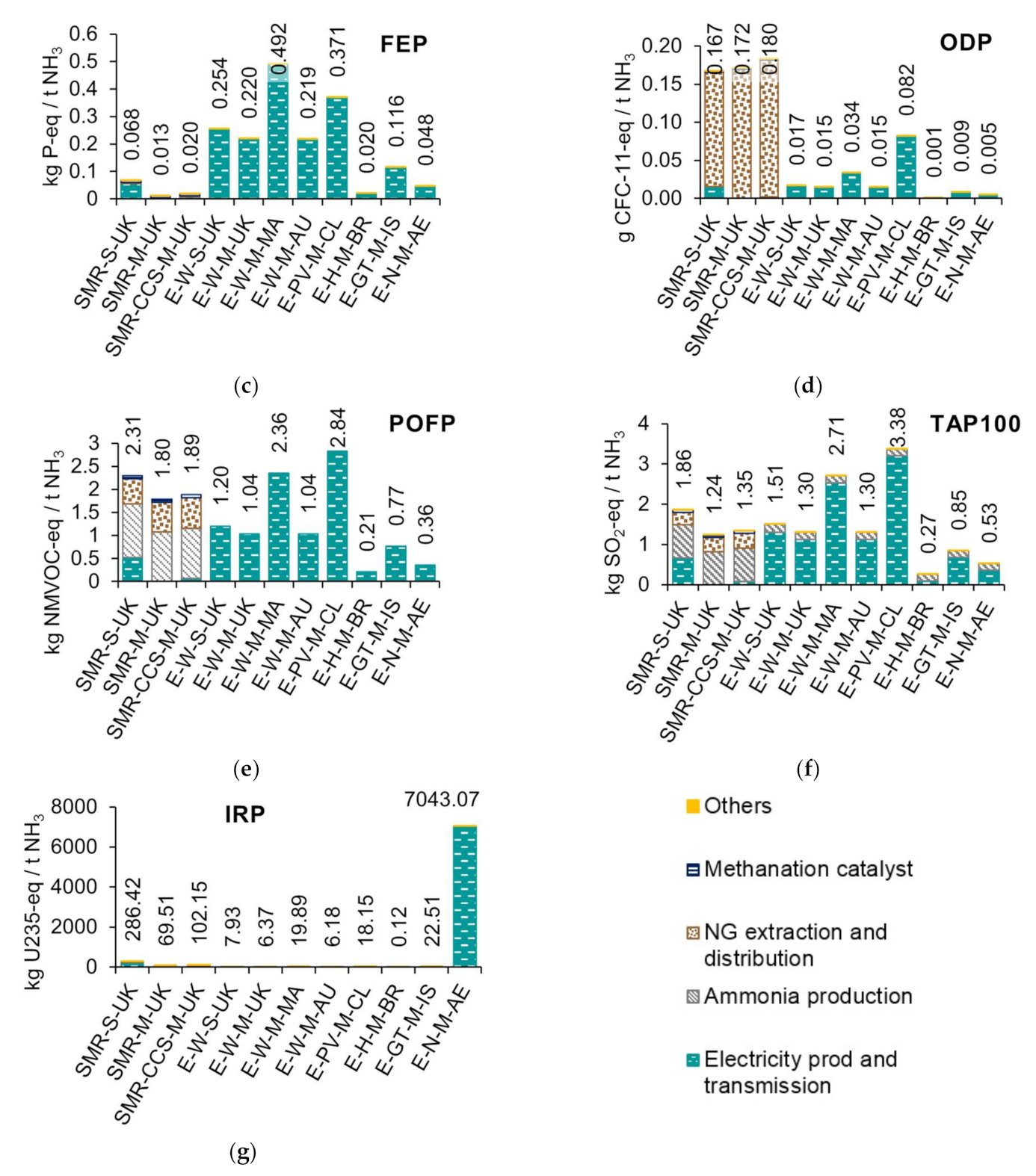
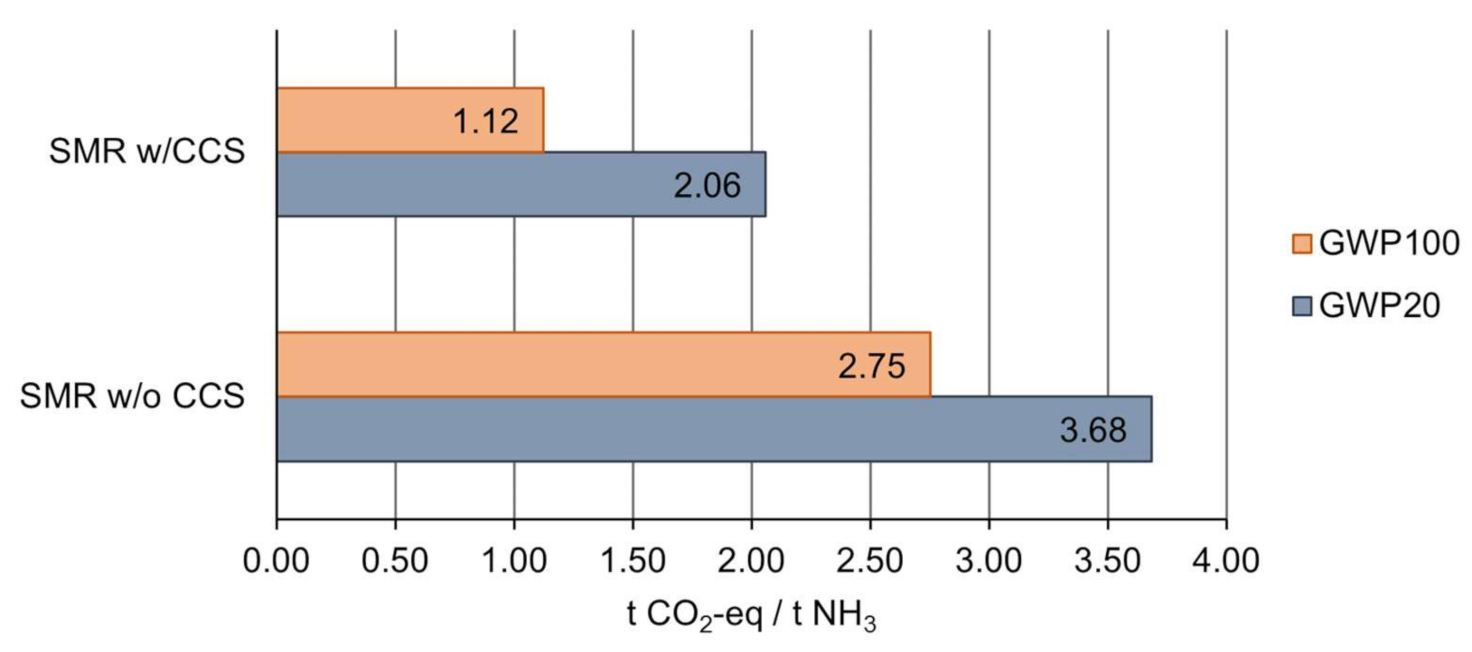
3.1. Life Cycle Impact Assessment of Ammonia Production
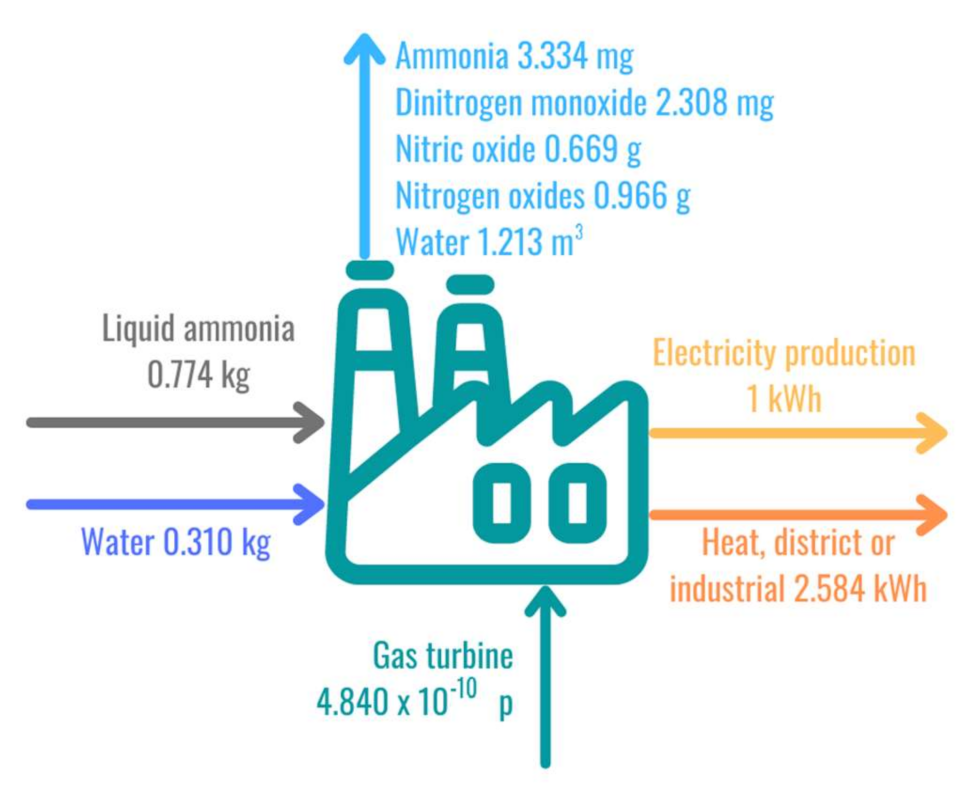
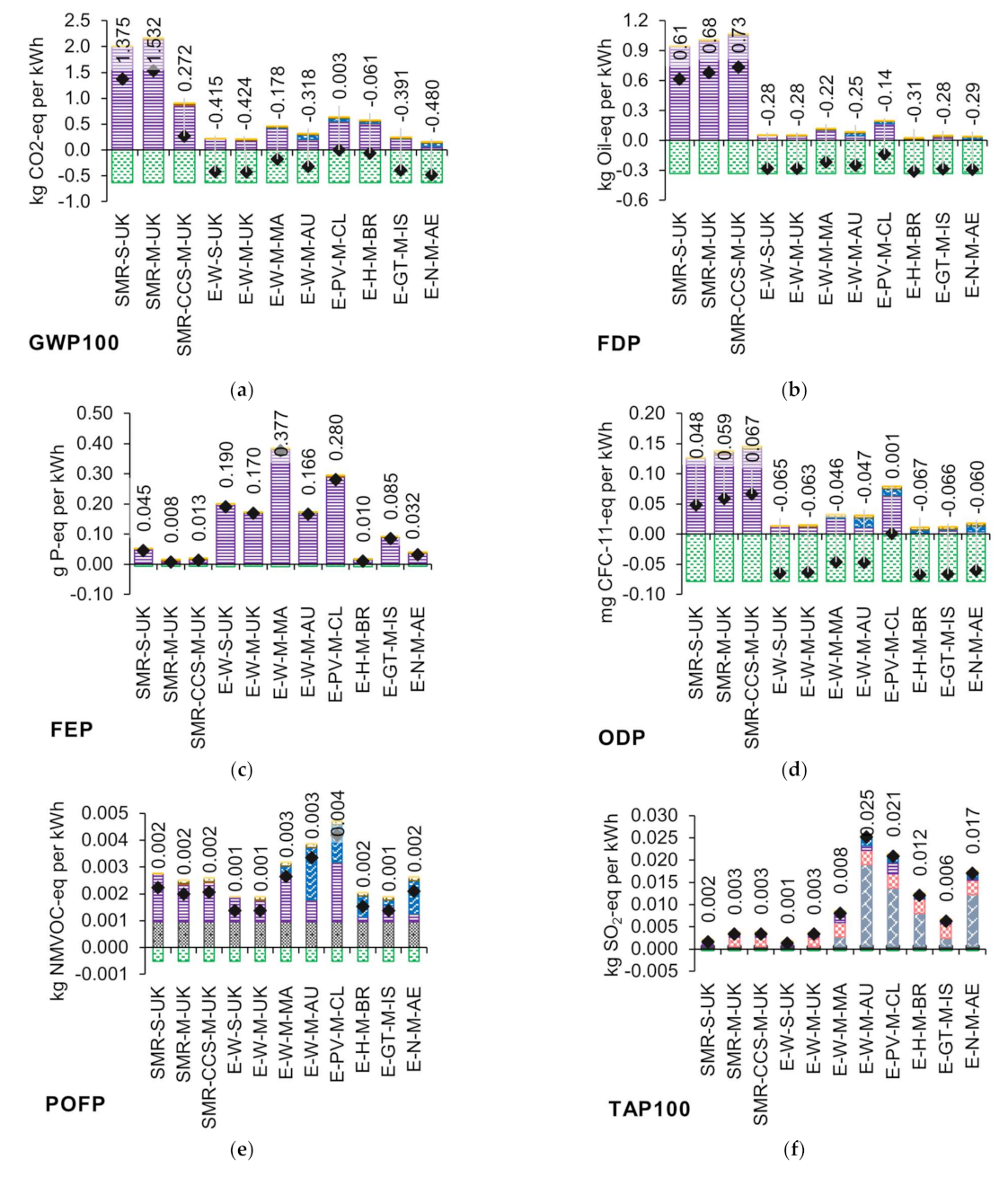
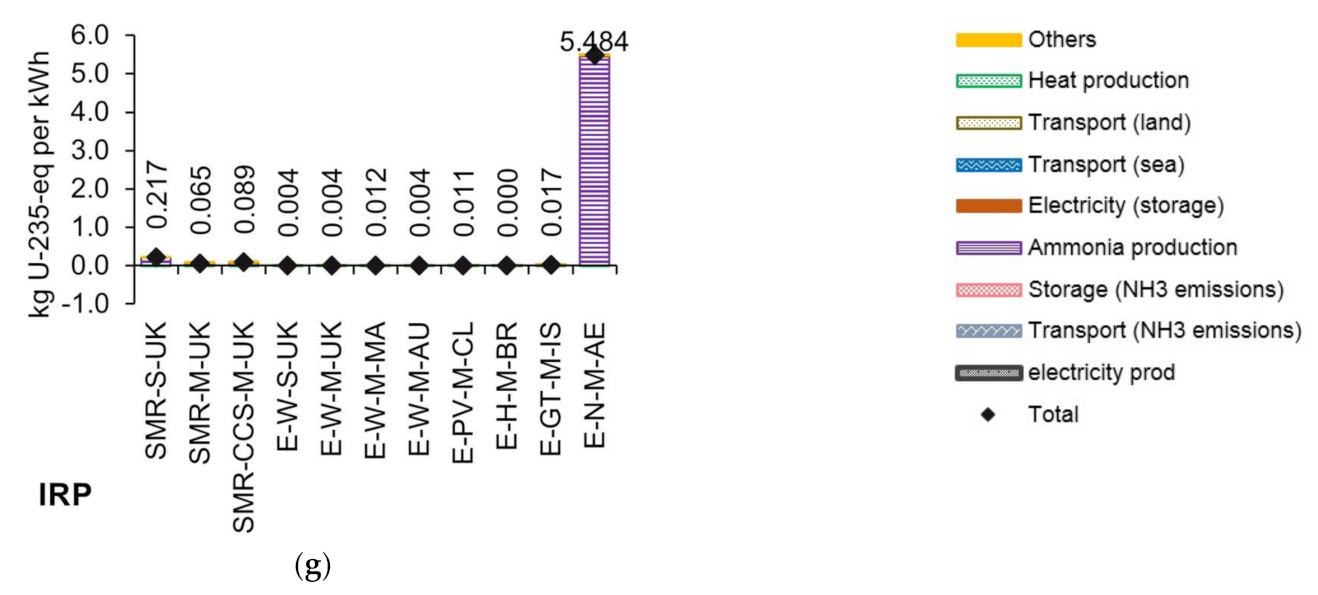
3.2. Life Cycle Impact Assessment of Ammonia-Based Electricity
3.3. Limitations and Recommendations for Further Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASU | air separation unit |
| CCS | carbon capture and storage |
| CFC | chlorofluorocarbon |
| CHP | combined heat and power |
| CO2 | carbon dioxide |
| FDP | fossil depletion potential |
| FEP | freshwater eutrophication potential |
| GHG | greenhouse gas |
| GWP | global warming potential |
| H2 | hydrogen |
| IRP | ionizing radiation potential |
| ISO | International Organization for Standardization |
| LCA | life cycle assessment |
| LCI | life cycle inventory |
| LCIA | life cycle impact assessment |
| N | nitrogen |
| NH3 | ammonia |
| NMVOC | non-methane volatile organic compounds |
| NO | nitrogen monoxide |
| NOX | nitrogen oxides |
| N2O | nitrous oxide |
| ODP | ozone depletion potential |
| P | phosphorous |
| POFP | photochemical oxidant formation potential |
| PV | photovoltaic |
| SMR | steam methane reforming |
| SO2 | sulphur dioxide |
| TAP | terrestrial acidification potential |
| UK | United Kingdom |
| U235 | uranium-235 |
References
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Elishav, O.; Lis, B.M.; Miller, E.M.; Arent, D.J.; Valera-Medina, A.; Dana, A.G.; Shter, G.E.; Grader, G.S. Progress and Prospective of Nitrogen-Based Alternative Fuels. Chem. Rev. 2020, 120, 5352–5436. [Google Scholar] [CrossRef] [PubMed]
- Valera-Medina, A.; Amer-Hatem, F.; Azad, A.K.; Dedoussi, I.C.; De Joannon, M.; Fernandes, R.X.; Glarborg, P.; Hashemi, H.; He, X.; Mashruk, S.; et al. Review on ammonia as a potential fuel: From synthesis to economics. Energy Fuels 2021, 35, 6964–7029. [Google Scholar] [CrossRef]
- Lhuillier, C.; Brequigny, P.; Lamoureux, N.; Contino, F.; Mounaïm-Rousselle, C. Experimental investigation on laminar burning velocities of ammonia/hydrogen/air mixtures at elevated temperatures. Fuel 2020, 263, 116653. [Google Scholar] [CrossRef] [Green Version]
