Enhancement of Biodiesel Production from High-Acid-Value Waste Cooking Oil via a Microwave Reactor Using a Homogeneous Alkaline Catalyst †
Abstract
1. Introduction
2. Chemicals, Equipment and Methods
2.1. Chemicals
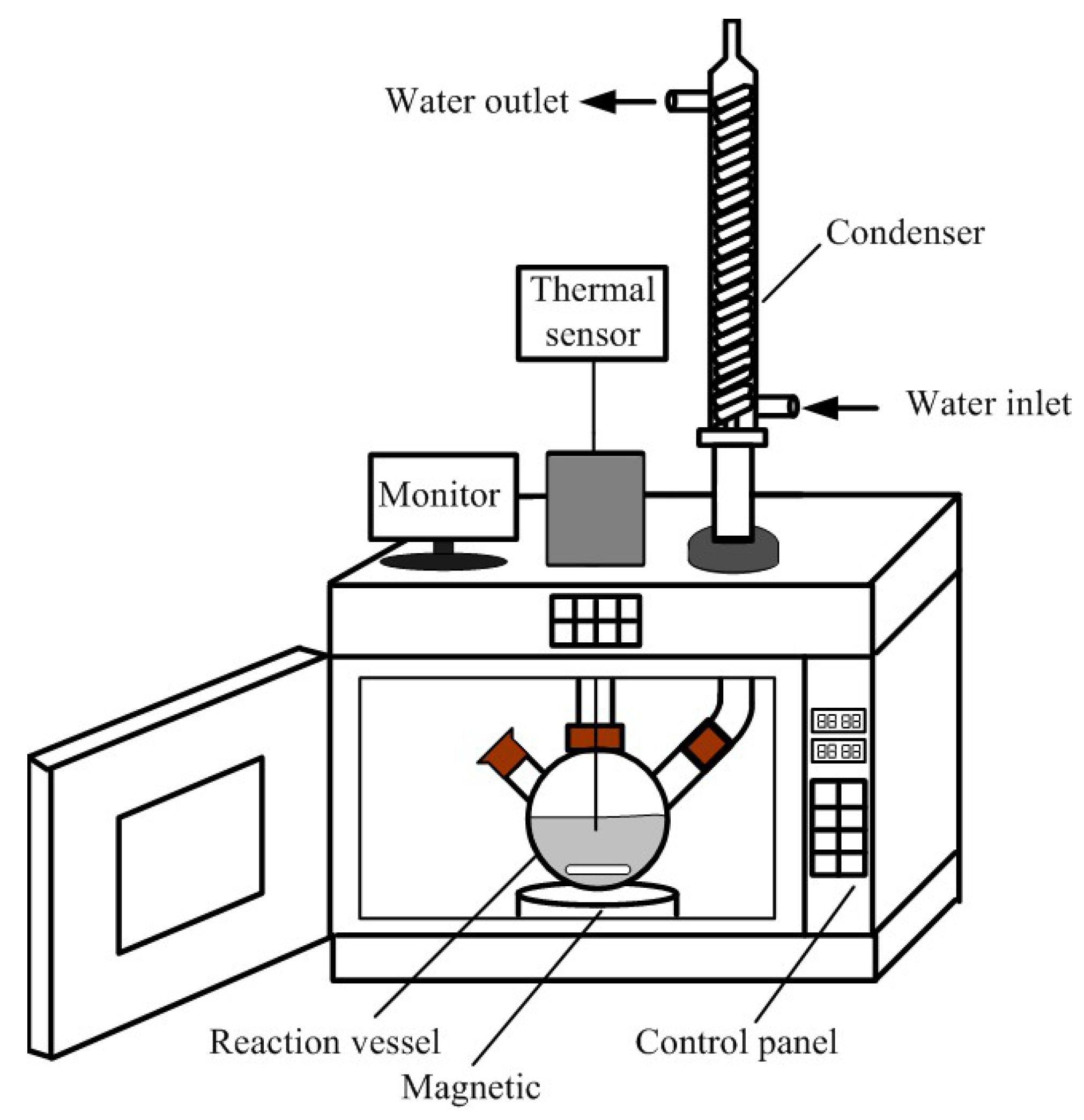
2.2. Equipment
2.3. Experimental Procedure.
2.4. Analytical Methods
3. Results and Discussion
3.1. Effects of Methanol-to-Oil Molar Ratio
3.2. Effects of Catalyst Amount
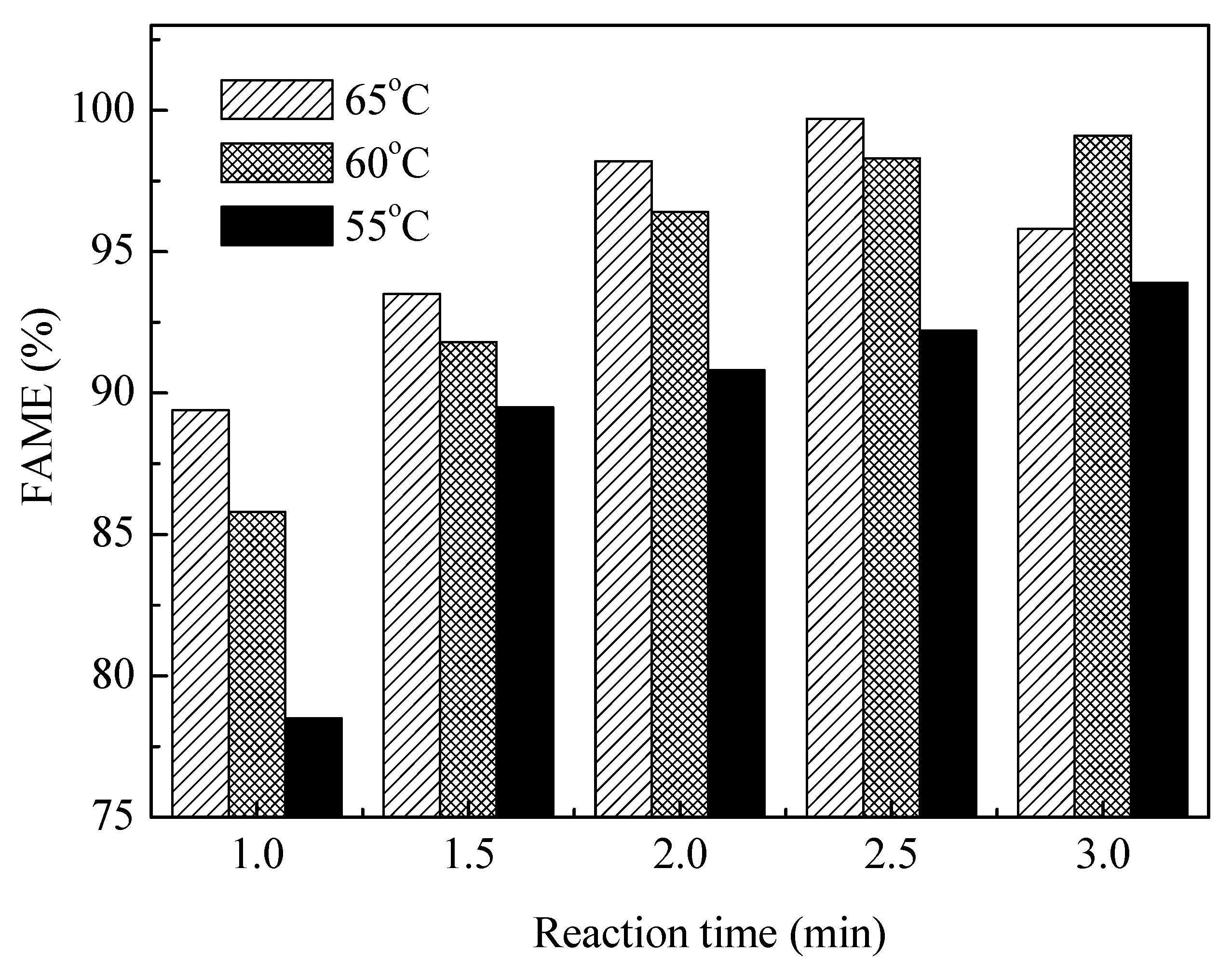
3.3. Effects of Reaction Temperature and Reaction Time
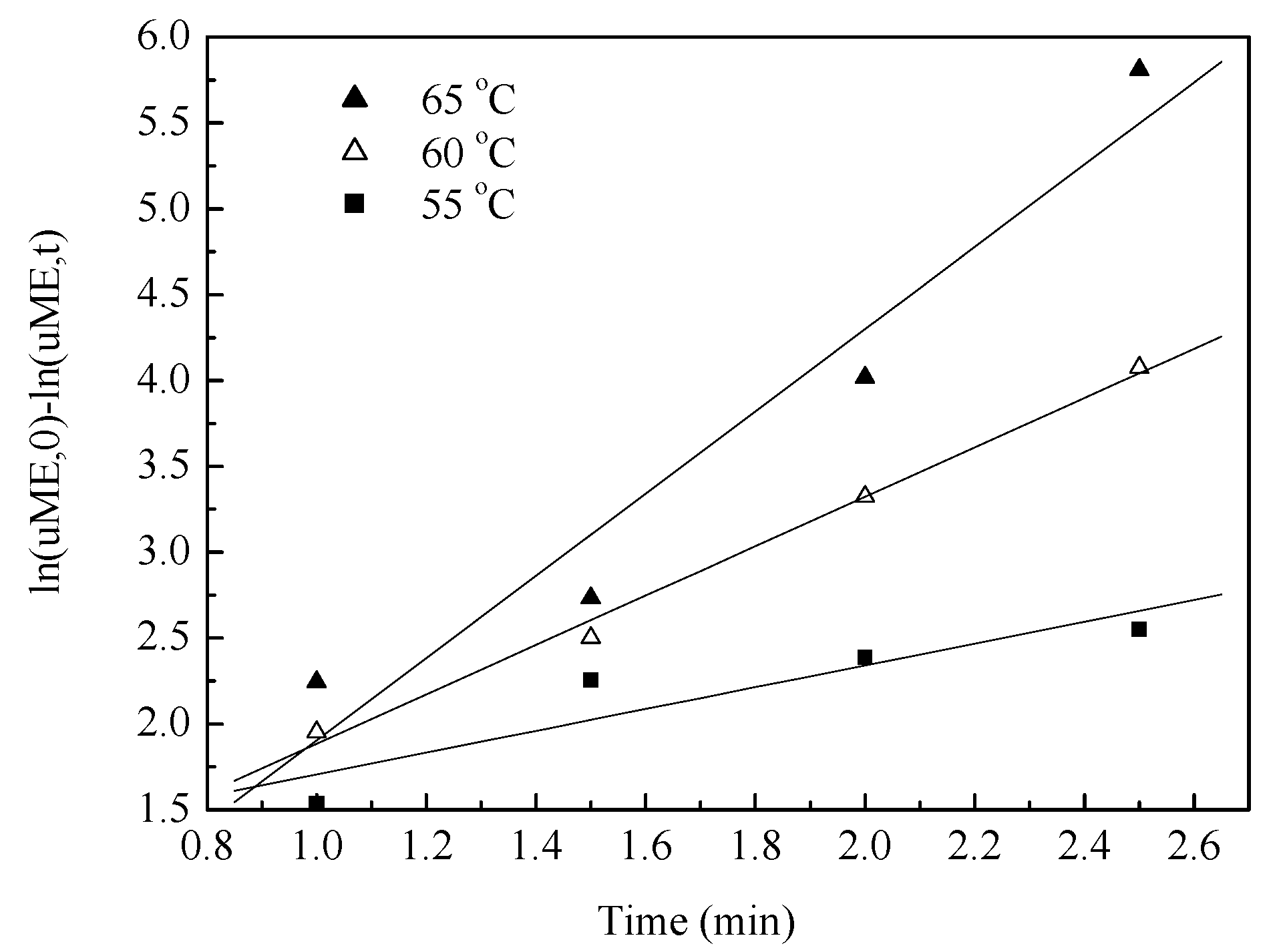
3.4. Kinetic Model
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez, D.E.; Goodwin, J.G.; Bruce, D.A. Transesterification of triacetin with methanol on solid acid and base catalysts. Appl. Catal. A Gen. 2005, 295, 97–105. [Google Scholar] [CrossRef]
- Xie, W.; Huang, X.; Li, H. Soybean oil methyl esters preparation using NaX zeolites loaded with KOH as a heterogeneous catalyst. Bioresour. Technol. 2007, 98, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.R.V.; Oshel, R.; Verkade, J.G. Room-temperature conversion of soybean oil and poultry fat to biodiesel catalyzed by nanocrystalline calcium oxides. Energy Fuels 2006, 20, 1310–1314. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Kuo, J.Y.; Hsieh, P.H.; Hou, S.S. Improving biodiesel conversions from blends of high-and low-acid-value waste cooking oils using sodium methoxide as a catalyst based on a high speed homogenizer. Energies 2018, 11, 2298. [Google Scholar] [CrossRef]
