Size- and Surface-Dependent Solubility of Cadmium Telluride in Aqueous Solutions
Abstract
1. Introduction
2. Experimental
2.1. Cadmium Telluride (CdTe) Sources
- Three sieved CdTe-powders with different maximal grain diameter d:
- (i)
- CdTe250 with d250 ≤ 250 µm, dmean,250 ≈ 4 to 5 µm (see below)
- (ii)
- CdTe840 with d840 ≤ 840 µm, dmean,840 ≈ 9 µm
- (iii)
- CdTe3000 with d3000 ≤ 3000 µm.
- CdTe3,m pieces from milled modules:
2.2. Samples for Leaching Experiments
- The untreated CdTe-powders from Sigma Aldrich as described in Section 2.1.
- Solar-cell-like activated CdTe-powder CdTeact as described in Section 2.3.
- Solar-cell-like etched CdTe-powder CdTeetch as described in Section 2.3.
- Solar-cell-like activated and etched CdTe-powder CdTeact+etch as described in Section 2.3.
- The milled module pieces CdTe3,m as described in Section 2.1.
2.3. Solar-Cell-Like Processing
2.3.1. Activated CdTe Powder CdTeact
2.3.2. Etched CdTe Powder CdTeetch
2.3.3. Activated and Etched CdTe Powder CdTeact+etch
2.4. Leaching Solutions and Sampling
- (a)
- pH4: 3.9 g citric acid with 4.5 g disodium hydrogen phosphate in 1000 mL deionized water.
- (b)
- pH7: 1.2 g citric acid with 9.6 g disodium hydrogen phosphate in 1000 mL deionized water.
- (c)
- pH10: 2.0 g sodium hydroxide in 1000 mL deionized water.
3. Leaching Results
3.1. Leaching of Untreated CdTe Powder with Different Grain Sizes in pH4
3.2. Leaching of Untreated CdTe Powder with Different Grain Sizes in pH7
3.3. Leaching of Untreated CdTe Powder with Different Grain Sizes in pH10
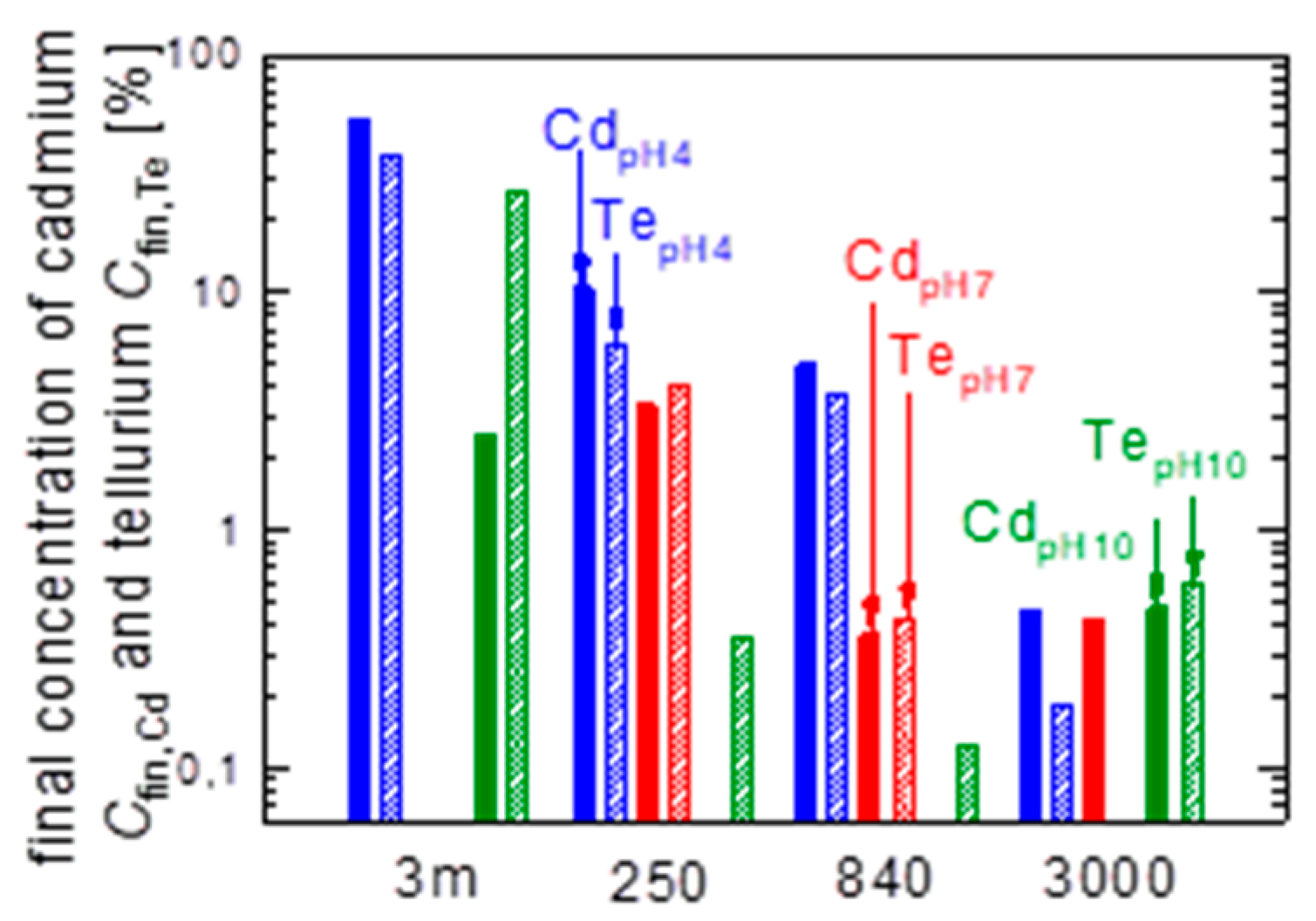
3.4. Maximum Values after t = 83 d
3.5. Comparison with Equation (2)
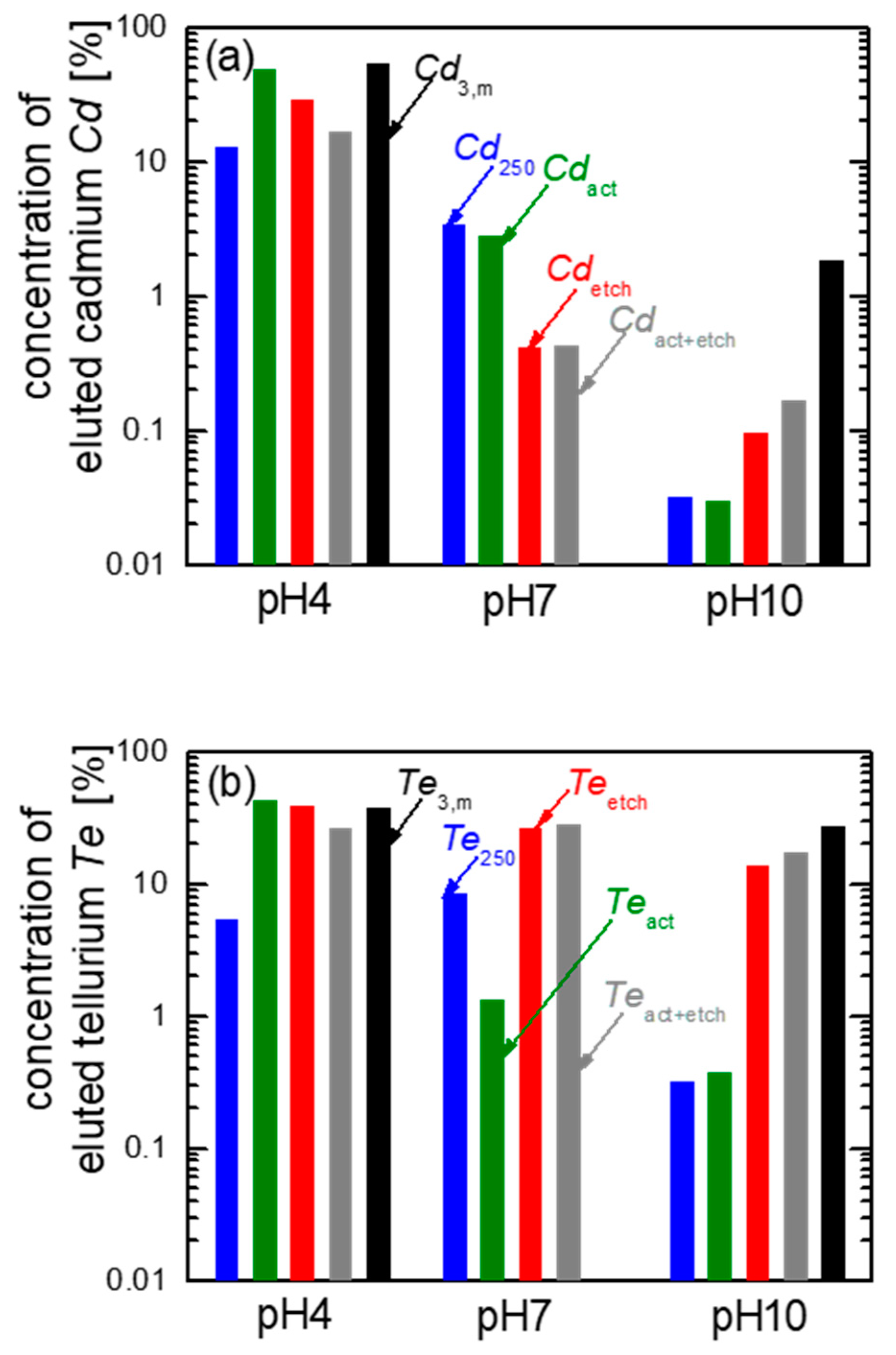
4. Leaching of ‘Solar-Cell-Like’ Processed CdTe Powder
- Powder CdTe250, untreated, initial powder (blue bars);
- Powder CdTe250, activated by CdCl2 (green bars);
- Powder CdTe250, etched in NP-etch (red bars);
- Powder CdTe250, activated and etched (grey bars);
- Milled module pieces from Ref. [6] (black bars).
