Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, J.; Li, J.; Li, X.; Xu, L.; Dong, Y.; Zeng, H. Quantum dot light-emitting diodes based on inorganic perovskite cesium lead halides (CsPbX3). Adv. Mater. 2015, 27, 7162–7167. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Y.; Zhang, S.; Cai, B.; Gu, Y.; Song, J.; Zeng, H. CsPbX3 quantum dots for lighting and displays: Room-temperature synthesis, photoluminescence superiorities, underlying origins and white light-emitting diodes. Adv. Funct. Mater. 2016, 26, 2435–2445. [Google Scholar] [CrossRef]
- Li, X.; Cao, F.; Yu, D.; Chen, J.; Sun, Z.; Shen, Y.; Zhu, Y.; Wang, L.; Wei, Y.; Wu, Y.; et al. All inorganic halide perovskites nanosystem: Synthesis, structural features, optical properties and optoelectronic applications. Small 2017, 13, 1603996. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.B.; Zaiats, G.; Wappes, I.; Kamat, P.V. CsPbBr3 solar cells: Controlled film growth through layer-by-layer quantum dot deposition. Chem. Mater. 2017, 29, 9767–9774. [Google Scholar] [CrossRef]
- Wang, Y.; Li, X.; Song, J.; Xiao, L.; Zeng, H.; Sun, H. All-inorganic colloidal perovskite quantum dots: A new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.-S.; Li, H.-Z.; Ge, J.; Li, H.-D.; Yin, Y.-C.; Wang, K.-H.; Chen, C.; Yao, J.-S.; Zhang, Q.; Yao, H.-B. Room temperature precipitated dual phase CsPbBr3–CsPb2Br5 nanocrystals for stable perovskite light emitting diodes. Nanoscale 2018, 10, 19262–19271. [Google Scholar] [CrossRef]
- Li, X.; Zhang, K.; Li, J.; Chen, J.; Wu, Y.; Liu, K.; Song, J.; Zeng, H. Heterogeneous nucleation toward polar-solvent-free, fast, and one-pot synthesis of highly uniform perovskite quantum dots for wider color gamut display. Adv. Mater. Interfaces 2018, 5, 1800010. [Google Scholar] [CrossRef]
- Huang, H.; Bodnarchuk, M.I.; Kershaw, S.V.; Kovalenko, M.V.; Rogach, A.L. Lead halide perovskite nanocrystals in the research spotlight: Stability and defect tolerance. ACS Energy Lett. 2017, 2, 2071–2083. [Google Scholar] [CrossRef]
- Kang, J.; Wang, L.-W. High defect tolerance in lead halide perovskite CsPbBr3. J. Phys. Chem. Lett. 2017, 8, 489–493. [Google Scholar] [CrossRef]