- Valera-Medina, A.; Banares-Alcantara, R. Techno-Economic Challenges of Green Ammonia as an Energy Vector; Valera-Medina, A., Banares-Alcantara, R., Eds.; Elsevier Inc.: London, UK, 2021; ISBN 978-0-12-820560-0. [Google Scholar]
- Fasihi, M.; Weiss, R.; Savolainen, J.; Breyer, C. Global potential of green ammonia based on hybrid PV-wind power plants. Appl. Energy 2021, 294, 116170. [Google Scholar] [CrossRef]
- Det Norske Veritas (DNV). Maritime Forecast to 2050. Energy Transition Outlook 2019; Det Norske Veritas (DNV): Bærum, Norway, 2018; p. 118. [Google Scholar]
- Raucci, C.; Bonello, J.-M.; Suarez De La Fuente, S.; Smith, T.; Sogaard, K. Aggregate Investment for the Decarbonisation of the Shipping Industry; UMAS: London, UK, 2020. [Google Scholar]
- Wei, Z.; En-ke, A. Life cycle analysis of coal-based ammonia fuel for vehicular application. J. China Coal Soc. 2010, 1, 46. [Google Scholar]
- Bicer, Y.; Dincer, I.; Zamfirescu, C.; Vezina, G.; Raso, F. Comparative life cycle assessment of various ammonia production methods. J. Clean. Prod. 2016, 135, 1379–1395. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle evaluation of hydrogen and other potential fuels for aircrafts. Int. J. Hydrogen Energy 2017, 42, 10722–10738. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle assessment of ammonia utilization in city transportation and power generation. J. Clean. Prod. 2018, 170, 1594–1601. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Clean fuel options with hydrogen for sea transportation: A life cycle approach. Int. J. Hydrogen Energy 2018, 43, 1179–1193. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I.; Vezina, G. Comprehensive Evaluation of NH3 Production and Utilization Options for Clean Energy Applications. In Proceedings of the AIChe Annual Meeting, Topical Conference: NH3 Energy, Minneapolis, MN, USA, 1 November 2017. [Google Scholar]
- Cox, B.; Treyer, K. Environmental and economic assessment of a cracked ammonia fuelled alkaline fuel cell for off-grid power applications. J. Power Sources 2015, 275, 322–335. [Google Scholar] [CrossRef]
- Razon, L.F.; Valera-Medina, A. A Comparative Environmental Life Cycle Assessment of the Combustion of Ammonia/Methane Fuels in a Tangential Swirl Burner. Front. Chem. Eng. 2021, 3, 1–13. [Google Scholar] [CrossRef]
- Xue, M.; Wang, Q.; Lin, B.-L.; Tsunemi, K. Assessment of Ammonia as an Energy Carrier from the Perspective of Carbon and Nitrogen Footprints. ACS Sustain. Chem. Eng. 2019, 7, 12494–12500. [Google Scholar] [CrossRef]
