An N-type Naphthalene Diimide Ionene Polymer as Cathode Interlayer for Organic Solar Cells
Abstract
1. Introduction
2. Materials and Methods
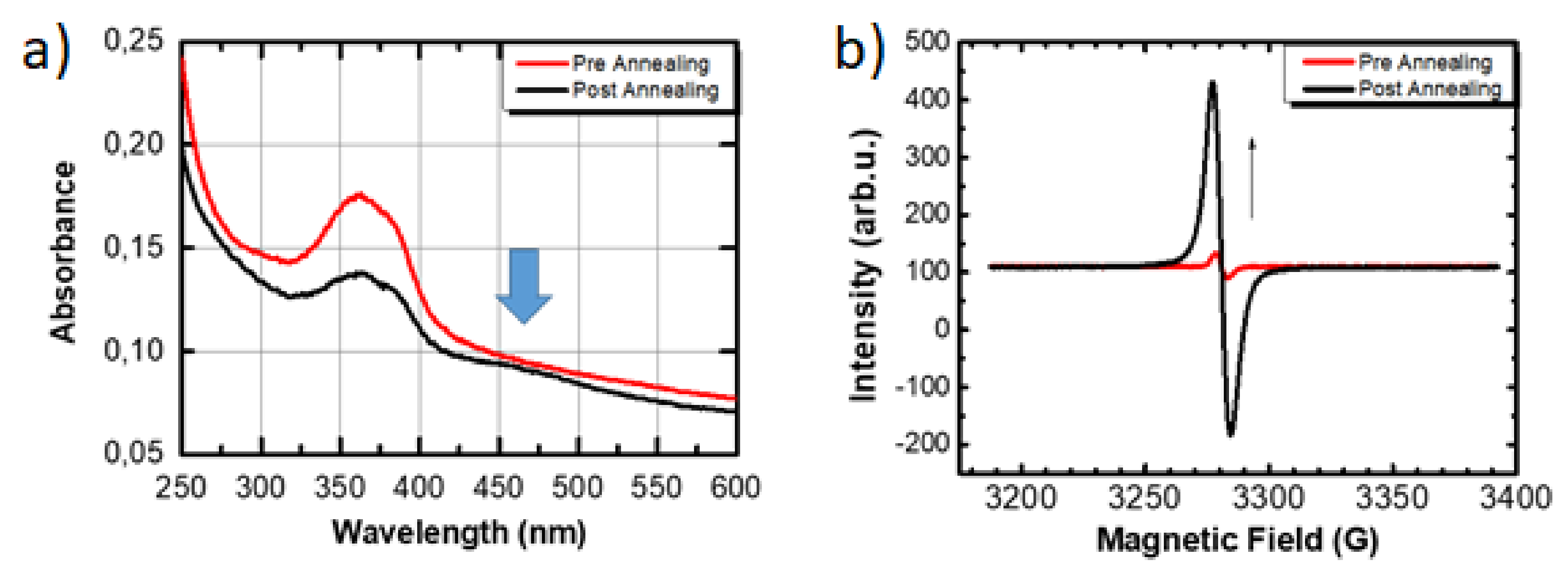
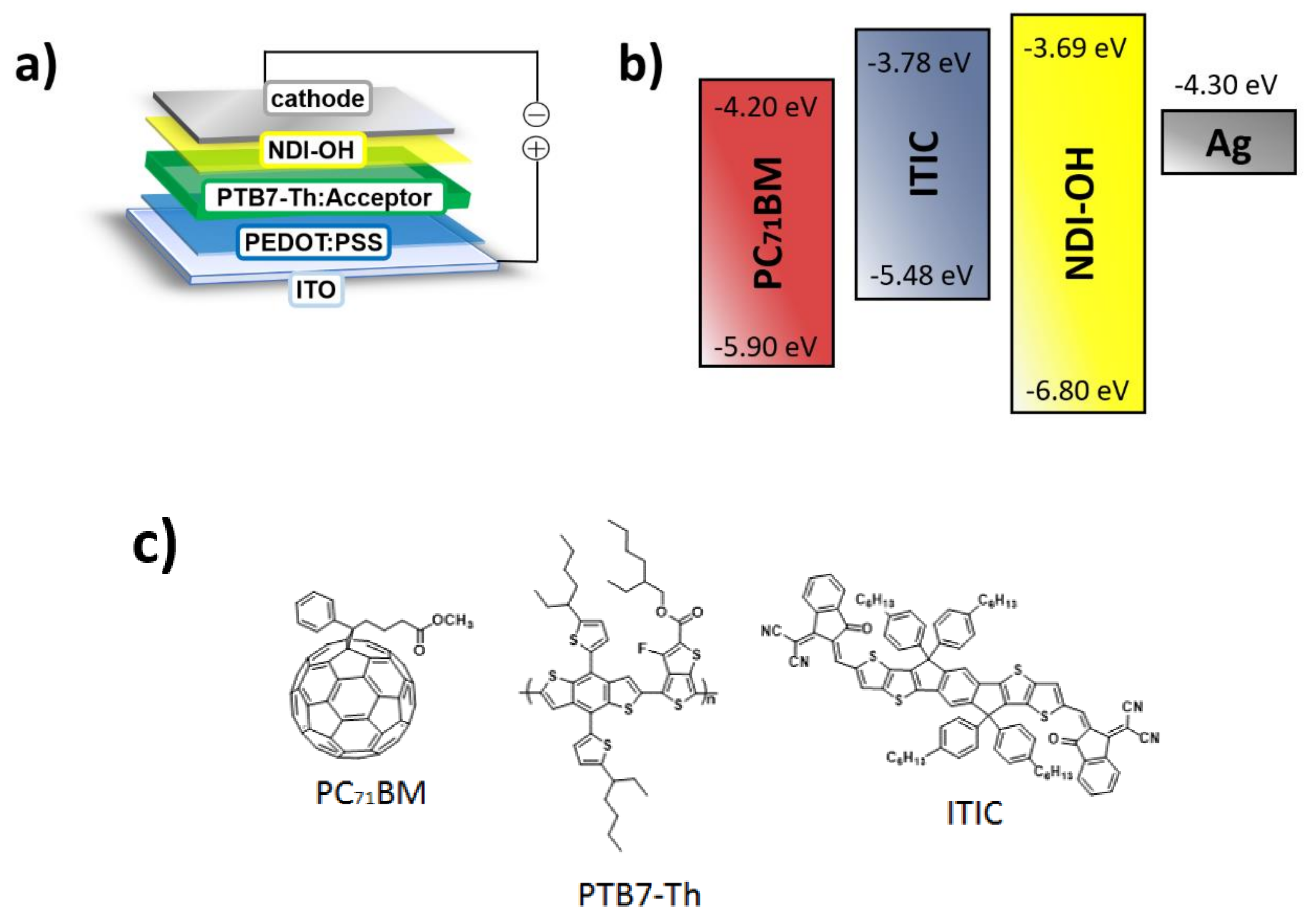
3. Results and Discussion
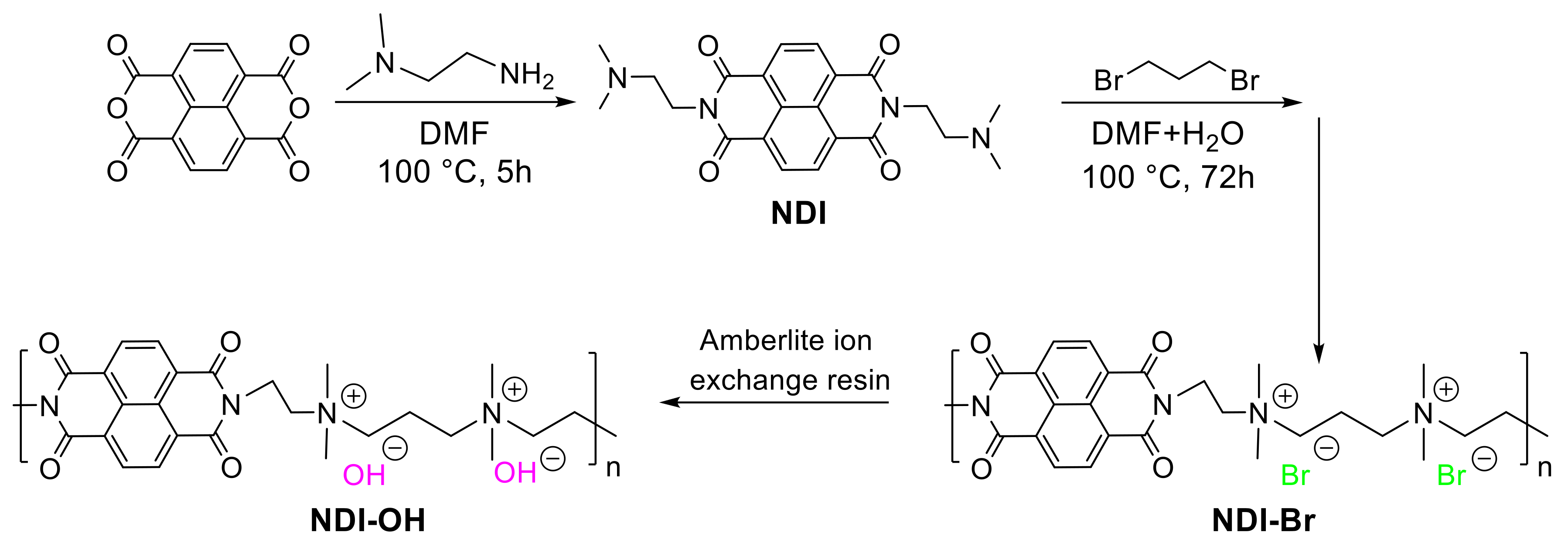
Material Synthesis and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer photovoltaic cells: Enhanced efficiencies via a network of internal donor-acceptor heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Ye, L.; Xiong, Y.; Zhang, Q.; Li, S.; Wang, C.; Jiang, Z.; Hou, J.; You, W.; Ade, H. Surpassing 10% efficiency benchmark for nonfullerene organic solar cells by scalable coating in air from single nonhalogenated solvent. Adv. Mater. 2018, 30, 1705485. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Fan, X.; Xu, B.; Yan, F.; Cui, H.; Wei, Q.; Peng, R.; Hong, L.; Huang, J.; Ge, Z. Solar Cells: Surpassing 10% Efficiency Benchmark for Nonfullerene Organic Solar Cells by Scalable Coating in Air from Single Nonhalogenated Solvent (Adv. Mater. 8/2018). Adv. Mater. 2018, 30, 1800075. [Google Scholar] [CrossRef] [PubMed]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and lightweight organic solar cells with high flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef]
- Wadsworth, A.; Moser, M.; Marks, A.; Little, M.S.; Gasparini, N.; Brabec, C.J.; Baran, D.; McCulloch, I. Critical review of the molecular design progress in non-fullerene electron acceptors towards commercially viable organic solar cells. Chem. Soc. Rev. 2019, 48, 1596. [Google Scholar] [CrossRef]
- Tai, Q.; Yan, F. Emerging semitransparent solar cells: Materials and device design. Adv. Mater. 2017, 29, 1700192. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef]