- Li, X.; Yu, D.; Cao, F.; Gu, Y.; Wei, Y.; Wu, Y.; Song, J.; Zeng, H. Healing all-inorganic perovskite films via recyclable dissolution–recyrstallization for compact and smooth carrier channels of optoelectronic devices with high stability. Adv. Funct. Mater. 2016, 26, 5903–5912. [Google Scholar] [CrossRef]
- Xia, H.; Wu, S.; Li, L.; Zhang, S. High binding ability ligand controlled formation of CsPbX3 (X = Cl/Br, Br, I) perovskite nanocrystals with high quantum yields and enhanced stability. RSC Adv. 2018, 8, 35973–35980. [Google Scholar] [CrossRef]
- Huang, H.; Susha, A.S.; Kershaw, S.V.; Hung, T.F.; Rogach, A.L. Control of emission color of high quantum yield CH3NH3PbBr3 perovskite quantum dots by precipitation temperature. Adv. Sci. 2015, 2, 1500194. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, B.; Zhang, J.; Gao, Y.; Zheng, Y.; Wang, K.; Sun, X.W. All-inorganic perovskite nanocrystals for high-efficiency light emitting diodes: Dual-phase CsPbBr3-CsPb2Br5 composites. Adv. Funct. Mater. 2016, 26, 4595–4600. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, L.; Shi, T.; Liao, G.; Tang, Z. Controllable synthesis of all inorganic lead halide Perovskite nanocrystals with various appearances in multiligand reaction system. Nanomaterials 2019, 9, 1751. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.; Kambhampati, P. Get the basics right: Jacobian conversion of wavelength and energy scales for quantitative analysis of emission spectra. J. Phys. Chem. Lett. 2013, 4, 3316–3318. [Google Scholar] [CrossRef]
- Lan, J.; Luo, L.; Wang, M.; Li, F.; Wu, X.; Wang, F. One pot gram-scale synthesis of CsPbBr3 nanocrystals and their application in green LED. J. Lumin. 2019, 210, 464–471. [Google Scholar] [CrossRef]
- Koolyk, M.; Amgar, D.; Aharon, S.; Etgar, L. Kinetics of cesium lead halide perovskite nanoparticle growth; focusing and de-focusing of size distribution. Nanoscale 2016, 8, 6403–6409. [Google Scholar] [CrossRef]
- Kim, S.H.; Man, M.T.; Lee, J.W.; Park, K.-D.; Lee, H.S. Influence of size and shape anisotropy on optical properties of CdSe quantum dots. Nanomaterials 2020, 10, 1589. [Google Scholar] [CrossRef]
- Kim, S.H.; Man, M.T.; Lee, H.S. Size and shell effects on CdSe quantum dots in binary ligand system. Appl. Sci. Converg. Technol. 2020, 29, 87–90. [Google Scholar] [CrossRef]