- Pfromm, P.H. Towards sustainable agriculture: Fossil-free ammonia. J. Renew. Sustain. Energy 2017, 9, 034702. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.; Hill, A.K.; Torrente-Murciano, L. Current and future role of Haber-Bosch ammonia in a carbon-free energy landscape. Energy Environ. Sci. 2020, 13, 331–344. [Google Scholar] [CrossRef]
- Keller, M.; Koshi, M.; Otomo, J.; Iwasaki, H.; Mitsumori, T.; Yamada, K. Thermodynamic evaluation of an ammonia-fueled combined-cycle gas turbine process operated under fuel-rich conditions. Energy 2020, 194, 116894. [Google Scholar] [CrossRef]
- Mashruk, S.; Xiao, H.; Valera-Medina, A. Rich-Quench-Lean model comparison for the clean use of humidified ammonia/hydrogen combustion systems. Int. J. Hydrogen Energy 2021, 46, 4472–4484. [Google Scholar] [CrossRef]
- Guteša Božo, M.; Mashruk, S.; Zitouni, S.; Valera-Medina, A. Humidified ammonia/hydrogen RQL combustion in a trigeneration gas turbine cycle. Energy Convers. Manag. 2021, 227, 113625. [Google Scholar] [CrossRef]
- Cesaro, Z.; Ives, M.; Nayak-Luke, R.; Mason, M.; Bañares-Alcántara, R. Ammonia to power: Forecasting the levelized cost of electricity from green ammonia in large-scale power plants. Appl. Energy 2021, 282, 116009. [Google Scholar] [CrossRef]
- ISO. ISO 14040:2006. Environmental Management—Life Cycle Assessment—Principles and Framework; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO. ISO 14044:2006. Environmental Management—Life Cycle Assessment—Requirements and Guidelines; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- Varun; Bhat, I.K.; Prakash, R. LCA of renewable energy for electricity generation systems—A review. Renew. Sustain. Energy Rev. 2009, 13, 1067–1073. [Google Scholar] [CrossRef]
- Barros, M.V.; Salvador, R.; Piekarski, C.M.; de Francisco, A.C.; Freire, F.M.C.S. Life cycle assessment of electricity generation: A review of the characteristics of existing literature. Int. J. Life Cycle Assess. 2020, 25, 36–54. [Google Scholar] [CrossRef]
- Ramirez, A.D.; Boero, A.; Rivela, B.; Melendres, A.M.; Espinoza, S.; Salas, D.A. Life cycle methods to analyze the environmental sustainability of electricity generation in Ecuador: Is decarbonization the right path? Renew. Sustain. Energy Rev. 2020, 134, 11. [Google Scholar] [CrossRef]