- Liao, C.Y.; Chen, Y.; Lee, C.C.; Wang, G.; Teng, N.W.; Lee, C.H.; Li, W.L.; Chen, Y.K.; Li, C.H.; Ho, H.L.; et al. Processing Strategies for an Organic Photovoltaic Module with over 10% Efficiency. Joule 2020, 4, 189–206. [Google Scholar] [CrossRef]
- Zhou, Y.; Kurosawa, T.; Ma, W.; Guo, Y.; Fang, L.; Vandewal, K.; Diao, Y.; Wang, C.; Yan, Q.; Reinspach, J. High performance all-polymer solar cell via polymer side-chain engineering. Adv. Mater. 2014, 26, 3767–3772. [Google Scholar] [CrossRef]
- Luo, Z.; Bin, H.; Liu, T.; Zhang, Z.G.; Yang, Y.; Zhong, C.; Qiu, B.; Li, G.; Gao, W.; Xie, D. Fine-tuning of molecular packing and energy level through methyl substitution enabling excellent small molecule acceptors for non-fullerene polymer solar cells with efficiency up to 12.54%. Adv. Mater. 2018, 30, 1706124. [Google Scholar] [CrossRef]
- Xu, X.; Bi, Z.; Ma, W.; Wang, Z.; Choy, W.C.H.; Wu, W.; Zhang, G.; Li, Y.; Peng, Q. Highly efficient ternary-blend polymer solar cells enabled by a nonfullerene acceptor and two polymer donors with a broad composition tolerance. Adv. Mater. 2017, 29, 1704271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Yu, R.; Liu, Z.; Peng, R.; Mi, D.; Hong, L.; Wei, Q.; Hou, J.; Kuang, Y.; Ge, Z. Ternary nonfullerene polymer solar cells with 12.16% efficiency by introducing one acceptor with cascading energy level and complementary absorption. Adv. Mater. 2018, 30, 1703005. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zhong, C.; Wu, H.; Yang, R.; Yang, W.; Huang, F.; Bazan, G.C.; Cao, Y.J. Origin of the enhanced open-circuit voltage in polymer solar cells via interfacial modification using conjugated polyelectrolytes. Mater. Chem. 2010, 20, 2617–2622. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Hsieh, C.H.; He, Y.; Hsu, C.S.; Li, Y. Combination of indene-C60 bis-adduct and cross-linked fullerene interlayer leading to highly efficient inverted polymer solar cells. J. Am. Chem. Soc. 2010, 132, 17381–17383. [Google Scholar] [CrossRef]