- Zhang, Y.; Dube, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Marchetti, J.M.; Miguel, V.U.; Errazu, A.F. Heterogeneous esterification of oil with high amount of free fatty acids. Fuel 2005, 86, 906–910. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Hou, S.S.; Kuo, J.Y.; Hsieh, P.H. Optimized conversion of waste cooking oil to biodiesel using calcium methoxide as catalyst under homogenizer system conditions. Energies 2018, 11, 2622. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Chang, L.W.; Hou, S.S. Study of solid calcium diglyceroxide for biodiesel production from waste cooking oil using a high speed homogenizer. Energies 2019, 12, 3205. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Kuo, J.Y.; Hsieh, S.A.; Hsieh, P.H.; Hou, S.S. Optimized conversion of waste cooking oil to biodiesel using modified calcium oxide as catalyst via a microwave heating system. Fuel 2020, 266, 117114. [Google Scholar] [CrossRef]
- Lertsathapornsuk, V.; Pairintra, R.; Aryusuk, K.; Krisnangkura, K. Microwave assisted in continuous biodiesel production from waste frying palm oil and its performance in a 100 kW diesel generator. Fuel Process. Technol. 2008, 89, 1330–1336. [Google Scholar] [CrossRef]
- Harrlngton, K.J.; D’Arey-Evans, C. Transesterification in situ of sunflower seed oil. Ind. Eng. Chem. Res. 1985, 24, 314–318. [Google Scholar] [CrossRef]