- Brennan, M.C.; Herr, J.E.; Nguyen-Beck, T.S.; Zinna, J.; Draguta, S.; Rouvimov, S.; Parkhill, J.; Kuno, M. Origin of the size-dependent stokes shift in CsPbBr3 perovskite nanocrystals. J. Am. Chem. Soc. 2017, 139, 12201–12208. [Google Scholar] [CrossRef] [PubMed]
- Campos-Gonzalez, E.; Rodriguez-Fragozo, P.; de la Cruz, G.G.; Santoyo-Salazar, J.; Zelaya-Angel, O. Synthesis of CdSe nanoparticles immersed in an organic matrix of amylopectin by means of rf sputtering. J. Cryst. Growth 2012, 338, 251–255. [Google Scholar] [CrossRef]
- Dutta, J.; Ajith, M.C.; Dutta, S.; Kadhane, U.R.; Kochupurackal, J.B.; Rai, B. An inherent instability study using ab initio computational methods and experimental validation of Pb(SCN)2 based perovskites for solar cell applications. Sci. Rep. 2020, 10, 15241. [Google Scholar] [CrossRef]
- Pan, J.; Quan, L.N.; Zhao, Y.; Peng, W.; Murali, B.; Sarmah, S.P.; Yuan, M.; Sinatra, L.; Alyami, N.M.; Liu, J.; et al. Highly efficient perovskite-quantum-dot light-emitting diodes by surface engineering. Adv. Mater. 2016, 28, 8718–8725. [Google Scholar] [CrossRef] [PubMed]
- Chukwuocha, E.O.; Onyeaju, M.C.; Harry, T.S.T. Theoretical studies on the effect of confinement on quantum dots using the Brus Equation. World J. Condens. Matter Phys. 2012, 2, 96–100. [Google Scholar] [CrossRef]