- Liao, S.H.; Jhuo, H.J.; Yeh, P.N.; Cheng, Y.S.; Li, Y.L.; Lee, Y.H.; Sharma, S.; Chen, S.A. Single junction inverted polymer solar cell reaching power conversion efficiency 10.31% by employing dual-doped zinc oxide nano-film as cathode interlayer. Sci. Rep. 2015, 4, 6813. [Google Scholar] [CrossRef]
- Jeong, M.; Jin, H.C.; Moon, D.K.; Kim, I.H. Small-Molecule Electrolyte: Simple Approach to Overcome Thickness Tolerance of Interlayer without Sacrificing the Performances of Polymer Solar Cells (Adv. Mater. Interfaces 18/2019). Adv. Mater. Interfaces 2019, 6, 1900797. [Google Scholar] [CrossRef]
- Hu, L.; Liu, Y.; Mao, L.; Xiong, S.; Sun, L.; Zhao, N.; Qin, F.; Jianga, Y.; Zhou, Y. Chemical reaction between an ITIC electron acceptor and an amine-containing interfacial layer in non-fullerene solar cells. J. Mater. Chem. A 2018, 6, 2273–2278. [Google Scholar] [CrossRef]
- Hu, L.; Xiong, S.; Wang, W.; Sun, L.; Qin, F.; Zhou, Y. Influence of Substituent Groups on Chemical Reactivity Kinetics of Nonfullerene Acceptors. J. Phys. Chem. C 2020, 124, 2307–2312. [Google Scholar] [CrossRef]
- Sorrentino, R.; Kozma, E.; Luzzati, S.; Po, R. Interlayers for non-fullerene based polymer solar cells: Distinctive features and challenges. Energy Environ. Sci. 2021. [Google Scholar] [CrossRef]
- Sun, K.; Zhang, S.; Li, P.; Xia, Y.; Zhang, X.; Du, D.; Isikgor, F.H.; Ouyang, J. Review on application of PEDOTs and PEDOT: PSS in energy conversion and storage devices. J. Mater. Sci. Mater. Electron. 2015, 26, 4438–4462. [Google Scholar] [CrossRef]