- pH4: The eluted Cd of the initial powder is Cd250 ≈ 10% (data in blue). Activation (green data) strongly increases the elution to Cdact ≈ 50%, whereas the etching (red data) and the combination of activation plus etching (grey data) decelerates the elution to Cdetch ≈ 30% and Cdact+etch ≈ 16%.
- pH7: All solar cell like processing steps decrease the elution in pH7, when compared to the unprocessed powder: the data in blue are still the highest for pH7.
- pH10: Solar cell-like processing leads to higher elution in pH10 with the highest value observed for the milled module pieces (see black data Cd3,m).
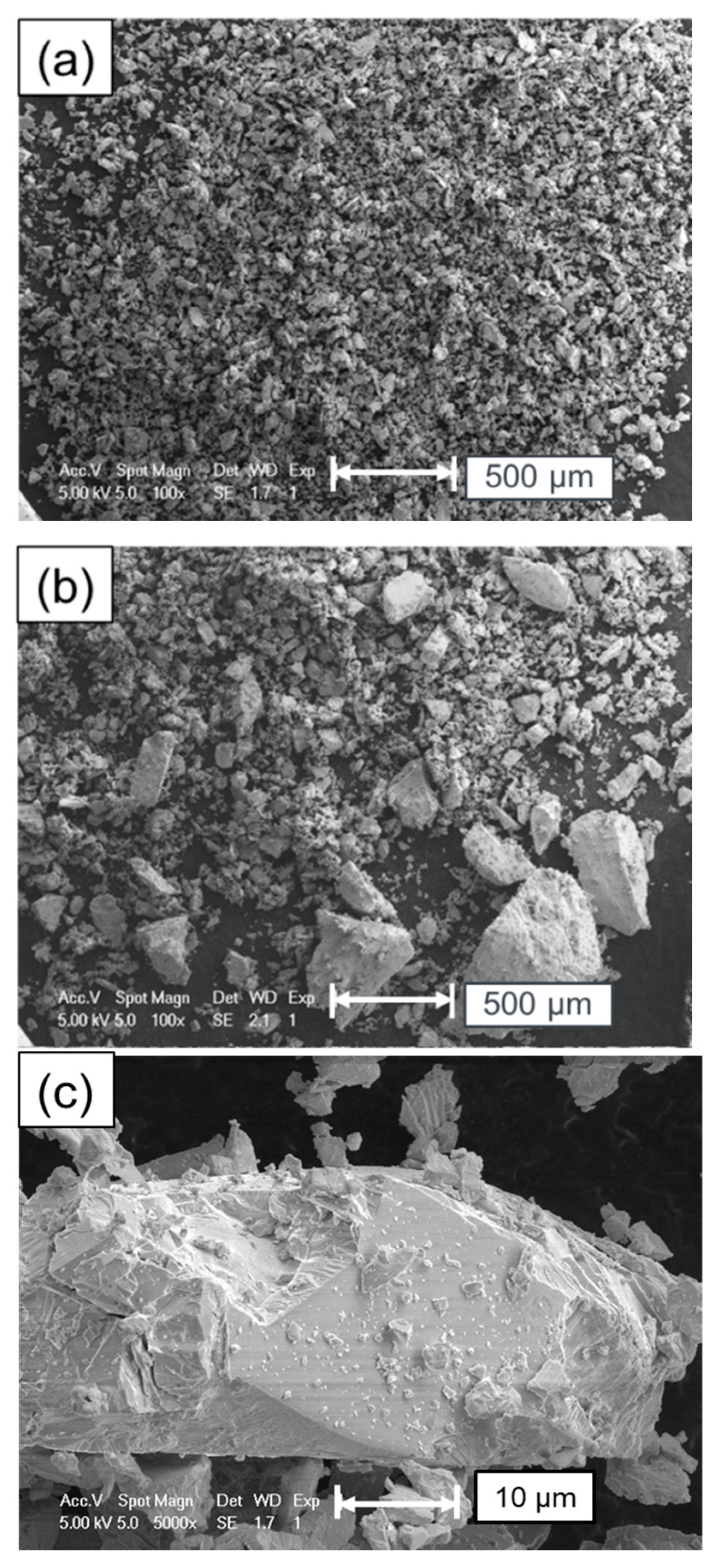