- Charoenchaitrakool, M.; Thienmethangkoon, J. Statistical optimization for biodiesel production from waste frying oil through two-step catalyzed process. Fuel Process. Technol. 2011, 92, 112–118. [Google Scholar] [CrossRef]
- Azcan, N.; Danisman, A. Alkali catalyzed transesterification of cottonseed oil by microwave irradiation. Fuel 2007, 86, 2639–2644. [Google Scholar] [CrossRef]
- Nayak, M.G.; Vyas, A.P. Optimization of microwave-assisted biodiesel production from Papaya oil using response surface methodology. Renew. Energy 2019, 138, 18–28. [Google Scholar] [CrossRef]
- Patil, P.D.; Gude, V.G.; Camacho, L.M.; Deng, S. Microwave-assisted catalytic transesterification of Camelina Sativa oil. Energy Fuels 2010, 2, 1298–1304. [Google Scholar] [CrossRef]
- Sharma, A.; Kodgire, P.; Kachhwaha, S.S.; Raghavendra, H.B.; Thakkar, K. Application of microwave energy for biodiesel production using waste cooking oil. Mater. Today 2018, 5, 23064–23075. [Google Scholar] [CrossRef]
- Hernando, J.; Leton, P.; Matia, M.P.; Novella, J.L.; Alvarez-Builla, J. Biodiesel and FAME synthesis assisted by microwaves: Homogeneous batch and flow processes. Fuel 2007, 86, 1641–1644. [Google Scholar] [CrossRef]
- Leadbeater, N.E.; Stencel, L.M. Fast, easy preparation of biodiesel using microwave heating. Energy Fuels 2006, 20, 2281–2283. [Google Scholar] [CrossRef]