- Proton Ventures B.V. Small Scale Ammonia Plant Design and Applications; Hands, R., Connock, L., Eds.; Nitrogen+Syngas: London, UK, 2018; pp. 1–10. [Google Scholar]
- European Commission. Reference Document on Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals -Ammonia, Acids and Fertilisers. The Best Available Tech. Ref. Doc. 2007, I, 513–527. [Google Scholar]
- Chisalita, D.; Petrescu, L.; Cormos, C. Environmental evaluation of european ammonia production considering various hydrogen supply chains. Renew. Sustain. Energy Rev. 2020, 130, 109964. [Google Scholar] [CrossRef]
- Al-Breiki, M.; Bicer, Y. Comparative life cycle assessment of sustainable energy carriers including production, storage, overseas transport and utilization. J. Clean. Prod. 2021, 279, 123481. [Google Scholar] [CrossRef]
- Al-Breiki, M.; Bicer, Y. Investigating the technical feasibility of various energy carriers for alternative and sustainable overseas energy transport scenarios. Energy Convers. Manag. 2020, 209, 112652. [Google Scholar] [CrossRef]
- Ecoinvent Ecoinvent 3.7.1 Database 2020. Available online: https://ecoinvent.org/the-ecoinvent-database/data-releases/ecoinvent-3-7-1/ (accessed on 30 January 2021).
- Bird, F.; Clarke, A.; Davies, P.; Surkovic, E. Ammonia: Zero-Carbon Fertiliser, Fuel and Energy Store. Policy Briefing; The Royal Society: London, UK, 2020; ISBN 9781782524489. [Google Scholar]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Ammonia Production Technologies. In Techno-Economic Challenges of Green Ammonia as an Energy Vector; Valera-Medina, A., Bañares-Alcántara, R., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 41–83. [Google Scholar] [CrossRef]
- Fúnez Guerra, C.; Reyes-Bozo, L.; Vyhmeister, E.; Jaén Caparrós, M.; Salazar, J.L.; Clemente-Jul, C. Technical-economic analysis for a green ammonia production plant in Chile and its subsequent transport to Japan. Renew. Energy 2020, 157, 404–414. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R.; Nayak-Luke, R. Optimization of green ammonia distribution systems for intercontinental energy transport. iScience 2021, 24, 102903. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- Reuters Australia Chases Green Ammonia for Power in Near-Term Hydrogen Push 2021. Available online: https://www.reuters.com/business/energy/australia-chases-green-ammonia-power-near-term-hydrogen-push-2021-05-13/ (accessed on 5 September 2021).