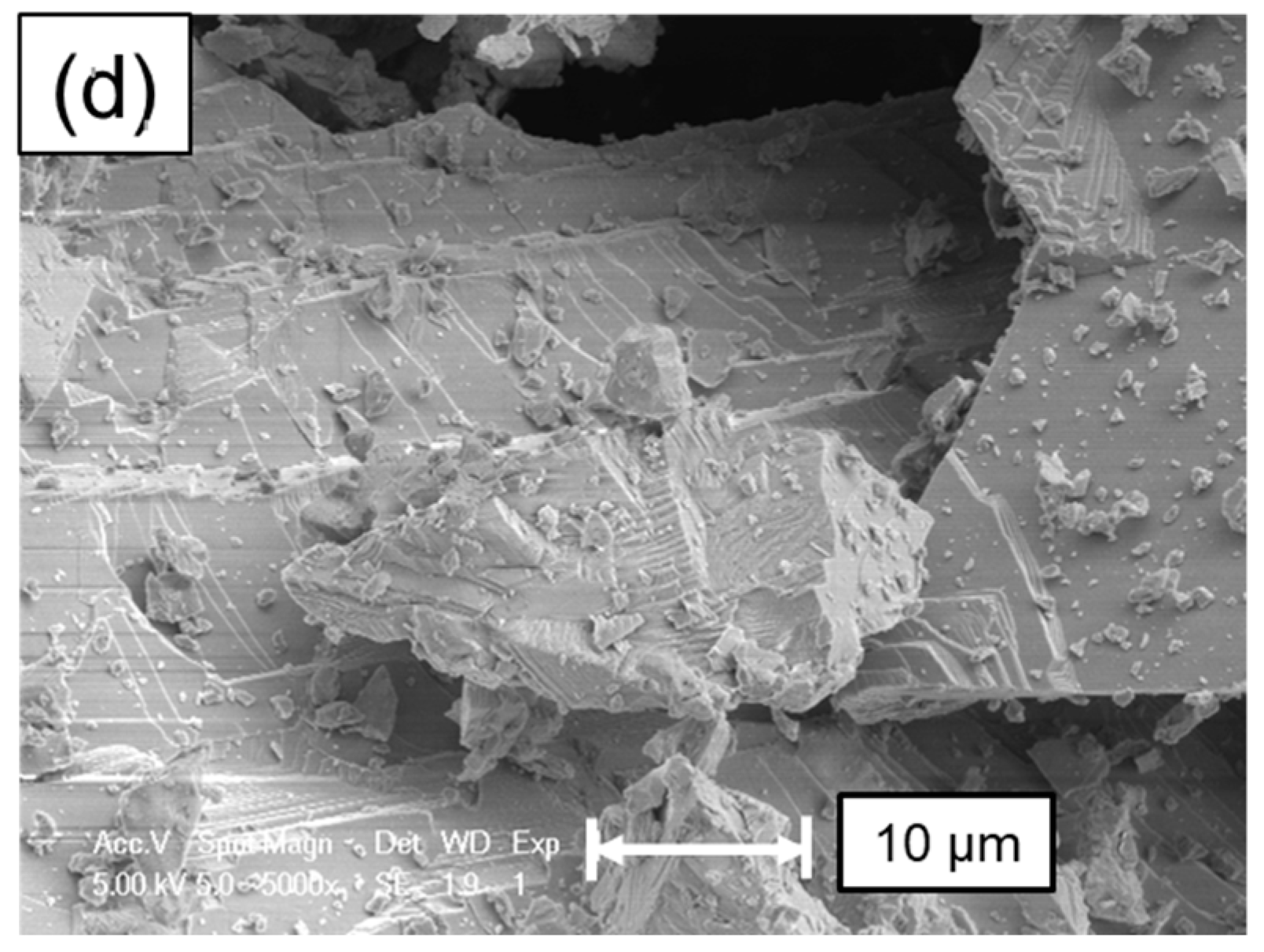
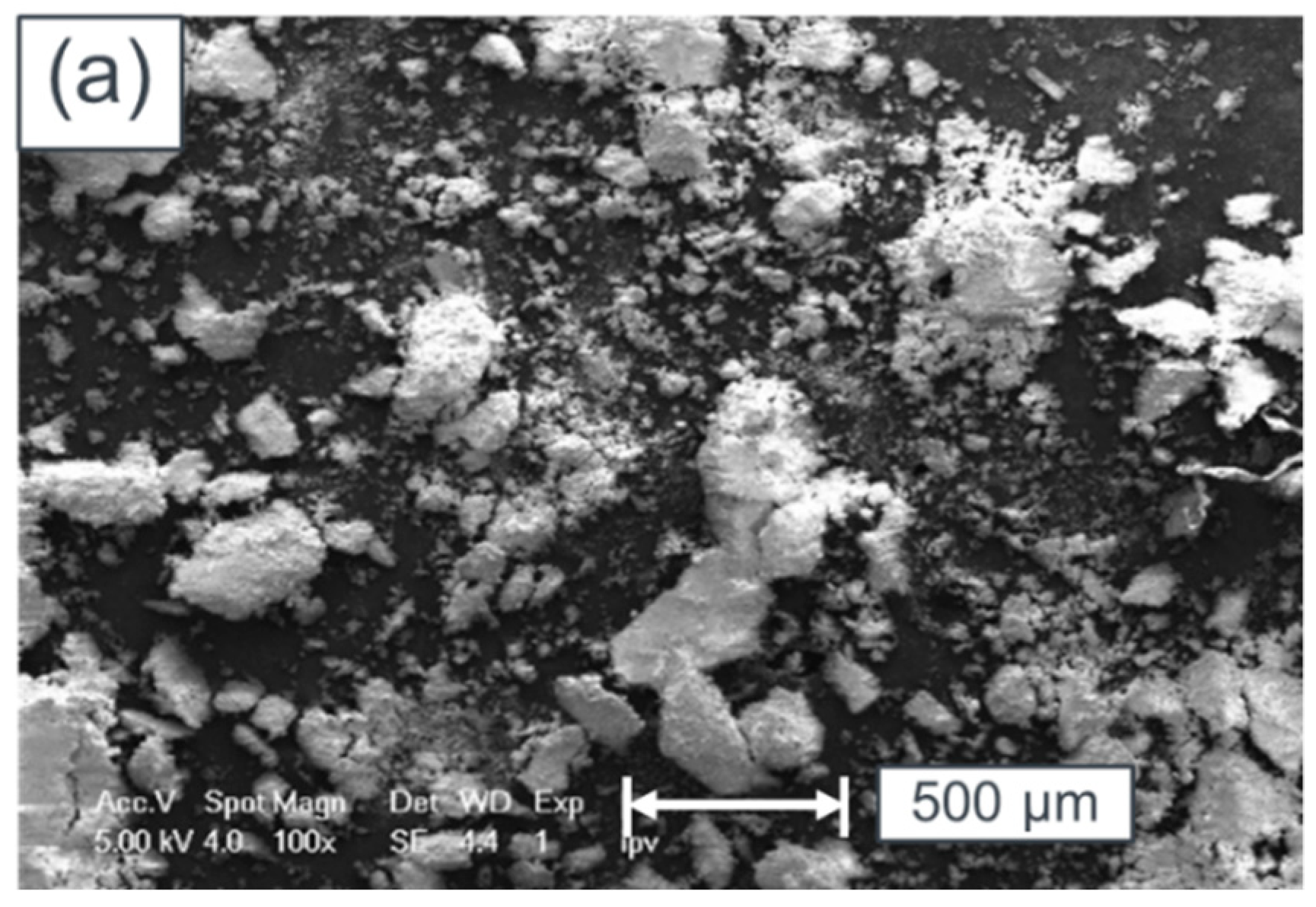
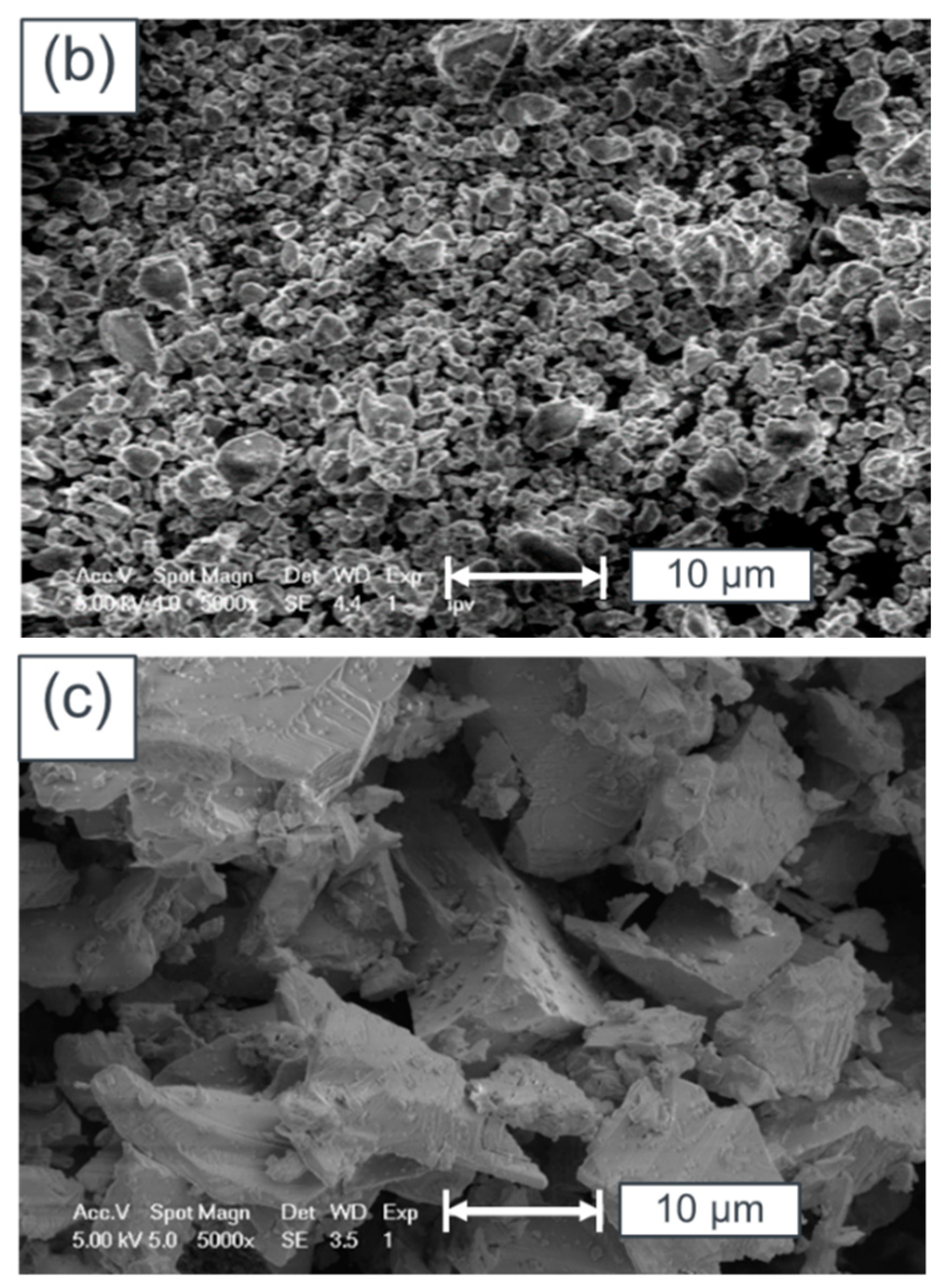
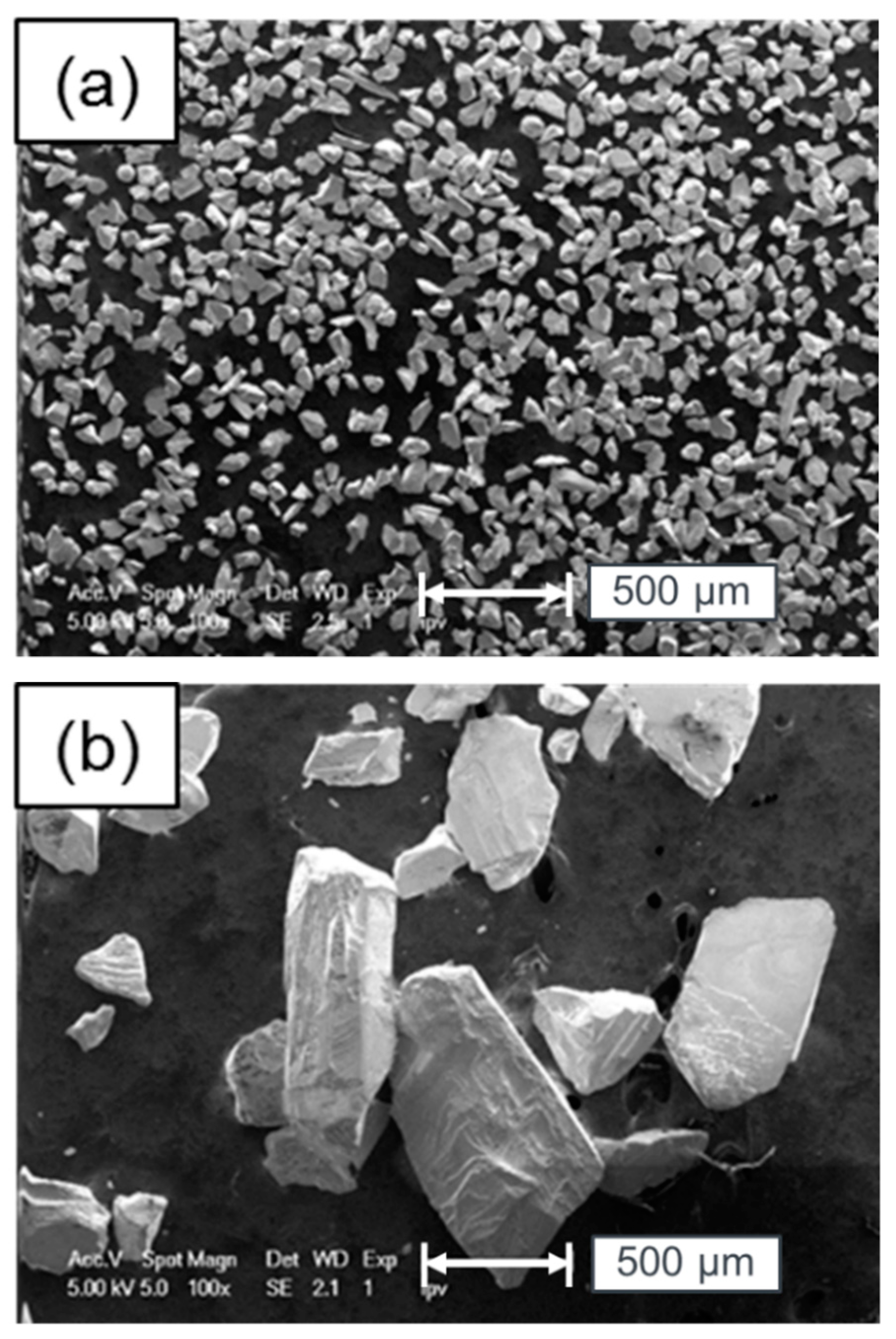
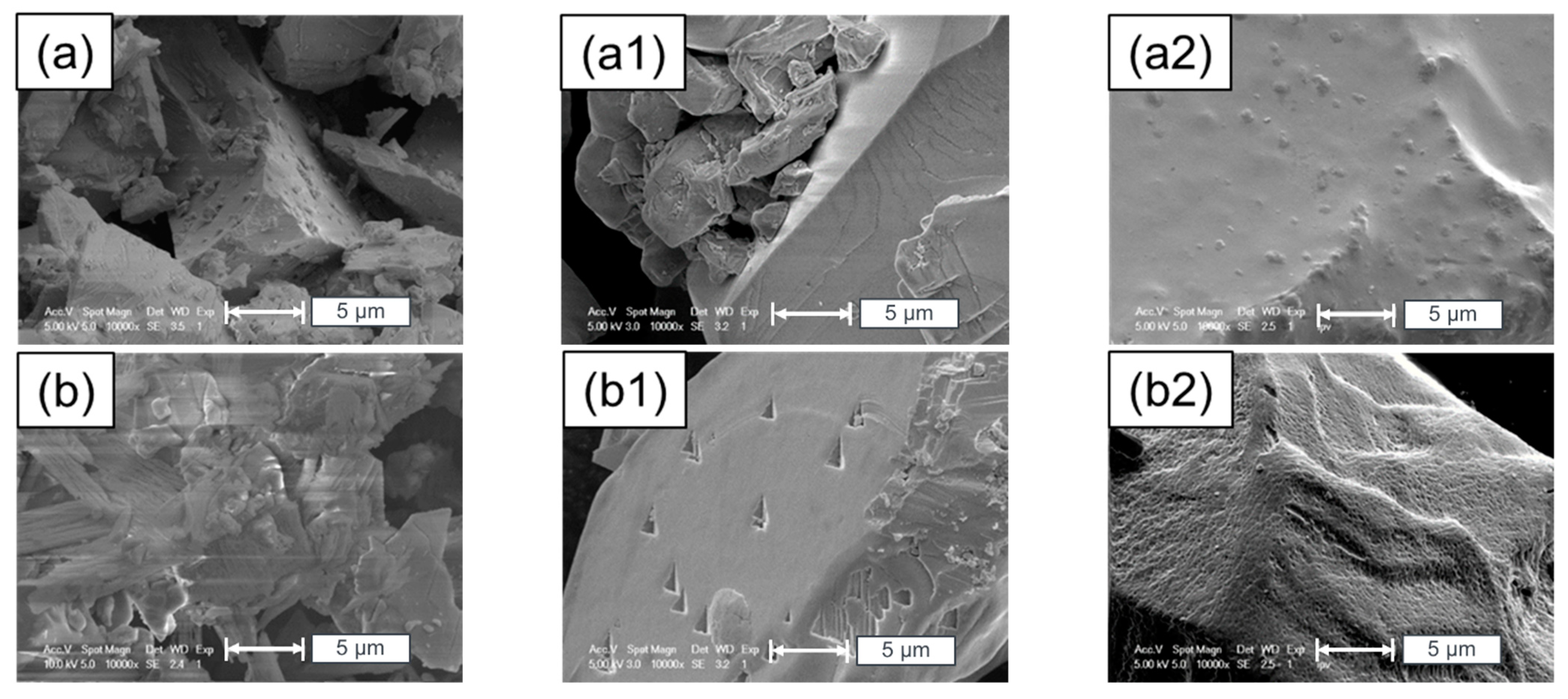
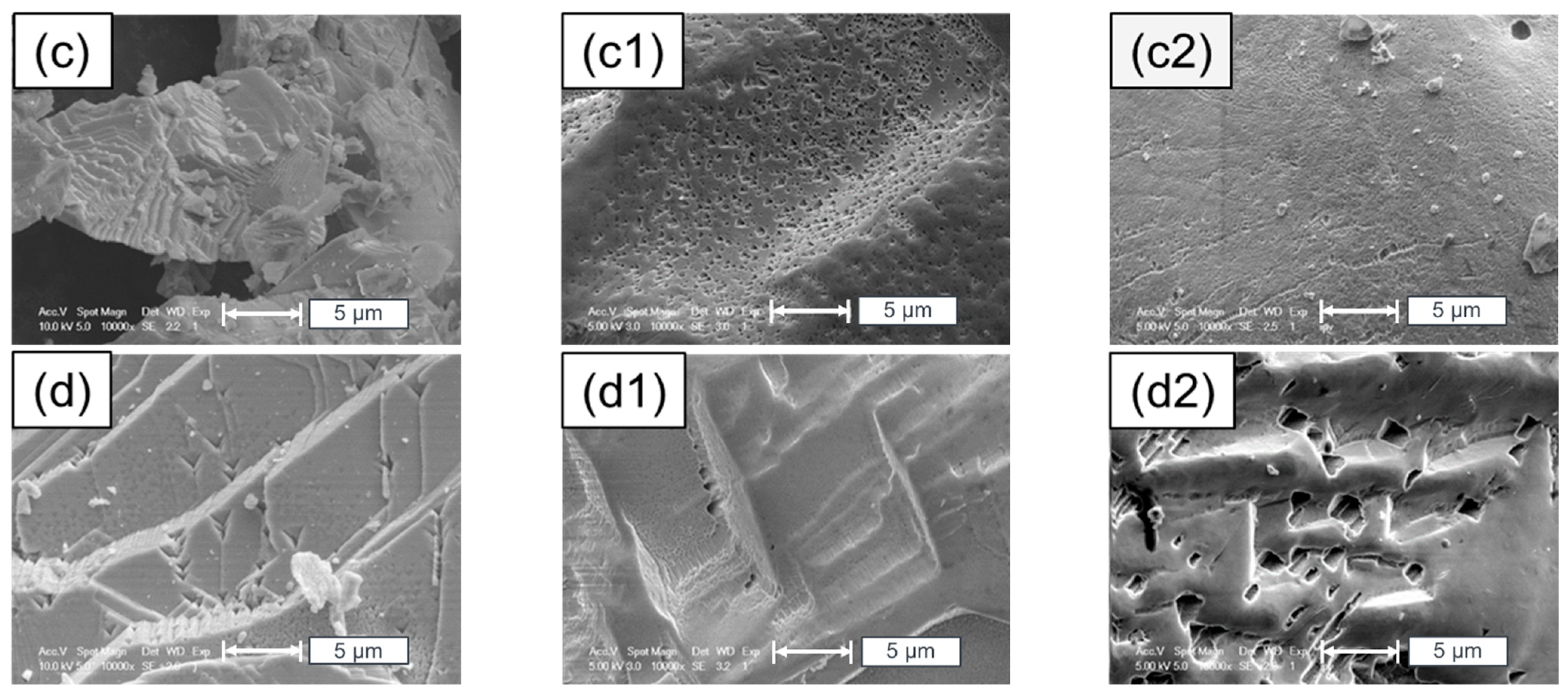
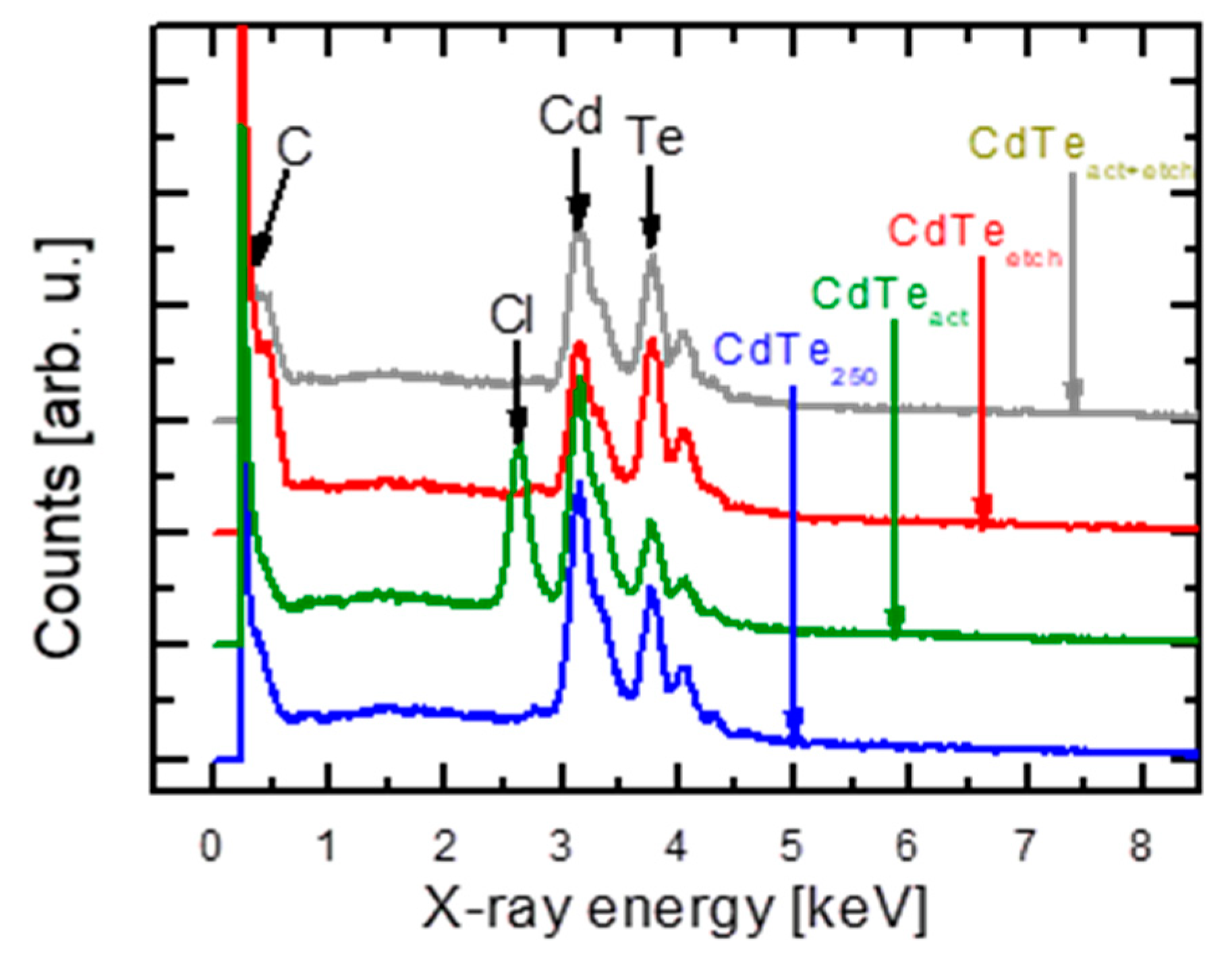
5. Surface Analysis of CdTe Powder
Surface Analysis of Leached and Processed CdTe Powder
6. Discussion
6.1. Thermodynamics and Surface Chemistry
6.2. Particle Size and Time-Dependent Elution
7. Conclusions
- The faster dissolution for the smaller particles is simply understood by their larger surface to volume ratio. The dissolution rate is proportional to the inverse diameter.