- Ratcliff, E.L.; Zacher, B.; Armstrong, N.R. Selective interlayers and contacts in organic photovoltaic cells. J. Phys. Chem. Lett. 2011, 2, 1337–1350. [Google Scholar] [CrossRef] [PubMed]
- Salim, T.; Yin, Z.; Sun, S.; Huang, X.; Zhang, H.; Lam, Y.M. Solution-processed nanocrystalline TiO2 buffer layer used for improving the performance of organic photovoltaics. Appl. Mater. Interfaces 2011, 3, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Stubhan, T.; Oh, H.; Pinna, L.; Krantz, J.; Litzov, I.; Brabec, C.J. Inverted organic solar cells using a solution processed aluminum-doped zinc oxide buffer layer. Org. Electron. 2011, 12, 1539–1543. [Google Scholar] [CrossRef]
- Seo, J.H.; Gutacker, A.; Sun, Y.; Wu, H.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A.J.; Bazan, G.C. Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J. Am. Chem. Soc. 2011, 133, 8416–8419. [Google Scholar] [CrossRef] [PubMed]
- Mai, C.K.; Schlitz, R.A.; Su, G.M.; Spitzer, D.; Wang, X.; Fronk, S.L.; Cahill, D.G.; Chabinyc, M.L.; Bazan, G.C. Side-chain effects on the conductivity, morphology, and thermoelectric properties of self-doped narrow-band-gap conjugated polyelectrolytes. J. Am. Chem. Soc. 2014, 136, 13478–13481. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous enhancement of open-circuit voltage, short-circuit current density, and fill factor in polymer solar cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef]
- Yang, T.; Wang, M.; Duan, C.; Hu, X.; Huang, L.; Peng, J.; Huang, F.; Gong, X. Inverted polymer solar cells with 8.4% efficiency by conjugated polyelectrolyte. Energy Environ. Sci. 2012, 5, 8208–8214. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A universal method to produce low–work function electrodes for organic electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, Y.; Xia, Y.; Xiong, K.; Cai, C.; Xia, L.; Hu, Z.; Zhang Huang, F.; Hou, L. One-step coating inverted polymer solar cells using a conjugated polymer as an electron extraction additive. J. Mater. Chem. A 2015, 3, 20500–20507. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, J.; Zheng, Q. Interfacial materials for organic solar cells: Recent advances and perspectives. Adv. Sci. 2016, 3, 1500362. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, S.; Li, S.; Yao, H.; Li, W.; Hou, J. A Self-Organized Poly (vinylpyrrolidone)-Based Cathode Interlayer in Inverted Fullerene-Free Organic Solar Cells. Adv. Mater. 2019, 31, 1804657. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, R.; Dong, S.; Lin, K.; Liu, J.; Huang, F.; Cao, Y. Quaternisation-polymerized N-type polyelectrolytes: Synthesis, characterisation and application in high-performance polymer solar cells. Mater. Horiz. 2017, 4, 88–97. [Google Scholar] [CrossRef]
- Wu, Z.; Sheng, C.; Dong, S.; Jiang, X.-F.; Siping, W.; Wu, H.; Yip, H.L.; Huang, F.; Cao, Y. n-Type water/alcohol-soluble naphthalene diimide-based conjugated polymers for high-performance polymer solar cells. J. Am. Chem. Soc. 2016, 138, 2004–2013. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Chen, Z.; Zhang, K.; Zheng, N.; Xie, R.; Liu, X.; Yang, X.; Huang, F.; Cao, Y. Self-Doped n-Type Water/Alcohol Soluble-Conjugated Polymers with Tailored Backbones and Polar Groups for Highly Efficient Polymer Solar Cells. Solar RRL 2017, 1, 61700055. [Google Scholar] [CrossRef]
- Kang, Q.; Ye, L.; Xu, B.; An, C.; Stuard, S.J.; Zhang, S.; Yao, H.; Ade, H.; Hou, J. A printable organic cathode interlayer enables over 13% efficiency for 1-cm2 organic solar cells. Joule 2019, 3, 227–239. [Google Scholar] [CrossRef]