- Arora, P.; Hoadley, A.F.A.; Mahajani, S.M.; Ganesh, A. Multi-objective optimization of biomass based ammonia production-Potential and perspective in different countries. J. Clean. Prod. 2017, 148, 363–374. [Google Scholar] [CrossRef]
- Boulakhbar, M.; Lebrouhi, B.; Kousksou, T.; Smouh, S.; Jamil, A.; Maaroufi, M.; Zazi, M. Towards a large-scale integration of renewable energies in Morocco. J. Energy Storage 2020, 32, 101806. [Google Scholar] [CrossRef]
- Atchison, J. 183,000 Tonnes per Year Green Ammonia in Morocco. Available online: https://www.ammoniaenergy.org/articles/183000-tonnes-per-year-green-ammonia-in-morocco/ (accessed on 5 September 2021).
- Armijo, J.; Philibert, C. Flexible production of green hydrogen and ammonia from variable solar and wind energy: Case study of Chile and Argentina. Int. J. Hydrogen Energy 2020, 45, 1541–1558. [Google Scholar] [CrossRef]
- Heynes, G. Green Hydrogen and Ammonia Mega Project Welcomed by Chilean Ministry of Energy. Available online: https://www.h2-view.com/story/green-hydrogen-and-ammonia-mega-project-welcomed-by-chilean-ministry-of-energy/?origin=mobileSearch (accessed on 5 September 2021).
- Reuters UAE’s only Nuclear Power Plant Begins Operations at 2nd Unit 2021. Available online: https://www.reuters.com/world/middle-east/uaes-only-nuclear-power-plant-begins-operations-2nd-unit-2021-08-27/ (accessed on 5 September 2021).
- Atchison, J. The Ammonia Wrap: Two New Large-Scale Ammonia Projects in the UAE and More-Ammonia Energy Association. Available online: https://www.ammoniaenergy.org/articles/the-ammonia-wrap-two-new-large-scale-ammonia-projects-in-the-uae-and-more/ (accessed on 5 September 2021).
- Osman, O.; Sgouridis, S. Optimizing the production of ammonia as an energy carrier in the UAE. In Proceedings of the 2018 5th International Conference on Renewable Energy: Generation and Applications (ICREGA), Al Ain, United Arab Emirates, 25–28 February 2018; pp. 277–280. [Google Scholar] [CrossRef]
- National Institute for Public Health and the Environment. ReCiPe 2016 v1.1; RIVM Report 2016-0104; National Institute for Public Health and the Environment: Bilthoven, The Netherlands, 2017; pp. 1–201.