- The time-dependent aggregation of the dissolved species with t0.43 is understood on the basis of a model that, originally, stems from pharmacy. The dissolution is controlled by the diffusion of Cd and Te in and across the surface of the spherical particles.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patterson, M.; Turner, A.; Sadeghi, M.; Marshall, R. Health, safety and environmental aspects of the use of cadmium compounds in thin film PV modules. Sol. Energy Mater. Sol. Cells 1994, 35, 305–310. [Google Scholar] [CrossRef]
- Fthenakis, V.M. Life cycle impact analysis of cadmium in CdTe PV production. Renew. Sustain. Energy Rev. 2004, 8, 303–334. [Google Scholar] [CrossRef]
- Kato, K.; Hibino, T.; Komoto, K.; Ihara, S.; Yamamoto, S.; Fujihara, H. A life-cycle analysis on thin-film CdS/CdTe PV modules. Sol. Energy Mater. Sol. Cells 2001, 67, 279–287. [Google Scholar] [CrossRef]
- Bohland, V.J.; Anisimov, I.I.; Dapkus, T.J.; Sasala, R.A.; Smigielski, K.A.; Kamm, K.D. Reclaiming Metallic Material from an Article Comprising a Non-Metallic Friable Substrate. U.S. Patent No. US 6129779 A, 10 October 2000. [Google Scholar]
- Wang, W.; Fthenakis, V. Kinetics study on separation of cadmium from tellurium in acidic solution media using ion-exchange resins. J. Hazard. Mater. 2005, 125, 80–88. [Google Scholar] [CrossRef]
- Zapf-Gottwick, R.; Koch, M.; Fischer, K.; Schwerdt, F.; Hamann, L.; Kranert, M.; Metzger, J.; Werner, J. Leaching Hazardous Substances out of Photovoltaic Modules. Int. J. Adv. Appl. Phys. Res. 2015, 2, 7–14. [Google Scholar] [CrossRef]
- Nover, J.; Zapf-Gottwick, R.; Feifel, C.; Koch, M.; Metzger, J.W.; Werner, J.H. Long-term leaching of photovoltaic modules. Jpn. J. Appl. Phys. 2017, 56, 08MD02. [Google Scholar] [CrossRef]
- Mitchell, K.W.; Fahrenbruch, A.L.; Bube, R.H. Photovoltaic determination of optical-absorption coefficient in CdTe. J. Appl. Phys. 1977, 48, 829–830. [Google Scholar] [CrossRef]
- Birkmire, R.W.; Candless, B.E. CdTe thin film technology: Leading thin film PV into the future. Curr. Opin. Solid State Mater. Sci. 2010, 14, 139–142. [Google Scholar] [CrossRef]
- Bonnet, D. Manufacturing of CSS CdTe solar cells. Thin Solid Films 2000, 361, 547–552. [Google Scholar] [CrossRef]
- Institut für Arbeitsschutz der DGUV. GESTIS-Stoffdatenbank. Available online: https://gestis.dguv.de/data?name=109355 (accessed on 8 January 2021).
- Terheggen, M.; Heinrich, H.; Kostorz, G.; Baetzner, D.; Romeo, A.; Tiwari, A. Analysis of Bulk and Interface Phenomena in CdTe/CdS Thin-Film Solar Cells. Interface Sci. 2004, 12, 259–266. [Google Scholar] [CrossRef]
- Bosio, A.; Menossi, D.; Mazzamuto, S.; Romeo, N. Manufacturing of CdTe thin film photovoltaic modules. Thin Solid Film. 2011, 519, 7522–7525. [Google Scholar] [CrossRef]
- Emziane, M.; DuRose, K.; Romeo, N.; Bosio, A.; Halliday, D. Effect of CdCl2 activation on the impurity distribution in CdTe/CdS solar cell structures. Thin Solid Film. 2005, 480, 377–381. [Google Scholar] [CrossRef]
- Rosenberg, R.J.; Zilliacus, R.; Lakomaa, E.L.; Rautiainen, A.; Mäkelä, A. Study of CdTe/CdS-thin films by isotope dilution, neutron activation analysis, inductively coupled plasma mass spectroscopy and secondary ion mass spectroscopy. Fresenius J. Anal. Chem. 1996, 354, 6–10. [Google Scholar] [CrossRef]
- MilliporeSigma. Available online: www.sigma-aldrich.com (accessed on 18 December 2020).
- Kraft, D.; Thissen, A.; Broetz, J.; Flege, S.; Campo, M.; Klein, A.; Jaegermann, W. Characterisation of tellurium layers for back contact formation on close to technology treated CdTe surfaces. J. Appl. Phys. 2003, 94, 3589–3598. [Google Scholar] [CrossRef]
- Zorn, M. Solubility of cadmium telluride. Bachelor’s Thesis, Institute for Photovoltaics, University of Stuttgart, Stuttgart, Germany, 2015. Chapter 4. [Google Scholar]
- Hajimammadov, R.; Fathi, N.; Bayramov, A.; Khrypunov, G.; Klochko, N.; Li, T. Effect of “CdCl2Treatment” on Properties of CdTe-Based Solar Cells Prepared by Physical Vapor Deposition and Close-Spaced Sublimation Methods. Jpn. J. Appl. Phys. 2011, 50, 05FH01. [Google Scholar] [CrossRef]
- McCandless, B.E.; Dobson, K.D. Processing options for CdTe thin film solar cells. Sol. Energy 2004, 77, 839–856. [Google Scholar] [CrossRef]
- Mack, S. Herstellung und Charakterisierung von CSS-CdTe-Dünnschicht-Solarzellen. Diploma’s Thesis, Friedrich-Schiller-Universität Jena, Jena, Germany, 2006. [Google Scholar]
- De Mello, W.Z.; de Almeida, M.D. Rainwater chemistry at the summit and southern flank of the Itatiaia massif, Southeastern Brazil. Environ. Pollut. 2004, 129, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Hochschule für Angewandte Wissenschaften (HAW) Hamburg. Available online: http://www.haw-hamburg.de/fileadmin/user_upload/FakLS/08LABORE/Chemie/Regenwasseranalyse/Regenwasser_Analyse_WS_12_13_Els.pdf (accessed on 1 September 2017).
- Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen (lanuv). Fachbericht 24, Beschaffenheit von Deponiesickerwässer. 2012. Available online: http://www.lanuv.nrw.de, (accessed on 1 September 2017).
- Marion, G.M.; Millero, F.J.; Camões, M.F.; Spitzer, P.; Feistel, R.; Chen, C.T. pH of seawater. Mar. Chem. 2011, 126, 89–96. [Google Scholar] [CrossRef]
- Alcademics. Available online: http://www.alcademics.com/2013/04/measuring-ph-of-mineral-waters.html (accessed on 1 September 2017).