- Liu, M.; Fan, P.; Hu, Q.; Russell, T.P.; Liu, Y. Aphthalene-Diimide-Based Ionenes as Universal Interlayers for Efficient Organic Solar Cells. Angew. Chem. Int. Ed. 2020, 59, 18131–18135. [Google Scholar] [CrossRef]
- William, S.R.; Long, T.E. Recent advances in the synthesis and structure–property relationships of ammonium ionenes. Prog. Polym. Sci. 2009, 34, 762–782. [Google Scholar] [CrossRef]
- Andric, G.; Boas, J.F.; Bond, A.M.; Fallon, G.D.; Ghiggino, K.P.; Hogan, C.F.; Hutchison, J.A.; Lee, M.A.; Langford, S.J.; Pilbrow, J.R.; et al. Spectroscopy of naphthalene diimides and their anion radicals. Aust. J. Chem. 2004, 57, 1011–1019. [Google Scholar] [CrossRef]
- Reilly, T.H., III; Hains, A.W.; Chen, H.Y.; Gregg, B.A. A Self-Doping, O2-Stable, n-Type Interfacial Layer for Organic Electronics. Adv. Energy Mater. 2012, 2, 455–460. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Hu, Z.; Xiao, J.; Zhao, W.; Li, H.-W.; Li, Q.-Y.; Tsang, S.-W.; Xu, Y.-X.; Zhang, K.; et al. Interface design for high-efficiency non-fullerene polymer solar cells. Energy Environ. Sci. 2017, 10, 1784–1791. [Google Scholar] [CrossRef]
- Luo, J.; Wu, H.; He, C.; Li, A.; Yang, W.; Cao, Y. Enhanced open-circuit voltage in polymer solar cells. Appl. Phys. Lett. 2009, 95, 043301. [Google Scholar]
- Bao, Q.; Liu, X.; Wang, E.; Fang, J.; Gao, E.; Braun, S.; Fahlman, M. Regular energetics at conjugated electrolyte/electrode modifier for organic electronics and their implications on design rules. Adv. Mater. Interfaces 2015, 2, 500204. [Google Scholar] [CrossRef]
- Bi, S.; Leng, X.; Li, Y.; Zheng, Z.; Zhang, X.; Zhang, Y.; Zhou, H. Interfacial modification in organic and perovskite solar cells. Adv. Mater. 2019, 31, 1805708. [Google Scholar] [CrossRef] [PubMed]
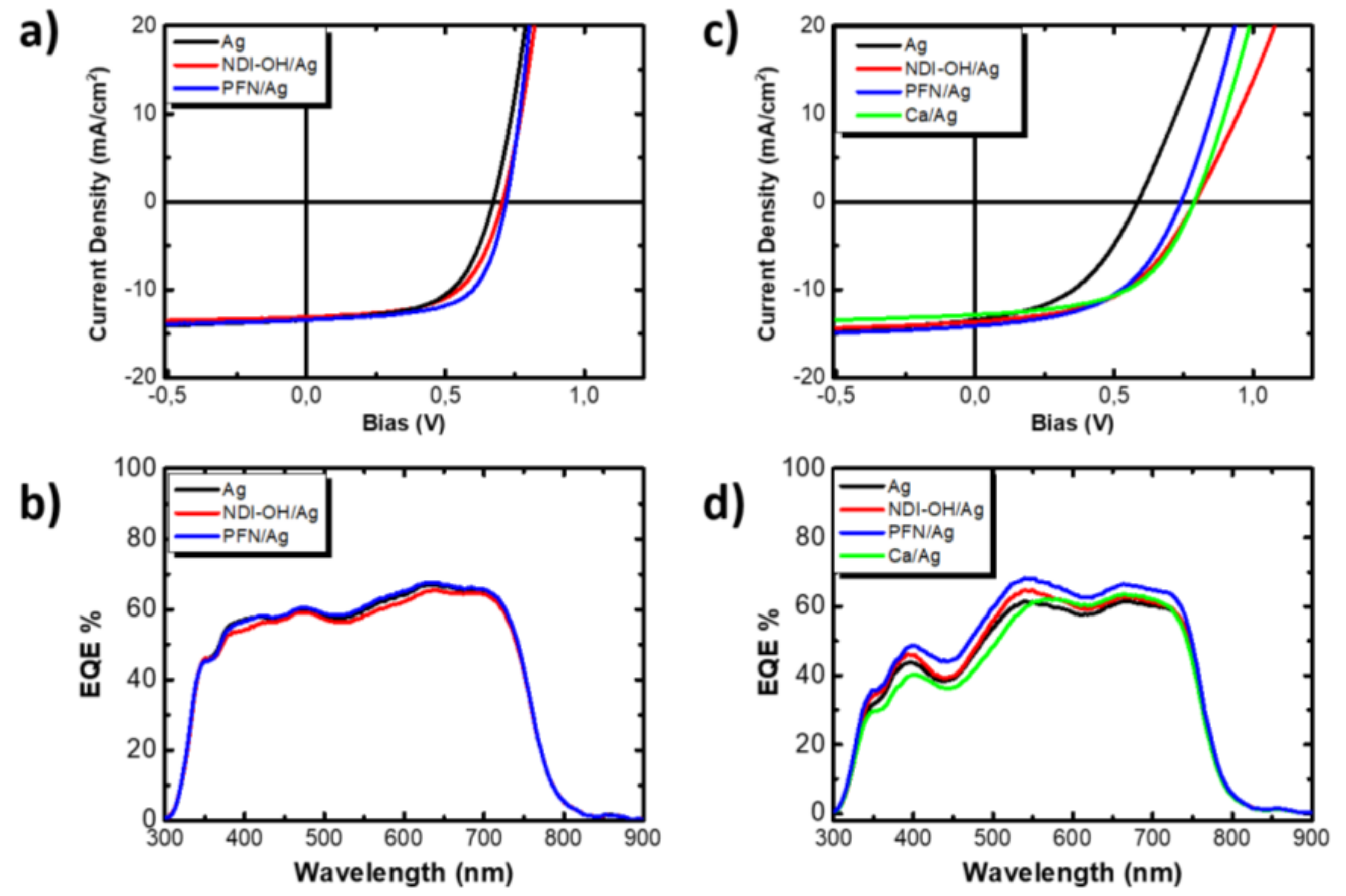

| Active Layer: PTB7-Th + PC71BM | VOC [V] | JSC [mA/cm2] | FF [%] | PCEmax/ PCEavg [%] | RS [Ω cm−2] | RSH [kΩ cm−2] |