- GreenDelta OpenLCA v1.10.3 2019. Available online: https://www.openlca.org/openlca/ (accessed on 30 January 2021).
- Singh, V.; Dincer, I.; Rosen, M.A. Life Cycle Assessment of Ammonia Production Methods. In Exergetic, Energetic and Environmental Dimensions; Elsevier: Amsterdam, The Netherlands, 2018; pp. 935–959. ISBN 9780128137352. [Google Scholar]
- Arora, P.; Sharma, I.; Hoadley, A.; Mahajani, S.; Ganesh, A. Remote, small-scale, ‘greener’ routes of ammonia production. J. Clean. Prod. 2018, 199, 177–192. [Google Scholar] [CrossRef]
- Ghavam, S.; Vahdati, M.; Wilson, I.A.G.; Styring, P. Sustainable Ammonia Production Processes. Front. Energy Res. 2021, 9, 1–19. [Google Scholar] [CrossRef]
- Tock, L.; Maréchal, F.; Perrenoud, M. Thermo-environomic evaluation of the ammonia production. Can. J. Chem. Eng. 2015, 93, 356–362. [Google Scholar] [CrossRef]
- Dufour, J.; Serrano, D.P.; Gálvez, J.L.; Moreno, J.; García, C. Life cycle assessment of processes for hydrogen production. Environmental feasibility and reduction of greenhouse gases emissions. Int. J. Hydrogen Energy 2009, 34, 1370–1376. [Google Scholar] [CrossRef]
- Atilhan, S.; Park, S.; El-Halwagi, M.M.; Atilhan, M.; Moore, M.; Nielsen, R.B. Green hydrogen as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100668. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Bicer, Y.; Dincer, I. Life cycle assessment of nuclear-based hydrogen and ammonia production options: A comparative evaluation. Int. J. Hydrogen Energy 2017, 42, 21559–21570. [Google Scholar] [CrossRef]
- Karaca, A.E.; Dincer, I.; Gu, J. A Comparative Life Cycle Assessment on Nuclear-Based Clean Ammonia Synthesis Methods. J. Energy Resour. Technol. 2020, 142, 102106. [Google Scholar] [CrossRef]
- WHO-World Health Organization Ionizing Radiation, Health Effects and Protective Measures. Available online: https://www.who.int/news-room/fact-sheets/detail/ionizing-radiation-health-effects-and-protective-measures (accessed on 12 September 2021).
- Bilgili, L. Comparative assessment of alternative marine fuels in life cycle perspective. Renew. Sustain. Energy Rev. 2021, 144, 110985. [Google Scholar] [CrossRef]
- Siddiqui, O.; Dincer, I. A comparative life cycle assessment of clean aviation fuels. Energy 2021, 234, 121126. [Google Scholar] [CrossRef]
- Al-Aboosi, F.Y.; El-Halwagi, M.M.; Moore, M.; Nielsen, R.B. Renewable ammonia as an alternative fuel for the shipping industry. Curr. Opin. Chem. Eng. 2021, 31, 100670. [Google Scholar] [CrossRef]
- Ayaz, S.K.; Altuntas, O.; Caliskan, H. Thermoecologic Assessment and Life Cycle–Based Environmental Pollution Cost Analysis of Microgas Turbine. J. Environ. Eng. 2020, 146, 04019096. [Google Scholar] [CrossRef]
- Hammond, G.P.; O’Grady, Á. The life cycle greenhouse gas implications of a UK gas supply transformation on a future low carbon electricity sector. Energy 2017, 118, 937–949. [Google Scholar] [CrossRef] [Green Version]