- Zeng, C.; Ramos-Ruiz, A.; Field, J.A.; Sierra-Alvarez, R. Cadmium telluride (CdTe) and cadmium sulfide (CdS) leaching behavior and surface chemistry in response to pH and O2. J. Environ. Manag. 2015, 154, 78–85. [Google Scholar] [CrossRef]
- Ramos-Ruiz, A.; Wilkening, J.V.; Field, J.A.; Sierra-Alvarez, R. Leaching of cadmium and tellurium from cadmium telluride (CdTe) thin-film solar panels under simulated landfill conditions. J. Hazard. Mater. 2017, 336, 57–64. [Google Scholar] [CrossRef]
- Hintz, R.J.; Johnson, K.C. The effect of particle size distribution on dissolution rate and oral absorption. Int. J. Pharm. 1989, 51, 9–17. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release. I. Fickian and non-Fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J. Control. Release 1987, 5, 23–26. [Google Scholar] [CrossRef]
- Crank, J. The Mathematics of Diffusion; Clarendon Press: Oxford, UK, 1875. [Google Scholar]
- Zanio, K. Chemical Diffusion in Cadmium Telluride J. Appl. Phys. 1970, 41, 1935–1940. [Google Scholar] [CrossRef]
- Kröger, F.A. The defect structure of CdTe. Rev. Phys. Appl. 1977, 12, 205–210. [Google Scholar] [CrossRef]
- Borsenberger, P.M.; Stevenson, D.A. Self-diffusion of Cd and Te in CdTe. J. Phys. Chem. Solids 1968, 29, 1277. [Google Scholar] [CrossRef]
- Shcherbak, L.; Kopach, O.; Fochuk, P.; Bolotnikov, A.E.; James, R.B. Empirical Correlations between the Arrhenius Parameters of Impurities’ Diffusion Coefficients in CdTe Crystals. J. Phase Equilibria Diffus. 2015, 36, 99–109. [Google Scholar] [CrossRef]
- Wilkin, R.T. Cadmium. In Monitored Natural Attenuation of Inorganic Contaminants in Ground Water; Ford, R.G., Wilkin, R.T., Puls, R.W., Eds.; U.S. Environmental Protection Agency: Ada, OK, USA, 2007; Volume 2, pp. 1–9. [Google Scholar]