- Chen, K.S.; Lin, Y.C.; Hsu, K.H.; Wang, H.K. Improving biodiesel yields from waste cooking oil by using sodium methoxide and a microwave heating system. Energy 2012, 38, 151–156. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H.; Chen, L.C. Ultrasonic mixing and closed microwave irradiation-assisted transesterification of soybean oil. Fuel 2010, 89, 3618–3622. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Lin, C.C.; Chang, Y.H. Microwave irradiation-assisted transesterification of soybean oil to biodiesel catalyzed by nanopowder calcium oxide. Fuel 2011, 90, 1963–1967. [Google Scholar] [CrossRef]
- Xie, W.; Peng, H.; Chen, L. Transesterification of soybean oil catalyzed by potassium loaded on alumina as a solid-base catalyst. Appl. Catal. A Gen. 2006, 300, 67–74. [Google Scholar] [CrossRef]
- Colucci, J.A.; Borrero, E.E.; Alape, F. Biodiesel from an alkaline transesterification reaction of soybean oil using ultrasonic mixing. J. Am. Oil Chem. Soc. 2005, 82, 525–530. [Google Scholar] [CrossRef]
- Hanh, H.D.; Dong, N.T.; Starvarache, C.; Okitsu, K.; Maeda, Y.; Nishimura, R. Methanolysis of triolein by low frequency ultrasonic irradiation. Energy Convers. Manag. 2008, 49, 276–280. [Google Scholar] [CrossRef]
- Zhang, L.; Xin, Z.; Liu, Z.; Ou, Y.; Ye, Z.; Li, Z.; Wei, G. Microwave-assisted catalytic transfer hydrogenation of biodiesel at constant microwave power. Fuel 2020, 270, 117510. [Google Scholar] [CrossRef]