- Bouman, E.A.; Ramirez, A.; Hertwich, E.G. Multiregional environmental comparison of fossil fuel power generation—Assessment of the contribution of fugitive emissions from conventional and unconventional fossil resources. Int. J. Greenh. Gas. Control 2015, 33, 1–9. [Google Scholar] [CrossRef]

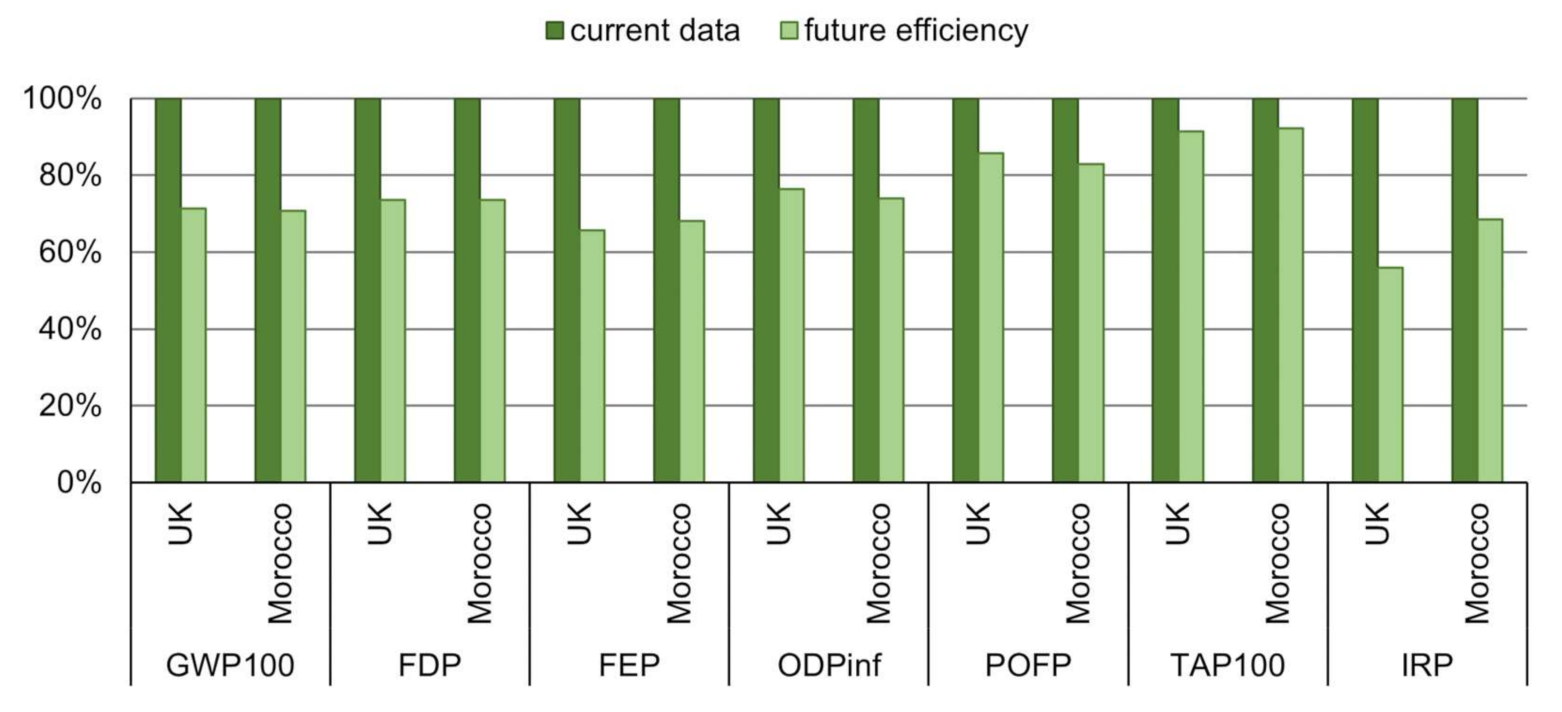
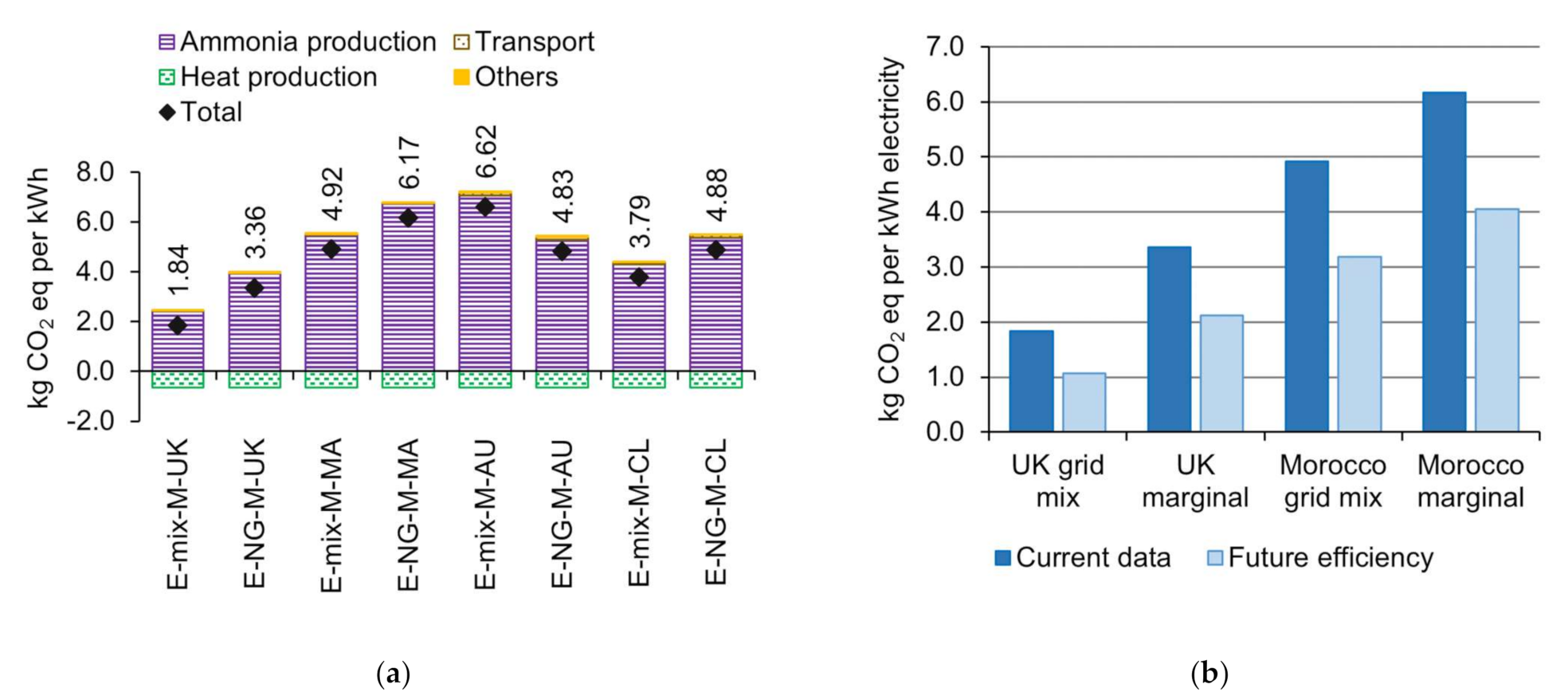
| Reference | System | Objective/Goal | System Boundaries | Functional Unit | Impact Categories |
|---|---|---|---|---|---|
| Bicer and Dincer [12] | Ammonia utilization in transportation and power generation. | To study the environmental impact of ammonia as fuel in passenger cars in cities and generation in a gas turbine power plant. | Manufacturing, operation, maintenance, and disposal of the vehicle. Construction of power plant, ammonia production (wind-based), transportation of ammonia, and power plant operation. | 1 traveled km and 1 MJ energy generated. | Abiotic depletion potential, acidification potential, global warming potential, ozone layer depletion potential. |
| Cox and Treyer [15] | A cracked ammonia fueled alkaline fuel cell. | To analyze the environmental and human health impacts of power production, for use at off-grid BTS, with a cracked ammonia-fueled AFC system and to compare with diesel generators. | Raw materials, fabrication, transport, use phase, fuel production, refurbishment, recycling, and final disposal. | 1 kWh of net electricity | Climate change, terrestrial acidification, human toxicity, particulate matter formation, metal depletion, non-renewable primary energy demand. |
| Razon and Valera-Medina [16] | Ammonia blends with methane for heat generation. | To compare the environmental impact of 14 scenarios for producing and combusting methane and ammonia to produce heat. | Ammonia production (7 different sources) and methane production (2 different sources), heat production. Transport was not included. | 1 MJ of heat. | Global warming potential, aquatic ecotoxicity, aquatic eutrophication, human toxicity, ionizing radiation, land occupation, mineral extraction, non-renewable energy, respiratory organics, ozone layer depletion, terrestrial acidification, terrestrial ecotoxicity, aquatic acidification. |
| Xue et al. [17] | Ammonia for power generation in fuel cells. | To determine the carbon footprint and nitrogen footprint when ammonia is used as an energy carrier. | Resources extraction, ammonia production, ammonia transport, fuel cell operation. | Carbon footprint (climate change) and nitrogen footprint. |
| Scenario Code | Description |
|---|---|
| SMR-S-UK | Methane to ammonia via steam methane reforming process, small capacity (20,000 t of NH3 per annum) in the UK. |
| SMR-M-UK | Methane to ammonia via steam methane reforming process, mid-capacity (100,000 t of NH3 per annum) in the UK. |
| SMR-CCS-M-UK | Methane to ammonia via steam methane reforming process, with carbon capture and storage, mid-capacity (100,000 t of NH3 per annum) in the UK. |
| E-W-S-UK | Power to ammonia via electrolysis process, electricity from wind, small capacity (20,000 t of NH3 per annum) in the UK. |