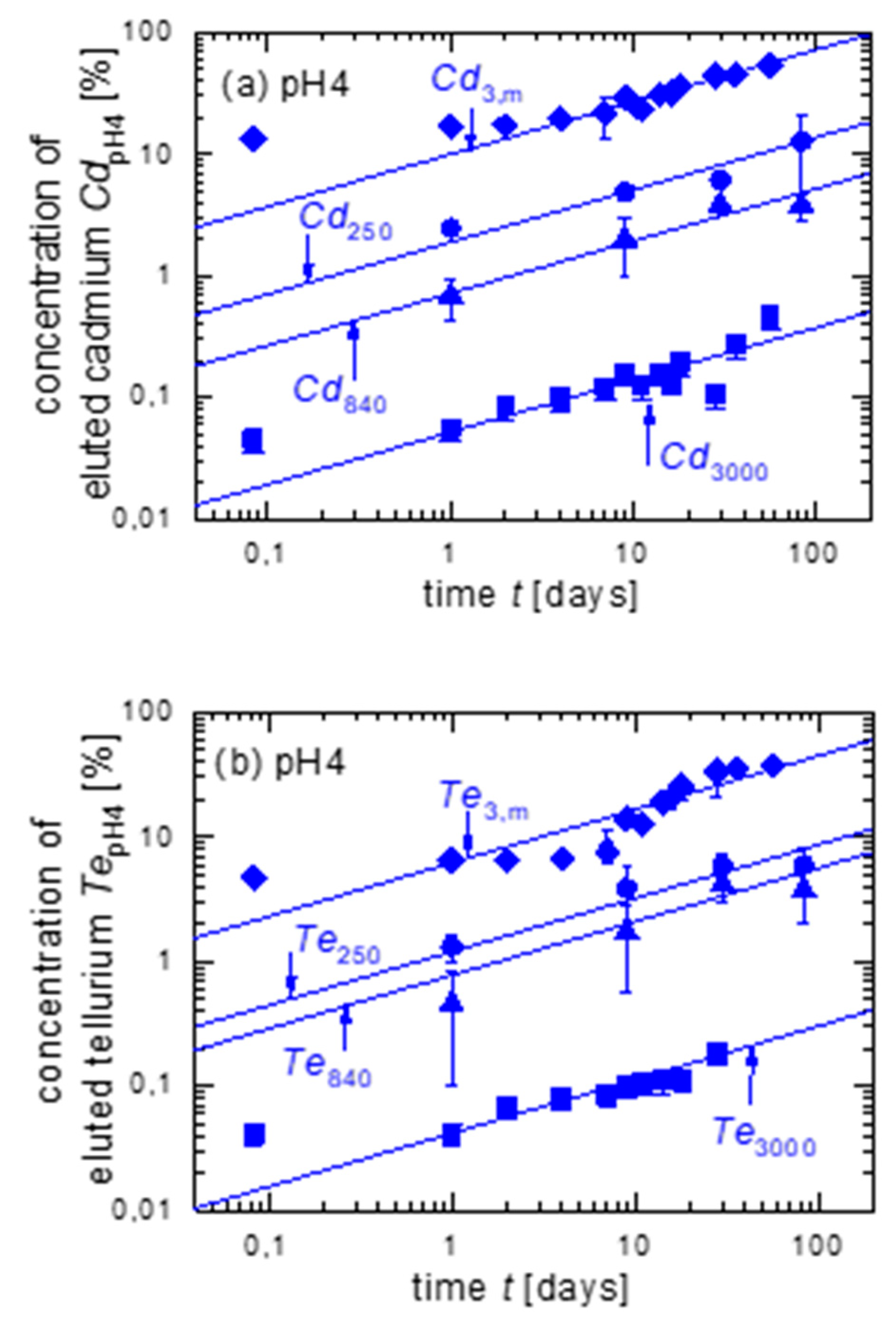
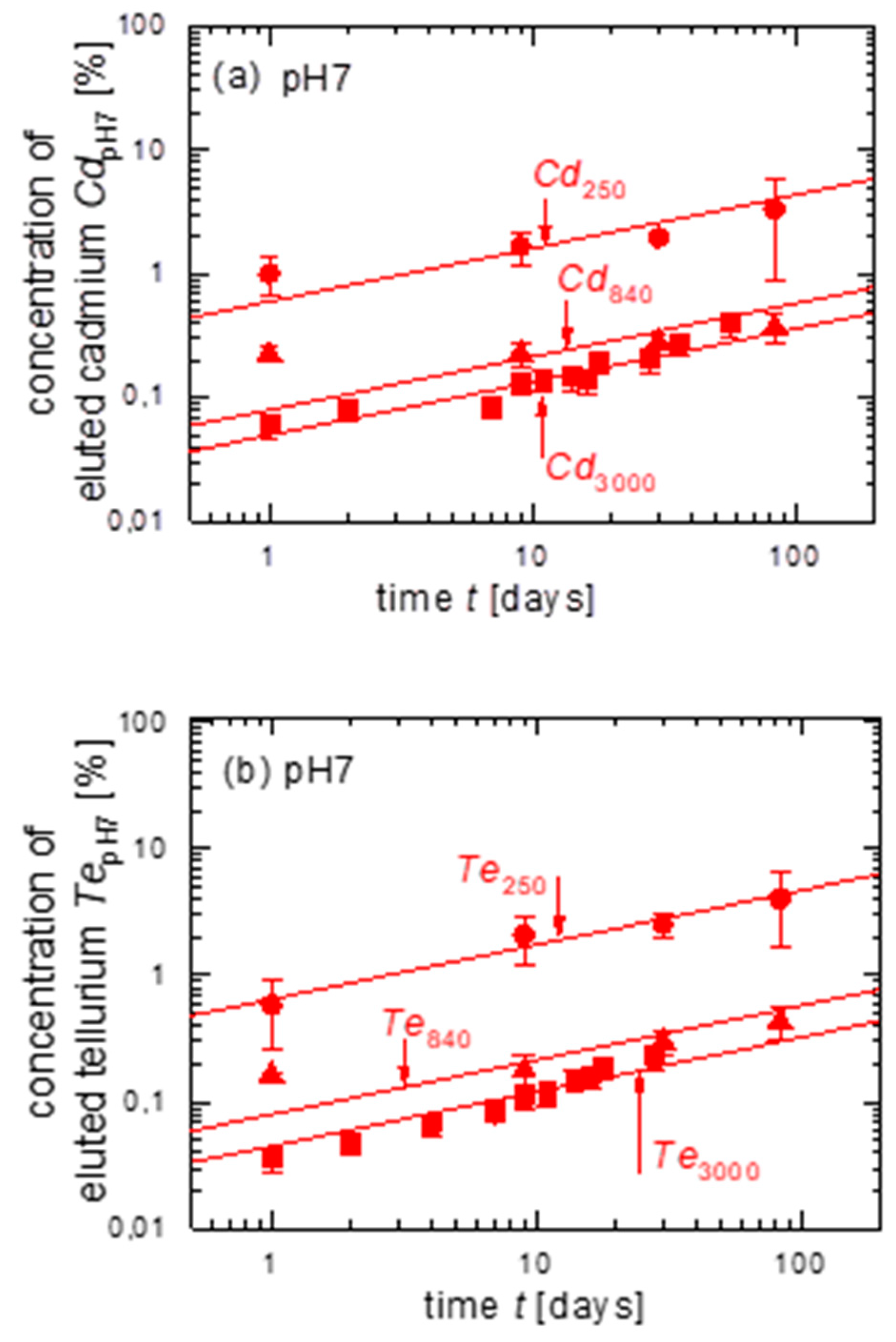
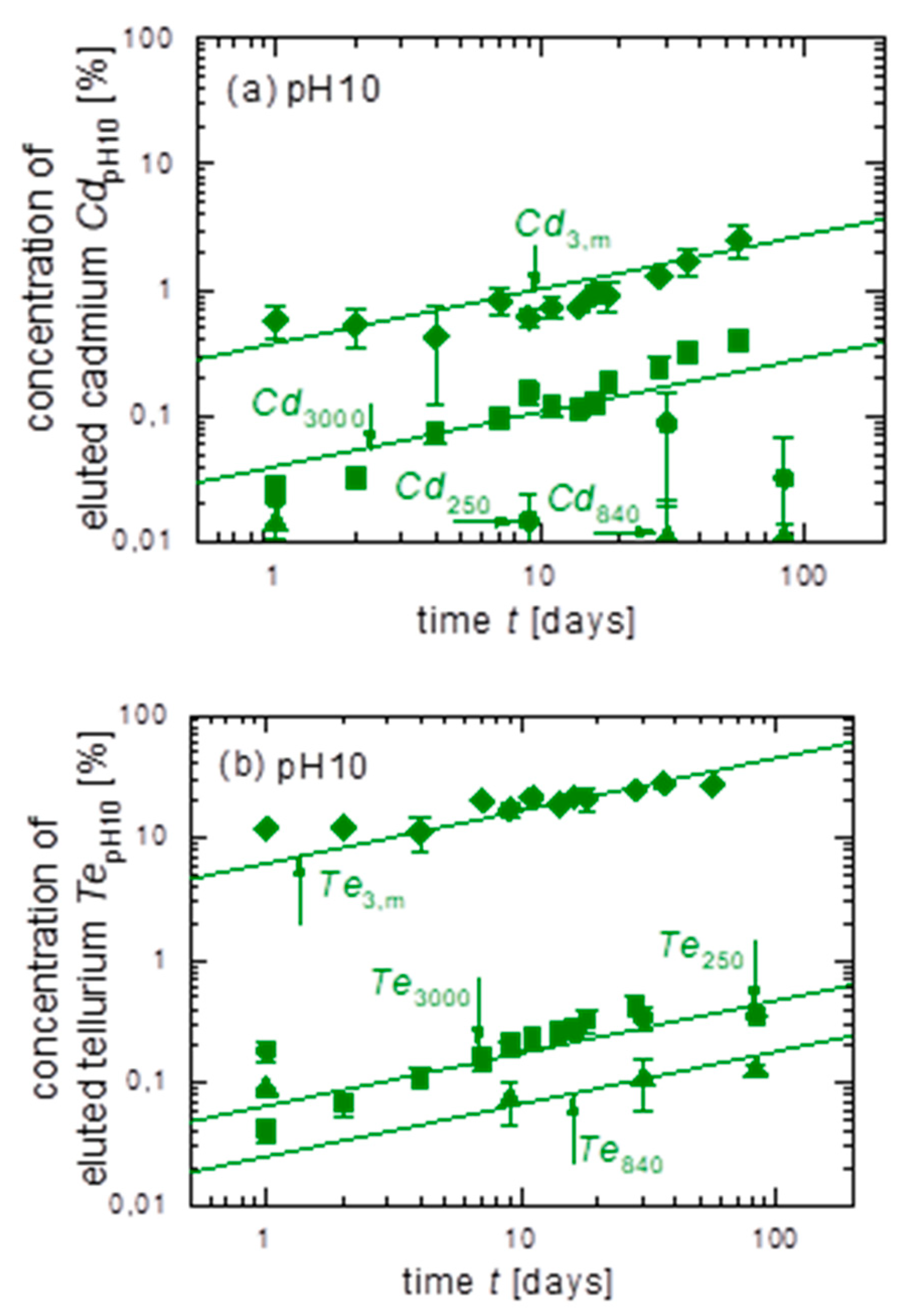
| Figure | Cd3,m/Cd250 | Cd250/Cd250 | Cd840/Cd250 | Cd3000/Cd250 | Te3,m/Te250 | Te250/Te250 | Te840/Te250 | Te3000/Te250 | |
| Equation (2) | - | 5 | 1 | 0.5 | <<0.5 | 5 | 1 | 0.5 | << 0.5 |
| pH4 | 3a | 5.4 | 1 | 0.4 | 0.03 | - | - | - | - |
| 3b | - | - | - | - | 5 | 1 | 0.5 | 0.03 | |
| pH7 | 4a | - | 1 | 0.15 | 0.1 | - | - | - | - |
| 4b | - | - | - | - | - | 1 | 0.15 | 0.1 | |
| pH10 | 5a | ≈ 500 | 1 | ≈ 1 | 4 | - | - | - | - |
| 5b | - | - | - | - | ≈ 100 | 1 | 0.4 | ≈ 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zapf-Gottwick, R.; Zorn, M.; Nover, J.; Koch, M.; Feifel, C.; Werner, J.H. Size- and Surface-Dependent Solubility of Cadmium Telluride in Aqueous Solutions. Energies 2021, 14, 398. https://doi.org/10.3390/en14020398
Zapf-Gottwick R, Zorn M, Nover J, Koch M, Feifel C, Werner JH. Size- and Surface-Dependent Solubility of Cadmium Telluride in Aqueous Solutions. Energies. 2021; 14(2):398. https://doi.org/10.3390/en14020398
Chicago/Turabian StyleZapf-Gottwick, Renate, Matthias Zorn, Jessica Nover, Michael Koch, Carolin Feifel, and Jürgen H. Werner. 2021. "Size- and Surface-Dependent Solubility of Cadmium Telluride in Aqueous Solutions" Energies 14, no. 2: 398. https://doi.org/10.3390/en14020398
APA StyleZapf-Gottwick, R., Zorn, M., Nover, J., Koch, M., Feifel, C., & Werner, J. H. (2021). Size- and Surface-Dependent Solubility of Cadmium Telluride in Aqueous Solutions. Energies, 14(2), 398. https://doi.org/10.3390/en14020398