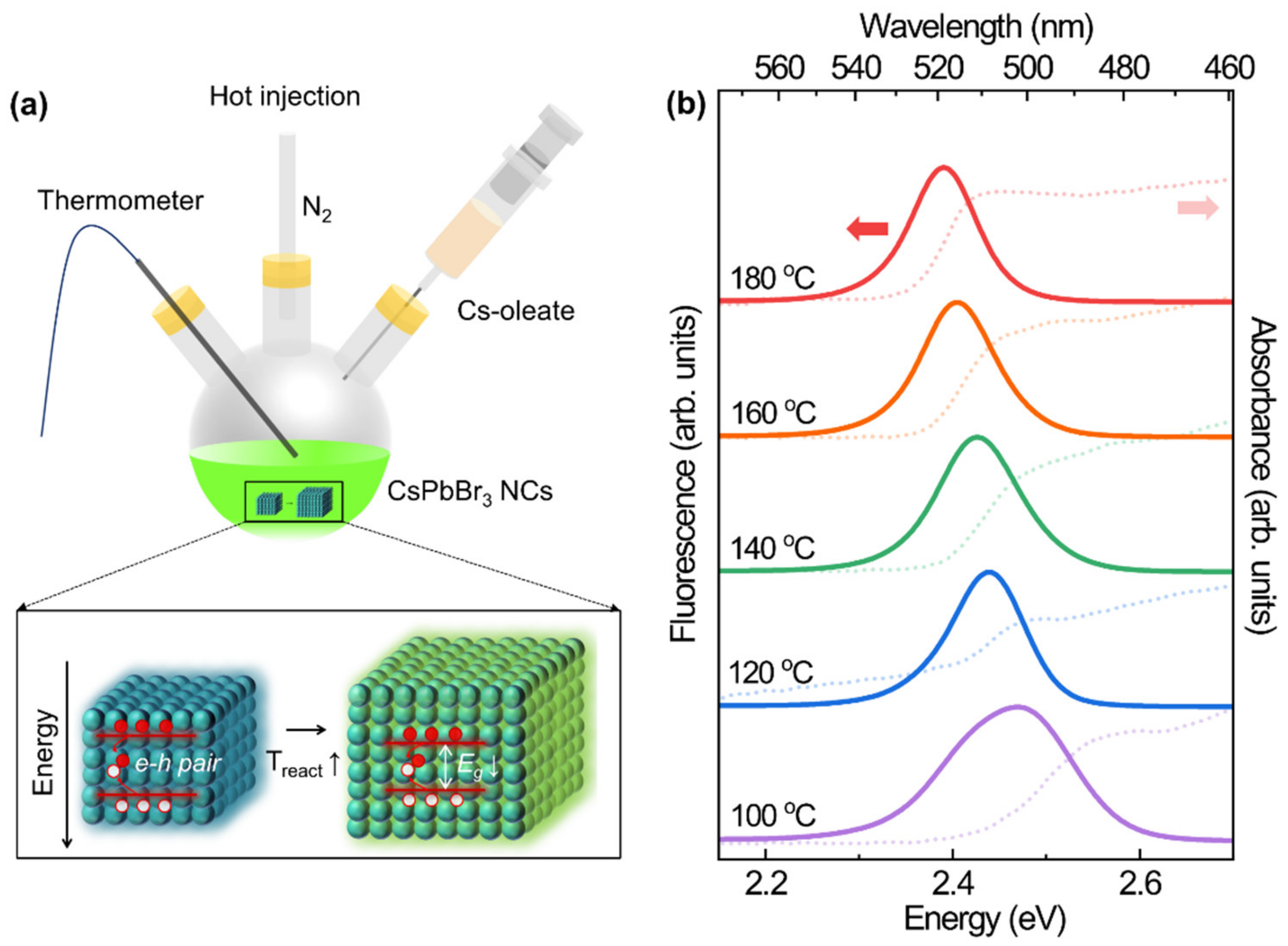
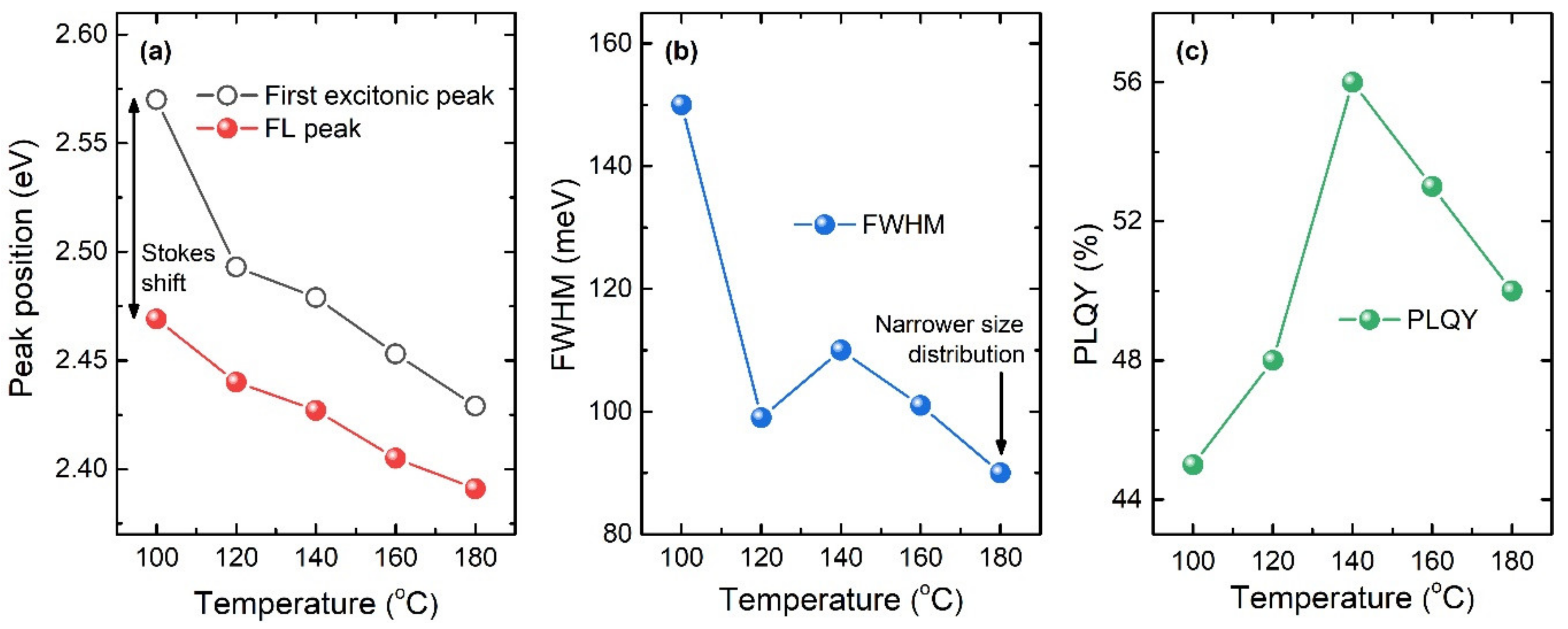
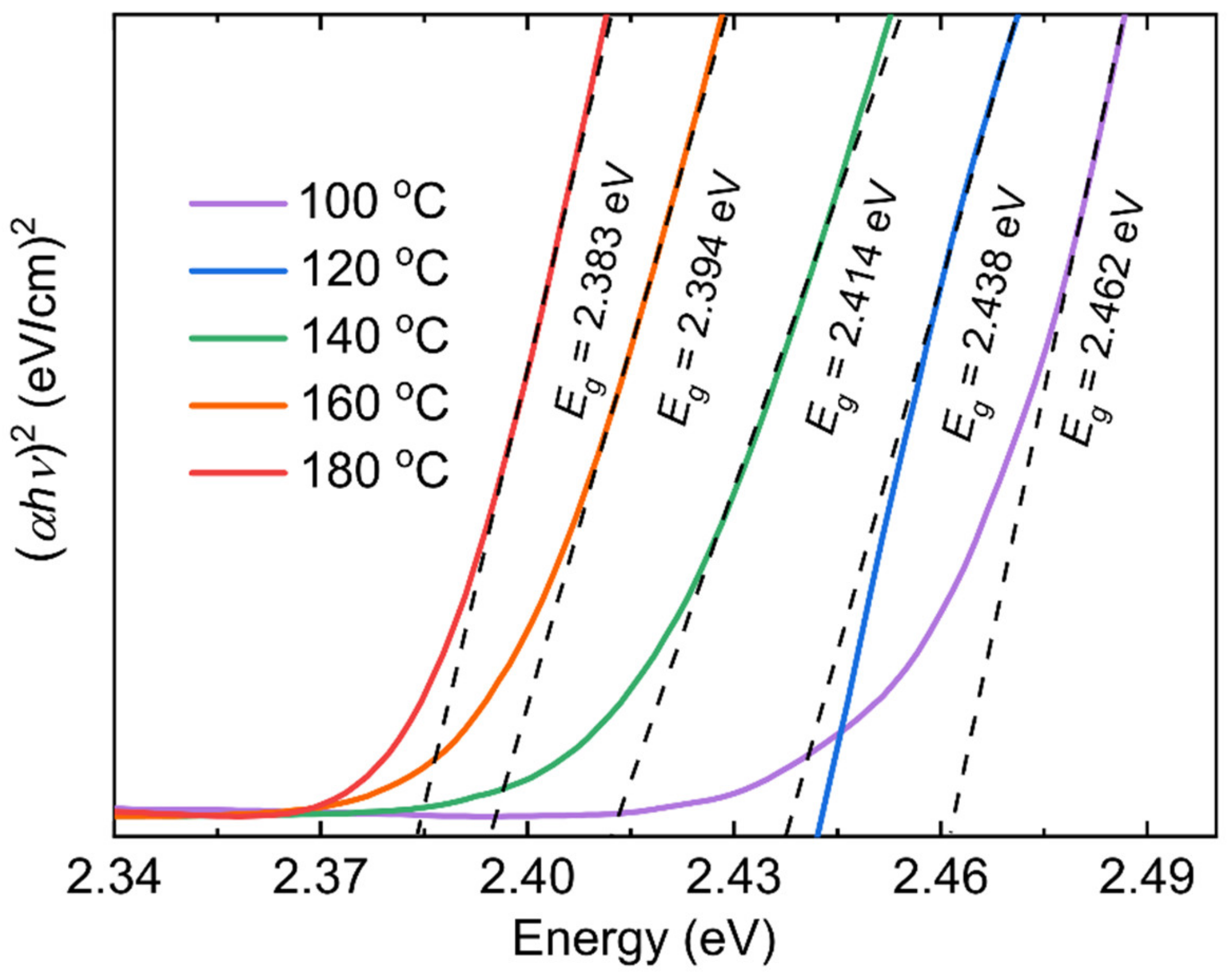
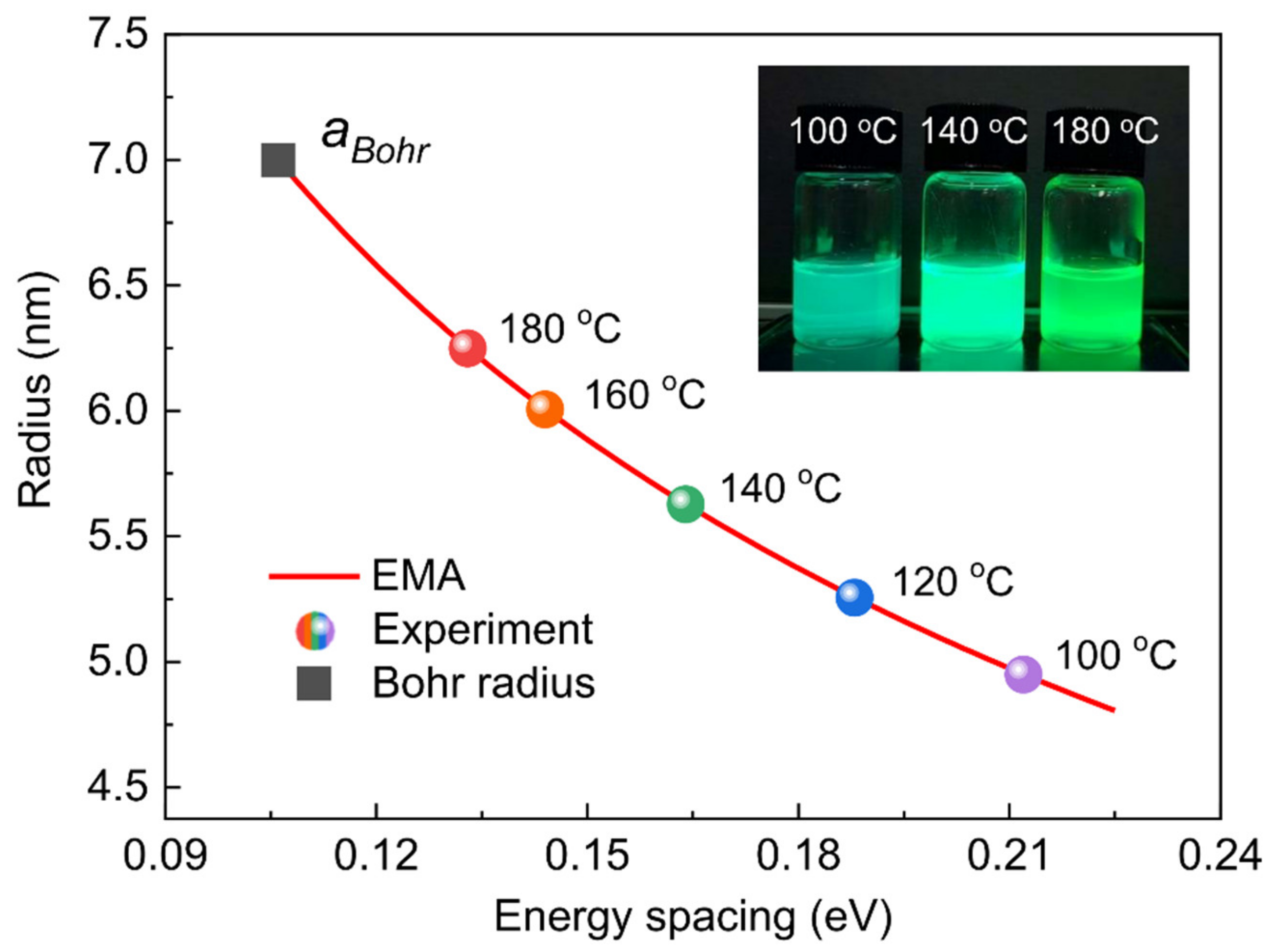
| Reaction Temperature (°C) | 100 | 120 | 140 | 160 | 180 |
|---|---|---|---|---|---|
| Energy Spacing (eV) | 0.21 | 0.19 | 0.16 | 0.14 | 0.13 |
| Diameter (nm) | 9.9 | 10.5 | 11.3 | 12.0 | 12.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.H.; Park, K.-D.; Lee, H.S. Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals. Energies 2021, 14, 275. https://doi.org/10.3390/en14020275
Kim SH, Park K-D, Lee HS. Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals. Energies. 2021; 14(2):275. https://doi.org/10.3390/en14020275
Chicago/Turabian StyleKim, Sung Hun, Kyoung-Duck Park, and Hong Seok Lee. 2021. "Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals" Energies 14, no. 2: 275. https://doi.org/10.3390/en14020275
APA StyleKim, S. H., Park, K.-D., & Lee, H. S. (2021). Growth Kinetics and Optical Properties of CsPbBr3 Perovskite Nanocrystals. Energies, 14(2), 275. https://doi.org/10.3390/en14020275