| E-W-M-UK | Power to ammonia via electrolysis process, electricity from wind, mid-capacity (100,000 t of NH3 per annum) in the UK. |
| E-W-M-MA | Power to ammonia via electrolysis process, electricity from wind, mid-capacity (100,000 t of NH3 per annum) in Morocco. |
| E-W-M-AU | Power to ammonia via electrolysis process, electricity from wind, mid-capacity (100,000 t of NH3 per annum) in Australia. |
| E-PV-M-CL | Power to ammonia via electrolysis process, electricity from photovoltaics, mid-capacity (100,000 t of NH3 per annum) in Chile. |
| E-H-M-BR | Power to ammonia via electrolysis process, electricity from hydropower, mid-capacity (100,000 t of NH3 per annum) in Brazil. |
| E-GT-M-IS | Power to ammonia via electrolysis process, electricity from geothermal, mid-capacity (100,000 t of NH3 per annum) in Iceland. |
| E-N-M-AE | Power to ammonia via electrolysis process, electricity from nuclear, mid-capacity (100,000 t of NH3 per annum) in the United Arab Emirates. |
| Impact Category | Indicator | Characterisation Factor | Unit |
|---|---|---|---|
| Climate change | Infra-red radiative forcing increase | Global warming potential—GWP100 | kg CO2-eq |
| Fossil resource scarcity | Upper heating value | Fossil depletion potential—FDP | kg oil-eq |
| Freshwater eutrophication | Phosphorus increase in freshwater | Freshwater eutrophication potential—FEP | kg P-eq |
| Ozone depletion | Stratospheric ozone decrease | Ozone depletion potential—ODPinf | kg CFC-11-eq |
| Photochemical oxidant formation | Tropospheric ozone increase | Photochemical oxidant formation potential—POFP | kg NMVOC-eq |
| Terrestrial acidification | Proton increase in natural soils | Terrestrial acidification potential—TAP100 | kg SO2-eq |
| Ionising radiation | Absorbed dose increase | Ionizing radiation potential—IRP | kg U235-eq |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boero, A.J.; Kardux, K.; Kovaleva, M.; Salas, D.A.; Mooijer, J.; Mashruk, S.; Townsend, M.; Rouwenhorst, K.; Valera-Medina, A.; Ramirez, A.D. Environmental Life Cycle Assessment of Ammonia-Based Electricity. Energies 2021, 14, 6721. https://doi.org/10.3390/en14206721
Boero AJ, Kardux K, Kovaleva M, Salas DA, Mooijer J, Mashruk S, Townsend M, Rouwenhorst K, Valera-Medina A, Ramirez AD. Environmental Life Cycle Assessment of Ammonia-Based Electricity. Energies. 2021; 14(20):6721. https://doi.org/10.3390/en14206721
Chicago/Turabian StyleBoero, Andrea J., Kevin Kardux, Marina Kovaleva, Daniel A. Salas, Jacco Mooijer, Syed Mashruk, Michael Townsend, Kevin Rouwenhorst, Agustin Valera-Medina, and Angel D. Ramirez. 2021. "Environmental Life Cycle Assessment of Ammonia-Based Electricity" Energies 14, no. 20: 6721. https://doi.org/10.3390/en14206721
APA StyleBoero, A. J., Kardux, K., Kovaleva, M., Salas, D. A., Mooijer, J., Mashruk, S., Townsend, M., Rouwenhorst, K., Valera-Medina, A., & Ramirez, A. D. (2021). Environmental Life Cycle Assessment of Ammonia-Based Electricity. Energies, 14(20), 6721. https://doi.org/10.3390/en14206721