- Gupta, A.R.; Rathod, V.K. Calcium diglyceroxide catalyzed biodiesel production from waste cooking oil in the presence of microwave: Optimization and kinetic studies. Renew. Energy 2018, 121, 757–767. [Google Scholar] [CrossRef]
- Lingfeng, C.; Guomin, X.; Bo, X.; Guangyuan, T. Transesterification of cottonseed oil to biodiesel by using heterogeneous solid basic catalysts. Energy Fuels 2007, 21, 3740–3743. [Google Scholar] [CrossRef]
- Tan, K.T.; Lee, K.T. Production of FAME by palm oil transesterification via supercritical methanol technology. Biomass Bioenergy 2009, 33, 1096–1099. [Google Scholar] [CrossRef]
- Koc, A.B. Ultrasonic monitoring of glycerol settling during transesterification of soybean oil. Bioresour. Technol. 2009, 100, 19–24. [Google Scholar]
- Satyanarayana, M.; Muraleedharan, C. A comparative study of vegetable oil methyl esters (biodiesels). Energy 2011, 36, 2129–2137. [Google Scholar] [CrossRef]
- Hanh, H.D.; Dong, N.T.; Okitsu, K.; Nishmura, R.; Maeda, Y. Biodiesel production through transesterification of triolein with various alcohols in an ultrasonic field. Renew. Energy 2009, 34, 766–768. [Google Scholar] [CrossRef]
- Anastopoulos, G.; Zannikou, Y.; Stournas, S.; Kalligeros, S. Transesterification of vegetable oils with ethanol and characterization of the key fuel properties of ethyl esters. Energies 2009, 2, 362–376. [Google Scholar] [CrossRef]
- Yang, F.X.; Su, Y.Q.; Li, X.H. The research on alkali-refining of Idesia polycarpa var. vestita bites. J. Northwest Univ. 2007, 35, 203–207. [Google Scholar]