|---|---|---|---|---|---|---|
| NDI-OH/Ag | 0.74 | 14.48 | 58 | 6.19 (5.86) | 8.76 | 832.63 |
| PFN/Ag | 0.73 | 14.16 | 64 | 6.60 (6.40) | 6.43 | 721.58 |
| Ag | 0.66 | 14.51 | 61 | 5.85 (5.72) | 8.12 | 591.12 |
| Active Layer: PTB7-Th + ITIC | VOC [V] | JSC [mA/cm2] | FF [%] | PCEmax/ PCEavg [%] | RS [Ω cm−2] | RSH [kΩ cm−2] |
|---|---|---|---|---|---|---|
| NDI-OH/Ag | 0.79 | 13.79 | 51 | 5.51 (5.06) | 15.07 | 471.91 |
| PFN/Ag | 0.73 | 14.09 | 51 | 5.29 (5.04) | 12.97 | 380.09 |
| Ag | 0.58 | 13.42 | 46 | 3.56 (3,38) | 15.64 | 221.99 |
| Ca/Ag | 0.79 | 12.84 | 54 | 5.47 (5.40) | 13.17 | 578.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorrentino, R.; Penconi, M.; Andicsová-Eckstein, A.; Scavia, G.; Švajdlenková, H.; Kozma, E.; Luzzati, S. An N-type Naphthalene Diimide Ionene Polymer as Cathode Interlayer for Organic Solar Cells. Energies 2021, 14, 454. https://doi.org/10.3390/en14020454
Sorrentino R, Penconi M, Andicsová-Eckstein A, Scavia G, Švajdlenková H, Kozma E, Luzzati S. An N-type Naphthalene Diimide Ionene Polymer as Cathode Interlayer for Organic Solar Cells. Energies. 2021; 14(2):454. https://doi.org/10.3390/en14020454
Chicago/Turabian StyleSorrentino, Roberto, Marta Penconi, Anita Andicsová-Eckstein, Guido Scavia, Helena Švajdlenková, Erika Kozma, and Silvia Luzzati. 2021. "An N-type Naphthalene Diimide Ionene Polymer as Cathode Interlayer for Organic Solar Cells" Energies 14, no. 2: 454. https://doi.org/10.3390/en14020454
APA StyleSorrentino, R., Penconi, M., Andicsová-Eckstein, A., Scavia, G., Švajdlenková, H., Kozma, E., & Luzzati, S. (2021). An N-type Naphthalene Diimide Ionene Polymer as Cathode Interlayer for Organic Solar Cells. Energies, 14(2), 454. https://doi.org/10.3390/en14020454