- He, H.; Sun, S.; Wang, T.; Zhu, S. Transesterification kinetics of soybean oil for production of biodiesel in supercritical methanol. J. Am. Oil Chem. Soc. 2007, 84, 399–404. [Google Scholar] [CrossRef]



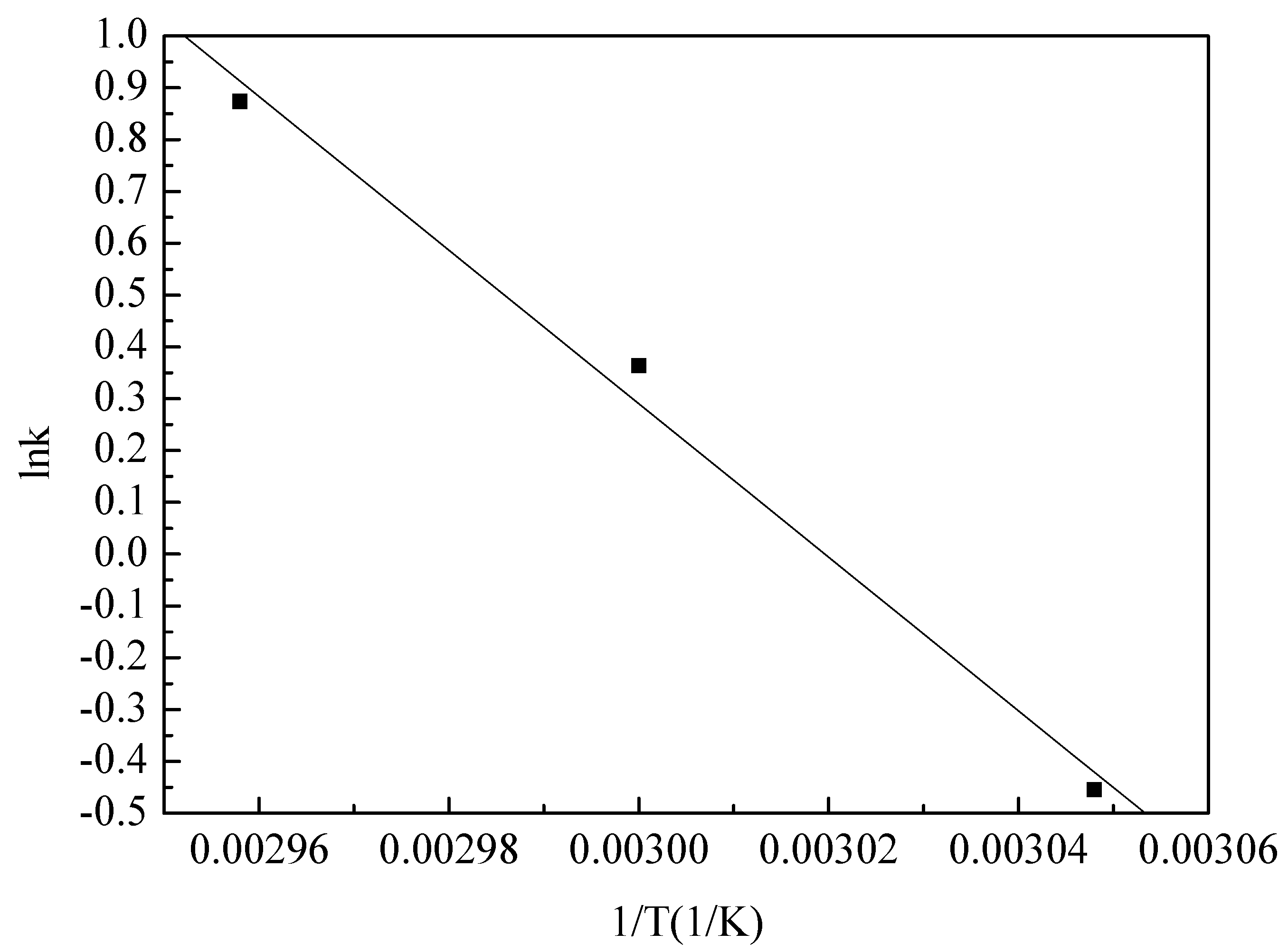
| Temperature (°C) | Reaction Rate Constant k (min−1) |
|---|---|
| 55 | 0.635 |
| 60 | 1.438 |
| 65 | 2.396 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, M.-C.; Liao, P.-H.; Lan, N.V.; Hou, S.-S. Enhancement of Biodiesel Production from High-Acid-Value Waste Cooking Oil via a Microwave Reactor Using a Homogeneous Alkaline Catalyst. Energies 2021, 14, 437. https://doi.org/10.3390/en14020437
Hsiao M-C, Liao P-H, Lan NV, Hou S-S. Enhancement of Biodiesel Production from High-Acid-Value Waste Cooking Oil via a Microwave Reactor Using a Homogeneous Alkaline Catalyst. Energies. 2021; 14(2):437. https://doi.org/10.3390/en14020437
Chicago/Turabian StyleHsiao, Ming-Chien, Peir-Horng Liao, Nguyen Vu Lan, and Shuhn-Shyurng Hou. 2021. "Enhancement of Biodiesel Production from High-Acid-Value Waste Cooking Oil via a Microwave Reactor Using a Homogeneous Alkaline Catalyst" Energies 14, no. 2: 437. https://doi.org/10.3390/en14020437
APA StyleHsiao, M.-C., Liao, P.-H., Lan, N. V., & Hou, S.-S. (2021). Enhancement of Biodiesel Production from High-Acid-Value Waste Cooking Oil via a Microwave Reactor Using a Homogeneous Alkaline Catalyst. Energies, 14(2), 437. https://doi.org/10.3390/en14020437





